Abstract
Paracetamol has a reasonable safety profile when taken in therapeutic doses. However, it could induce hepatotoxicity and even more severe fatal acute hepatic damage when taken in an overdose. The green alga, Dunaliella salina was investigated for hepatoprotective and antioxidant activity against paracetamol-induced liver damage in rats. Male albino Wistar rats overdosed with paracetamol showed liver damage and oxidative stress as indicated by significantly (P<0.05) increased serum levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total and direct bilirubin, malondialdehyde, cholesterol and nitric oxide. At the same time, there were decreased activities of serum superoxide dismutase and total antioxidant capacity compared with the control group. Treatment with D. salina methanol extract at doses of 500 and 1000 mg/kg body weight or silymarin could significantly (P<0.05) decrease the liver damage marker enzymes, total and direct bilirubin, malondialdehyde, cholesterol and nitric oxide levels and increase the activities of superoxide dismutase and total antioxidant capacity in serum when compared with paracetamol intoxicated group. Liver histopathology also showed that D. salina reduced the centrilobular necrosis, congestion and inflammatory cell infiltration evoked by paracetamol overdose. These results suggest that D. salina exhibits a potent hepatoprotective effect on paracetamol-induced liver damage in rats, which may be due to both the increase of antioxidant enzymes activity and inhibition of lipid peroxidation.
Keywords: Dunaliella salina, paracetamol, hepatotoxicity, antioxidant, reactive oxygen species
Dunaliella salina is a unicellular marine phytoplankton that belongs to the phylum Chlorophyta. Unlike other green algae, Dunaliella does not contain a rigid cell wall but has a thin elastic plasma membrane that responds rapidly to changes in osmotic pressure by changes in cell volume[1]. Therefore, it can physically withstand three- to four-fold increases or decreases in osmotic pressure, shrinking or swelling in response, respectively[2]. However, Dunaliella is a unique species of microalgae that has evolved to live in extreme environmental conditions comprising a major constituent of all natural hypersaline environments[3,4]. D. salina is considered as one of the most salt tolerant life forms, which have adapted to very high ultraviolet (UV) radiation. It is thought that their carotene content functions as a sunscreen protecting chlorophyll and DNA from harmful UV irradiation[5].
Under stress conditions such as high light intensity, increased temperature, high salinity and nutrient deficiency, Dunaliella accumulates significant amounts of β-carotene. Dunaliella is one of the richest natural producers of carotenoid, producing up to 15% of its dry weight under suitable conditions[6,7]. Until today, β-carotene remains the major natural product harvested from D. salina. Common uses of β-carotene include food colouring, additives to multivitamin preparations, health-food products, cosmetics and as a precursor of vitamin A[8,9]. The potential ability of carotenoids to act as antioxidants and immunomodulatory agents has led to more active research investigating their application for the prevention of human cancers[8]. Therefore, D. salina could be used as a source of antioxidant to improve free radical scavenging activities in the body and protect cells from oxidative damage.
In recent years, attention has been focused upon the role of biotransformation of chemicals to highly reactive metabolites that initiate cellular toxicity. Several chemicals, including clinically used drugs, can cause cellular damage through metabolic activation of the chemical to highly reactive compounds such as free radicals, carbenes and nitrenes[10,11,12]. Free radicals have been implicated in the aetiology of many degenerative diseases such as cancer, cataract, coronary heart disease, stroke, rheumatoid arthritis, diabetes, Alzheimer's disease and ageing process[13,14,15,16,17]. Paracetamol, the widely used analgesic antipyretic drug, though considered a safe drug, it produces hepatic necrosis and renal failure when given in high doses[18,19]. Oxidative stress was reported to play a fundamental role in the pathogenesis of paracetamol-induced liver damage[20]. Therefore, the search for new bioactive products with antioxidant activity is necessary to overcome paracetamol-induced hepatic oxidative damage. The present study aims to investigate the antioxidant activity of methanol extract of D. salina in paracetamol-induced hepatotoxicity by studying its effect on serum liver function and oxidative stress biomarkers, as well as on hepatic histopathology of the rat.
