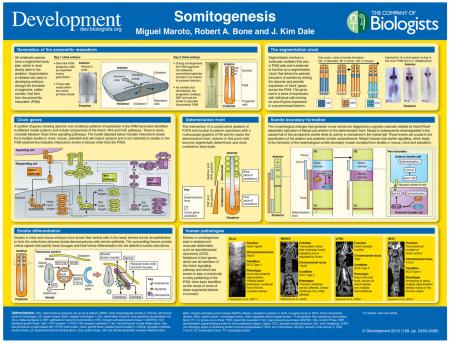
Summary
A segmented body plan is fundamental to all vertebrate species and this bestows both rigidity and flexibility on the body. Segmentation is initiated through the process of somitogenesis. This article aims to provide a broad and balanced cross-species overview of somitogenesis and to highlight the key molecular and cellular events involved in each stage of segmentation. We highlight where our understanding of this multifaceted process relies on strong experimental evidence as well as those aspects where our understanding still relies largely on models.
Keywords: Determination front, Gastrulation, Segmentation clock
Introduction
A characteristic feature of the vertebrate body plan is a segmented body axis, most clearly seen in the skeleton. Segmentation is initiated very early in the developing embryo through the formation of segments called somites, which later give rise to vertebrae and skeletal muscle, as well as to some dermis (Dequeant and Pourquie, 2008). During somitogenesis, the unsegmented paraxial or presomitic mesoderm (PSM) progressively segments into bilaterally symmetrical epithelial somites in an anterior to posterior direction. This is a rhythmic process with a periodicity that matches that of a molecular oscillator acting in PSM cells (Dequeant and Pourquie, 2008). This oscillator is believed to function as a segmentation ‘clock’ that drives the periodic formation of somites (Cooke and Zeeman, 1976). Both the periodicity and final number of somites are species-specific characteristics. Here, and in the accompanying poster, we provide an overview of somitogenesis, highlighting the molecular and cellular events involved in each stage of the segmentation process.
Gastrulation: generation of the PSM
One of the first morphological landmarks to form in the developing embryo is the blastopore or site of gastrulation. In some, but not all, vertebrates, this site is termed the primitive streak. In chick, this structure forms during the first few hours of development at the midline of the developing embryo. Gastrulation describes the movement of cells towards the blastopore/primitive streak and their subsequent ingression through this structure to generate the three germ layers of the embryo (ectoderm, mesoderm and endoderm) from which all embryonic tissues will derive. These movements involve an epithelial to mesenchymal transition (EMT) driven by the action of the fibroblast growth factor (FGF)-regulated transcription factor Snail. The activities of Snail and Sox3 (SRY-box containing 3) in the primitive streak are mutually repressive and maintain the balance of ectodermal progenitors in the epiblast and mesendodermal progenitors that ingress through the blastopore/streak (Acloque et al., 2011).
In chick and mouse, it has been shown that stem-like progenitor cells for certain tissues, such as the paraxial mesoderm (from which somites derive), are specified and reside within a domain of the primitive streak (Selleck and Stern, 1991; Psychoyos and Stern, 1996; Cambray and Wilson, 2007; McGrew et al., 2008). These progenitors divide and the cells they generate migrate out from the streak to take up a position in the posterior PSM (Selleck and Stern, 1991; Psychoyos and Stern, 1996; Cambray and Wilson, 2007; McGrew et al., 2008). Their new identity is characterised by the expression of the PSM-specific marker Tbx6 (T-box 6). As somites bud off the anterior end of the PSM, they are constantly replenished by cells entering the posterior end of the PSM from the site of gastrulation (Dequeant and Pourquie, 2008).
The segmentation clock
The segmentation clock drives the dynamic and periodic mRNA expression of a number of so-called ‘clock’ genes [such as those encoding the bHLH transcription factors Hairy1 and Hairy2 in chick, hairy and enhancer of split 1 (Hes1) and Hes7 in mouse, Hairy and enhancer of split-related 1 (Her1) and Her7 in zebrafish] across the PSM in a posterior to anterior fashion, with a periodicity that matches somite formation (Pourquie, 2011; Gibb et al., 2010). The wave of expression is not due to cell movement but to individual cells turning on and off gene expression in a synchronised and periodic fashion (Palmeirim et al., 1997). This is an intrinsic property of the PSM tissue. Once the wave reaches the anterior limit of the PSM, a somite pair buds off and a new wave of expression is initiated in the posterior PSM.
