Abstract
Various published data show that in patients with metastatic melanoma, high-dose interleukin-2 (IL2) is associated with 5-year survival rates of 15% from treatment initiation. We previously reported a median survival of 15.6 months, and a 20% 5-year survival rate for 150 patients who were treated with inpatient IL2 (Cancer Biother Radiopharm 2012;27:337). In the current study, we sought to determine whether treatment with active specific immunotherapy (ASI) with patient-specific tumor stem cell vaccines derived from autologous tumor cell (TC) lines contributed to the survival result. Existing databases revealed that 32/149 IL2-treated patients also received ASI, while 117 did not. ASI was given within 12 months of IL2 therapy in 19/32 patients. Patients who received IL2 plus ASI had better overall survival (p<0.001) with longer median survival (39.5 vs. 12.0 months) and a higher 5-year survival rate (39% vs. 13%). Survival was better even after exclusion of 55 IL2-alone patients who died before 12 months of follow-up (p=0.12). In subset analyses, survival was longer for 25 patients who received ASI after IL2 than for 7 who received ASI before IL2 (5-year survival 46% vs. 14%, p<0.001) and for 16 patients who received a dendritic cell/TC-based ASI compared with 16 injected with irradiated TC (p=0.17). This retrospective study suggests that receipt of IL2 followed by a patient-specific melanoma stem cell vaccine is associated with better survival than IL2 alone.
Key words: : active specific immunotherapy, cancer stem cells, dendritic cells, IL2, melanoma, vaccines
Introduction
Metastatic melanoma is still a therapeutic challenge despite recent regulatory approval of oral enzyme inhibitors that target V600 BRAF mutations, such as vemurafinib,1–3 dabrafenib,4,5 trametinib that targets MEK,5,6 and the monoclonal antibody ipilimumab that blocks the CTLA4 checkpoint molecule on T lymphocytes.7,8 It is widely anticipated that one or more monoclonal antibodies directed against the programmed death 1 (PD-1) checkpoint molecule9,10 or its ligand (PDL-1),11 also will obtain approval. Before availability of these agents, high-dose inpatient interleukin-2 (IL2) regimens were considered the treatment of choice in those patients who were physically fit enough for such therapy.12,13 We documented a dramatic decline in the use of IL2 in the treatment of metastatic melanoma in recent years,14 but also suggested that IL2 should still be the treatment of choice in patients who are medically fit enough for such treatment. This suggestion was based on the documentation of a surprisingly high 20% 5-year survival rate for 150 patients treated with inpatient IL2 regimens during 1987–2010, and the lack of long-term follow-up data for the newer therapies.
In a 185-patient randomized trial, administering the HLA-A2–restricted gp100 peptide with high-dose IL2 was associated with a higher response rate, better progression-free survival, and better overall survival compared with high-dose IL2 alone.15 For more than 20 years, we have been testing cancer vaccines using short-term autologous term cell lines as the antigen source.16 Knowing that many of our IL2 patients had also received treatment with these vaccines as active specific immunotherapy (ASI), made us wonder if vaccine treatment had contributed to the 20% 5-year survival rate observed in patients who had received IL2.
Patients and Methods
As previously described, the subject population consisted of metastatic melanoma patients who received high-dose, inpatient IL2 during 1987–2010.14 IL2-treated patients were identified from clinical trial accrual lists of the Cancer Biotherapy Research Group (CBRG) and the Hoag Cancer Center for protocols that had been approved by institutional review boards for the protection of human subjects, and/or identified retrospectively from pharmacy logs and financial billing records with a waiver of consent under the Common Rule for the protection of human subjects and a waiver of authorization under the Health Insurance Portability and Accountability Act (HIPAA). The inpatient intravenous IL2 regimens included high-dose bolus IL2,12 continuous infusion IL2,17 and a hybrid schedule of bolus and continuous infusion IL2.18 Patients who had been treated with ASI were identified from the Hoag Cancer Center and CBRG data sets for clinical trials that have been previously reported.19–21
ASI products utilized autologous tumor cell (TC) antigens derived from short-term cell cultures.16,22 The first ASI product consisted of irradiated autologous TC administered with various adjuvants, especially interferon-γ and granulocyte macrophage colony-stimulating factor (GM-CSF) (BB-IND 9212).19 The second ASI product consisted of autologous dendritic cells (DC) pulsed with irradiated autologous TC suspended in GM-CSF (DC/TC) (BB-IND 8554).20 TC and DC/TC subsequently were compared in a randomized trial in which both products were suspended in GM-CSF.21 In all three trials, the schedule of subcutaneous vaccine injections was weekly for 3 weeks and then monthly for 5 months. TC patients received a median of 10 million cells (range 2–24 million) per injection; whereas DC/TC patients received a median of 15 million cells (range 4–35 million) per injection.