MATERIALS AND METHODS
Algal sample was collected from a highly saline concentrating pond (crystallizer pond) present at the solar saltern of Port Fouad, Egypt during summer 2010. In this pond, salinity reaches to ~340 g/l and is characterised by the presence of bloom of D. salina in red colour[21]. The collected sample was examined and identified microscopically according to Butcher[22]. D. salina was cultivated in a natural medium enriched by 40 mg/l KNO3; 26 mg/l KH2PO4; 6 mg/l Fe-EDTA according to Yamaoka et al.[23]. Cells of D. salina were harvested by centrifugation at 3500 rpm for 15 min and washed twice with distilled water for complete removal of culture medium then dried at 45-50° for 48 h. The dried material was mixed with methanol and sonicated to break the cell wall, then placed on the shaker platform for 24 h for cold extraction. The filtrate was evaporated by rotary evaporator at 30-35° and the mass obtained was dissolved in distilled water as a vehicle and employed for further experiments.
Paracetamol (acetaminophen) was purchased from Eipico Co., 10th of Ramadan City, Egypt. Paracetamol was suspended in pathogen-free normal distilled water before use. Silymarin was purchased from Sedico, Pharmaceutical Co., 6th October City, Egypt. All the diagnostic kits assaying hepatic function tests, the levels of lipid peroxidation and antioxidants were obtained from Bio-Diagnostic Co., Giza, Egypt. All other chemicals were of analytical grade.
Male albino rats of Wistar strain weighing between 100 and 130 g (8 weeks old) were purchased from the Animal House Colony of the National Research Center, Dokki, Cairo, Egypt. The animals were housed in polypropylene cages and maintained at 25±2° under 14/10 h dark and light cycle. They were allowed free access to standard pellet diet and water ad libitum. The animals were acclimatised for one week under laboratory conditions. Ethical clearance for handling the animals was obtained from the ethical committee constituted for the purpose.
Acute toxicity studies:
Acute oral toxicity was conducted according to the method of Organisation for Economic Co-operation and Development (OECD)[24]. Animals were kept fasting providing only water, after which the algal extract was administered orally by gastric tube in different gradual doses (1-5 g/kg), and observed for any toxic symptoms and mortality for 72 h.
Experimental design:
Thirty male Wistar rats were divided into five groups of six rats each. Group I rats received distilled water for 7 days and served as a vehicle control. The animals in the group II served as paracetamol-intoxicated control and were given single oral administration of paracetamol (3 g/kg), 1 h after distilled water administration. Groups III, IV and V received methanol extract of D. salina (500, 1000 mg/kg) and silymarin (200 mg/kg)[25], respectively, once daily for seven consecutive days followed by a single oral administration of paracetamol (3 g/kg), 1 h after the last algal dose administration.
After 48 h of paracetamol administration, rats were anesthetised with diethyl ether. Blood of each rat was collected by puncturing retro-orbital plexus in sterilised dry centrifuge tube and allowed to coagulate for 30 min at 37°. The clear sera obtained after centrifugation (3000 rpm for 15 min) were used for further biochemical estimation. After blood collection, the animals of all groups were sacrificed by cervical decapitation and liver specimens were harvested for histopathological examination.
