Abstract
Cork tree, (Sonneratia caseolaris L.), family Sonneratiaceae, is a typical plant in mangroves. It is widespread in tropical and subtropical tideland throughout the World. It is reported to possess many medicinal properties. For searching new pharmacological activities of Cork tree, the total phenolic contents, antioxidant activities and the electric eel acetylcholinesterase inhibitions and the kinetics of extracts of various plant parts were determined. The graphs of trolox equivalent antioxidant capacity and ferric reducing antioxidant power of all extracts showed good linearity with P-value of slopes less than 0.05. The methanol extract of calyxs by maceration method and methanol extract of stamen by soxhlet method presented moderate trolox equivalent antioxidant capacity values. For ferric reducing antioxidant power assay, all extracts gave fair to low antioxidant activities. The tacrine, stamen extract and seed extract by maceration using methanol showed noncompetitive inhibition on acetylcholinesterase activity. While, luteolin, luteolin glycoside and calyx extract and seed extract by boiling using water presented partial noncompetitive inhibition on acetylcholinesterase activity.
Keywords: Total phenolics, antioxidant, anticholinesterase, kinetics, Sonneratia caseolaris
Cork tree, (Sonneratia caseolaris L.), family Sonneratiaceae, is found along deep muddy river banks, mangroves forest and river mouths. It has cone shaped pneumatophores. The tree has ultrafiltration technique to exclude salt, so it can tolerate various level of salinity of water in its habitat. Its stem and branch are used for firewood, building boats, posts of bridges and houses. It can absorb, accumulate, distribute and circulate heavy metal as Cu, Pb, Zn, Cr and Ni in mangrove community[1]. The Cork tree flowers are edible as vegetable with nampriks (spicy dish). Its sour tasting young berry fruits are edible. The Thai traditional medicine indicates that the half ripe fruits are used to relieve cough, the ripe fruits are used as anthelmintic drug and the fermented fruit juice is said to be useful in arresting haemorrhage. The Cork tree is also used as an astringent and antiseptic in Bangladesh[2]. Some chemical constituents that were isolated from Cork tree leaves were fatty acids, hydrocarbons, pectin, sugars[3], flavonoids such as luteolin and luteolin-7-O-β-glucoside[4], sterols[5], triterpenoids and benzene carboxylic acid derivatives[6]. The (-)-R-nyasol, (-)-R-4΄-O-methylnyasol and maslinic acid in Cork tree are responsible for its cytotoxic activity[7]. One of the species in family Sonneratiaceae that is related to Cork tree is Lamphaen (Thai common name) or Sonneratia ovata Backer. Its wood is used as firewood and dyestuff. Its sour fruit is edible and is applied as medicine in poultices to relieve sprain[8].
The antioxidant therapy and acetylcholinesterase (AChE) inhibitor have been shown to be beneficial to Alzheimer's disease (AD), which link to a deficiency in production of neurotransmitter acetylcholine[9,10]. The Desmodium gangeticum, which has been used in Ayurveda for treating neurological symptoms showed antioxidant activity and efficacy in amelioration of AD symptoms via nootropic activity and deterioration of AChE activity[11]. So the phenolic compounds from fruits, vegetables and herb extracts have been exploited because of their potential antioxidative properties[9,12]. The aim of this study was to assess the antioxidant capacities of phenolic compounds of the extracts derived from various plant parts of Cork tree and Lamphaen fruit by trolox equivalent antioxidant capacity (TEAC) and ferric reducing antioxidant power (FRAP) and to demonstrate the inhibition of electric eel AChE enzyme of these extracts, which was used for searching new pharmacological activities of Cork tree.
MATERIALS AND METHODS
The various parts of Cork tree, (S. caseolaris), and young unripe fruit of S. ovata were collected from Aumpawa, Samutsongkhram province, Thailand by researcher of Faculty of Pharmacy, Silpakorn University in September-October, 2008. The dry voucher specimens were deposited in the Department of Pharmacognosy, Silpakorn University in Nakhon-Pathom, Thailand.
ABTS2−, 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonate), was obtained as sulfonic acid from Sigma, St. Louis, USA. Trolox, (+/−)–6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid, 97%, was purchased from Aldrich, Steinheim, Germany. Potassium persulfate, ferrous sulfate (FeSO4·7H2O) and sodium acetate purchased from Asia Pacific Specialty Chemicals Limited, Seven Hills, Australia. Sodium carbonate and sulphuric acid were purchased from Ajax Finechem, Seven Hills, Australia. Folin–Ciocalteu reagent and ferric chloride (FeCl3·6H2O) was purchased from CarLo ErbaReagenti, Milano, Italy. 2,4,6-tri-pyridyl-s-triazine (TPTZ) from Fluka Chemie GmbH, Switzerland. Absolute ethanol, methanol, ethylacetate and dichloromethane were purchased from Merck, Darmstadt, Germany. Bidistilled water was produced by our laboratory. Electric eel AChE type V-S, acetylthicoholine iodide (ASCh), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) and tacrine HCl (9-amino-1,2,3,4-tetrahydroacridine hydrochloride hydrate) were purchased from Sigma-Aldrich, St. Louis, MO, USA.
