Abstract
Neuroendocrine tumors (NETs) are rare neoplasms characterized by overexpression of somatostatin receptors (SSTRs). Functional imaging plays a crucial role in management of NETs. Recently, positron emission tomography/computed tomography (PET/CT) with 68Gallium (68Ga)-labeled somatostatin analogues has shown excellent results for imaging of NETs and better results than conventional SSTR scintigraphy. In this review we have discussed the utility of 68Ga-labeled somatostatin analogue PET/CT in NETs for various established and potential indications. In addition we have also shared our own experience from a tertiary care center in India.
Keywords: 68Gallium-labeled [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-NaI3-octreotide; 68Gallium-labeled [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-Phe1-Tyr3-Octreotide; 68Gallium-labeled [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-Tyr3-Octreotate; Neuroendocrine tumor; PET/CT; somatostatin receptor
INTRODUCTION
Neuroendocrine tumors (NETs) are rare tumors arising from the neuroendocrine cells dispersed through the body derived from the neural crest. The incidence of these tumors appears to be rising. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database indicates an increase in the reported annual age-adjusted incidence of NETs from 1.09/100,000 (1973) to 5.25/100,000 (2004).[1] This may be in part due to the improvement in imaging and biochemical methods for detection of NETs. These tumors can originate from endocrine glands such as the pituitary and adrenal medulla, as well as endocrine cell clusters in the thyroid or the pancreas and widely dispersed endocrine cells in the gastrointestinal and respiratory tract as well as skin.[2] As these tumors belong to the amine precursor uptake and decarboxylation (APUD) cell system, they can concentrate and secrete a wide variety of amines and peptides. The presence of hormone syndromes related to secreted amine/hormone production, allows the differentiation of NET into functional (33-50% of cases) or nonfunctional subgroups. Another characteristic feature of NET cells is the expression of several receptors in high quantities.[3] Apart from location, NETs are also graded according to proliferation activity (G1: Ki67 < 2%, G2: Ki67 2-20%, and G3: Ki67 > 20%) which can have strong impact on prognosis and therapy.[4]
Because of the small lesion size, variable anatomical location, and low metabolic rate; conventional imaging of such tumors is often difficult. Computed tomography (CT), ultrasound (US), and magnetic resonance imaging (MRI) are often unable to characterize or sometimes unable to detect such tumors.[5] Therefore, functional imaging plays a crucial role in management of NETs. Somatostatin receptor scintigraphy (SRS) is an important tool for imaging of NETs and has been shown to be superior as compared to other morphological imaging modalities, for the detection of both primary NET and their metastatic lesions in a landmark study by Krenning et al., with more than 1,000 patients.[6] A few years back, novel 68Gallium (68Ga) labeled somatostatin analogues were developed as positron emission tomography (PET) tracers for NETs and have shown excellent results. In this review we will discuss the methods and implications of PET with these 68Ga-labeled somatostatin analogues for imaging of NETs and share our experience in this regard [Table 1].
Table 1.
Brief overview of patients who underwent 68Ga-labeled analogue PET/CT for known or suspected NET
PRINCIPLE OF IMAGING WITH 68GA-LABELED SOMATOSTATIN ANALOGUES
These 68Ga-labeled somatostatin analogues are generally short peptide analogues of somatostatin which are linked to the positron emitter 68Ga by a bifunctional chelate, usually 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). The 68Ga-DOTA-peptides bind to the somatostatin receptors (SSTRs) overexpressed on NETs cells. Six different SSTRs have been identified.[7] These are SSTR1, 2A, 2B, 3, 4, and 5. These SSTRs are G-protein coupled transmembrane receptors and are internalized after binding to specific ligand.[7] Among these SSTR2 and 5 are predominantly overexpressed in NETs, while normal tissue majorly express SSTR3 and 5. Three major 68Ga-DOTA-peptides are currently available for imaging:68Ga-DOTA-Phe1-Tyr3-Octreotide (TOC), 68Ga-DOTA-NaI3-Octreotide (NOC), and 68Ga-DOTA-Tyr3-Octreotate (TATE). The main difference among these three tracers (DOTA-TOC, DOTA-NOC, and DOTA-TATE) is their variable affinity to SSTR subtypes.[8] All of them can bind to SSTR2 and SSTR5, while only DOTA-NOC shows good affinity for SSTR3.[9] This has clinical implication in the form that a wide spectrum ligand (68Ga-DOTA-NOC) may be preferred for imaging. However, there is currently no evidence of a clinical impact of these differences in SSTR binding affinity, and therefore no preferential use of one compound over the others can be advised.[10]
ADVANTAGES OVER CONVENTIONAL SRS
With the advent of 68Ga-DOTA peptide PET/CT there is a trend toward shifting from conventional scintigraphy to PET/CT. Many studies have already shown the superiority of 68Ga-DOTA peptide PET/CT over conventional SRS for imaging NETs.[11,12] This is because 68Ga-DOTA peptide PET/CT offers several advantages over conventional SRS. Firstly, the synthesis of 68Ga-DOTA peptides is relatively easy and economical, and does not require a cyclotron. On the other hand, the production of 111In-Octreotide requires a cyclotron and is relatively costly. Secondly, PET/CT imaging requires less time than SRS (2 h, instead of the 4 plus 24 h acquisition). Thirdly, the higher spatial resolution of the PET as compared to the single photon emission computed tomography (SPECT) (3-6 mm versus 10-15 mm), providing better visualization of small lesions. Fourthly, 68Ga-DOTA-petides have about ten-fold higher affinity for SSTRs as compared to 111In-Octreotide. Also, the 68Ga-DOTA-NOC has broad spectrum affinity for SSTRs (SSTR2, 3, and 5) as compared to 111In-Octreotide (SSTR2 only). Finally, PET provides the possibility of quantification of the tracer uptake in a given region of interest. This can be achieved by measuring the standardized uptake value (SUVmax) which can be used for response monitoring and prognostication.[13,14]
SYNTHESIS OF 68GA-LABELED SOMATOSTATIN ANALOGUES
The synthesis process is relatively easy. 68Ga can be easily eluted from a commercially available 68Ge/68Ga generator. At our center we have a 30-50 mCi 68Ge/68Ga generator (Cyclotron Co. Ltd.; Obninsk, Russia). The long half-life of the mother radionuclide 68Ge (270.8 days) makes it possible to use the generator for approximately 6-12 months depending on use and can be eluted as early as every 3 h.[15] 68Ga (T1/2 = 68 min) is a positron emitter with 89% positron emission and negligible gamma emission (3.2%). For labeling, the 68Ge/68Ga generator is eluted using 0.1 M HCL. The eluent is loaded onto a cation exchange cartridge to preconcentrate and prepurify (using 80% acetone/0.15 M HCL). Purified 68Ga is then directly eluted with 97.7% acetone/0.05 M HCL into the reaction vial containing 30-50 μg of DOTA-TOC/DOTA-NOC. Synthesis is carried out at approximately 126°C for 10-15 min. This is followed by removal of labeled peptide from unlabeled peptide using reverse phase C-18 column with 400 μl of ethanol. This solution is further diluted with normal saline and passed through 0.22 μm filter to get sterile preparation for injection. Radiolabeling yields of >95% can usually be achieved within 15 min. The radiation exposure to the radiochemist is within limits prescribed.[16] With availability of automated modules the synthesis has become safer.
IMAGING PROTOCOL OF 68GA-LABELED SOMATOSTATIN ANALOGUE PET/CT
Guidelines are available with respect to PET/CT imaging with 68Ga-DOTA-peptides.[17] The discontinuation of somatostatin analogue treatment before PET/CT is desired but not mandatory and has been shown not to influence results.[18] Fasting is not required. The recommended dose of 68Ga-DOTA-peptides is usually 132-222 MBq (4-6 mCi), but should not be less than 100 MBq.[17] PET/CT is acquired 45-60 min post injection, with the general consensus that best images are obtained at 60 min. Images are acquired from skull (must include the pituitary gland) to mid-thigh. Additional views can be taken as and when required. Use of intravenous contrast during CT part of PET/CT is controversial, with few studies advocating their use.[19] At our center we do not routinely use intravenous CT contrast and reserve its use in selected cases. The images are reconstructed using iterative reconstruction using standard protocols.