Liver function test:
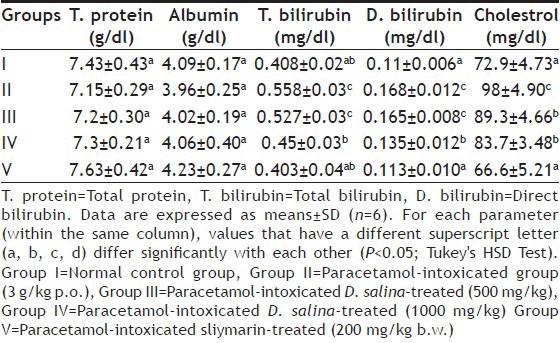
Standard colorimetric methods were used to estimate aspartate aminotransferase (AST) and alanine aminotransferase (ALT) by measuring concentration of oxaloacetate hydrazone and the pyruvate hydrazones formed with 2,4-dinitrophenyl-hydrazine, respectively. The colour was measured at 546 nm according to Reitman and Frankel[26]. To determine the activity of alkaline phosphatase (ALP), serum was incubated with disodium phenyl phosphate and buffered at pH 10 for 15 min at 37°. The hydrolytic products, phenol is condensed with 4-amino antipyrine and then oxidised with alkaline ferric cyanide to give a complex, which is measured photometically at 510 nm[27]. Standard methods were used to estimate total protein[28], albumin[29], T-bilirubin and D-bilirubin[30]. Concentration of cholesterol was determined using the method described by Searcy and Bergquist[31].
Determination of serum malondialdehyde, nitric oxide and antioxidant biomarkers:
Malondialdehyde (MDA) was assayed colorimetrically according to Ohkawa et al.[32] by using trichloroacetic acid 10% and thiobarbituric acid 0.67%. Thiobarbituric acid reactive substances (TBARS) were determined by measuring the absorbance at 535 nm and expressed as MDA formed. Activities of superoxide dismutase (SOD) and total antioxidant capacity (TAC) were assayed using the method of Nishikimi et al.[33] and Koracevic et al.[34], respectively. Nitric oxide (NO) determined wherein acid medium in the presence of nitrite and the formed nitrous acid diazotise sulphanilamide is combined with N-(1–naphthyl) ethylenediamine. The resulting dye has a bright reddish purple colour, which can be measured calorimetrically at 540 nm[35].
Histopathology study:
Liver samples were dissected out, excised from the experimental animals of each group, washed with the normal saline, fixed in 10% formalin and processed for paraffin embedding following the microtome technique. The sections were taken at 5 μ thickness, processed in alcohol-xylene series and were stained with alum-haematoxylin and eosin. The sections were examined microscopically for the evaluation of histopathological changes.
Statistical analysis:
The data were first tested for normality and variance homogeneity, prior to any further statistical analysis. The data were found normally distributed, and the variances were homogeneous. Therefore, the data were statistically evaluated by one-way analysis of variance (ANOVA) test to determine overall effects of the treatments using statistical package SPSS Version 17.0. Post individual comparison was carried out with Tukey's multiple comparison test (Tukey's HSD). Results were presented as mean±SD of six animals. The values of P≤0.05 were considered statistically significant.
RESULTS
Acute toxicity studies:
The oral acute toxicity test showed no lethality or signs of toxicity for D. salina up to a dose level of 5 g/kg and were considered as safe. Therefore, 500 and 1000 mg/kg/day of the extract were the doses selected for evaluation of hepatoprotective and antioxidant activity in vivo.
Effect of D. salina extract on serum liver enzymes:
Data pertaining to the levels of liver marker enzymes in serum of the experimental rats are presented in fig. 1, other nonenzymatic serum liver biomarkers; total protein, albumin, cholesterol, total and direct bilirubin are tabulated in Table 1. The different biochemical parameters, except for total protein and albumin, registered a significant (P<0.05) rise in serum of paracetamol overdosed rats (group II) as compared with the normal control (group I). Otherwise, no significant differences were observed for total protein and albumin. The effect of D. salina extract on serum AST, ALT, ALP, cholesterol, total and direct bilirubin in rats intoxicated with paracetamol showed significant (P<0.05) dose dependent decline as compared with paracetamol intoxicated groups. The degree of protection by methanol extract of D. salina (1000 mg/kg) was either similar for some parameters (AST, ALT and T-bilirubin) or close for others (ALP and D-bilirubin), with that of the standard drug; silymarin.