Survival was calculated from the date on which IL2 therapy was initiated to the date of death or date of last follow-up up to 5 years. Actuarial survival curves were generated to compare the subsets of patients treated with IL2 alone to those treated with IL2+ASI. Demographic features were compared by the Student's T test for averages and by the chi square test for frequencies. Additional subset comparisons within the vaccine cohort were based on whether vaccine was given before or after IL2, and whether patients had received TC or DC/TC vaccine. The Mantel-Haenszel log rank test was used to compare differences between survival curves.
Results
Table 1 shows demographic and prognostic features for these patients by treatment cohort. As part of this analysis, an effort was made to determine the clinical stage of patients at the time they received IL2. One patient was found to have been treated for stage 2 disease rather than recurrent stage 3 or stage 4, and therefore was excluded from this analysis. Of the 149 IL2-treated metastatic melanoma patients, 32 also received ASI during the course of their disease. Because of limitations of registry data, stage could not be assigned for 20 patients, and serum levels of lactic dehydrogenase were rarely known; hence, they were not used to modify the stage of patients with clinical M1A or M1B disease. A higher proportion of IL2+ASI patients had more favorable stages of disease (recurrent stage III, M1A, or M1B) compared with the IL2 cohort (p=0.057). Performance status was ECOG 0 or 1 in most patients at the time they received IL2. The proportions of females were similar (p=0.30). The patients who received ASI were younger at the time of IL2 treatment. Median and 5-year survival rates were 15.5 months and 26% for 73 patients aged <52 years at the time of IL2 compared with 14.0 months and 13% for 76 patients aged ≥52 years at the time of IL2 (p=0.12).
Table 1.
Characteristics of Patients Who Received Interleukin-2 with or Without Active Specific Immunotherapy with Antigens from Autologous Proliferating Tumor Cells
| IL2 | IL2+ASI | |
|---|---|---|
| Total number of patients | 117 | 32 |
| Median age when received IL2 | 53 years | 46 years |
| Female | 56 (48%) | 12 (38%) |
| Regionally recurrent stage III | 11 (11%) | 8 (25%) |
| M1A | 12 (12%) | 8 (25%) |
| M1B | 13 (13%) | 2 (6%) |
| M1C | 61 63%) | 15 (44%) |
| Unknown stage | 20 (17%) | 0 |
Lactic dehydrogenase levels were not used to modify stage.
IL2, interleukin-2; ASI, active specific immunotherapy; M1A, distant lymph node and/or soft tissue metastases; M1B, lung metastases with or without soft tissue metastases; M1C, visceral metastases.
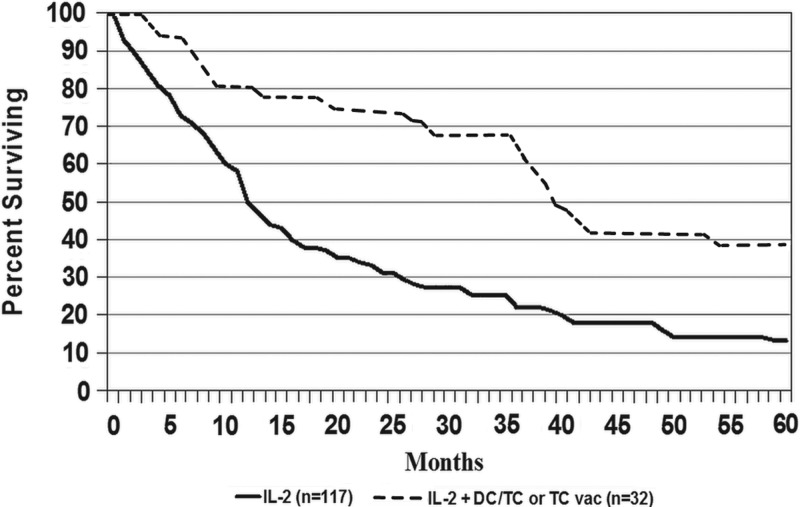
Figure 1 shows that survival was better for patients who received IL2+ASI compared with those who received IL2 alone. Median follow-up was more than 5 years for surviving patients; among survivors, 12/13 IL2+ASI and 11/20 IL2 patients had been followed at least 5 years. Median survivals were 39.5 and 12.0 months, and 5-year survival rates were 39% and 13%, respectively (p<0.001).