NORMAL BIODISTRIBUTION AND DOSIMETRY
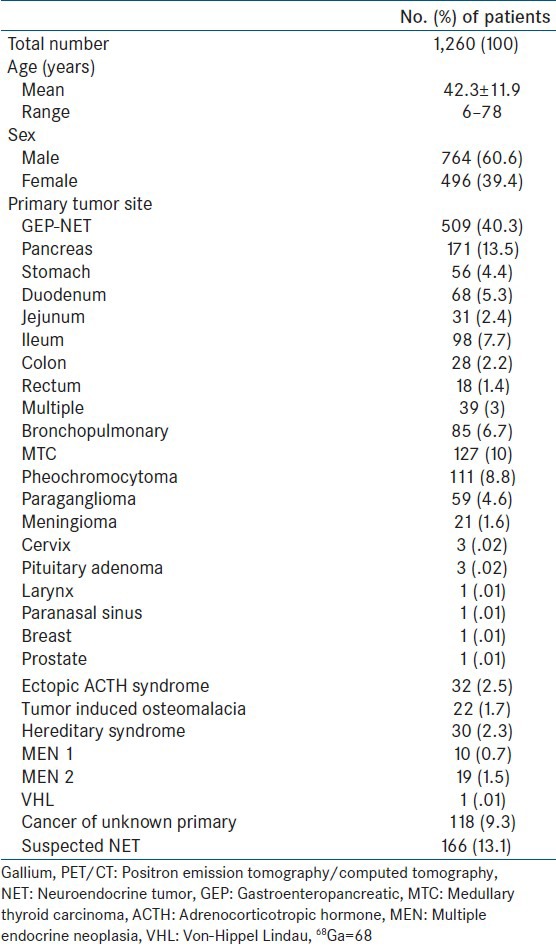
As 68Ga-DOTA peptide binds to cell surface SSTRs, it is physiologically distributed in organs which normally express high levels of SSTRs.[20] It is important to have knowledge of the physiologic tracer distribution before attempting to interpret the pathologic sites of uptake. Normal tracer uptake is seen in the pituitary, salivary glands, thyroid, liver, spleen, adrenals, pancreas, kidneys, ureters, and bladder [Figure 1]. The spleen shows the highest tracer uptake, while the uptake in liver is usually variable and mild. Uptake in exocrine pancreas is a problem, is variable, and can lead to false positive results.[21] In general, pancreatic uptake similar to liver is usually physiological.[22] Another pitfall is physiological uptake in adrenal glands which might interfere with diagnosis of adrenal NETs. The dosimetry of 68Ga-DOTA-peptides is still under evaluation. The whole body effective dose usually varies between 1.7 and 2.5 × 10-2 mSv/MBq and the urinary system receives the highest absorbed dose.[23]
Figure 1.
Maximum intensity projection image of 68Gallium-labeled-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-NaI3-octreotide (68Ga-DOTANOC) positron emission tomography (PET) done in a 45-year-old male after resection of an ileal carcinoid reveals normal radiotracer distribution in pituitary gland, spleen, liver, bilateral adrenal glands, kidneys, ureters, and urinary bladder
GASTROENTEROPANCREATIC NETS (GEP-NETS)
68Ga-DOTA peptide PET/CT has been shown to be extremely useful for imaging of GEP-NETs. The majority of these tumors contain high number of SSTRs, homogeneously distributed throughout the tumor, and expressed at both primary and metastatic sites.[24] The utility of 68Ga-DOTA peptide PET/CT is well-established and can influence many aspects of GEP-NET management including staging patients with already diagnosed NETs, detection of sites of recurrence in patients with treated NETs (restaging), diagnosis of patients suspected of having NET based on clinical features or biochemical evidence of hormone excess, selection of potential candidates for cold somatostatin analogue or peptide receptor radionuclide therapy (PRRT), and monitoring response to therapy in such patients.
Diagnosis, staging and restaging
A recent meta-analysis by Treglia et al., evaluated 16 studies comprising 567 patients with GEP and thoracic NETs.[25] The pooled sensitivity and specificity of 68Ga-DOTA peptide PET or PET/CT in detecting NETs were 93% (95% confidence interval (CI): 91-95%) and 91% (95% CI: 82-97%), respectively, on per patient-based analysis. They advised that this accurate technique should be considered as first-line diagnostic imaging methods in patients with suspicious thoracic and/or GEP NETs. Ambrosini et al., reviewed their experience of imaging GEP-NETs in 1,239 patients.[26] The sensitivity was 92% and specificity was 98% for the detection of NET. The mean SUVmax of positive lesions was 22.8 ± 18.6 (2.2-150.0), reflecting high SSTR expression by GEP-NETs. Our experience has been similar [Figures 2 and 3]. In a prospective analysis of 109 patients done at our center, 68Ga-DOTA-NOC PET/CT has shown a sensitivity and specificity of 78.3 and 92.5% for primary tumor and 97.4 and 100% for metastases, respectively.[27] It changed the management strategy in 21 patients (19%) and supported management decisions in 32 patients (29%). It was better than conventional imaging modality for the detection of both primary tumor (P < 0.001) and metastases (P < 0.0001). In that study 68Ga-DOTA-NOC PET/CT was superior to conventional imaging for the detection of lymph node (P < 0.0001) and bone (P = 0.002), but not liver metastases (P = 1.000). These findings were similar to those reported by Putzer et al.[28] Kumar et al., from our center prospectively compared 68Ga-DOTA-TOC PET/CT and contrast enhanced CT (CECT) for diagnosis and staging of 20 patients with pancreatic NET.[29] The detection rate of CECT was lower than 68Ga-DOTA-TOC PET-CT, both for primary tumor (20 vs 15) and metastatic disease (13 vs 7). Another of our studies addressed subgroup of gastrinoma patients with negative or equivocal CECT findings.[30] 68Ga-DOTA-NOC PET/CT showed a detection rate of 68% overall, 92.8% in those with equivocal CT findings and 36.4% in those with negative CT. Diagnostic performance of 68Ga-DOTA-NOC PET/CT was superior in patients with equivocal CECT findings than that in patients with negative CECT (P = 0.010). Frilling et al., have also demonstrated the superiority of 68Ga-DOTA-TOC PET/CT over conventional imaging (CT/MRI) in GEP-NETs.[31] In that series of 52 patients, PET/CT altered the treatment plan in 31 (59.6%) patients.
Figure 2.
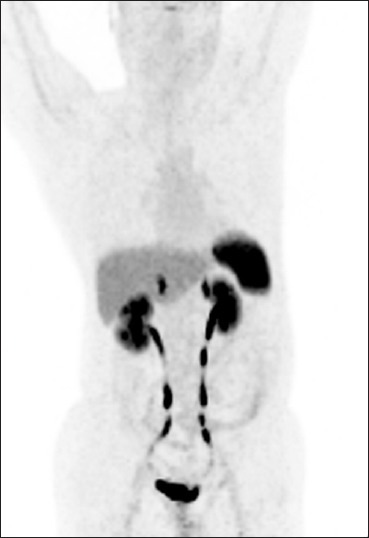
A 60-year-old man, diagnosed case of duodenal carcinoid underwent 68Ga-DOTANOC PET/computed tomography (CT) for evaluation of suspected liver metastasis. Maximum intensity projection PET image (a) shows intense tracer uptake in right upper part of abdomen (bold arrow) and focal areas of tracer uptake in liver (arrow). Transaxial images show circumferential duodenal thickening (b and c, bold arrow) with increased tracer uptake. Also noted small foci of increased tracer uptake in liver in PET-CT (E, arrow), with no corresponding lesion on noncontrast CT (d), suspicious for metastasis. This liver lesion was confirmed to be metastatic on contrast CT
Figure 3.