Components of the Notch pathway share this dynamic expression profile in the PSM in a variety of vertebrate species (Pourquie, 2011; Gibb et al., 2010). In addition, genes from the Wnt and FGF pathways have also been shown to cycle across the PSM of the mouse (Palmeirim et al., 1997; Aulehla et al., 2003; Ishikawa et al., 2004; Niwa et al., 2007; Dale et al., 2006; Dequeant et al., 2006). Recent data suggest that these activities could also be dynamic in the posterior end of the PSM of other vertebrate species, suggesting their potential involvement in the initiation of the oscillations (Krol et al., 2011). At least in the case of the Notch pathway, the generation and maintenance of oscillations along the PSM have been shown to rely on negative-feedback loops driven by unstable negative regulators of the pathway that are encoded by the clock genes: Hes7 in the mouse (Bessho et al., 2003) and Lunatic fringe (Lfng) in the chicken (Dale et al., 2003). Notch signalling is crucial for both clock gene oscillations and somite formation in the mouse (Ferjentsik et al., 2009). In addition, Notch is required to synchronise oscillations between neighbouring cells (Herrgen et al., 2010; Ozbudak and Lewis, 2008).
A major unresolved issue in the field is to identify the molecular mechanism by which the periodicity of the oscillations is regulated. There is some evidence in mouse and chick to suggest that Wnt activity plays a role in ensuring that the oscillations occur with the correct periodicity (Gibb et al., 2009). Downregulation of Wnt signalling could also be involved in the final arrest of oscillations in the anterior PSM, where levels of nuclear β-catenin are significantly reduced compared with the rest of the PSM (Aulehla et al., 2008).
There is clear evidence of crosstalk between the FGF, Wnt and Notch pathways in the control of the oscillations, although there is still much to be learned about the molecular level at which these interactions are established (Pourquie, 2011). It is noteworthy that striped Notch expression also occurs in arthropods (McGregor et al., 2009) such as spiders (Stollework et al., 2003), centipedes (Chipman and Akam, 2008) and cockroaches (Pueyo et al., 2008). As yet, there is no evidence that a clock governs segmentation in these species; however, this striped expression raises the possibility that Notch could be part of a key ancestral mechanism for segmentation. Furthermore, oscillations in the expression of Notch target genes are not unique to overtly segmented tissues; Hes1 expression oscillates in a wide variety of cell lines and also in neural progenitor cell populations (Shimojo et al., 2008; Hirata et al., 2002). Although evidence to indicate that the oscillations serve a ‘clock’-like function is still to come, these observations do suggest a more global implication for the clock, and studies of the oscillations that sweep the PSM could help us to understand the clock mechanism as a whole.
The determination front
In the PSM, a posterior-anterior gradient of Fgf8 and nuclear β-catenin expression is opposed by an anterior-posterior gradient of retinoic acid (RA) activity (Aulehla et al., 2003; Aulehla et al., 2008; Dubrulle et al., 2001; Sawada et al., 2001; Aulehla and Pourquie, 2010; Diez del Corral and Storey, 2004). The intersection of these two gradients is believed to provide a transition point, known as the ‘determination front’, at which cells in the PSM can initiate their segmentation programme (Dubrulle et al., 2001). Cells posterior to the determination front are maintained in a non-determined state by FGF activity (Dubrulle et al., 2001). The current model holds that the size of each somite is defined by the number of mesodermal cells that pass the determination front between these two opposing signalling domains during one cycle of the segmentation clock. Changes in FGF signalling levels can change the length of the somite that forms subsequently from the affected PSM tissue and is believed to be due to alteration in the position of the determination front (Dubrulle et al., 2001; Sawada et al., 2001).
Somite boundary formation
The morphological changes that eventually generate the new somite at the anterior end of the PSM are triggered by a genetic cascade driven by the wave of Notch activity as it moves anteriorly. This cascade begins with activation of mesoderm posterior 2 (Mesp2) by Notch in a Tbx6-dependent manner in a one-somite domain just anterior to the determination front (Saga, 2007; Sasaki et al., 2011; Oginuma et al., 2008). In the cells located posterior to the determination front, Mesp2 expression is inhibited by FGF activity (Sasaki et al., 2011). The domain of Mesp2 expression is then refined to just the anterior half of the prospective somite due to the loss of Tbx6 via the action of the Ripply repressor (Takahashi et al., 2010). This refining of the domain of Mesp2 activity is crucial in establishing somite polarity, which is in turn essential for later patterning of the skeleton and especially the vertebrae, as these are formed from the posterior half of one somite and the anterior half of the caudally adjacent somite (Christ et al., 2007).
