Abstract
Ca deposition by isolated matrix vesicles from fetal calf growth plate cartilage and by a deoxycholate extract from matrix vesicles that included their phosphatase was studied under defined in vitro conditions. Electron microscopy showed that after removal of deoxycholate and lyophilization of the vesicle extract, new vesicles were reconstituted, often with multiple membrane layers. Both intact calf vesicles and reconstituted vesicles initiated Ca deposition maximally when supplied with ATP, GTP, CTP, or UTP. Only nucleoside triphosphates supported Ca deposition well; mono- and diphosphoesters, although hydrolyzed, were ineffective as substrates. Nucleoside triphosphates supported Ca deposition even if the final [Ca] X [P] reached in the reaction mixture was below a metastable level (3.5 mM2), suggesting that matrix vesicles or reconstituted vesicles promote calcification by localizing Ca or PO4 or both. ATP or GTP supported Ca deposition readily at concentrations ranging from 0.25 to 1.0 mM but, at 2.5 and 5.0 mM, Ca deposition was inhibited. The ATPase of intact matrix vesicles and reconstituted vesicles was stimulated by addition of Ca2+ and Mg2+. Ca deposition did not require additional Mg2+. These results lend support to the hypothesis that matrix vesicles and their phosphatases play an important role in mineralization.
Full text
PDF
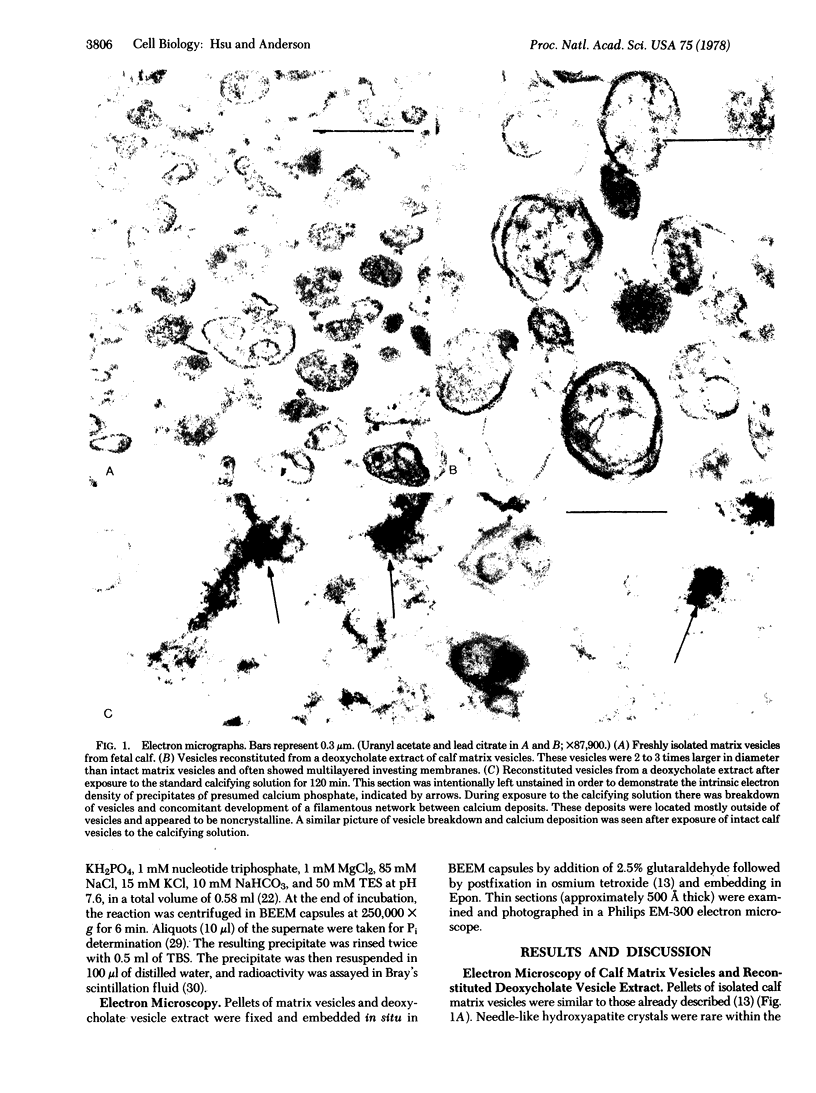
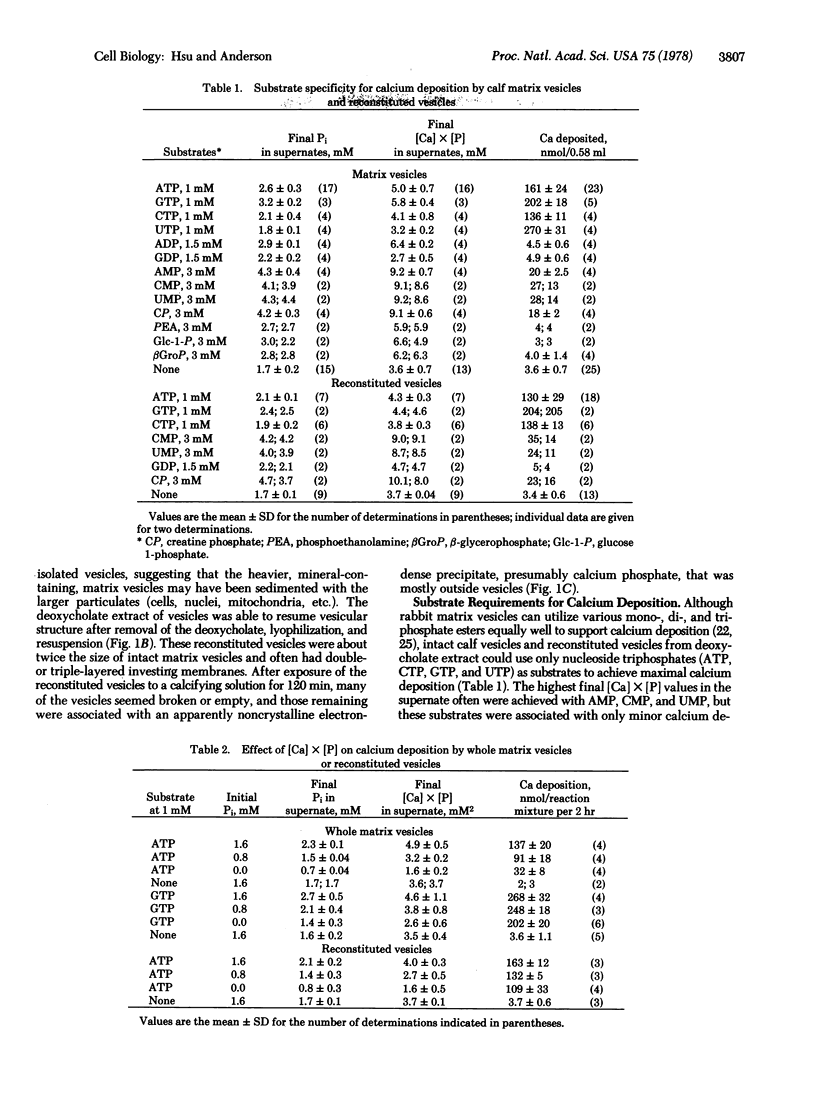
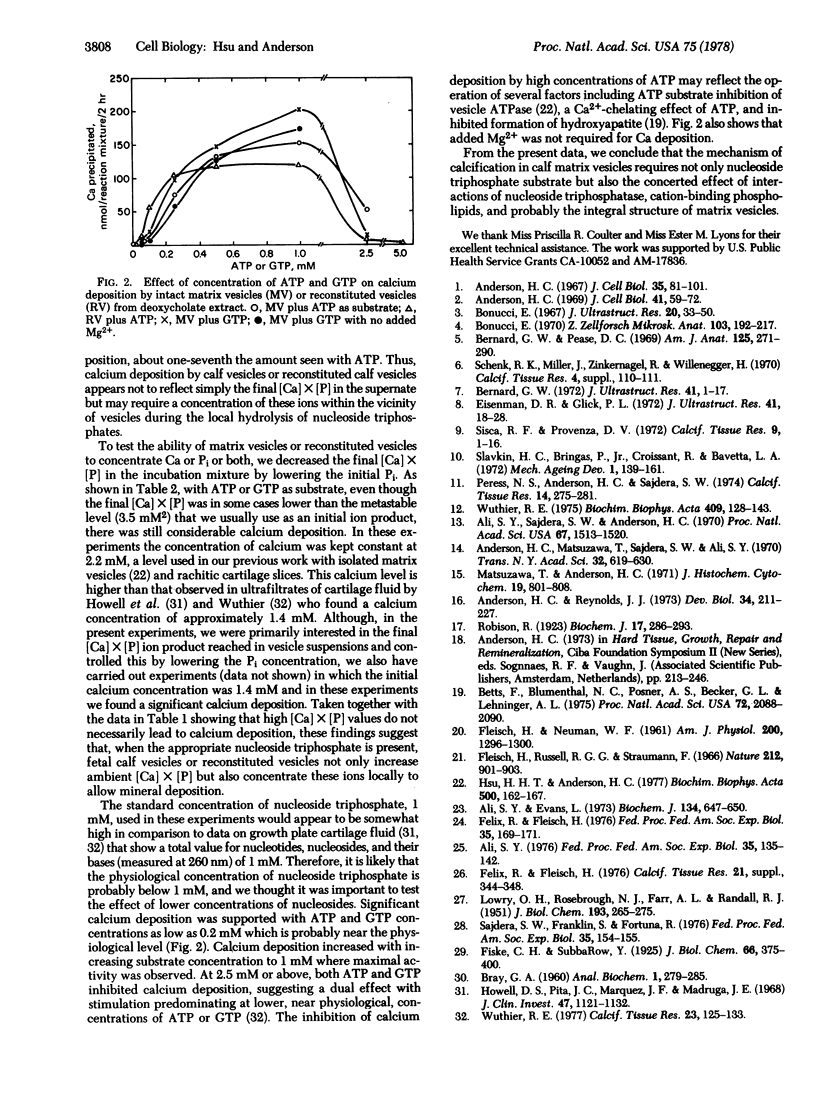
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y. Analysis of matrix vesicles and their role in the calcification of epiphyseal cartilage. Fed Proc. 1976 Feb;35(2):135–142. [PubMed] [Google Scholar]