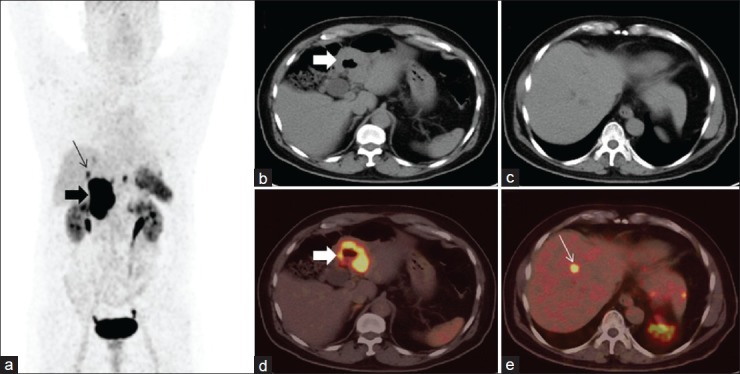
A 50-year-old male, operated case of gastrinoma of stomach, presented with recurrent abdominal pain and raised serum gastrin levels. CT findings were suspicious for recurrence in thickened gastric folds. 68Ga-DOTANOC PET/CT was done for restaging. Maximum intensity projection PET image (a) shows a focal area of increased radiotracer uptake in abdomen near midline (arrow), confirmed as positive portal lymph node on PET/CT (b-d, arrow). No abnormal radiotracer uptake was noted in region of stomach
Suspected NET
An important subgroup of these patients present with clinical, biochemical, or imaging suspicion of NET. In these patients a histopathological diagnosis of NET is still not available. Given the high sensitivity and specificity of 68Ga-DOTA-peptide PET/CT in these patients it can be employed to confirm or rule out NET. Ambrosini et al., have shown high sensitivity of 89.5% and specificity of 100% for 68Ga-DOTA-NOC PET/CT in patients with clinical/biochemical/radiological suspicion of NET.[32] In that population, increased blood markers and clinical signs/symptoms were associated with the lowest frequency of true-positive findings, highlighting that NETs are frequently suspected but rarely diagnosed. On the contrary, a positive radiological finding was more commonly associated with positive 68Ga-DOTA-NOC PET/CT. The authors concluded that 68Ga-DOTA-NOC PET/CT in not routinely indicated in patients with clinical/biochemical suspicion of NET. Another similar study by Haug et al., on the contrary, advocated the use of 68Ga-DOTA-TATE PET/CT in these patients.[33] 68Ga-DOTA-TATE PET/CT showed a sensitivity of 81% and specificity of 90% in their study. Our experience is similar. We did a retrospective analysis of 164 patients with suspected NET based on clinical/biochemical/imaging findings. In that series 68Ga-DOTA-NOC PET/CT showed a sensitivity of 94.8% and specificity of 86.5%. The accuracy of PET-CT was 90.4% in patients with clinical signs/symptoms, 86.7% in those with raised biochemical markers, and 92.7% in those with suspicious imaging findings. We must remember the threshold for imaging in patients with suspected NET varies from center to center and hence no definite guideline can be provided at present. However, it appears that in appropriately selected patient population the yield can be high as reported by Haug et al.,[33] and our experience.
Selection of therapy and monitoring response
A major role of 68Ga-DOTA-peptide therapy is selection of patients for SSTR based therapy with cold or radiolabeled somatostatin analogues. In a study by Miederer et al., in 18 patients, 68Ga-DOTA-TOC PET/CT scans were quantified by SUV calculations and correlated to a cell membrane-based SSTR2-immunohistochemistry (IHC) score (0-3).[34] They found that negative IHC scores were consistent with SUV values below 10, and all scores of 2 and 3 specimens corresponded with high SUV values (above 15). This validates the use of 68Ga-DOTA-peptide PET/CT for selection of somatostatin analogues (cold/PRRT) therapy as high uptake is associated with high levels of SSTR expression. The uptake of somatostatin analogues has been shown to be dependent on a number of variables; the most important among these is cellular differentiation.[35] The system proposed for GEP-NETs by the European Neuroendocrine Tumor Society (ENETS) and also now recommended by the World Health Organization (WHO) uses either mitotic rate or Ki-67 labeling index.[36] Ki-67 index is calculated by using MIB-1 monoclonal antibody against the Ki-67 antigen. The MIB-1 labeling index is the fraction of tumor cells that are labeled by Ki-67. Tumors with higher Ki-67 expression are associated with poorer prognosis. Adams et al., have showed a linear relationship between higher proliferative rate (Ki-67) and uptake of the glucose metabolic tracer 18F-Fluorodeoxyglucose (18F-FDG).[37] Such patients with high 18F-FDG uptake, and thus a high Ki-67 index and cellular proliferation will respond poorly to somatostatin analogues but might respond to chemotherapy. A comparison of 68Ga-DOTANOC and 18F-FDG studies done at our center in 26 patients has shown that well-differentiated GEP-NETs with low Ki-67 index have higher tumor uptake, while uptake on 18F-FDG PET is higher in poorly differentiated tumors. Therefore, at our center we routinely perform both 18F-FDG and 68Ga-DOTANOC PET/CT in patients with metastatic NETs as this combination can provide insights into both therapeutic strategy and prognosis. In addition, 68Ga-DOTANOC PET/CT can also be used for monitoring response to treatment in GEP-NETs, although the results have been variable.[13,38]
Prognosis
The prognostic ability of 68Ga-DOTA-peptides PET/CT results from its inverse association with cellular proliferation.[39] As NET becomes more aggressive, it loses its ability of SSTR expression. Campana et al., have demonstrated the prognostic value of SUV on 68Ga-DOTA-NOC in patients with NET.[14] A SUVmax ≥ 19.3 was found to be a significant predictor of survival on multivariate analysis. Haug et al., on the other hand found change in tumor-to-spleen SUV ratio (ΔSUVT/S) to be an independent predictor of progression free survival after PRRT.[13] In their study, ΔSUVT/S was superior to ΔSUVmax for prediction of outcome. In our analysis of 40 patients with NETs, we found SUVmax on 68Ga-DOTA-NOC PET/CT and histopathological grades to be significantly associated with progression free survival on multivariate analysis. The SUVmax cutoff obtained in our study was 4, which was less than that reported by Campana et al.[14] Heterogeneity between the patient populations might have caused this difference.
PULMONARY NETS
Pulmonary NETs are second most common site for NETs after GEP-NETs and account for 22-27% of such tumors. The WHO classification of pulmonary NETs classifies these neoplasms in order of increasing malignant potential into typical carcinoids, atypical carcinoids, and large cell and small cell NETs.[40] Most of these are typical carcinoids with metastases in only 15% and a high 5 year survival rate of over 90%.[41] While typical carcinoids are commonly seen in young adults, the less common atypical carcinoids are more frequent in elderly and are more often associated with metastasis.[42] The differentiation of pulmonary NETs is associated with SSTR expression, with better differentiated tumors showing higher SSTR expression.[43] Many studies in the past have explored 68Ga-labeled somatostatin analogue PET/CT in patients with pulmonary NETs, often in conjunction with 18F-FDG. Ambrosini et al., evaluated 68Ga-DOTA-NOC PET/CT in 11 patients with bronchial carcinoid.[44] PET/CT detected at least one lesion in nine of 11 patients and was negative in two. PET/CT and CECT were discordant in eight of 11 patients. On a clinical basis, PET/CT provided additional information in nine of 11 patients leading to the changes in the clinical management of three of nine patients. Jindal et al., form our center found 68Ga-DOTA-TOC PET/CT to be very useful for detection of pulmonary carcinoids and commented that it can play an important role in management of such tumors.[45] Kayani et al., compared 68Ga-DOTATATE and 18F-FDG PET/CT in 18 patients with pulmonary NET.[46] In that series, typical carcinoids showed significantly higher uptake of 68Ga-DOTA-TATE and significantly less uptake of 18F-FDG than did tumors of higher grade (P = 0.002 and 0.005). In addition, 68Ga-DOTA-TATE was superior to 18F-FDG for discriminating endobronchial tumor from distal collapsed lung. We at our center found similar results. In a prospective study at our center, the SUVmax in typical carcinoids on 68Ga-DOTA-TOC-PET/CT was significantly higher (SUVmax, 8.8-66) compared with atypical carcinoids (SUVmax, 1.1-18.5; P = 0.002).[47] It appears that different uptake patterns on 68Ga-DOTA-TOC PET/CT and 18F-FDG PET/CT and the ratio of SUVmax may be helpful in differentiating between typical and atypical carcinoids.
METASTATIC NET WITH UNKNOWN PRIMARY
NETs account for about 2-4% of carcinoma of unknown primary site (CUP) and are often mentioned separately because this entity belongs to a treatable subset.[48] Identification of the primary site is of prime importance as many aspects of tumor management are dependent on it, ranging from disease prognosis, treatment outcome, and survival rates. Morphological imaging, though routinely performed, may not be very useful because of their low sensitivity for NETs. Conventional SRS has been explored to detect occult primary sites in patients with metastatic GEP-NETs with a detection rate of 39%.[49] Prasad et al., were the first to evaluate the role of 68Ga-DOTA-NOC PET/CT for CUP-NET.[50] They demonstrated that 68Ga-DOTA-NOC PET-CT was able to localize the primary tumor in 59% of the patients. Moreover, there was change in management in 10% of the patients. The experience from our center is similar [Figure 4]. In a prospective evaluation in 20 patients, we found that 68Ga-DOTANOC PET-CT was able to localize the primary tumor in 12/20 (60%) patients.[51] The most common site of primary was midgut. Even in patients where no primary tumor was localized, additional sites of metastatic disease were observed when compared to conventional imaging, mostly in lymph nodes and bones. There was a change in management in 3/20 patients (15%), who underwent surgery. In the remaining 17 patients, demonstration of SSTR expression by PET-CT made them suitable candidate for PRRT.
Figure 4.