FIG. 1.
Survival curves for patients with metastatic melanoma who were treated with inpatient interleukin-2 (IL2)±active specific immunotherapy with autologous melanoma stem cell antigens. ASI, active specific immunotherapy.
| 0 | 1 year | 2 years | 3 years | 4 years | 5 years | ||
|---|---|---|---|---|---|---|---|
| IL2+ASI | Surviving | 32 | 27 | 24 | 21 | 14 | 13 |
| Censored | 0 | 0 | 1 | 0 | 0 | 0 | |
| Dead | 0 | 6 | 1 | 0 | 7 | 1 | |
| IL2 | Surviving | 117 | 57 | 34 | 24 | 19 | 11 |
| Censored | 0 | 5 | 0 | 0 | 1 | 3 | |
| Dead | 0 | 55 | 24 | 12 | 5 | 5 |
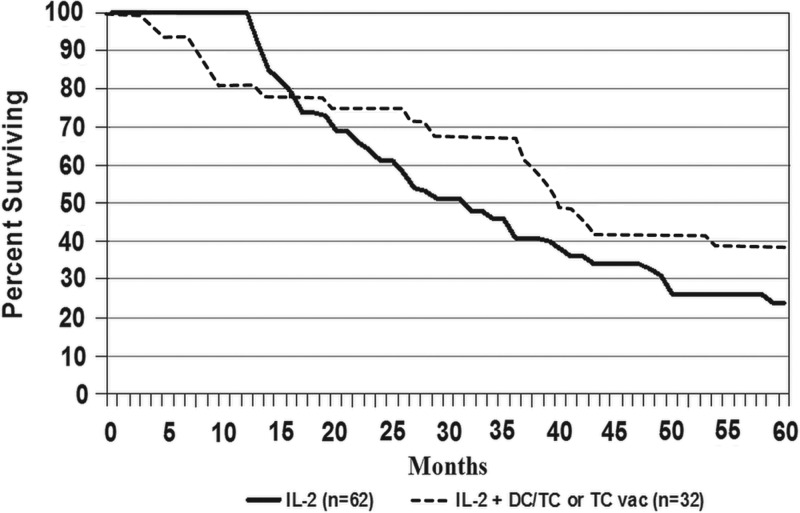
Because of the potential for bias, because of deaths of IL2 patients before an opportunity to receive vaccine, an analysis was performed that excluded all 55 patients in the IL2-alone arm who died before 12 months of follow-up, leaving 62 patients to compare with the 32 who received IL2+ASI. As shown in Figure 2, despite the advantage that survival for IL2 alone was now 100% at 12 months, the curves were already crossing at 16 months and survival was still better in the IL2+ASI cohort, with median survivals of 39.5 versus 29.8 months and 5-year survival rates of 39% versus 24% (p=0.12).
FIG. 2.
Survival curves for patients with metastatic melanoma who were treated with inpatient IL2±ASI with autologous melanoma stem cell antigens, but excluding all 55 L2-alone patients who died in less than 12 months.
Of the 32 patients treated with IL2+ASI, 16 received injections of TC and 16 received injections of DC/TC. Survival was longer for patients who received DC/TC (median 5 years vs. 3 years, 5-year survival, 50% vs. 28% (p=0.17), which is consistent with other comparisons of these two approaches.20,21 Of the 32 patients who received IL2+ASI, 7 received ASI before IL2 and 25 received ASI after receiving IL2. Of the seven who received ASI before IL2, five received ASI within the year before IL2, and one of each were treated 2.5 and 3.3 years earlier. Of the 25 patients who received ASI after IL2, 14 received ASI within a year of IL2, 7 between 1 and 2 years after IL2, 2 received ASI between 2 and 3 years after IL2, and 2 between 3.0 and 3.5 years later. Survival was longer for patients who received IL2 before receiving ASI (median 43 vs. 8 months, 5-year survival 48% vs. 14%, p<0.001).
