Abstract
Investigation of the endemic Madagascan plant Nematostylis anthophylla (Rubiaceae) for antiproliferative activity against the A2780 ovarian cancer cell line led to the isolation of the known triterpene saponin randianin (1) and the two new bioactive triterpene saponins 2″-O-acetylrandianin (2) and 6″-O-acetylrandianin (3). The structures of the two new compounds were elucidated based on analysis of their 1D and 2D NMR spectra, and mass spectrometric data. The three isolated triterpene saponins displayed moderate but selective antiproliferative activity, with IC50 values of 1.2, 1.7 and 2.2 μM, respectively, against the A2780 ovarian cancer, but only weak inhibition of the proliferation of A2058 melanoma and the H522 lung cancer cell lines.
Keywords: Triterpene saponin, Antiproliferative activity, Selectivity, Nematostylis anthophylla (Rubiaceae)
Introduction
As part of our engagement in an International Cooperative Biodiversity Group (ICBG) program, we are focusing on the search for antiproliferative natural products from both the tropical dry forests and the rainforests of Madagascar [1-3]. The A2780 human ovarian cancer cell line is used as the primary screen because it is a stable and yet relatively drug-sensitive cell line and gives reproducible results. As a part of this research, an EtOH extract from the roots of Nematostylis anthophylla (Rubiaceae) from the Highlands of Central Madagascar exhibited antiproliferative activity against the A2780 cell line, with an IC50 value of 6.9 μg/ml. The Rubiaceae family is a large family of 630 genera and about 13,000 species found worldwide [4]. This family is a rich source of indole alkaloids, terpenoids and anthraquinones, all of which are well-known for their broad range of bioactivity, including antimicrobial, antimalarial, antidiabetic, vasorelaxant, cytotoxic, antioxidant, and anti-inflammatory activities among others [5-9]. Since Nematostylis is one of the many genera of the Rubiaceae family that have not been systematically investigated for their phytochemical composition, the ethanol extract of Nematostylis anthophylla was selected for bioassay-guided fractionation to isolate its active components.
Results and Discussion
Isolation of Bioactive Compounds
An EtOH extract of the roots of Nematostylis anthophylla was subjected to liquid-liquid partitioning to give an active n-BuOH fraction with an IC50 value of 2.2 μg/ml. Bioassay-guided separation, including LH-20 size-exclusion, HP-20 Diaion and silica gel normal-phase chromatography, was used to obtain three bioactive compounds comprising the known triterpene saponin randianin (1) and the two new related glycosides 2″-O-acetylrandianin (2) and 6″-O-acetylrandianin (3). All three compounds had moderate antiproliferative activity against A2780 ovarian cancer cells, with IC50 values of 2.2 μM, 1.2 μM and 1.7 μM, respectively. Herein, we report the structural elucidation and antiproliferative properties of the two new isolates.
Identification of Compounds 1 and 2
Compound 1 was identified as randianin (oleanolic acid-3-O-β-d-glucopyranosyl- (1→3)-β-d-glucopyranoside) by comparison of its chemical and spectroscopic data with those reported in the literature for the aglycone [10] and the glycoside [11].
Compound 2, [α]21D +12° (c 1.2, MeOH), was isolated as a light yellow solid. Its positive ion HRESIMS revealed peaks for cationized molecules at m/z 845.4692 [M+Na]+ and 861.4618 [M+K]+, corresponding to a molecular formula of C44H70O14. The observation of a carbonyl absorption at 1734 cm−1 in the IR spectrum, a 13C NMR resonance at δ(C) 170.7 ppm, and a singlet signal at δ(H) 1.98 ppm in the 1H NMR spectrum suggested the presence of an acetyl group. Meanwhile, its glycosidic nature was corroborated by the presence of two anomeric proton signals at δ(H) 4.83 and 5.43 ppm. In addition to the methyl and carbonyl carbons of the acetyl group, there were 42 carbon signals in the 13C NMR spectrum, among which 30 carbon signals were assigned to a triterpenoid aglycone and the remaining 12 carbons to a disaccharide moiety. The 1H NMR spectrum of 2 indicated that the aglycone had seven methyl groups, with three-proton singlets at δ(H) 0.80, 0.89, 0.97, 1.00, 1.03, 1.27 and 1.33, and one olefinic proton at δ(H) 5.49. Correspondingly, signals for seven methyl carbons at δ(C) 15.8, 17.2, 17.8, 24.1, 26.6, 28.5 and 33.7 ppm, and for two olefinic carbons at δ(C) 122.9 and 145.2 ppm, were observed in the 13C NMR spectrum (Table 1). The presence of a carbonyl absorption at 1689 cm−1 and a broad hydroxyl absorption at 3453 cm−1 in its IR spectrum, together with a 13C NMR resonance at δ(C) 180.6 ppm, supported the presence of a carboxylic acid group.