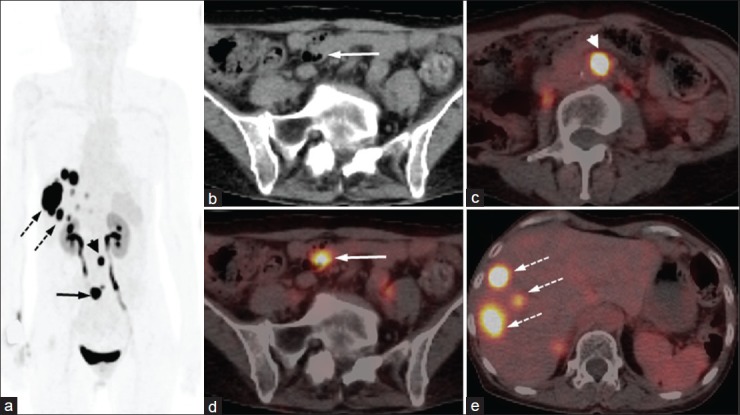
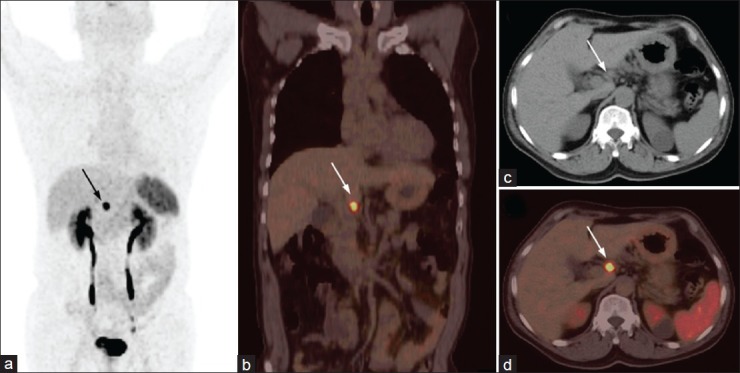
A 35-year-old female presenting with recurrent pain abdomen and multiple hepatic space occupying lesions on ultrasound. Fine needle aspiration cytology from liver lesions demonstrated metastatic neuroendocrine tumor (NET). 68Ga-DOTANOC PET/CT was done to localize the primary. Maximum intensity projections PET image (a) showed multiple liver lesions (broken arrows) along with two discrete foci in abdomen (arrow and arrowhead). Axial CT (b) and PET/CT (c) images of the abdomen revealed focal tracer uptake in ileum with minimal wall thickening (arrow). Also noted are 68Ga-DOTANOC avid retroperitoneal lymph node metastasis (d, arrowhead) and multiple liver metastases (e, broken arrows). The ileal lesion was proven to be carcinoid at histopathology
MEDULLARY CARCINOMA THYROID
Medullary thyroid carcinoma (MTC) is a NET originating in the parafollicular cells (C cells) of the thyroid, which are derived from the neural crest. MTC secretes calcitonin as well as other polypeptides such as carcinoembryonic antigen (CEA) which can be used as tumor markers. The reported prevalence is 3-12% of thyroid cancers and may occur in either sporadic (75-80% of cases) or inherited forms (20-25%), which include multiple endocrine neoplasia (MEN) types IIA and IIB and isolated familial MTC.[52] Lymph nodes are the most common site of metastases throughout the clinical course[53] followed by bones, liver, and lungs.[54] Surgery remains the primary mode of treatment.[55] Residual/recurrent tumor after surgery is usually suggested by elevated basal serum calcitonin and CEA.[56] Localization of recurrent tumor is extremely difficult even with high resolution morphological imaging and a wide array of radiopharmaceuticals such as 99mTc (V)-Dimercaptosuccinic acid, 99mTc-Sestamibi, and 131/123I-Metaiodobenzylguanidine have been evaluated with variable success.[57,58] 18F-FDG PET/CT has been shown to be a useful imaging tool in such patients, though the results have been variable. A recent meta-analysis by Cheng et al., showed pooled sensitivities of 0.68 (95% CI: 0.64-0.72) for 18FDG PET and 0.69 (95% CI: 0.64-0.74) for 18FDG PET/CT.[59]
MTC cells are also known to express SSTRs owing to their neuroendocrine origin and behavior.[60] Conventional SRS with 111In-pentriotide have been used in MTC with variable success.[61] More recently, PET/CT with 68Ga-DOTA-peptides has been evaluated in MTC [Figure 5]. Conry et al., compared the accuracy of 68Ga-DOTA-TATE and 18F-FDG PET/CT for detection of recurrent MTC and mapping the extent of disease in 18 patients.[62] Per patient based sensitivity of 72.2% for 68Ga-DOTA-TATE versus 77.8% for 18F-FDG PET/CT was seen and the difference was not significant. While 18F-FDG PET/CT detected more lesions, in 10 patients a discordant tracer pattern of per-region and/or per-lesion distribution of recurrent disease was observed. The authors concluded that the role of two tracers is complimentary. We have prospectively compared 68Ga-DOTA-NOC and 18F-FDG PET/CT in 41 patients with recurrent MTC.[63] In our study, 68Ga-DOTA-NOC PET/CT proved superior to 18F-FDG PET-CT with a higher sensitivity (75.61 vs 63.4%). However, the difference was not statistically significant (P = 0.179). 68Ga-DOTA-NOC PET/CT was superior to 18F-FDG PET-CT for detecting recurrence in cervical lymph nodes (P < 0.001), but not for other sites. Discordance was observed in 25% patients between the two imaging agents, mainly for lymph nodal lesions. Although, no cutoff for serum calcitonin could be obtained for disease detection on PET/CT, values > 500 pg/ml was more commonly associated with distant metastasis. At present it appears wise to evaluate patients with recurrent MTC using dual tracers (68Ga-DOTA-NOC and 18F-FDG) and their role appears complimentary in such patients.[64] There is small difference between our study and that by Conry et al.,[62] which might be because of the different receptor affinity profile of tracers used. 68Ga-DOTA-NOC has an affinity profile for broader SSTR subtypes: SSTR2, SSTR3, and SSTR5; whereas 68Ga-DOTA-TATE is more active at SSTR2 and SSTR3.[9]
Figure 5.
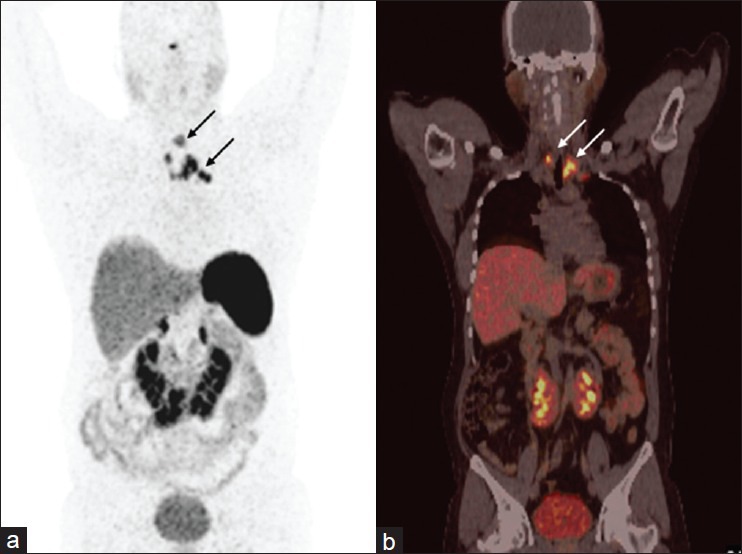
A 31-year-old male with medullary carcinoma thyroid post total thyroidectomy, central neck dissection, and right side radical neck dissection. He presented with rising calcitonin level. 68Ga-DOTANOC PET/CT was done for restaging. Maximum intensity projection PET image (a) revealed presence of multiple focal areas of increased radiotracer uptake (arrows) in cervical and high mediastinal region, confirmed as SSTR positive cervical and high mediastinal lymph nodes on PET/CT (b, arrows). Resurgery confirmed the diagnosis. In addition, horseshoe kidney was incidentally detected on PET/CT
PHEOCHROMOCYTOMA/PARAGANGLIOMA
Paragangliomas are tumors that develop from endocrine cells derived from pluripotent neural crest stem cells and are associated with neurons of the autonomic nervous system. Those developing from adrenal medulla are most common (~90%) and called pheochromocytoma.[64] Pheochromocytomas are a feature of certain disorders with an autosomal dominant pattern of inheritance (e.g. MEN2) in about one-fourth of unselected cases.[65] They are rare (~1%), but treatable cause of hypertension. About 10-20% of these tumors are malignant. Paragangliomas may also arise anywhere from the sympathetic nervous system or the parasympathetic nervous system. While those arising from sympathetic nervous system (abdominothoracic paraganglioma) are frequently associated with catecholamine overproduction, those arising from parasympathetic system (head and neck paraganglioma) rarely do so.[66] Paragangliomas are familial in 9% cases.[67] They can be multicentric in 10% sporadic cases and 32% of familial cases.[68] Precise localization of these tumors is mandatory for management as surgery is the mainstay of treatment.