Discussion
This retrospective analysis suggests that patients with recurrent regional melanoma or distant metastatic melanoma, treated with inpatient, high-dose IL2 regimens, also being treated with ASI during the course of disease, are associated with better survival. The results are consistent with those from the randomized trial of IL2 with or without gp100 peptide vaccine in which addition of vaccine was associated with better survival.15 Such vaccines may be providing a direct therapeutic benefit, or be acting synergistically with immune cells that have been affected by IL2. This may explain the higher than expected 19% 5-year survival rate that was observed in our series for 149 IL2-treated patients who had regional recurrent metastases or distant metastases.14 With regard to the therapeutic benefit of the ASI in successive trials, we observed a 29% 5-year survival rate for 74 melanoma patients treated with s.c. injections of TC,19 and a 54% 5-year survival rate for 54 patients treated with DC/TC.20 In a randomized trial, DC/TC plus GM-CSF was superior to TC plus GM-CSF, and survival in each treatment arm was similar to the results seen in the earlier single-arm trials.21 The clinical course of 2 of the 32 patients who received both ASI and IL2 supports the supposition that the apparent benefit of ASI was not just because of the selection bias for patients who were doing well after receiving IL2; both patients experienced disease progression during the evaluation period after IL2. As previously reported in detail,21 one patient who experienced disease progression immediately after completion of IL2, subsequently responded to DC/TC.23 She had presented with metastases to the spine, lung, and rectum, progressed in several sites, including the brain, despite surgery, radiation, and chemotherapy, and then developed additional sites of metastases after 2 months of IL2. She underwent resection of gallbladder and abdominal wall metastases after IL2, and already had new soft tissue sites of disease at the time she started DC/TC 5 months after completing IL2. She achieved a complete remission,23 and was still alive and disease-free 5 years after starting the vaccine. Another patient who was previously reported,20 had presented with lung and brain metastases, had progressed following IL2, and was started on ASI following partial resection of an adrenal metastasis. ASI was interrupted for irradiation of the adrenal mass, and then the schedule of vaccines was completed. She was disease free for more than 6 years and is known to have survived more than 10 years after receipt of IL2 and vaccine.
It has been apparent that even in patients healthy enough to receive IL2, the long-term survival benefits of IL2 are limited, and in the absence of randomized trial data, the long-term survival benefit associated with higher doses of IL2 has been questioned. In a retrospective comparison of contemporary metastatic melanoma patients matched for several characteristics, we found no difference in survival for those who received IL2 compared with those who did not.24 However, this could have been because of more favorable prognostic features in the control group, such as number and sites of metastases, variables that were not always available for matching, using cancer registry data for comparison with patients who received IL2 in clinical trials. Nevertheless, the 5-year survival rates of 26% to 31% from the date of diagnosis of metastatic disease were higher than expected in view of published 5-year survival rates for patients with metastatic melanoma that consistently were reportedly less than 10%.25 We recently determined that 11/60 of the IL2-treated patients in that analysis also received ASI compared with 7/60 of the matched controls (no IL2). The 20% 5-year survival rates from date of IL2 therapy in these studies suggested a benefit for a number of patients who had not obtained an objective response.14,24 What is provocative about the current retrospective review is that patients, who received IL2+ASI in the form of a TC or DC/TC vaccine, had a 5-year survival rate that was three times higher than patients who received only IL2, but the latter's 5-year survival rate of 13% is similar to the survival rate reported by various investigators for high-dose IL2 therapy.15,24–26
IL2 has been used as a standard therapy to treat melanoma ever since its regulatory approval for the treatment of metastatic renal cell cancer in 1992. Approval for marketing of IL2 in melanoma was granted in 1998 based on the data pooled from 270 patients accrued between 1985 and 1993 on eight different clinical trials.12 The objective response rate was 16% with 6% complete responses, but those were quite durable. However, at a median follow-up over 62 months, median survival for the entire population was only 11.4 months, and overall 5-year survival for all patients appeared to be about 15%.12 With longer follow-up, median survival increased to 12 months, but 5-year survival was unchanged.26 Recently, investigators from Boston and Houston pooled their experience with high-dose bolus IL2 therapy in 208 patients, with stage IV or unresectable stage III disease, who were treated between 2003 and 2009.27 In this group, the response rate was 19% with 6% CR and 5-year survival was projected to be 39%, although median follow-up for survivors was only 2.5 years; thus, the actual 5-year survival rate may have been considerably less.