The new somite boundary is formed at the anterior tip of the PSM, where the cells have acquired an elevated level of expression of adhesion molecules such as E-cadherin and neural cell adhesion molecule (NCAM) (Thorsteindóttir et al., 2011). Mesp2 is again instrumental in this process by inducing Eph-ephrin signalling activity that participates in the epithelialisation of somite boundary cells and the creation of the new furrow (Watanabe et al., 2009; Barrios et al., 2003). This process involves clustering of integrin α5 at the somite boundary, which in turn recruits fibronectin-based extracellular matrix to the forming border (Girós et al., 2011; Jülich et al., 2009).
Somite differentiation
From the moment the new epithelial somite is made, it starts to mature and differentiate. The surrounding tissues are instrumental in providing signals that direct specification of these different tissues from the naïve somite and in their further differentiation into the definitive somitic derivatives: the sclerotome, the dermatome and the myotome (Christ et al., 2007; Yusuf and Brand-Saberi, 2006). Thus, cells in the ventral part of the somite de-epithelialise to form the sclerotome, which eventually goes on to form the vertebrae of the skeleton and the tendon progenitor known as the syndetome. This process is influenced by the levels of notochord-derived sonic hedgehog (Shh), which are highest in the ventral half-somite where they act to induce the expression of paired box 1 (Pax1) and Pax9 in the sclerotome cells (Christ et al., 2007; Yusuf and Brand-Saberi, 2006). The dermomyotome cells from the dorsal part of the somite remain epithelial. Wnt1/3a from the neural tube and Wnt8c from the ectoderm participate in the induction of Pax3 and Pax7 expression in the dermomyotome (Christ et al., 2007; Yusuf and Brand-Saberi, 2006). Cells from the tips of the dermomyotome then give rise to the underlying myotome, which starts to express the myogenic factors Myf5 (myogenic factor 5) and MyoD (myogenic differentiation 1), and eventually generates the epaxial (back) and hypaxial (body wall) muscles and part of the dermis of the back. Neurotrophin 3 (NTF3) from the neural tube induces specification of the dermatome, the precursor of dermis tissue (Yusuf and Brand-Saberi, 2006).
Anterior-posterior identity
Vertebrae have distinct morphologies depending on their location along the anterior-posterior body axis, and it is the expression of Hox genes that provides the basis for this specification. Hox genes encode transcription factors that are expressed in restricted domains along the embryonic axis and different combinations of genes give rise to different kinds of vertebrae. The relationship between the physical order of the genes on the chromosome and the temporal activation and spatial extent of expression domains is known as spatial and temporal collinearity. During gastrulation in chick, Hox genes are sequentially expressed in the ingressing epiblast cells and they act to specify the axial identity of the paraxial mesoderm cells, and hence the developing vertebrae, as they form (Iimura and Pourquie, 2006; Iimura et al., 2009).
Human pathologies
Somitogenesis is a critical process in humans. When this process goes awry due to the presence of teratogenic agents or congenital mutations, it leads to the generation of skeletal and muscular deformities. The aetiology of most of these problems is still unknown. However, human mutations in four genes known to play a crucial role in early patterning of the PSM [LFNG, MESP2, HES7 and delta-like 3 (DLL3)] have been identified as the cause of some of these segmental defects (Turnpenny et al., 2007; Sparrow et al., 2010; Whittock et al., 2004; Sparrow et al., 2006). Through studying somitogenesis in animal models, we can hope to increase our knowledge of how this process is regulated at the molecular level, which will allow inferences to be made as to the molecular basis of human segmentation. The study of somitogenesis is therefore of great interest to medical science.