Table 1.
NMR Spectroscopic Data for 1-3 in Pyridine-d5 (1H-500 MHz, 13C-125 MHz)
| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | |
| 1a | 1.21-1.25 m | 39.0, CH2 | 1.21-1.25 m | 39.0, CH2 | 1.22-1.26 m | 39.0, CH2 |
| 1b | 1.39-1.42 m | 1.37-1.40 m | 1.39-1.42 m | |||
| 2a | 1.75-1.78 m | 26.8, CH2 | 1.76-1.79 m | 26.9, CH2 | 1.74-1.77 m | 26.8, CH2 |
| 2b | 2.14-2.18 m | 2.15-2.19 m | 2.14-2.18 m | |||
| 3 | 3.36 dd (4.4, 11.9) | 89.3, CH | 3.36 dd (4.4, 11.9) | 89.3, CH | 3.36 dd (4.4, 11.7) | 89.4, CH |
| 4 | - | 40.1, C | - | 40.1, C | - | 40.1, C |
| 5 | 0.76-0.80 m | 56.1, CH | 0.76-0.79 m | 56.1, CH | 0.78-0.82 m | 56.1, CH |
| 6a | 1.21-1.25 m | 18.8, CH2 | 1.22-1.25 m | 18.8, CH2 | 1.23-1.26 m | 18.8, CH2 |
| 6b | 1.45-1.49 m | 1.46-1.50 m | 1.46-1.50 m | |||
| 7a | 1.78-1.82 m | 33.6, CH2 | 1.78-1.82 m | 33.6, CH2 | 1.78-1.82 m | 33.6, CH2 |
| 7b | 1.85-1.87 m | 1.85-1.87 m | 1.85-1.87 m | |||
| 8 | - | 39.8, C | - | 39.8, C | - | 39.8, C |
| 9 | 1.65 brt (8.9) | 48.3, CH | 1.64 brt (8.9) | 48.4, CH | 1.65 brt (8.9) | 48.3, CH |
| 10 | - | 37.3, C | - | 37.3, C | - | 37.3, C |
| 11 | 1.88-1.92 m | 24.1, CH2 | 1.88-1.92 m | 24.1, CH2 | 1.88-1.92 m | 24.1, CH2 |
| 12 | 5.50 t (3.3) | 122.8, CH | 5.49 t (3.3) | 122.9, CH | 5.50 t (3.3) | 122.8, CH |
| 13 | - | 145.3, C | - | 145.2, C | - | 145.3, C |
| 14 | - | 42.5, C | - | 42.6, C | - | 42.5, C |
| 15a | 1.18-1.21 m | 28.7, CH2 | 1.18-1.21 m | 28.7, CH2 | 1.18-1.21 m | 28.7, CH2 |
| 15b | 2.02-2.05 m | 2.02-2.05 m | 2.02-2.05 m | |||
| 16a | 1.76-1.79 m | 24.1, CH2 | 1.75-1.78 m | 24.1, CH2 | 1.76-1.79 m | 24.1, CH2 |
| 16b | 2.18-2.21 m | 2.17-2.20 m | 2.18-2.21 m | |||
| 17 | - | 47.0, C | - | 47.1, C | - | 47.0, C |
| 18 | 3.32 dd (4.1, 14.0) | 42.4, CH | 3.32 dd (4.1, 14.0) | 42.4, CH | 3.32 dd (4.0, 13.9) | 42.4, CH |
| 19a | 1.28-1.31 m | 46.9, CH2 | 1.28-1.31 m | 46.9, CH2 | 1.28-1.31 m | 46.9, CH2 |