A 42% objective response rate was reported among 31 patients treated with a combination of gp100 peptide vaccine and high-dose IL2.28 Subsequently, the Cytokine Working Group treated 131 patients during three separate trials with this combination, but recorded a response rate of only 16.5% among 121 evaluable patients.29 Median survival was about 15 months and 15% of patients remained alive 30 to 70 months after starting treatment. A 21-center randomized trial involving 185 patients with stage IV or locally advanced stage III cutaneous melanoma was conducted during 2000 to 2007 to determine whether gp100 was adding benefit to IL2.15 In this trial, the response rate as determined by central review was only 6% for IL2 alone compared with 16% for IL2 plus gp100 vaccine, and progression-free survival was longer with addition of gp100 (median 3.9 vs. 1.7 months, p=0.008). Median survival was also longer (26 months vs. 11 months), but 5-year survival was projected to be only 15% in both arms.
Results of randomized trials of antimelanoma vaccines have failed to provide justification for regulatory approval, but interest in such approaches persists because of their limited toxicities and potential to induce an enduring tumor-specific immune response.30,31 It appears unlikely that vaccine products will replace any of the effective systemic antimelanoma products as a monotherapy for the treatment of measurable melanoma, but they may add to the long-term survival of patients treated with other agents, including IL2, antibodies targeting T-lymphocyte checkpoint pathways, and enzyme inhibitors that target intracellular signal transduction pathways.30,31 The results from our retrospective analysis and the prospective results of the IL2 plus gp100 randomized trial15 are consistent with this concept. However, in another large randomized trial, gp100 added no benefit to ipilimumab in patients with metastatic melanoma,7 but an earlier report described a surprisingly high 44% response rate to interferon α after previous treatment with an allogeneic vaccine product in 18 patients with metastatic melanoma, and a median survival of more than 32 months in the responders.32 The magnitude of benefit appeared much greater in our study than for gp100 plus IL2.15,29 This could be due to better prognostic features in our cohort of patients, although all had to have been healthy enough to be candidates for IL2. Another possible explanation is that the patient-specific, polyvalent DC/TC vaccine approach may be associated with greater efficacy than a peptide vaccine.
Whether the longer survival observed in our metastatic melanoma patients who received IL2+ASI is due to the vaccine or the result of unintended bias is uncertain. Limitations of this study include a variety of unintended biases that can be present in any retrospective review. Patients in the IL2+ASI cohort tended to be younger and have lower stage disease, and both of these were associated with a trend for survival benefit. It is unclear how many of these patients had a therapeutic plan to utilize both IL2 and a patient-specific vaccine. Such a treatment plan was not included in any of the clinical trials in which any of these patients enrolled. Because it typically took several months to establish a cell line and prepare a vaccine product, most patients who progressed and deteriorated rapidly would not have had a chance to receive vaccine treatment. The patients who received vaccine may represent a better prognostic group in terms of tumor burden, but we know that very few of these patients were felt to have responded to their IL2-based therapy, and most received vaccine either within the year before or within a year after receiving IL2. There is also certainly a bias in that, most patients who progressed rapidly after IL2 were probably not considered appropriate candidates for vaccine immunotherapy. These concerns led us to perform the analysis that excluded all patients who received only IL2, but were censored alive or dead before 12 months of follow-up.
Acknowledgments
This work was supported by the CBRG and Hoag Hospital.
Disclosure Statement
Dr. Dillman has recently served as a consultant to Prometheus, Inc. (San Diego, CA), the owners of IL2. He serves as the chief medical officer for California Stem Cell, Inc. (Irvine, CA), and has stock options from that company, which now owns the intellectual property associated with the autologous vaccines described in this article. Dr. Dillman and/or other immediate family members have stock in other companies that make antimelanoma products, including Bristol-Myers Squibb, Glaxo-Smith-Kline, Merck, and Pfizer, participate in a speakers' bureau for Genentech. Other authors declare that they have no proprietary, financial, professional, or other personal interests in any product, service, and/or company that could be construed as influencing the position presented in this article.