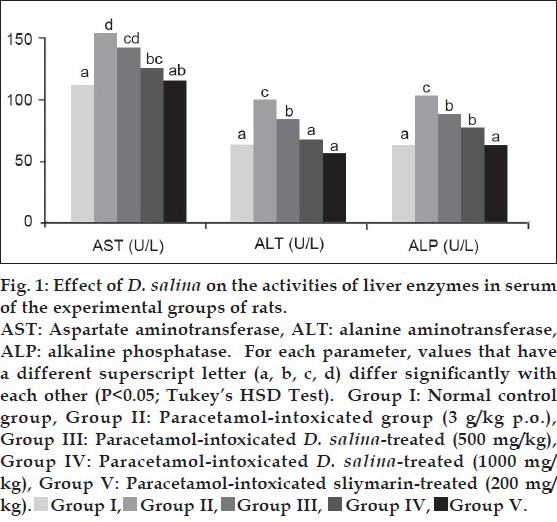
TABLE 1.
EFFECT OF D. SALINA ON THE ACTIVITY OF LIVER NONENZYMATIC BIOMARKERS IN SERUM OF EXPERIMENTAL GROUPS OF RATS
Effect of D. salina Extract on serum antioxidant and lipid peroxidation
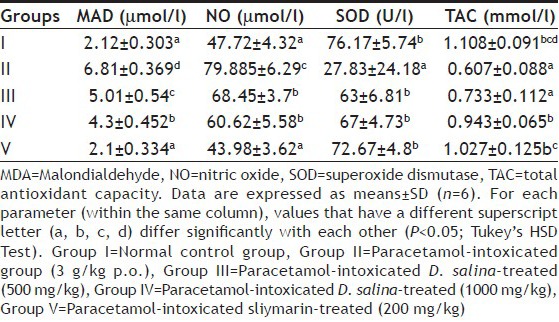
Table 2 shows the effect of D. salina extract on lipid peroxidation, NO and antioxidant biomarkers (SOD, TAC) against paracetamol-induced oxidative stress in rats. Treatment of experimental animals with paracetamol produced a significant (P<0.05) decline in the serum antioxidant markers such as TAC and SOD, while it caused increase in the levels of the primary product of lipid peroxidation; MDA and NO in comparison to the normal control group. Pretreatment of rats with the two doses of D. salina showed antioxidative properties by causing significant (P<0.05) elevation in the activity of TAC and SOD, whereas significantly (P<0.05) inhibited the elevation of MAD and NO. The elimination of oxidative stress by D. salina extract at the dose of 1000 mg/kg and silymarin was almost statistically similar.
TABLE 2.
EFFECT OF D. SALINA ON LIPID PEROXIDATION, NITRIC OXIDE AND ANTIOXIDANT BIOMARKERS IN SERUM OF EXPERIMENTAL GROUPS OF RATS
Histopathological changes in liver:
Histopathological examination of liver tissue of rats received distilled water (normal control group) showed apparently normal hepatic tissue; the hepatic cells are radially placed and each cell has a large spherical nucleus and granular cytoplasm without any injury (fig. 2a). Liver tissue of rats treated with paracetamol revealed congestion of the portal vein with mild to moderate periportal area infiltration with some inflammatory cells mainly macrophages and lymphocytes (fig. 2b). Liver of animal administered the D. salina extract at dose of 500 mg/kg and intoxicated with paracetamol revealed the same portal vein congestion, but the periportal hepatocytes have milder vacuolar degeneration with centrally located nuclei (fig. 2c). Treatment with D. salina extract at dose of 1000 mg/kg improved liver condition and showed very mild portal vein congestion and leucocytic infiltration with no vacuolation of the periportal hepatocytes (fig. 2d). This situation was comparable to that of animal liver treated with slymarin (fig. 2e).
Fig. 2.