Preparation of Cork tree extract:
The various parts of Cork tree powder (stamen (anther and filament), calyx of flower, meat of fruit, meat of ripe fruit, skin of fruit and persistent calyx of fruit, seed, pneumatophore and leaf) were macerated in methanol, soxhlet extracted using methanol, ethylacetate, dichloromethane, digested using water then dried with spray-dryer or freeze-dryer and squeezed then dried with spray-dryer or freeze-dryer. Then 100 g of each type of powder was macerated in 400 ml methanol separately and were shaken at room temperature for 24 h and then filtered. The filtrate was evaporated until dry under vacuum. For soxhlet method, the 50 g powder was extracted with methanol or ethylacetate or dichloromethane for 4 h.
All dried crude extracts from maceration and soxhlet methods were kept in temperature controlled chamber at 4°. The young fruit of Lamphaen powder were macerated in methanol.
Quantitative of total polyphenols:
The total phenolic was measured using Folin–Ciocalteu method as described by Kumazawa et al.[13]. The 0.5 ml extract solution (10 μg/ml) was mixed with 0.5 ml Folin–Ciocalteu reagent and 0.5 ml 10% Na2CO3. The mixture was vigorously shaken and incubated for 1 h at room temperature. The absorbance was measured at 760 nm (Agilent 8453E UV/Vis Spectroscopy, USA). The standard curve of polyphenol was prepared by using standard gallic acid (2-8 mg/l). The total polyphenols were expressed in gallic acid equivalent (GAE) in g/100 g of crude extract and g/100 g of dried plant.
Trolox equivalent antioxidant capacity assay:
The TEAC assay procedure was done following method of Re et al.[14], with some modification by mixing 1:1 volume of 7 mM ABTS2− and 4.9 mM potassium persulfate solution. The mixed solution was kept at room temperature for 16 h in dark chamber. The ABTS·+ solution was diluted with water to equilibrate its absorbance (A) to 0.7 (±0.02) unit at 734 nm by spectrometer. The 50 μl of 0-0.5 mg/ml trolox or 0-10 mg/ml sample solutions were reacted with 3 ml ABTS+ solution. The absorbances at 760 nm were measured by spectrometer at 6 min after mixing (n=4). The standard curve of trolox solution was linear between 0 and 17.27 μg/ml. The result expressed as %inhibition as compared with the reaction of solvent and the TEAC was calculated as the ratio of %inhibition of sample to %inhibition of trolox at the same concentration.
Ferric reducing antioxidant power assay:
The FRAP assay was measured by method of Halvorsen et al.[15] and Niemeyer and Metzler[16]. FRAP reagent was prepared by mixing 100 ml 0.3 M sodium acetate buffer, pH 3.6; 10 ml 0.01 M TPTZ in 0.04 M HCl and 10 ml 0.02 M FeCl3·6H2O. The standard curve was linear between 0.25 and 1.50 mM of 50% methanol FeSO4·7H2O solutions. The standard gallic acid (0-50 μg/ml) and sample extracts (0-667 μg/ml) were prepared for antioxidant measurement. The 4.5 ml FRAP reagent was mixed with 450 μl bidistilled water and 150 μl of various concentration of gallic acid or plant extract solutions. The mixtures were incubated for 30 min and monitored the absorbance by spectrometer at 595 nm (n=3). The result expressed as GEAC (gallic acid equivalent capacity assay). The GEAC is a ratio of the FeSO4 concentration that is equivalent to the oxidation capacity of extract to the FeSO4 concentration and equivalent to the oxidation capacity of gallic acid.