References
- 1.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010;363:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild , Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ascierto PA, Minor D, Ribas A, et al. Phase II Trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013;31:3205. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2010;367:1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2010;367:107. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Thomas LO, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated melanoma. N Engl J Med 2011;364:26. [DOI] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (Anti-PD-1) in melanoma. N Engl J Med 2013;369:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: An overview. Oncology 2009;23:488. [PMC free article] [PubMed] [Google Scholar]
- 14.Dillman RO, Barth NM, VanderMolen LA, et al. Should high-dose interleukin-2 still be the preferred treatment for patients with metastatic melanoma? Cancer Biother Radiopharm 2012;27:337. [DOI] [PubMed] [Google Scholar]
- 15.Schwartzentruber DJ, Lawson DH, Richards JM, et al. Gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011;364:2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillman RO, Nayak SK, Beutel L. Establishing in vitro cultures of autologous tumor cells for use in active specific immunotherapy. J Immunother 1993;14:65. [DOI] [PubMed] [Google Scholar]
- 17.Dillman RO, Church C, Oldham RK, et al. Inpatient continuous infusion interleukin-2 in 788 patients with cancer: The National Biotherapy Study Group experience. Cancer 1993;71:2358. [DOI] [PubMed] [Google Scholar]
- 18.Dillman RO, Wiemann MC, VanderMolen LA, et al. Hybrid high-dose bolus/continuous infusion interleukin-2 in patients with metastatic melanoma: A phase II trial of the Cancer Biotherapy Research Group (formerly the National Biotherapy Study Group). Cancer Biother Radiopharm 1997;12:249. [DOI] [PubMed] [Google Scholar]
- 19.Dillman RO, DePriest C, DeLeon C, et al. Patient-specific vaccines derived from autologous tumor cell lines as active specific immunotherapy: Results of exploratory phase I/II trials in patients with metastatic melanoma. Cancer Biother Radiopharm 2007;22:309. [DOI] [PubMed] [Google Scholar]
- 20.Dillman RO, Selvan SR, Schiltz PM, et al. Phase II trial of dendritic cells loaded with antigens from self-renewing, proliferating autologous tumor cells as patient-specific anti-tumor vaccines in patients with metastatic melanoma: Final Report. Cancer Biother Radiopharm 2009;24:311. [DOI] [PubMed] [Google Scholar]
- 21.Dillman RO, Cornforth AN, Depriest C, et al. Tumor stem cell antigens as consolidative active specific immunotherapy: A randomized phase II trial of dendritic cells versus tumor cells in patients with metastatic melanoma. J Immunother 2012;35:641. [DOI] [PubMed] [Google Scholar]
- 22.Selvan SR, Carbonell DJ, Fowler AW, et al. Establishment of stable cell lines for personalized melanoma cell vaccine. Melanoma Res 2010;20:280. [DOI] [PubMed] [Google Scholar]
- 23.Dillman RO, Nanci AA, Williams ST, et al. Durable complete response of refractory, progressing metastatic melanoma after treatment with a patient-specific vaccine. Cancer Biother Radiopharm 2010;25:553. [DOI] [PubMed] [Google Scholar]
- 24.Dillman RO, O'Connor AA, Simpson L, et al. Does continuous-infusion interleukin-2 increase survival in metastatic melanoma? Am J Clin Oncol 2003;26:141. [DOI] [PubMed] [Google Scholar]
- 25.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: Long-term survival update. Cancer J Sci Am 2000;6(suppl 1):S11. [PubMed] [Google Scholar]
- 27.Joseph RW, Sullivan R, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother 2012;35:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 1998;4:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosman JA, Carrillo C, Urba WJ, et al. Three phase II Cytokine Working Group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HAL-A2-positive advanced melanoma. J Clin Oncol 2008;26:2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dillman RO. Melanoma vaccines: Trials and tribulations. Vaccine Dev Ther 2013;3:57 [Google Scholar]
- 31.Dillman RO, Cornforth AN, Nistor G. Cancer stem cell antigen-based vaccines: The preferred strategy for active specific immunotherapy of metastatic melanoma? Expert Opin Biol Ther 2013;13:643. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell MS, Jakowatz J, Harel W, et al. Increased effectiveness of interferon alfa-2b following active specific immunotherapy for melanoma. J Clin Oncol 1994;12:402. [DOI] [PubMed] [Google Scholar]