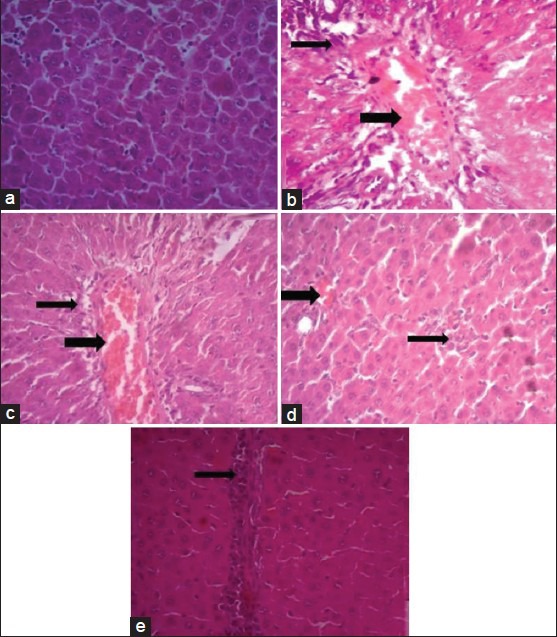
Effect of D. salina on the liver histopathological photomicrographs of the experimental groups of rats.
Histopathological photomicrographs (×400) of livers of various groups stained with haematoxylin and eosin. (a) Normal architecture of rat liver, (b) Necrosis and hepatocellular fatty degeneration (eccentric nuclei) in acetaminophen intoxicated liver and congestion of portal vein and peri-portal infiltration of inflammatory cells, (c) Lesser damage of hepatocytes and low index of necrosis (centrally located nuclei) in D. salina-500 mg/kg pretreated group, (d) Minimal damage of hepatocytes and very low index of necrosis in D. salina-1000 mg/kg pretreated group and mild congestion, (e) Very lesser damage of hepatocytes and low index of necrosis in silymarin pretreated group, narrow arrows refer to inflammatory cells infiltration, wide arrows refer to congestion of portal vein.
DISCUSSION
Although remarkably safe of paracetamol at therapeutic doses, excessive amounts cause centrilobular hepatic necrosis, leading to acute liver failure. The hepatotoxicity of paracetamol has been attributed to the formation of a highly reactive toxic electrophile, N-acetyl-p-benzoquinoneimine (NAPQI) by cytochrome P-450[36]. NAPQI is initially detoxified by conjugation with reduced glutathione (GSH) and excretion in urine[37]. When the rate of NAPQI formation exceeds the rate of detoxification by GSH, it oxidises tissue macromolecules such as lipid or -SH group of proteins. Lipid peroxidation is an autocatalytic process, which is a common consequence of cell death.
In acute toxicity study, LD50 was estimated to be >5000 mg/kg. Hence, one-fifth and one-tenth of the LD50, (500 and 1000 mg/kg) were selected to evaluate the effect of D. salina against praracetamol-induced liver injury in the study.
The liver enzymes, AST and ALT are cytoplasmic in origin and released into the blood after hepatic cell damage. However, ALT is more specific to the liver and is deemed a better parameter for detecting liver injury because high level of AST indicates liver damage as well as cardiac infarction and muscle injury[38]. Furthermore, serum ALP and bilirubin levels are related to the function of hepatic cell. Increase in ALP level is due to increased synthesis, in the presence of increasing biliary pressure[39].
In agreement with previous studies[40], the animals treated with a dose of 3 g/kg of paracetamol showed a significant hepatic damage at 24 h after dosing, as elicited by the significant (P<0.05) elevated levels of hepato-specific serum markers, AST, ALT and ALP as well as total and direct bilirubin. Pretreatment with D. salina extract was protective, as indicated by significant (P<0.05) reduction of all the previous parameters. The normalisation of the above enzyme levels in rats treated with the algal dose of 1000 mg/kg was comparable with that observed for silymarin, which clearly establishes the hepatoprotective effect of this dose. This indicates that administration of D. salina extract at the high dose might be able to induce regeneration of liver cells, reducing the leakage of the above enzymes into the blood.