Anticholinesterase assay:
The Cork tree extracts, which showed good antioxidant activities, were selected for finding their 50% inhibitory concentration (IC50) to stop AChE activities as followed. The AChE activity was measured in vitro by the method of Ellman et al.[17]. The assay contained 1 ml of mixture of 0.25 mM ASCh and 0.25 mM DTNB in 50 mM sodium phosphate buffer pH 8 and 200 μl of various concentration of AChE. The final volume was adjusted to 3 ml with 50 mM sodium phosphate buffer pH 8. The absorbances were measured at 412 nm by spectrometer at 0, 0.5 min then every 1 min interval within 15.5 min. Then the product formation was calculated for each AChE concentration. The results revealed that 73.73 ng/ml AChE was proper concentration for finding IC50 and kinetic experiments. For studying, the standard tacrine (0-0.067 μg/ml) or luteolin (0-0.33 μg/ml) or luteolin glycoside (0-0.33 μg/ml) or sample extract (0-134 μg/ml) solution and the 73.73 ng/ml AChE were pre-incubated for 10 min before the addition of ASCh. The IC50 was estimated by the method described by Kamal et al.[18] and Alhomida et al.[19].
Estimation of anticholinesterase kinetic parameters:
Michaelis constants (Km) were determined by means of (1) substrate concentration at 1/2Vmax of V versus substrate concentration plot and (2) Lineweaver–Burk plot over ASCh concentration range of 0.025-0.25 mM (1/ASCh = 4-40/mM), while V and Vmax were velocity and maximum velocity, respectively. The kinetic values were applied by transforming data of Lineweaver–Burk plot, Dixon plot, 1/Vmaxapp versus extract concentration plot, 1/Vmaxiapp versus 1/ASCh concentration plot, and slope of Dixon plot versus 1/ASCh concentration. The Vmaxapp was the maximum apparent velocity of the AChE at the given concentration of extract (inhibitor). The Vmaxapp was obtained from the intersection at ordinate of Lineweaver–Burk plot. The Vmaxiapp was the maximum apparent velocity of the AChE in the presence of extract at the given concentration of ASCh and Vmaxiapp was obtained from the intersection at ordinate of Dixon plot.
Statistical analysis:
The graphs were plotted by using MS Excel® Software 2010 (Microsoft Corp.). The values of the correlation coefficient, slope, intercept and their standard errors were obtained by linear and non-linear regression analysis using this program.
RESULTS AND DISCUSSION
The percent yield of extracts and the amount of total phenolics in Cork tree and S. ovata extracts are shown in Table 1. The Cork tree gave the highest percent yield of extract when it was macerated with methanol. The soxhlet method that used methanol for extracting gave highest percent yield of extracts above that of using dichloromethane and ethylacetate. The stamen gave higher percent yield of extract than the other plant parts. The maceration and soxhlet extraction methods using methanol revealed higher total phenolic contents than the other extraction methods when calculated on both crude extracts and dried plants basis. The soxhlet extraction using dichloromethane yielded the lowest total phenolics extraction efficiency. The extracts of stamen contained high amount of total phenolic contents when compared with the extract from other plant parts, especially, when it was extracted by maceration and soxhlet methods using methanol. When compared with S. ovata fruit, meat of Cork tree fruit contained higher total phenolics content. These might be concluded that percent yield and amount of total phenolics of Cork tree extracts depended upon type of plant parts, extraction method and extractant.
TABLE 1.
PERCENT YIELD, TOTAL PHENOLS AND ANTIOXIDANT ACTIVITIES OF VARIOUS PART OF CORK TREE AND S. OVATA FRUIT EXTRACTS
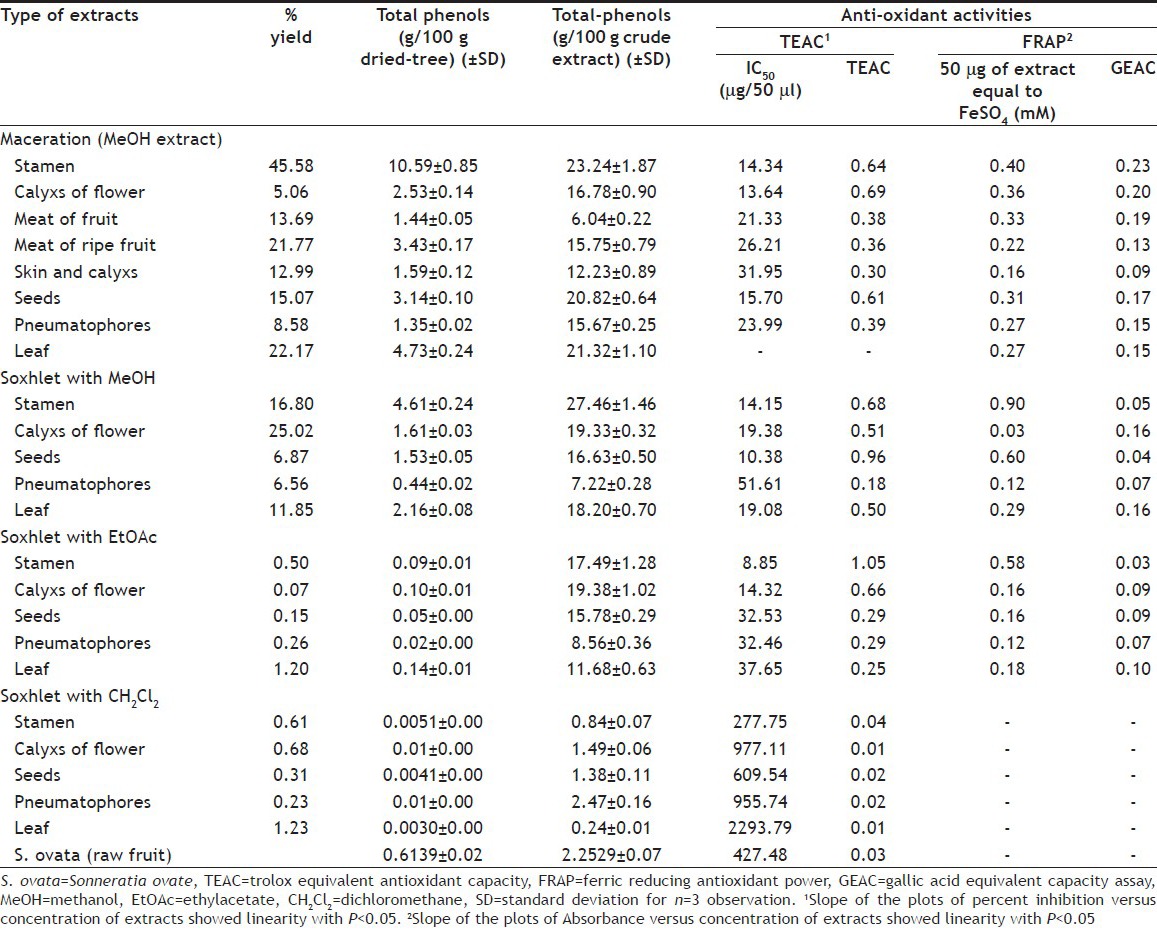