The diagnosis of pheochromocytoma is established biochemically by measuring the level of urinary and plasma catecholamines and their metabolites (24-h total metanephrine and/or catecholamine).[69] Imaging is important for the localization of tumor and excluding possibility of multifocal lesions before surgery. CT or MRI provide excellent morphologic details and have high sensitivity in the depiction of pheochromocytoma, but their specificity is low. 123/131I-Metaiodobenzylguanidine (MIBG) scintigraphy is currently the functional imaging method of choice for the localization of pheochromocytomas and paragangliomas. It provides high sensitivity and specificity, but is not without limitations.[70] From in vitro and in vivo studies, it has been established that SSTR 2, 3, and 4 are expressed in pheochromocytoma and paraganglioma.[71] Usually the expression of SSTR receptors is increased in malignant pheochromocytomas and paragangliomas.[72] Previous studies with 111In-Octerotide have shown higher sensitivity for detecting metastatic pheochromocytoma than for detecting benign pheochromocytoma, but the overall sensitivity remains low (~30%).[72] Limited literature is available with respect to 68Ga-DOTA-peptide imaging in pheochromocytoma and paraganglioma, majority from our center. Win et al., compared 68Ga-DOTA-TATE PET with 123I-MIBG in five patients with pheochromocytoma and showed that 68Ga-DOTA-TATE PET showed more lesions, with higher uptake and better resolution.[73] Maurice et al., compared 68Ga-DOTA-TATE PET with 123I-MIBG in 15 patients with pheochromocytoma/paraganglioma.[74] They recommended that 68Ga-DOTA-TATE PET should be used as the first line investigation for paraganglioma and metastatic disease. In the largest study till date, Naswa et al., from our center showed the superiority of 68Ga-DOTA-NOC PET/CT over 131I-MIBG in 35 patients with pheochromocytoma/paraganglioma.[75] 68Ga-DOTA-NOC PET/CT showed a diagnostic accuracy of 97.1% on per-patient and 98% on lesion-wise analysis [Figure 6]. No significant relationship was however observed between the degree of tracer uptake (SUVmax) and lesion size and no difference was seen between adrenal and extra-adrenal lesions. A combination of 68Ga-DOTA-NOC PET/CT and 18F-FDG PET/CT is able to preoperatively characterize indeterminate adrenal masses.[76] Naswa et al., have also shown the utility of 68Ga-DOTA-NOC PET/CT for imaging of carotid body chemodectoma, by demonstrating additional lesions or metastasis.[77] A recent study by Sharma et al., from our center has shown the superiority of 68Ga-DOTA-NOC PET/CT over conventional imaging (CT/MRI) and 131I-MIBG in head and neck paraganglioma.[78] In that series of 26 patients, 68Ga-DOTA-NOC PET/CT showed more lesions as compared to 131I-MIBG (P < 0.0001) and conventional imaging (P = 0.015). More importantly, a combination of CT/MRI and 131I-MIBG scintigraphy detected only 53/78 (67.9%) lesions and was also inferior to PET/CT (P < 0.0001). Other PET tracers like 18F-FDG, 18F-FDOPA, and 11C-hyroxyephidrine have been evaluated with variable results in pheochromocytoma/paraganglioma and their role viz-à-viz 68Ga-DOTA-peptides needs to be evaluated.[79]
Figure 6.
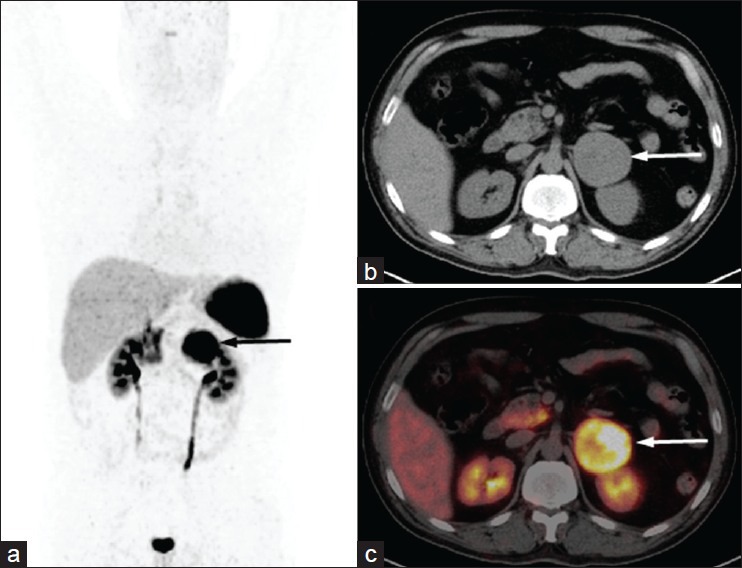
A 28-year-old male with uncontrolled hypertension and left adrenal mass. Urinary metanephrine was mildly elevated. He underwent 68Ga-DOTANOC PET/CT for characterization of the adrenal mass. MIP PET image (a) show intense tracer uptake in left suprarenal region (arrow). Transaxial CT (b) and PET/CT (c) images showed increased tracer uptake in the large left suprarenal mass with central necrosis (arrow) suggesting pheochromocytoma. Postoperative histopathology confirmed pheochromocytoma
HEREDITARY SYNDROMES WITH NET
A wide variety of hereditary syndromes can present with NET. These include MEN syndromes (1 and 2), familial paraganglioma syndrome, von-Hippel Lindau (VHL) syndrome, succinate dehydrogenase (SDH) mutation, and neurofibromatosis type 1. MEN 1 syndrome is the most common and GEP-NETs are often associated. They are usually functional and commonly include gastrinomas (60%) and insulinomas (10%), although carcinoid tumors are also known to occur.[80] MEN2 syndrome on other hand is associated with MTC and pheochromocytoma.[81] As most of these tumors express SSTRs, 68Ga-DOTA-peptide PET/CT can play an important role in management of these disorders. Froeling et al., evaluated and reported the utility of 68Ga-DOTA-TOC PET/CT in 21 patients with MEN1 syndrome.[82] PET/CT was superior to contrast CT for detection of NET lesions (P < 0.001) and impacted therapeutic strategy in almost half of the patients. Our experience is similar [Figure 7]. It appears to be especially useful in asymptomatic relatives of index patients. Further evaluation of 68Ga-DOTA-peptides in these hereditary syndromes is warranted.
Figure 7.
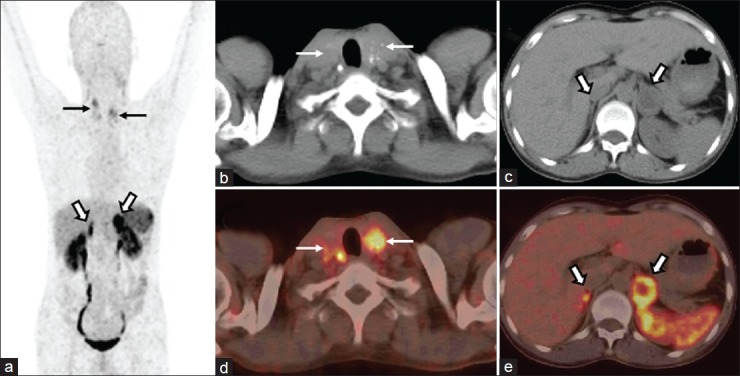
A 35-year-old man, suspected case of MEN 2A syndrome, with known bilateral adrenal masses and cervical lymphadenopathy underwent 68Ga-DOTANOC PET/CT for characterization of the lesions. MIP PET (a) image shows intense tracer uptake in bilateral cervical (arrows) and adrenal regions (bold arrows). Transaxial CT (b) and PET/CT (c) images showed increased tracer uptake in the bilateral calcified thyroid masses (bold arrows). Also noted were bilateral adrenal masses with increased tracer uptake (d and e, arrows). The diagnosis of MEN 2A was confirmed on genetic analysis
OTHER NETS
68Ga-DOTA-peptide PET/CT has been shown to be useful for a wide range of other rare tumors of neuroendocrine origin. These include pituitary adenoma, hemangioblastoma, meningioma, melanoma, and others.[83,84,85,86] It has also been employed for locating the primary tumor in patients with tumor induced osteomalacia and ectopic adrenocorticotropic hormone (ACTH) producing tumors. A recent study by Clifton-Bligh et al., have shown the utility of 68Ga-DOTA-TATE PET/CT imaging in six patients with tumor induced osteomalacia.[87] Our experience is similar, with PET/CT being able to show culprit tumor in a significant proportion of these patients. No systemic study is available regarding utility of 68Ga-DOTA-peptide PET/CT in ectopic ACTH producing tumor. Results from our center have also not been too encouraging. Only four of our patients (of 32) so far have shown localization (lungs in three patients, pancreas in one). Further studies are required in future addressing these tumors.