Living organisms have developed complex antioxidant systems to counteract reactive oxygen species (ROS) and to reduce their damage. The sum of endogenous and food-derived antioxidants represents the total antioxidant activity of the system. The cooperation among different antioxidants provides greater protection against attack by reactive oxygen or nitrogen species, than any single compound alone. Thus, the TAC may provide more relevant biological information compared with that obtained by the measurement of individual components, as it considers the cumulative effect of all antioxidants present in plasma and body fluids[40]. Furthermore, SOD forms a crucial part of the cellular antioxidant defense mechanism. It removes superoxide (O2−) by converting it to H2O2, which can be rapidly converted to water by catalase and glutathione peroxide (GPx)[41]. It is also known that MDA is one of the end products in the lipid peroxidation process[42]. However, oxidative stress results in toxicity when the generated free radicals exceed the cell's capacity for their removal. In the present study, the increase in MDA and decrease in both TAC and SOD levels in rats administrated with paracetamol suggest enhancement of lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms to prevent the formation of excessive free radicals. Treatment with D. salina extract significantly reversed these changes.
Alteration of biomembrane lipid profile disturbs its fluidity and increases microviscosity of the membrane as a result of cholesterol increasing, which leads to cellular rigidity[43]. NO is a highly reactive oxidant produced by liver parenchymal and nonparenchymal cells from L-arginine via an inducible form of NO synthase. Overproduction of NO in the liver has been implicated as an important event in endotoxin shock and in other models of hepatic inflammation and injury[44]. Intoxication of rats with paracetamol may have altered membrane structure and function as suggested by the increases in cholesterol and NO. However, pretreatment of rats with extracts of D. salina inhibited the alteration of lipid membranes and hence prevented alterations in the levels of cholesterol and NO. These results suggest that methanol extract of D. salina play a role in peroxidation by inhibiting free radical attacks on biomembranes.
Histological sections of liver showed that centrilobular necrosis, the pathognomonic feature of hepatotoxicity, which appeared in paracetamol-intoxicated rats, was strikingly reduced in D. salina pretreated rats. Furthermore, the congestion and inflammatory cell infiltration evoked by paracetamol was considerably decreased by D. salina indicating its possible antihepatotoxic action.
In conclusion, D. salina extract could be considering as a potential source of natural antioxidant with hepatoprotective activity. The hepatoprotective effect of D. salina may be due to the presence of carotenoids in the extract, which is known for their antioxidant activity. Further detailed investigations on this algae are needed in order to identify and isolate the hepatoprotective components in the extract and to justify its use in the treatment of liver disorders. Finally, the education of the public and medical profession is needed to increase awareness of the potential toxic effects of paracetamol overdose.
ACKNOWLEDGEMENTS
A special acknowledgement is owed to Dr. Abdelazim Ibrahim, the lecturer of Pathology, College of Veterinary Medicine, Suez Canal University, for his tremendous help and advice in histopathological examination.