The result of antioxidant activities of Cork tree extracts by TEAC assay presented good linearity with coefficient (r2) between 0.9452 and 0.9993, except the coefficient of leaf extract by soxhlet method using dichloromethane (r2 = 0.4978). The significance levels of all slopes were less than 0.05. The extracts by maceration method using methanol showed higher antioxidant activity than other extraction methods. Table 1 shows that the IC50 of the maceration extracts of calyx (flower), stamen and seed were quite low, which equalled to 13.64, 14.34 and 15.70 μg/50 μl and their TEAC values were 0.69, 0.64 and 0.61, respectively. The antioxidant activities of methanol extracts derived from soxhlet extraction presented good results, especially the seed, stamen and calyx extracts and their TEAC values were 0.96, 0.68 and 0.51, respectively. The ethylacetate extracts by soxhlet method also indicated good antioxidant activities. As seen in Table 1, the ethylacetate extracts of stamen and calyx (flower) presented TEAC equalled to 1.05 and 0.66, respectively. All Cork tree parts extracted by soxhlet using dichloromethane gave low TEAC values in range of 0.01-0.04. This may suggest that the extractive power of solvent and methods of extraction showed effect on kind of extracted substances which affected the antioxidant activities. The total phenolic contents and antioxidant activities of the extracts from various Cork tree's parts, which were derived from boiling water and squeeze methods and then spraying or freezing dry, have been reported by our group[20], which are mentioned here in brief. Most plant part extracts of Cork tree derived from boiling-spray-dried revealed close antioxidant activities to that of maceration, which the extracts from stamen, calyx (flower) and leaf gave TEAC values equalled to 0.64, 0.61 and 0.66, respectively. The boiling-freeze-dried extracts of stamen and calyx indicated moderate antioxidant activities and their TEAC values were 0.42 and 0.50, respectively. For the squeeze method, the spray-dried extracts of fruit showed moderate activities (TEAC=0.49), and the freeze-dried extracts of stamen, calyx and fruit showed moderate activities with TEAC=0.58, 0.57 and 0.58, respectively. The other plant part extracts from boiling and squeeze methods, which are not mentioned here, indicated low TEAC value. The S. ovata fruit extract indicated TEAC value equalled to 0.03, which was rather low compared with that of Cork tree. From TEAC assay, the maceration extraction was the best method when considering the antioxidant activities of all plant part extracts. The soxhlet method was also the good extraction method, but the antioxidant activities of extracts from this method depended on the solvent of extraction. The maceration and soxhlet extraction using methanol showed quite equal antioxidant activities, except the activity of soxhlet extraction of seed that was higher. The stamen also gave good antioxidant activity when it was extracted by soxhlet using ethylacetate. Bunyapraphatsara et al. reported that IC50 of antioxidant activities of the Cork tree fruit extract calculated from DPPH radical reaction is 4.17 μg/ml[21]. From this study, the meat of fruit and meat of ripe fruit showed IC50 of antioxidant activities calculated from ABTS radical reaction equalled to 426 and 524.20 μg/ml, respectively. And the stamen, calyx of flower and seed extracts indicated quite good activity in scavenge stable radical, ABTS·+, under almost investigated extraction techniques. The relationship between TEAC (y) and total phenolic content (x) of crude extracts could be confirmed by linear regression analysis, which had correlation coefficients (r2) range of between 0.0002 and 0.47 (Table 2). These r2 suggested that 47, 31 and 45% of antioxidant activities of Cork tree extracts were derived from maceration and soxhlet extraction using methanol and using ethylacetate accessed from phenolic compounds, respectively.