Perspectives
There are multiple aspects of the process of somitogenesis that are clearly important areas to focus on in the future as they could be of key relevance to our understanding of the astonishing variation in body plan found among vertebrates. This knowledge would also contribute to the elucidation of the aetiologies of human pathologies associated with defective segmentation. The oscillatory expression of clock genes is initiated in the progenitor cells of the primitive streak concomitant with the onset of gastrulation. It is still unknown how the oscillatory mechanism is first established in these progenitor cells. It also remains unclear to what extent the Notch, FGF and Wnt pathways interact in the segmentation clock, at the determination front and, more precisely, in the interconnection between these two phenomena, and how conserved this crosstalk is among the different vertebrate species. Another aspect that requires further understanding is the regulation of the clock periodicity. The roles of Fgf8 and Wnt3a in regulating both the determination front and clock gene expression highlight a paradox that remains unresolved: the determination front appears to rely on caudorostral graded expression of pathway components, whereas the oscillator relies on the dynamic expression of target genes sweeping across the same tissue. Finally, although progress has been made recently to further our understanding of the molecular mechanism by which the final number of somites/segments is determined in different vertebrate species (Gomez et al., 2008; Schroter and Oates, 2010; Tenin et al., 2010), much remains to be learned.
In summary, a wealth of information and data has amassed over the last decade pertaining to the molecular regulation of certain aspects of somitogenesis, but we still have a long way to go in understanding how these different pieces of the puzzle fit together to achieve the correct temporal and spatial orchestration of a segmented body axis.
Acknowledgements
We thank Peter Turnpenny, Sally Dunwoodie, Hum. Mol. Genet. and Dev. Dyn. for allowing us to use images from their papers (Turnpenny et al., 2007; Sparrow et al., 2010; Whittock et al., 2004; Sparrow et al., 2006); and Guy Wiedermann for the cLfng in situ image.
Funding
Research in the laboratory of J.K.D. is supported by the Wellcome Trust and the UK Medical Research Council (MRC) and J.K.D. is a Royal Society University Research Fellow. R.A.B. is an MRC-funded PhD student.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Development at a Glance
A high-resolution version of the poster is available for downloading in the online version of this article at http://dev.biologists.org/content/139/14/2453.full
References
- Acloque H, Ocaña OH, Matheu A, Rizzotti K, Wise C, Lovell-Badge R, Nieto MA. Reciprocal repression between Sox3 and Snail transcription factors defines embryonic territories at gastrulation. Dev. Cell. 2011;21:546–558. doi: 10.1016/j.devcel.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulehla A, Pourquie O. Signaling gradients during paraxial mesoderm development. Cold Spring Harb. Perspect. Biol. 2010;2:a000869. doi: 10.1101/cshperspect.a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Wiegrebe W, Baubet V, Wahl MB, Deng C, Taketo M, Lewandoski M, Pourquie O. A beta-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat. Cell Biol. 2008;10:186–193. doi: 10.1038/ncb1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr. Biol. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Bessho Y, Hirata H, Masamizu Y, Kageyama R. Periodic repression by the bHLH factor Hes7 is an essential mechanism for the somite segmentation clock. Genes Dev. 2003;17:1451–1456. doi: 10.1101/gad.1092303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambray N, Wilson V. Two distinct sources for a population of maturing axial progenitors. Development. 2007;134:2829–2840. doi: 10.1242/dev.02877. [DOI] [PubMed] [Google Scholar]
- Chipman A, Akam M. The segmentation cascade in the centipede Strigamia maritima: involvement of the Notch pathway and pair-rule gene homologues. Dev. Biol. 2008;319:160–169. doi: 10.1016/j.ydbio.2008.02.038. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Scaal M. Amniote somite derivatives. Dev. Dyn. 2007;236:2382–2396. doi: 10.1002/dvdy.21189. [DOI] [PubMed] [Google Scholar]
- Cooke J, Zeeman EC. A Clock and Wavefront model for the control of the number of repeated structures during animal morphogenesis. J. Theor. Biol. 1976;58:455–476. doi: 10.1016/s0022-5193(76)80131-2. [DOI] [PubMed] [Google Scholar]