CONCLUSION
68Ga-labeled somatostatin analogue PET/CT has emerged as an important imaging tool for NET. It can influence many aspects of management of such tumors and has the potential to be the first-line imaging investigation for their evaluation, especially for GEP-NETs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25:458–511. doi: 10.1210/er.2003-0014. [DOI] [PubMed] [Google Scholar]
- 3.Jensen RT. Endocrine tumors of the gastrointestinal tract and pancreas. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005. pp. 2220–31. [Google Scholar]
- 4.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: A review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 5.Ramage JK, Davies AH, Ardill J, Bax N, Caplin M, Grossman A, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumors. Gut. 2005;54(Suppl 4):iv1–16. doi: 10.1136/gut.2004.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In- DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: The Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–31. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 7.Cescato R, Schulz S, Waser B, Eltschinger V, Rivier JE, Wester HJ, et al. Internalization of sst2, sst3, and sst5 receptors: Effects of somatostatin agonists and antagonists. J Nucl Med. 2006;47:502–11. [PubMed] [Google Scholar]
- 8.Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34:982–93. doi: 10.1007/s00259-006-0317-x. [DOI] [PubMed] [Google Scholar]
- 9.Wild D, Mäcke HR, Waser B, Reubi JC, Ginj M, Rasch H, et al. 68Ga-DOTANOC: A first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging. 2005;32:724. doi: 10.1007/s00259-004-1697-4. [DOI] [PubMed] [Google Scholar]
- 10.Kabasakal L, Demirci E, Ocak M, Decristoforo C, Araman A, Ozsoy Y, et al. Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2012;39:1271–7. doi: 10.1007/s00259-012-2123-y. [DOI] [PubMed] [Google Scholar]
- 11.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. [68Ga] DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 12.Kowalski J, Henze M, Schuhmacher J, Mäcke HR, Hofmann M, Haberkorn U. Evaluation of positron emission tomography imaging using [68Ga]-DOTA-D Phe (1)-Tyr (3)-octreotide in comparison to [111In]-DTPAOC SPECT. First results in patients with neuroendocrine tumors. Mol Imaging Biol. 2003;5:42–8. doi: 10.1016/s1536-1632(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 13.Haug AR, Auernhammer CJ, Wängler B, Schmidt GP, Uebleis C, Göke B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010;51:1349–56. doi: 10.2967/jnumed.110.075002. [DOI] [PubMed] [Google Scholar]
- 14.Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AM, Santini D, et al. Standardized uptake values of (68) Ga-DOTANOC PET: A promising prognostic tool in neuroendocrine tumors. J Nucl Med. 2010;51:353–9. doi: 10.2967/jnumed.109.066662. [DOI] [PubMed] [Google Scholar]
- 15.Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48:1741–8. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi DK, Snehlata, Dwivedi AK, Lochab SP, Kumar R, Naswa N, et al. Radiation exposure to nuclear medicine personnel handling positron emitters from Ge-68/Ga-68 generator. Indian J Nucl Med. 2011;26:86–90. doi: 10.4103/0972-3919.90258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. Procedure guidelines for PET/CT tumor imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga- DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37:2004–10. doi: 10.1007/s00259-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 18.Haug AR, Rominger A, Mustafa M, Auernhammer C, Göke B, Schmidt GP, et al. Treatment with octreotide does not reduce tumor uptake of 68Ga-DOTATATE as measured by PET/CT in patients with neuroendocrine tumors. J Nucl Med. 2011;52:1679–83. doi: 10.2967/jnumed.111.089276. [DOI] [PubMed] [Google Scholar]
- 19.Ruf J, Schiefer J, Furth C, Kosiek O, Kropf S, Heuck F, et al. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: Spotlight on the CT phases of a triple-phase protocol. J Nucl Med. 2011;52:697–704. doi: 10.2967/jnumed.110.083741. [DOI] [PubMed] [Google Scholar]
- 20.Boy C, Heusner TA, Poeppel TD, Redmann-Bischofs A, Unger N, Jentzen W, et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1-sst5) expression in normal human tissue: Correlation of sst2 mRNA and SUVmax. Eur J Nucl Med Mol Imaging. 2011;38:1224–36. doi: 10.1007/s00259-011-1760-x. [DOI] [PubMed] [Google Scholar]
- 21.Castellucci P, Pou Ucha J, Fuccio C, Rubello D, Ambrosini V, Montini GC, et al. Incidence of increased 68Ga-DOTANOC uptake in the pancreatic head in a large series of extrapancreatic NET patients studied with sequential PET/CT. J Nucl Med. 2011;52:886–90. doi: 10.2967/jnumed.111.088328. [DOI] [PubMed] [Google Scholar]
- 22.Al-Ibraheem A, Bundschuh RA, Notni J, Buck A, Winter A, Wester HJ, et al. Focal uptake of 68Ga-DOTATOC in the pancreas: Pathological or physiological correlate in patients with neuroendocrine tumors? Eur J Nucl Med Mol Imaging. 2011;38:2005–13. doi: 10.1007/s00259-011-1875-0. [DOI] [PubMed] [Google Scholar]
- 23.Eberlein U, Lassmann M. Dosimetry of [(68) Ga]-labeled compounds. Appl Radiat Isot. 2012 doi: 10.1016/j.apradiso.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 24.Reubi JC, Kvols L, Krenning E, Lamberts SW. In vitro and in vivo detection of somatostatin receptors in human malignant tissues. Acta Oncol. 1991;30:463–8. doi: 10.3109/02841869109092402. [DOI] [PubMed] [Google Scholar]
- 25.Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and Gastroenteropancreatic neuroendocrine tumors: A meta-analysis. Endocrine. 2012;42:80–7. doi: 10.1007/s12020-012-9631-1. [DOI] [PubMed] [Google Scholar]
- 26.Ambrosini V, Campana D, Tomassetti P, Fanti S. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S52–60. doi: 10.1007/s00259-011-1989-4. [DOI] [PubMed] [Google Scholar]
- 27.Naswa N, Sharma P, Kumar A, Nazar AH, Kumar R, Chumber S, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: A prospective single-center study. AJR Am J Roentgenol. 2011;197:1221–8. doi: 10.2214/AJR.11.7298. [DOI] [PubMed] [Google Scholar]
- 28.Putzer D, Gabriel M, Henninger B, Kendler D, Uprimny D, Dobrozemsky G, et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J Nucl Med. 2009;50:1214–21. doi: 10.2967/jnumed.108.060236. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R, Sharma P, Garg P, Karunanithi S, Naswa N, Sharma R, et al. Role of (68) Ga-DOTATOC PET-CT in the diagnosis and staging of pancreatic neuroendocrine tumors. Eur Radiol. 2011;21:2408–16. doi: 10.1007/s00330-011-2199-y. [DOI] [PubMed] [Google Scholar]
- 30.Naswa N, Sharma P, Soundararajan R, Karunanithi S, Nazar AH, Kumar R, et al. Diagnostic performance of somatostatin receptor PET/CT using (68) Ga-DOTANOC in gastrinoma patients with negative or equivocal CT findings. Abdom Imaging. 2012;38:552–60. doi: 10.1007/s00261-012-9925-z. [DOI] [PubMed] [Google Scholar]
- 31.Frilling A, Sotiropoulos GC, Radtke A, Malago M, Bockisch A, Kuehl H, et al. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann Surg. 2010;252:850–6. doi: 10.1097/SLA.0b013e3181fd37e8. [DOI] [PubMed] [Google Scholar]