TABLE 2.
RELATIONSHIP BETWEEN TOTAL PHENOLIC CONTENT AND TEAC OR GEAC CALCULATED AS CORRELATION COEFFECIIENT (r2)
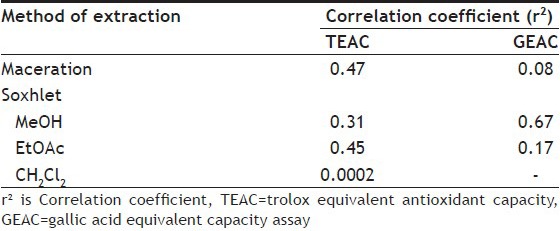
From TEAC assay, the extracts of Cork tree derived from maceration and soxhlet (methanol and ethylacetate) extraction, which indicated moderate to good activities, were chosen to test with FRAP assay (Table 1). The GEAC values were calculated from the linear regression plots between absorbance and concentration of extracts. The plots presented good linearity with coefficient (r2) between 0.9883 and 0.9998, and their significance levels of all slopes were less than 0.05. The result of FRAP assay showed that the chosen extracts indicated fair to low activities for reducing Fe3+ to Fe2+. Their GEAC values were in range of 0.06-0.23. The maceration and boiling (results have been reported[20]) then freeze-dried or spray-dried extracts presented close power of antioxidant activities. While soxhlet extraction using methanol and ethyl acetate gave extracts that presented low GEAC values, the stamen was part of the Cork tree and its macerated extract gave higher GEAC value than other plant part extracts derived from any extraction methods. These results were alike the results of the TEAC assay where the stamen revealed moderate antioxidant activities. The stamen was also contained the highest total phenolic content. Hence, GEAC values might relate to total phenolic content. Then the relationship between GEAC (y) and total phenolic content (x) of crude extracts was calculated by linear regression analysis, which revealed correlation coefficients (r2) range of between 0.08 and 0.67 (Table 2). These r2 suggested that 8-67% of the reducing activity of Cork tree accessed from phenolic compounds. However, great variability in antioxidant activities were found among different patterns of antioxidant capacity of used method[22]. The TEAC and GEAC results implied that Cork tree showed better scavenging radical activities than reducing power and these activities might not come from their phenolic contents only.
From antioxidant results, the extracts by maceration and boiling-spray-dried methods were chosen for testing anticholinesterase activities. The plot between ΔA and different AChE concentrations with 15.5 min incubatory showed linearity relationship (r2>0.95). The relationships between ΔA and AChE concentrations within 3 min incubatory indicated good linearity (r2>0.99) with P-values less than 0.05. From this plot, the optimum concentration and incubation time of AChE were selected at 73.73 ng/ml and 3 min, respectively, and they were chosen to provide further kinetic studies of AChE inhibitor.