- Dale JK, Maroto M, Dequeant ML, Malapert P, Pourquie O. Periodic Notch inhibition by Lunatic Fringe underlies the chick segmentation clock. Nature. 2003;421:275–278. doi: 10.1038/nature01244. [DOI] [PubMed] [Google Scholar]
- Dale JK, Malapert P, Chal J, Vilhais-Neto G, Maroto M, Johnson T, Jayasinghe S, Trainor P, Herrmann B, Pourquie O. Oscillations of the Snail genes in the presomitic mesoderm coordinate segmental patterning and morphogenesis in vertebrate somitogenesis. Dev. Cell. 2006;10:355–366. doi: 10.1016/j.devcel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Dequeant ML, Pourquie O. Segmental patterning of the vertebrate embryonic axis. Nat. Rev. Genet. 2008;9:370–382. doi: 10.1038/nrg2320. [DOI] [PubMed] [Google Scholar]
- Dequeant ML, Glynn E, Gaudenz K, Wahl M, Chen J, Mushegian A, Pourquie O. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science. 2006;314:1595–1598. doi: 10.1126/science.1133141. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Storey KG. Opposing FGF and retinoid pathways: a signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. BioEssays. 2004;26:857–869. doi: 10.1002/bies.20080. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, McGrew MJ, Pourquie O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–232. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- Ferjentsik Z, Hayashi S, Dale JK, Bessho Y, Herreman A, De Strooper B, del Monte G, de la Pompa JL, Maroto M. Notch is a critical component of the mouse somitogenesis oscillator and is essential for the formation of the somites. PLoS Genet. 2009;5:e1000662. doi: 10.1371/journal.pgen.1000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb S, Zagorska A, Melton K, Tenin G, Vacca I, Trainor P, Maroto M, Dale JK. Interfering with Wnt signalling alters the periodicity of the segmentation clock. Dev. Biol. 2009;330:21–31. doi: 10.1016/j.ydbio.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb S, Maroto M, Dale JK. The segmentation clock moves up a notch. Trends Cell Biol. 2010;20:593–600. doi: 10.1016/j.tcb.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girós A, Grgur K, Gossler A, Costell M. α5β1 integrin-mediated adhesion to fibronectin is required for axis elongation and somitogenesis in mice. PLoS One. 2011;6:e22002. doi: 10.1371/journal.pone.0022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Ozbudak EM, Wunderlich J, Baumann D, Lewis J, Pourquie O. Control of segment number in vertebrate embryos. Nature. 2008;454:335–339. doi: 10.1038/nature07020. [DOI] [PubMed] [Google Scholar]
- Herrgen L, Ares S, Morelli LG, Schröter C, Jülicher F, Oates AC. Intercellular coupling regulates the period of the segmentation clock. Curr. Biol. 2010;20:1244–1253. doi: 10.1016/j.cub.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298:840–843. doi: 10.1126/science.1074560. [DOI] [PubMed] [Google Scholar]
- Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442:568–571. doi: 10.1038/nature04838. [DOI] [PubMed] [Google Scholar]
- Iimura T, Denans N, Pourquie O. Establishment of Hox vertebral identities in the embryonic spine precursors. Curr. Top. Dev. Biol. 2009;88:201–234. doi: 10.1016/S0070-2153(09)88007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Kitajima S, Takahashi Y, Kokubo H, Kanno J, Inoue T, Saga Y. Mouse Nkd1, a Wnt antagonist, exhibits oscillatory gene expression in the PSM under the control of Notch signalling. Mech. Dev. 2004;121:1443–1453. doi: 10.1016/j.mod.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Jülich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- Krol AJ, Roellig D, Dequeant ML, Tassy O, Glynn E, Hattem G, Mushegian A, Oates AC, Pourquie O. Evolutionary plasticity of segmentation clock networks. Development. 2011;138:2783–2792. doi: 10.1242/dev.063834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Pechmann M, Schwager EE, Damen WGM. An ancestral regulatory network for posterior development in arthropods. Commun. Integr. Biol. 2009;2:174–176. doi: 10.4161/cib.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew M, Sherman A, Lillico SG, Ellard FM, Radcliffe PA, Gilhooley HJ, Mitrophanous KA, Cambray N, Wilson V, Sang H. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–2299. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- Niwa Y, Masamizu Y, Nakayama R, Deng CX, Kageyama R. The initiation and propagation of Hes7 oscillation are cooperatively regulated by Fgf and Notch signaling in the somite segmentation clock. Dev. Cell. 2007;13:298–304. doi: 10.1016/j.devcel.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Oginuma M, Niwa Y, Chapman DL, Saga Y. Mesp2 and Tbx6 cooperatively create periodic patterns coupled with the clock machinery during mouse somitogenesis. Development. 2008;135:2555–2562. doi: 10.1242/dev.019877. [DOI] [PubMed] [Google Scholar]