- Ali S. Y., Evans L. The uptake of [Ca]calcium ions by matrix vesicles isolated from calcifying cartilage (Short Communication). Biochem J. 1973 Jun;134(2):647–650. doi: 10.1042/bj1340647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y., Sajdera S. W., Anderson H. C. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H. C. Electron microscopic studies of induced cartilage development and calcification. J Cell Biol. 1967 Oct;35(1):81–101. doi: 10.1083/jcb.35.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H. C., Matsuzawa T., Sajdera S. W., Ali S. Y. Membranous particles in calcifying cartilage matrix. Trans N Y Acad Sci. 1970 May;32(5):619–630. doi: 10.1111/j.2164-0947.1970.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Anderson H. C., Reynolds J. J. Pyrophosphate stimulation of calcium uptake into cultured embryonic bones. Fine structure of matrix vesicles and their role in calcification. Dev Biol. 1973 Oct;34(2):211–227. doi: 10.1016/0012-1606(73)90351-5. [DOI] [PubMed] [Google Scholar]
- Anderson H. C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969 Apr;41(1):59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard G. W., Pease D. C. An electron microscopic study of initial intramembranous osteogenesis. Am J Anat. 1969 Jul;125(3):271–290. doi: 10.1002/aja.1001250303. [DOI] [PubMed] [Google Scholar]
- Bernard G. W. Ultrastructural observations of initial calcification in dentine and enamel. J Ultrastruct Res. 1972 Oct;41(1):1–17. doi: 10.1016/s0022-5320(72)90034-2. [DOI] [PubMed] [Google Scholar]