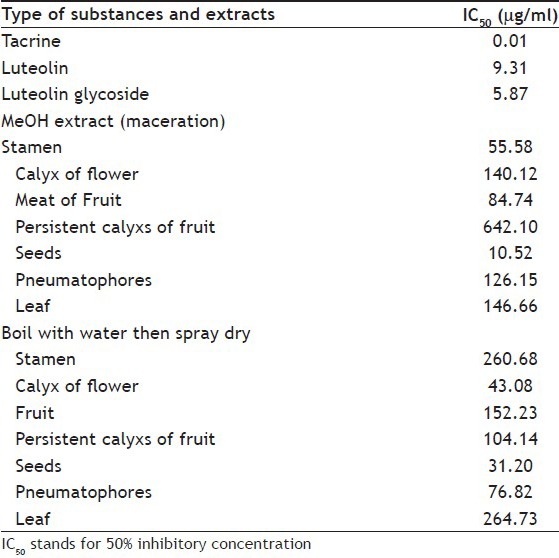
For kinetic study, tacrine was chosen for positive control. The luteolin and luteolin glycoside, which were found in Cork tree extracts[4], were selected to find out their kinetics. The IC50 values of AChE inhibition by tacrine, luteolin, luteolin glycoside and the chosen Cork tree extracts are presented in Table 3. The seed extracts by maceration method showed better AChE inhibition than other part of Cork tree extracts and its IC50 values was close to that of luteolin. The stamen, meat of fruit, seed and leaf extracts by maceration extraction using methanol presented lower IC50 values than that came from boiling with water. The calyx (flower and fruit) and pneumatophore extracts by boiling method presented lower IC50 than that derived from maceration. For further kinetic study of AChE inhibition, the extracts which gave low IC50 as maceration extracts of stamen and seed, and the boiling-spray-dried extracts of calyx (flower) and seed were chosen.
TABLE 3.
IC50 VALUES OF ANTICHOLINESTERASE
For kinetic study, the plots between percent activity of enzyme and concentration of tacrine, luteolin, luteolin glycoside and the chosen Cork tree extracts (plot data did not demonstrate) indicated that they were reversible enzyme inhibitors, since the slopes of graphs gradually decrease by increasing concentration[23]. The plot between product and time of tacrine, luteolin, luteolin glycoside and the chosen Cork tree extracts (plot data did not demonstrate) gave information that all of them were time-dependent inhibition, since the product formation was reduced upon the time. From Lineweaver–Burk plots (plot data did not demonstrate) indicated that tacrine, the stamen and seed extracts by maceration method were noncompetitive inhibitors. While luteolin, luteolin glycoside and calyx (flower) and seed extracts by boiling-spray-dried method presented that they were partial noncompetitive inhibitors as the nature of graph mentioned by Kamal[18]. From Dixon plot and secondary replot of Dixon plot (plot data did not demonstrate) showed that the inhibition of tacrine, the stamen and seed extracts by maceration method were mixed noncompetitive inhibition subtype as the nature of graph mention by Tipton[24]. While the data from Dixon plot of luteolin, luteolin glycoside and calyx (flower) and seed extracts by boiling-spray-dried method did not give any concluded information in the range of concentrations that were used in this study.
The estimate of kinetic values of this experiment is shown in Table 4. There was some report that revealed that the high Km value corresponded to low affinity of acethylchloinesterase to substrate[25]. From our results, tacrine, luteolin and luteolin glycoside presented low Km values that reflected their high affinity to AChE. These were confirmed by their low IC50 values. The sequence of average Km values of selected extracts from high to low were boiled calyx (flower), macerated seed, macerated stamen and boiled seed, respectively. However, the seed extract by maceration using methanol showed the lowest IC50 of all selected extracts, and the following sequences of IC50 were boiled seed, boiled calyx (flower) and macerated stamen. These meant that Km of the extracts did not relate to their IC50. The data indicated that the IC50 of selected extracts corresponded to inhibition constant (Ki), which represented the proportion between inhibited enzyme and inhibitor–enzyme complex. The plot of logIC50 vs logKi gave r2 equal to 0.75 (when Ki came from slope of Lineweaver–Burk VS (tacrine or sample extract) plot) and to 0.82 (Ki from KIapps vs 1/[ASCh] plot). When KI is dissociation constant of AChE–ASCh-inhibitor complex into AChE–ASCh complex+inhibitor. This implied that 75-80% of IC50 of the extracts corresponded to Ki. The γKm represents the dissociation constant of AChE–ASCh-inhibitor complex into AChE-inhibitor complex+ASCh. The low γKm value meant the higher stability of AChE-inhibitor complex than AChE–ASCh-inhibitor complex. Prior study by Alhomida et al. has shown that tacrine combines to human retinal AChE at stage of AChE–ASCh complex to produce AChE–ASCh-tacrine complex[19]. For our study, the γKm value of tacrine was 0.22 mM, which was higher than that of luteolin, luteolin glycoside and the extract of boiled seed. This seem to suggest that luteolin, luteolin glycoside and the extract of boiled seed preferred to combine at stage of AChE to produce AChE-inhibitor complex, which were stable than AChE–ASCh-inhibitor complex. While the extracts of macerated stamen, macerated seed and boiled calyx (flower) had high γKm values, so they may combine into complex as that of tacrine.
TABLE 4.