- 32.Ambrosini V, Campana D, Nanni C, Cambioli S, Tomassetti P, Rubello D, et al. Is 68Ga-DOTA-NOC PET/CT indicated in patients with clinical, biochemical or radiological suspicion of neuroendocrine tumor? Eur J Nucl Med Mol Imaging. 2012;39:1278–83. doi: 10.1007/s00259-012-2146-4. [DOI] [PubMed] [Google Scholar]
- 33.Haug AR, Cindea-Drimus R, Auernhammer CJ, Reincke M, Wängler B, Uebleis C, et al. The Role of 68Ga-DOTATATE PET/CT in suspected neuroendocrine tumors. J Nucl Med. 2012;53:1686–92. doi: 10.2967/jnumed.111.101675. [DOI] [PubMed] [Google Scholar]
- 34.Miederer M, Seidl S, Buck A, Scheidhauer K, Wester HJ, Schwaiger M, et al. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:48–52. doi: 10.1007/s00259-008-0944-5. [DOI] [PubMed] [Google Scholar]
- 35.Ezziddin S, Logvinski T, Yong-Hing C, Ahmadzadehfar H, Fischer HP, Palmedo H, et al. Factors predicting tracer uptake in somatostatin receptor and MIBG scintigraphy of metastatic gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2006;47:223–33. [PubMed] [Google Scholar]
- 36.Rindi G, Villanacci V, Ubiali A. Biological and molecular aspects of gastroenteropancreatic neuroendocrine tumors. Digestion. 2000;62:19–26. doi: 10.1159/000051851. [DOI] [PubMed] [Google Scholar]
- 37.Adams S, Baum R, Rink T, Schumm-Dräger PM, Usadel KH, Hör G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumors. Eur J Nucl Med. 1998;25:79–83. doi: 10.1007/s002590050197. [DOI] [PubMed] [Google Scholar]
- 38.Gabriel M, Oberauer A, Dobrozemsky G, Decristoforo C, Putzer D, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. 2009;50:1427–34. doi: 10.2967/jnumed.108.053421. [DOI] [PubMed] [Google Scholar]
- 39.Kimura N, Miura W, Noshiro T, Miura Y, Ookuma Y, Nagura H. Ki-67 is an indicator of progression of neuroendocrine tumors. Endocr Pathol. 1994;5:223–8. doi: 10.1007/BF02921490. [DOI] [PubMed] [Google Scholar]
- 40.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumors. Eur Respir J. 2001;18:1059–68. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 41.Morandi U, Casali C, Rossi G. Bronchial typical carcinoid tumors. Semin Thorac Cardiovasc Surg. 2006;18:191–8. doi: 10.1053/j.semtcvs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Srirajaskanthan R, Toumpanakis C, Karpathakis A, Marelli L, Quigley AM, Dusmet M, et al. Surgical management and palliative treatment in bronchial neuroendocrine tumors: A clinical study of 45 patients. Lung Cancer. 2009;65:68–73. doi: 10.1016/j.lungcan.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Belhocine T, Foidart J, Rigo P, Najjar F, Thiry A, Quatresooz P, et al. Fluorodeoxyglucose positron emission tomography and somatostatin receptor scintigraphy for diagnosing and staging carcinoid tumors: Correlations with the pathological indexes p53 and Ki-67. Nucl Med Commun. 2002;23:727–34. doi: 10.1097/00006231-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Ambrosini V, Castellucci P, Rubello D, Nanni C, Musto A, Allegri V, et al. 68Ga-DOTA-NOC: A new PET tracer for evaluating patients with bronchial carcinoid. Nucl Med Commun. 2009;30:281–6. doi: 10.1097/MNM.0b013e32832999c1. [DOI] [PubMed] [Google Scholar]
- 45.Jindal T, Kumar A, Venkitaraman B, Dutta R, Kumar R. Role of (68) Ga-DOTATOC PET/CT in the evaluation of primary pulmonary carcinoids. Korean J Intern Med. 2010;25:386–91. doi: 10.3904/kjim.2010.25.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kayani I, Conry BG, Groves AM, Win T, Dickson J, Caplin M, et al. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J Nucl Med. 2009;50:1927–32. doi: 10.2967/jnumed.109.066639. [DOI] [PubMed] [Google Scholar]
- 47.Jindal T, Kumar A, Venkitaraman B, Meena M, Kumar R, Malhotra A, et al. Evaluation of the role of [18F] FDG-PET/CT and [68Ga] DOTATOC-PET/CT in differentiating typical and atypical pulmonary carcinoids. Cancer Imaging. 2011;15:70–5. doi: 10.1102/1470-7330.2011.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hainsworth JD, Spigel DR, Litchy S, Greco FA. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuro-endocrine carcinoma: A Minnie Pearl Cancer Research Network Study. J Clin Oncol. 2006;24:3548–54. doi: 10.1200/JCO.2005.05.0575. [DOI] [PubMed] [Google Scholar]
- 49.Savelli G, Lucignani G, Seregni E, Marchianò A, Serafini G, Aliberti G, et al. Feasibility of somatostatin receptor scintigraphy in the detection of occult primary gastro-entero-pancreatic (GEP) neuroendocrine tumors. Nucl Med Commun. 2004;25:445–9. doi: 10.1097/00006231-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP. Detection of unknown primary neuroendocrine tumors (CUP-NET) using 68Ga-DOTANOC receptor PET-CT. Eur J Nucl Med Mol Imaging. 2010;37:67–77. doi: 10.1007/s00259-009-1205-y. [DOI] [PubMed] [Google Scholar]
- 51.Naswa N, Sharma P, Kumar A, Soundararajan R, Kumar R, Malhotra A, et al. 68 Ga-DOTANOC PET/CT in patients with carcinoma of unknown primary of neuroendocrine origin. Clin Nucl Med. 2012;37:245–51. doi: 10.1097/RLU.0b013e31823ea730. [DOI] [PubMed] [Google Scholar]
- 52.Leboulleux S, Baudin E, Travagli JP, Schlumberger M. Medullary thyroid carcinoma. Clin Endocrinol (Oxf) 2004;61:299–310. doi: 10.1111/j.1365-2265.2004.02037.x. [DOI] [PubMed] [Google Scholar]
- 53.Dralle H, Damm I, Scheumann GF, Kotzerke J, Kupsch E. Frequency and significance of cervicomediastinal lymph node metastases in medullary thyroid carcinoma: Results of a compartment-oriented microdissection method. Henry Ford Hosp Med J. 1992;40:264–7. [PubMed] [Google Scholar]
- 54.Bergholm U, Adami HO, Bergstrom R, Johansson H, Lundell G, Telenius-Berg M, et al. Clinical characteristics in sporadic and familial medullary thyroid carcinoma: A nationwide study of 249 patients in Sweden from 1959 through 1981. Cancer. 1989;63:1196–204. doi: 10.1002/1097-0142(19890315)63:6<1196::aid-cncr2820630626>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 55.Scollo C, Baudin E, Travagli JP, Caillou B, Bellon N, Leboulleux S, et al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2003;88:2070–5. doi: 10.1210/jc.2002-021713. [DOI] [PubMed] [Google Scholar]
- 56.DeLellis RA, Rule AH, Spiler I, Nathanson L, Tashjian AH, Jr, Wolfe HJ. Calcitonin and carcinoembryonic antigen as tumor markers in medullary thyroid carcinoma. Am J Clin Pathol. 1978;70:587–94. doi: 10.1093/ajcp/70.4.587. [DOI] [PubMed] [Google Scholar]
- 57.Ugur O, Kostakğlu L, Güler N, Caner B, Uysal U, Elahi N, et al. Comparison of 99mTc (V)-DMSA, 201Tl and 99mTc-MIBI imaging in the follow-up of patients with medullary carcinoma of the thyroid. Eur J Nucl Med. 1996;23:1367–71. doi: 10.1007/BF01367593. [DOI] [PubMed] [Google Scholar]
- 58.Gao Z, Biersack H, Ezziddin S, Logvinski T, An R. The role of combined imaging in metastatic medullary thyroid carcinoma: 111In-DTPA-octreotide and 131I/123I-MIBG as predictors for radionuclide therapy. J Cancer Res Clin Oncol. 2004;130:649–56. doi: 10.1007/s00432-004-0588-1. [DOI] [PubMed] [Google Scholar]
- 59.Cheng X, Bao L, Xu Z, Li D, Wang J, Li Y. 18F-FDG-PET and 18F-FDG-PET/CT in the detection of recurrent or metastatic medullary thyroid carcinoma: A systematic review and meta-analysis. J Med Imaging Radiat Oncol. 2012;56:136–42. doi: 10.1111/j.1754-9485.2012.02344.x. [DOI] [PubMed] [Google Scholar]