- Betts F., Blumenthal N. C., Posner A. S., Becker G. L., Lehninger A. L. Atomic structure of intracellular amorphous calcium phosphate deposits. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2088–2090. doi: 10.1073/pnas.72.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonucci E. Fine structure and histochemistry of "calcifying globules" in epiphyseal cartilage. Z Zellforsch Mikrosk Anat. 1970;103(2):192–217. doi: 10.1007/BF00337312. [DOI] [PubMed] [Google Scholar]
- Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967 Sep;20(1):33–50. doi: 10.1016/s0022-5320(67)80034-0. [DOI] [PubMed] [Google Scholar]
- Eisenmann D. R., Glick P. L. Ultrastructure of initial crystal formation in dentin. J Ultrastruct Res. 1972 Oct;41(1):18–28. doi: 10.1016/s0022-5320(72)90035-4. [DOI] [PubMed] [Google Scholar]
- FLEISH H., NEUMAN W. F. Mechanisms of calcification: role of collagen, polyphosphates, and phosphatase. Am J Physiol. 1961 Jun;200:1296–1300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- Felix R., Fleisch H. Role of matrix vesicles in calcification. Fed Proc. 1976 Feb;35(2):169–171. [PubMed] [Google Scholar]
- Felix R., Fleisch H. The role of matrix vesicles in calcification. Calcif Tissue Res. 1976 Aug;21 (Suppl):344–348. [PubMed] [Google Scholar]
- Fleisch H., Russell R. G., Straumann F. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature. 1966 Nov 26;212(5065):901–903. doi: 10.1038/212901a0. [DOI] [PubMed] [Google Scholar]
- Howell D. S., Pita J. C., Marquez J. F., Madruga J. E. Partition of calcium, phosphate, and protein in the fluid phase aspirated at calcifying sites in epiphyseal cartilage. J Clin Invest. 