ESTIMATED KINETIC VALUES BY VARIOUS PLOTS AND REPLOTS
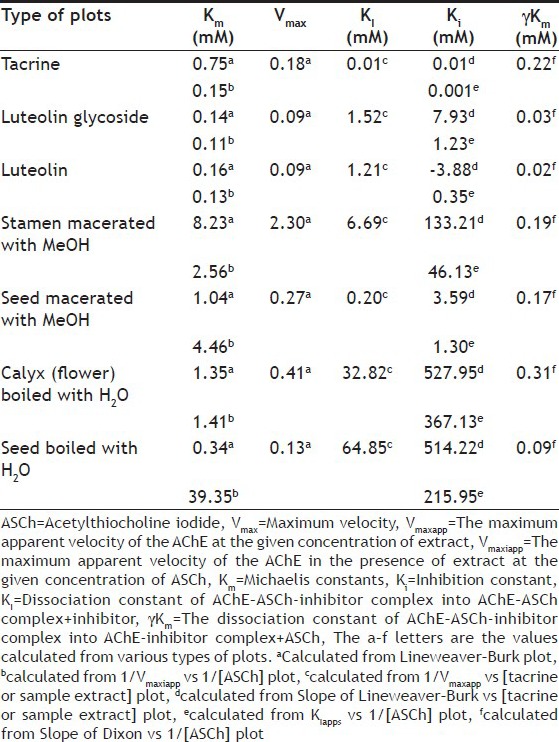
The 8-morpholinooctylphysostigmine and 8-(cis-2,6-dimethylmorpholino)octylphysostigmine, the heptylphysostigmine derivative, have been undergone clinical evaluation against Alzheimer's disease. The mechanisms of action of these two compounds in inhibition of AChE of electric eel AChE (type V) are noncompetitive inhibition[26]. The luteolin and luteolin-7-O-rutinoside has been reported inhibition on AChE and butyrylcholinesterase, respectively[27,28]. However, the kinetic, of them, have not been reported. From this experiment, the luteolin and luteolin glycoside, which were found in Cork tree, presented moderate IC50 values against electric eel AChE and their kinetic are shown in Table 4.
From this study, the methanol extract of Cork tree seed by maceration method showed moderate antioxidant activities, low IC50 value close to luteolin and it presented noncompetitive inhibition effect on electric eel AChE. It revealed possibility to form complex with AChE as tacrine did. This might suggest that the methanol extract of Cork tree seed had potential as AChE inhibitor. And it is possible that this extract might present beneficial medicinal properties, which needs further studies for proving their advantages in detail.
ACKNOWLEDGEMENTS
The authors wish to thank the Silpakorn Research and Development Institute for grant support and Faculty of Pharmacy, Silpakorn University, Nakhon-Pathom, Thailand for the facilities.
Footnotes
Wetwitayaklung, et al.: Antioxidant and Anticholinesterase Activities of Sonneratia caseolaris Extracts
REFERENCES
- 1.Zan Q, Wang Y, Wang B. Accumulation and cycle of heavy metal in Sonneratia apetala and S. caseolaris mangrove community at Futian of Shenzhen. Huan Jing Ke Xue. 2002;23:81–8. [PubMed] [Google Scholar]
- 2.Ghani A. 2nd ed. Dhaka: Asiatic Society of Bangladesh; 2003. Medicinal plants of Bangladesh with chemical constituents and used. [Google Scholar]
- 3.Xu J, Lin P, Meguro S, Kawachi S. Phytochemical research on mangrove plants. 1. Lipids and carbohydrates in propagules of ten mangrove species of China. Mokuzai Gakkaishi. 1997;43:875–81. [Google Scholar]
- 4.Sadhy SK, Ahmed F, Ohtsuki T, Ishibashi M. Flavonoids from Sonneratia caseolaris. J Nat Med. 2006;60:264–5. doi: 10.1007/s11418-006-0029-3. [DOI] [PubMed] [Google Scholar]
- 5.Hogg RW, Gillan FT. Fatty acids, sterols and hydrocarbons in the leaves of eleven species of mangrove. Phytochemistry. 1984;23:93–9. [Google Scholar]
- 6.Tian M, Dai H, Li X, Wang B. Chemical constituents of marine medicinal mangrove plant Sonneratia caseolaris. Chin J Oceanol Limnol. 2009;27:288–96. [Google Scholar]
- 7.Bandaranayake WM. Traditional and medicinal use of mangroves. Mangroves Salt Marshes. 1998;2:133–48. [Google Scholar]
- 8.Othman B. Sonneratia ovata Backer. In: Faridah Hanum I, Van der Maesen LJ., editors. Plant Resources of South-East Asia No. 11. Auxiliary Plants. Bogor, Indonesia: Prosea Foundation; 1997. pp. 242–4. [Google Scholar]
- 9.Kim JK, Bae H, Kim MJ, Choi SJ, Cho HY, Hwang HJ, et al. Inhibitory effect of Poncirus trifoliate on acetyl cholinesterase and attenuating activity against trimethyltin induced learning and memory impairment. Biosci Biotechnol Biochem. 2009;73:1105–12. doi: 10.1271/bbb.80859. [DOI] [PubMed] [Google Scholar]