Footnotes
Madkour and Abdel-Daim: Hepatoprotective and Antioxidant Activity of Dunaliella salina
REFERENCES
- 1.Shariati M, Lilley RM. Loss of intracellular glycerol from Dunaliella by electroporation at constant osmotic pressure: Subsequent restoration of glycerol content and associated volume changes. Plant Cell Environ. 1994;17:1295–304. [Google Scholar]
- 2.Avron M, Ben-Amotz A. Boca Raton: CRC Press; 1992. Dunaliella: Physiology, biochemistry, and biotechnology; p. 240. [Google Scholar]
- 3.Shariati M, Hadi M. Isolation, purification and identification of three unicellular green alga species of Dunaliella salina, Dunaliella parva and Dunaliella pseudosalina from salt marsh of Gave-Khoni of Isfahan-Iran. Iran J Biol. 2000;9:45–54. [Google Scholar]
- 4.Phadwal K, Singh PK. Isolation and characterization of an indigenous isolate of Dunaliella sp. for β-carotene and glycerol production from a hypersaline lake in India. J Basic Microbiol. 2003;43:423–29. doi: 10.1002/jobm.200310271. [DOI] [PubMed] [Google Scholar]
- 5.Ben -Amotz A, Shaish A, Avron M. Mode of action of massively accumulated β-carotene of Dunaliella bardawil in protecting the algae against damage by excess irradiation. Plant Physiol. 1989;91:1040–3. doi: 10.1104/pp.91.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseini Tafreshi A, Shariati M. Pilot culture of three strains of Dunaliella salina for β-carotene production in open ponds in the center region of Iran. World J Microbiol Biotechnol. 2006;22:1003–6. [Google Scholar]
- 7.Hadi MR, Shariati M, Afsharzadeh S. Microalgal biotechnology: Carotenoid and glycerol production by the green algae Dunaliella isolated from the Gave-Khoni salt marsh, Iran. Biotechnol Bioprocess Eng. 2008;13:540–44. [Google Scholar]
- 8.Chidambara Murthy KN, Vanitha A, Rajesha J, Mahadeva Swamy M, Sowmya PR, Ravishankar GA. In vivo antioxidant activity of carotenoids from Dunaliella salina: A green microalga. Life Sci. 2005;76:1381–90. doi: 10.1016/j.lfs.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Dufosse L, Galaup P, Yaron A, Arad SM, Blanc P, Chidambara Murthy KN, et al. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci Technol. 2005;16:389–406. [Google Scholar]
- 10.Gupta M, Mazumder UK, Kumar ST, Periyasamy G, Kumar SR. Antioxidant and hepatoprotective effects of Bauhinia racemosa against paracetamol and carbon tetra-chloride induced liver damage in rats. Iran J Pharm Ther. 2004;3:12–20. [Google Scholar]
- 11.Azab S, Abdel-Daim M, Eldahshan O. Phytochemical, cytotoxic, hepatoprotective and antioxidant properties of Delonix regia leaves extract. Med Chem Res. 2013;22:4269–77. [Google Scholar]
- 12.Abdel-Daim MM, Abuzead SM, Halawa SM. Protective Role of Spirulina platensis against acute Deltamethrin-induced toxicity in Rats. PLoS One. 2013;8:e72991. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atawodi SE. Antioxidant potentials of African plants. Afr J Biotechnol. 2005;4:128–33. [Google Scholar]
- 14.Abdel-Daim M, Funasaka Y, Kamo T, Ooe M, Matsunaka H, Yanagita E, et al. Effect of chemical peeling on photocarcinogenesis. J Dermatol. 2010;37:864–72. doi: 10.1111/j.1346-8138.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Daim M, Funasaka Y, Kamo T, Ooe M, Matsunaka H, Yanagita E, et al. Preventive effect of chemical peeling on ultraviolet induced skin tumor formation. J Dermatol Sci. 2010;60:21–8. doi: 10.1016/j.jdermsci.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Funasaka Y, Abdel-Daim M, Kawana S, Nishigori C. Effect of chemical peeling on the skin in relation to UV irradiation. Exp Dermatol. 2012;21(Suppl 1):31–5. doi: 10.1111/j.1600-0625.2012.01500.x. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell SR. Coronary artery diseased-free radical damage, antioxidant protection and the role of homocysteine. Basic Res Cardiol Suppl. 2000;1:65–71. doi: 10.1007/s003950070012. [DOI] [PubMed] [Google Scholar]
- 18.Abraham P. Oxidative stress in paracetamol-induced pathogenesis: (I) Renal damage. Indian J Biochem Biophys. 2005;42:59–62. [PubMed] [Google Scholar]