1968 May;47(5):1121–1132. doi: 10.1172/JCI105801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. H., Anderson H. C. A simple and defined method to study calcification by isolated matrix vesicles. Effect of ATP and vesicle phosphatase. Biochim Biophys Acta. 1977 Nov 7;500(1):162–172. doi: 10.1016/0304-4165(77)90056-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuzawa T., Anderson H. C. Phosphatases of epiphyseal cartilage studied by electron microscopic cytochemical methods. J Histochem Cytochem. 1971 Dec;19(12):801–808. doi: 10.1177/19.12.801. [DOI] [PubMed] [Google Scholar]
- Peress N. S., Anderson H. C., Sajdera S. W. The lipids of matrix vesicles from bovine fetal epiphyseal cartilage. Calcif Tissue Res. 1974 May 28;14(4):275–281. doi: 10.1007/BF02060301. [DOI] [PubMed] [Google Scholar]
- Robison R. The Possible Significance of Hexosephosphoric Esters in Ossification. Biochem J. 1923;17(2):286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdera S. W., Franklin S., Fortuna R. Matrix vesicles of bovine fetal cartilage: metabolic potential and solubilization with detergents. Fed Proc. 1976 Feb;35(2):154–155. [PubMed] [Google Scholar]
- Schenk R. K., Müller J., Zinkernagel R., Willenegger H. Ultrastructure of normal and abnormal bone repair. Calcif Tissue Res. 1970;(Suppl):110–111. doi: 10.1007/BF02152377. [DOI] [PubMed] [Google Scholar]
- Sisca R. F., Provenza D. V. Initial dentin formation in human deciduous teeth. An electron microscope study. Calcif Tissue Res. 1972;9(1):1–16. doi: 10.1007/BF02061941. [DOI] [PubMed] [Google Scholar]
- Wuthier R. E. Electrolytes of isolated epiphyseal chondrocytes, matrix vesicles, and extracellular fluid. Calcif Tissue Res. 1977 Jun 28;23(2):125–133. doi: 10.1007/BF02012777. [DOI] [PubMed] [Google Scholar]
- Wuthier R. E. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochim Biophys Acta. 1975 Oct 21;409(1):128–143. doi: 10.1016/0005-2760(75)90087-9. [DOI] [PubMed] [Google Scholar]