- 10.Loizzo MR, Tundis R, Menichini F, Menichini F. Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: An update. Curr Med Chem. 2008;12:1209–28. doi: 10.2174/092986708784310422. [DOI] [PubMed] [Google Scholar]
- 11.Joshi H, Parle M. Antiamnestic effects of Demodium gangeticum in mice. Yakugaku Zasshi. 2006;126:795–804. doi: 10.1248/yakushi.126.795. [DOI] [PubMed] [Google Scholar]
- 12.Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 2001;49:2774–9. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- 13.Kumazawa S, Taniguchi M, Suzuki Y, Shimura M, Kwon MS, Nakayama T. Antioxidant activity of polyphenols in carob pods. J Agric Food Chem. 2002;50:373–7. doi: 10.1021/jf010938r. [DOI] [PubMed] [Google Scholar]
- 14.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Anitoxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 15.Halvorsen BL, Holte K, Myhtad MC, Barikmo I, Hvattum E, Remberg SF, et al. A systematic screening of total antioxidants in dietary plants. J Nutr. 2002;132:461–71. doi: 10.1093/jn/132.3.461. [DOI] [PubMed] [Google Scholar]
- 16.Niemeyer HB, Metzler M. Differences in the antioxidant activity of plant and mammalian lignans. J Food Eng. 2003;56:255–6. [Google Scholar]
- 17.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 18.Kamal MA, Greig NH, Alhomida AS, Al-Jafari AA. Kinetics of human acetylcholinesterase inhibition by the novel experimental Alzheimer therapeutic agent, tolserine. Biochem Pharmacol. 2000;60:561–70. doi: 10.1016/s0006-2952(00)00330-0. [DOI] [PubMed] [Google Scholar]
- 19.Alhomida AS, Al-Rajhi AA, Kamal MA, Al-Jafari AA. Kinetic analysis of the toxicological effect of tacrine (Cognex®) on human retinal acetylcholinesterase activity. Toxicology. 2000;147:33–9. doi: 10.1016/s0300-483x(00)00177-3. [DOI] [PubMed] [Google Scholar]
- 20.Phaechamud T, Yodkhum K, Limmatvapirat C, Wetwitayaklung P. Morphology, thermal and antioxidative properties of water extracts from Sonneratia caseolaris (L.) Engl. prepared with freeze drying and spray drying. Res J Pharm Biol Chem Sci. 2012;3:725–39. [Google Scholar]
- 21.Bunyapraphatsara N, Srisukh V, Jutiviboonsuk A, Sornlek P, Thongbainoi W, Chuakul W, et al. Vegetable from the mangrove areas. Thai J Phytopharm. 2002;9:1–12. [Google Scholar]
- 22.Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat Res. 2005;579:200–13. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Bisswanger H. Weinheim: Wiley-VCH verlag GmbH; 2002. Enzyme Kinetics: Principles and Methods. [Google Scholar]
- 24.Tipton KF. Patterns of enzyme inhibition. In: Engel PC, editor. Enzymology LabFax. Oxford: Bios Scientific Publisher; 1996. pp. 115–74. [Google Scholar]
- 25.Fournier D, Muero A. Modification of acetylcholinesterase as a mechanism of resistance to insecticides. Comp Biochem Physiol. 1994;108C:19–31. [Google Scholar]
- 26.Perola E, Cellai L, Lamba D, Filocamo L, Brufani M. Long chain analogs of physostigmine as potential drugs for Alzheimer's disease: New insights into the mechanism of action in the inhibition of acetylcholinesterase. Biochim Biophys Acta. 1997;1343:41–50. doi: 10.1016/s0167-4838(97)00133-7. [DOI] [PubMed] [Google Scholar]
- 27.Jeung SK, Yim HJ, Kim HM, Shin SG, Han SJ, Park JH, et al. Isolation of acetylcholinesterase inhibitors from the flowers of Chrysanthemum indicum Linne. FASEB J. 2007;21:lb189. [Google Scholar]
- 28.Orhan I, Kartal M, Tosun F, Sener B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z Naturforsch C. 2007;62:829–32. doi: 10.1515/znc-2007-11-1210. [DOI] [PubMed] [Google Scholar]