- 19.Ahmed M, Khater MR. Evaluation of the protective effect of Ambrosia maritime extract on acetaminophen-induced liver damage. J Ethnopharmacol. 2001;75:169–74. doi: 10.1016/s0378-8741(00)00400-1. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan C, Williams WM, Ray MB, Chen TS. Prevention of acetaminophen-induced liver toxicity by 2(R,S)-n-propylthiazolidine-4(R)-carboxylic acid in mice. Biochem Pharmacol. 2001;61:245–52. doi: 10.1016/s0006-2952(00)00558-x. [DOI] [PubMed] [Google Scholar]
- 21.Madkour FF, Gaballah MM. Phytoplankton assemblage of a solar saltern in Port Fouad, Egypt. Oceanologia. 2012;45:687–700. [Google Scholar]
- 22.Butcher RW. Ser. 4. London: H.M. Stationery Office; 1959. An introductory account of the smaller alga of British coastal waters, I. Introduction and Chlorophyceae; p. 74. [Google Scholar]
- 23.Yamaoka Y, Takimura O, Fuse H, Kamimura K. â-Carotene production by Dunaliella salina in led-batch and semi-continuous cultures under nutrient supplement. Seibutsu-Kogaku Kaishi. 1994;72:111–4. [Google Scholar]
- 24.Paris: OECD; 2001. OECD, Guideline for Testing of Chemicals-Acute Oral Toxicity-Acute Toxic Class Method. [Google Scholar]
- 25.Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Investig. 2002;22:51–65. [Google Scholar]
- 26.Reitman S, Frankel AS. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin Pathol. 1957;28:53–6. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Kind PR, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrin. J Clin Pathol. 1954;7:322–6. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 29.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 30.Walters M, Gerade H. Ultramicromethod for determination of conjugated and total bilirubin in serum or plasma. Microchem J. 1970;15:231–31. [Google Scholar]
- 31.Searcy RL, Bergquist A. A new colour reaction for the quantitation of serum cholesterol. Clin Chim Acta. 1960;5:192–9. doi: 10.1016/0009-8981(60)90035-8. [DOI] [PubMed] [Google Scholar]
- 32.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 33.Nishikimi M, Roa NA, Yogi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–54. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 34.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–61. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery HA, Dymock JF. The determination of nitrite in water. Analyst. 1961;86:414–6. [Google Scholar]
- 36.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinoneimine a cytochrome P-450 mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA. 1984;81:1327–30. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermeulen NP, Bessems JG, Van de Streat R. Molecular aspects of paracetamol-induced hepatotoxicity and its mechanism based prevention. Drug Metab Rev. 1992;24:367–407. doi: 10.3109/03602539208996298. [DOI] [PubMed] [Google Scholar]
- 38.Drotman R, Lawhan G. Serum enzymes are indications of chemical induced liver damage. Drug Chem Toxicol. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 39.Muriel P, Garcipiana T. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol. 1992;12:439–42. doi: 10.1002/jat.2550120613. [DOI] [PubMed] [Google Scholar]
- 40.Kuriakose GC, Kurup MG. Antioxidant and hepatoprotective activity of Aphanizomenon flos-aquae Linn against paracetamol intoxication in rats. Indian J Exp Biol. 2010;48:1123–30. [PubMed] [Google Scholar]
- 41.Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: Where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 42.Kurata M, Suzuki M, Agar NS. Antioxidant systems and erythrocyte life span in mammals. Comp Biochem Physiol B. 1993;106:477–87. doi: 10.1016/0305-0491(93)90121-k. [DOI] [PubMed] [Google Scholar]
- 43.Cooper RA, Durocher JR, Leslie MH. Decreased fluidity of red cell membrane lipids in a beta lipoproteinemia. J Clin Invest. 1977;60:115–21. doi: 10.1172/JCI108747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner CR, Heck DE, Yang CS, Thomas PE, Zhang XJ, DeGeorge GL, et al. Role of nitric oxide in acetaminophen-induced hepatotoxicity in the rat. Hepatology. 1998;27:748–54. doi: 10.1002/hep.510270316. [DOI] [PubMed] [Google Scholar]