- Ozbudak EM, Lewis J. Notch signalling synchronizes the zebrafish segmentation clock but is not needed to create somite boundaries. PLoS Genet. 2008;4:e15. doi: 10.1371/journal.pgen.0040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeirim I, Henrique D, Ish-Horowicz D, Pourquie O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- Pourquie O. Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell. 2011;145:650–663. doi: 10.1016/j.cell.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychoyos D, Stern C. Fates and migratory routes of primitive streak cells in the chick embryo. Development. 1996;122:3263–3273. doi: 10.1242/dev.122.5.1523. [DOI] [PubMed] [Google Scholar]
- Pueyo JI, Lanfear R, Couso JP. Ancestral Notch-mediated segmentation revealed in the cockroach Periplaneta americana. Proc. Natl. Acad. Sci. USA. 2008;105:16614–16619. doi: 10.1073/pnas.0804093105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y. Segmental border is defined by the key transcription factor Mesp2, by means of suppression of Notch activity. Dev. Dyn. 2007;236:1450–1455. doi: 10.1002/dvdy.21143. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Kiso M, Kitagawa M, Saga Y. The repression of Notch signalling occurs via the stabilization of mastermind-like 1 by Mesp2 and is essential for somitogenesis. Development. 2011;138:55–64. doi: 10.1242/dev.055533. [DOI] [PubMed] [Google Scholar]
- Sawada A, Shinya M, Jiang YJ, Kawakami A, Kuroiwa A, Takeda H. Fgf/MAPK signalling is a crucial positional cue in somite boundary formation. Development. 2001;128:4873–4880. doi: 10.1242/dev.128.23.4873. [DOI] [PubMed] [Google Scholar]
- Schroter C, Oates AC. Segment number and axial identity in a segmentation clock period mutant. Curr. Biol. 2010;20:1254–1258. doi: 10.1016/j.cub.2010.05.071. [DOI] [PubMed] [Google Scholar]
- Selleck M, Stern C. Fate mapping and cell lineage of Hensen’s node in the chick embryo. Development. 1991;112:615–626. doi: 10.1242/dev.112.2.615. [DOI] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in Notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Sparrow D, Chapman G, Wouters MA, Whittock NV, Ellard S, Fatkin D, Turnpenny PD, Kusumi K, Sillence D, Dunwoodie SL. Mutation of the LUNATIC FRINGE gene in humans causes spondylocostal dysostosis with a severe vertebral phenotype. Am. J. Hum. Genet. 2006;78:28–37. doi: 10.1086/498879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow DB, Sillence D, Wouters MA, Turnpenny PD, Dunwoodie SL. Two novel missense mutations in HAIRY-AND-ENHANCER-OF-SPLIT-7 in a family with spondylocostal dysostosis. Eur. J. Hum. Genet. 2010;18:674–679. doi: 10.1038/ejhg.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollework A, Schoppmeier M, Damen WG. Involvement of Notch and Delta genes in spider segmentation. Nature. 2003;423:863–865. doi: 10.1038/nature01682. [DOI] [PubMed] [Google Scholar]
- Takahashi J, Ohbayashi A, Oginuma M, Saito D, Mochizuki A, Saga Y, Takada S. Analysis of Ripply1/2 deficient mouse embryos reveals a mechanism underlying the rostro-caudal patterning within a somite. Dev. Biol. 2010;342:134–145. doi: 10.1016/j.ydbio.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Tenin G, Wright D, Ferjentsik Z, Bone R, McGrew MJ, Maroto M. The chick somitogenesis oscillator is arrested before all paraxial mesoderm is segmented into somites. BMC Dev. Biol. 2010;10:1–12. doi: 10.1186/1471-213X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteindóttir S, Deries M, Cachaco AS, Bajanca F. The extracellular matrix dimension of skeletal muscle development. Dev. Biol. 2011;354:191–207. doi: 10.1016/j.ydbio.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Turnpenny P, Alman B, Cornier AS, Giampietro PF, Offiah A, Tassy O, Pourquie O, Kusumi K, Dunwoodie S. Abnormal vertebral segmentation and the notch signalling pathway in man. Dev. Dyn. 2007;236:1456–1474. doi: 10.1002/dvdy.21182. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc. Natl. Acad. Sci. USA. 2009;106:7467–7472. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittock N, Sparrow DB, Wouters MA, Sillence D, Ellard S, Dunwoodie SL, Turnpenny PD. Mutated MESP2 causes spondylocostal dysostosis in humans. Am. J. Hum. Genet. 2004;74:1249–1254. doi: 10.1086/421053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf F, Brand-Saberi B. The eventful somite: patterning, fate determination and cell division in the somite. Anat. Embryol. 2006;211(Suppl. 1):21–30. doi: 10.1007/s00429-006-0119-8. [DOI] [PubMed] [Google Scholar]