- 60.Zatelli MC, Piccin D, Tagliati F, Bottoni A, Luchin A, Vignali C, et al. Selective activation of somatostatin receptor subtypes differentially modulates secretion and viability in human medullary thyroid carcinoma primary cultures: Potential clinical perspectives. J Clin Endocrinol Metab. 2006;91:2218–24. doi: 10.1210/jc.2006-0334. [DOI] [PubMed] [Google Scholar]
- 61.Baudin E, Lumbroso J, Schlumberger M, Leclere J, Giammarile F, Gardet P, et al. Comparison of octreotide scintigraphy and conventional imaging in medullary thyroid carcinoma. J Nucl Med. 1996;37:912–6. [PubMed] [Google Scholar]
- 62.Conry BG, Papathanasiou ND, Prakash V, Kayani I, Caplin M, Mahmood S, et al. Comparison of (68) Ga-DOTATATE and (18) F-fluorodeoxyglucose PET/CT in the detection of recurrent medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2010;37:49–57. doi: 10.1007/s00259-009-1204-z. [DOI] [PubMed] [Google Scholar]
- 63.Naswa N, Sharma P, Suman Kc S, Lata S, Kumar R, Malhotra A, et al. Prospective evaluation of 68Ga-DOTA-NOC PET-CT in patients with recurrent medullary thyroid carcinoma: Comparison with 18F-FDG PET-CT. Nucl Med Commun. 2012;33:766–74. doi: 10.1097/MNM.0b013e3283541157. [DOI] [PubMed] [Google Scholar]
- 64.Gifford RW, Jr, Manger WM, Bravo EL. Pheochromocytoma. Endocrinol Metab Clin North Am. 1994;23:387–404. [PubMed] [Google Scholar]
- 65.Neumann HP, Berger DP, Sigmund G, Blum U, Schmidt D, Parmer RJ, et al. Pheochromocytomas, multiple endocrine neoplasia type 2, and von Hippel-Lindau disease. N Engl J Med. 1993;329:1531–8. doi: 10.1056/NEJM199311183292103. [DOI] [PubMed] [Google Scholar]
- 66.Hinerman RW, Amdur RJ, Morris CG, Kirwan J, Mendenhall WM. Definitive radiotherapy in the management of paragangliomas arising in the head and neck: A 35- year experience. Head Neck. 2008;30:1431–8. doi: 10.1002/hed.20885. [DOI] [PubMed] [Google Scholar]
- 67.Grufferman S, Gillman MW, Pasternak LR, Peterson CL, Young WG., Jr Familial carotid body tumors: Case report and epidemiologic review. Cancer. 1980;46:2116–22. doi: 10.1002/1097-0142(19801101)46:9<2116::aid-cncr2820460934>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 68.Kliewer KE, Wen DR, Cancilla PA, Cochran AJ. Paragangliomas: Assessment of prognosis by histologic, immunohistochemical, and ultrastructural techniques. Hum Pathol. 1989;20:29–39. doi: 10.1016/0046-8177(89)90199-8. [DOI] [PubMed] [Google Scholar]
- 69.Kudva YC, Sawka AM, Young WF., Jr Clinical review 164: The laboratory diagnosis of adrenal pheochromocytoma: The Mayo Clinic experience. J Clin Endocrinol Metab. 2003;88:4533–9. doi: 10.1210/jc.2003-030720. [DOI] [PubMed] [Google Scholar]
- 70.Khafagi FA, Shapiro B, Fig LM, Mallette S, Sisson JC. Labetalol reduces 131I MIBG uptake by pheochromocytoma and normal tissues. J Nucl Med. 1989;30:481–9. [PubMed] [Google Scholar]
- 71.Ueberberg B, Tourne H, Redmann A, Walz MK, Schmid KW, Mann K, et al. Differential expression of the human somatostatin receptor subtypes sst1 to sst5 in various adrenal tumors and normal adrenal gland. Horm Metab Res. 2005;37:722–8. doi: 10.1055/s-2005-921092. [DOI] [PubMed] [Google Scholar]
- 72.van der Harst E, de Herder WW, Bruining HA, Bonjer HJ, de Krijger RR, Lamberts SW, et al. (123) I metaiodobenzylguanidine and (111) In octreotide uptake in begnign and malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86:685–93. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- 73.Win Z, Al-Nahhas A, Towey D, Todd JF, Rubello D, Lewington V. 68Ga-DOTATATE PET in neuroectodermal tumors: First experience. Nucl Med Commun. 2007;28:359–63. doi: 10.1097/MNM.0b013e32808ea0b0. [DOI] [PubMed] [Google Scholar]
- 74.Maurice JB, Troke R, Win Z, Ramachandran R, Al-Nahhas A, Naji M, et al. A comparison of the performance of 68Ga-DOTATATE PET/CT and 12≥I-MIBG SPECT in the diagnosis and follow-up of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39:1266–70. doi: 10.1007/s00259-012-2119-7. [DOI] [PubMed] [Google Scholar]
- 75.Naswa N, Sharma P, Nazar AH, Agarwal KK, Kumar R, Ammini AC, et al. Prospective evaluation of 68Ga-DOTA-NOC PET-CT in phaeochromocytoma and paraganglioma: Preliminary results from a single centre study. Eur Radiol. 2012;22:710–9. doi: 10.1007/s00330-011-2289-x. [DOI] [PubMed] [Google Scholar]
- 76.Naswa N, Sharma P, Soundararajan R, Patnecha M, Lata S, Kumar R, et al. Pre-operative characterisation of indeterminate large adrenal masses with dual tracer PET-CT using 18F FDG and 68Ga-DOTANOC: Initial results. Diagn Interv Radiol. 2012;19:294–8. doi: 10.5152/dir.2013.048. [DOI] [PubMed] [Google Scholar]
- 77.Naswa N, Kumar A, Sharma P, Bal C, Malhotra A, Kumar R. Imaging carotid body chemodectomas with 68Ga-DOTA-NOC PET-CT. Br J Radiol. 2012;85:1140–5. doi: 10.1259/bjr/17448792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharma P, Thakar A, Suman KCS, Dhull VS, Singh H, Naswa N, et al. 68Ga-DOTANOC PET-CT for baseline evaluation of patients with head and neck paraganglioma. J Nucl Med. 2013;54:841–7. doi: 10.2967/jnumed.112.115485. [DOI] [PubMed] [Google Scholar]
- 79.Taïeb D, Neumann H, Rubello D, Al-Nahhas A, Guillet B, Hindié E. Modern nuclear imaging for paragangliomas: Beyond SPECT. J Nucl Med. 2012;53:264–74. doi: 10.2967/jnumed.111.098152. [DOI] [PubMed] [Google Scholar]
- 80.Thakker RV. Multiple endocrine neoplasia type 1. In: De Groot LJ, Jameson JL, editors. Endocrinology. 4th ed. Philadelphia: Saunders; 2000. pp. 2503–17. [Google Scholar]
- 81.Wohllk N, Schweizer H, Erlic Z, Schmid KW, Walz MK, Raue F, et al. Multiple endocrine neoplasia type 2. Best Pract Res Clin Endocrinol Metab. 2010;24:371–87. doi: 10.1016/j.beem.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Froeling V, Elgeti F, Maurer MH, Scheurig-Muenkler C, Beck A, Kroencke TJ, et al. Impact of Ga-68 DOTATOC PET/CT on the diagnosis and treatment of patients with multiple endocrine neoplasia. Ann Nucl Med. 2012;26:738–43. doi: 10.1007/s12149-012-0634-z. [DOI] [PubMed] [Google Scholar]
- 83.Naswa N, Das CJ, Sharma P, Karunanithi S, Bal C, Kumar R. Ectopic pituitary adenoma with empty sella in the setting of MEN-1 syndrome: Detection with 68Ga-DOTANOC PET/CT. Jpn J Radiol. 2012;30:783–6. doi: 10.1007/s11604-012-0117-0. [DOI] [PubMed] [Google Scholar]
- 84.Ambrosini V, Campana D, Allegri V, Opocher G, Fanti S. 68Ga-DOTA-NOC PET/CT detects somatostatin receptors expression in von hippel-lindau cerebellar disease. Clin Nucl Med. 2011;36:64–5. doi: 10.1097/RLU.0b013e3181fef14a. [DOI] [PubMed] [Google Scholar]
- 85.Afshar-Oromieh A, Giesel FL, Linhart HG, Haberkorn U, Haufe S, Combs SE, et al. Detection of cranial meningiomas: Comparison of 68Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging. 2012;39:1409–15. doi: 10.1007/s00259-012-2155-3. [DOI] [PubMed] [Google Scholar]
- 86.Brogsitter C, Zöphel K, Wunderlich G, Kämmerer E, Stein A, Kotzerke J. Comparison between F-18 fluorodeoxyglucose and Ga-68 DOTATOC in metastasized melanoma. Nucl Med Commun. 2013;34:47–9. doi: 10.1097/MNM.0b013e32835ae4ed. [DOI] [PubMed] [Google Scholar]
- 87.Clifton-Bligh RJ, Hofman MS, Duncan E, Sim IeW, Darnell D, Clarkson A, et al. Improving diagnosis of tumor-induced osteomalacia with gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab. 2013;98:687–94. doi: 10.1210/jc.2012-3642. [DOI] [PubMed] [Google Scholar]