| 19b | 1.82-1.84 m | 1.82-1.84 m | 1.82-1.84 m | |||
| 20 | - | 31.3, C | - | 31.3, C | - | 31.3, C |
| 21a | 1.49-1.52 m | 34.6, CH2 | 1.49-1.52 m | 34.6, CH2 | 1.49-1.52 m | 34.6, CH2 |
| 21b | 1.82-1.84 m | 1.82-1.84 m | 1.82-1.84 m | |||
| 22a | 1.45-1.49 m | 33.6, CH2 | 1.45-1.49 m | 33.6, CH2 | 1.46-1.50 m | 33.5, CH2 |
| 22b | 2.05-2.08 m | 2.05-2.08 m | 2.05-2.08 m | |||
| 23 | 1.27 s | 17.8, CH3 | 1.27 s | 17.8, CH3 | 1.32 s | 17.8, CH3 |
| 24 | 0.89 s | 28.5, CH3 | 0.89 s | 28.5, CH3 | 1.01 s | 28.5, CH3 |
| 25 | 0.82 s | 15.8, CH2 | 0.80 s | 15.8, CH2 | 0.82 s | 15.8, CH2 |
| 26 | 1.00 s | 17.4, CH3 | 1.00 s | 17.2, CH3 | 1.00 s | 17.3, CH3 |
| 27 | 1.33 s | 26.5, CH3 | 1.33 s | 26.6, CH3 | 1.33 s | 26.5, CH3 |
| 28 | - | 180.7, C | - | 180.6, C | - | 180.7, C |
| 29 | 1.03 s | 24.1, CH3 | 1.03 s | 24.1, CH3 | 1.02 s | 24.1, CH3 |
| 30 | 0.97 s | 33.7, CH3 | 0.97 s | 33.7, CH3 | 0.97 s | 33.6, CH3 |
| C-3-Glucose | ||||||
| 1′ | 4.91 d (7.8) | 106.7, CH | 4.83 d (7.8) | 107.1, CH | 4.91 d (7.6) | 106.7, CH |
| 2′ | 4.09-4.11 m | 74.8, CH | 3.96-4.02 m | 74.4, CH | 4.05-4.08 m | 74.7, CH |
| 3′ | 4.23 t (8.8) | 89.3, CH | 4.15 t (8.8) | 89.3, CH | 4.18 t (8.9) | 89.7, CH |
| 4′ | 4.11-4.14 m | 70.2, CH | 4.04-4.08 m | 70.7, CH | 4.11 t (9.3) | 70.0, CH |
| 5′ | 3.92-3.98 m | 78.3, CH | 3.89-3.93 m | 78.1, CH | 3.92-3.98 m | 78.3, CH |
| 6′a | 4.32 d (11.3) | 62.9, CH | 4.26 d (11.4) | 63.1, CH | 4.32 dd (6.2, 11.8) | 62.9, CH |
| 6′b | 4.51 d (11.0) | 4.48 dd (2.1, 11.5) | 4.52 dd (2.2, 11.8) | |||
| C-3′-Glucose | ||||||
| 1″ | 5.32 d (7.8) | 106.3, CH | 5.43 d (8.1) | 103.7, CH | 5.25 d (7.9) | 106.3, CH |
| 2″ | 4.02-4.05 m | 75.9, CH | 5.66 dd (8.1, 9.1) | 75.7, CH | 4.03-4.05 m | 75.7, CH |
| 3″ | 4.26 t (9.1) | 75.8, CH | 4.31 t (9.1) | 76.6, CH | 4.22 t (9.1) | 75.7, CH |
| 4″ | 4.20 t (9.2) | 72.0, CH | 4.20 t (9.2) | 72.3, CH | 4.01 t (9.1) | 71.9, CH |
| 5″ | 4.07-4.09 m | 79.1, CH | 4.07-4.10 m | 79.1, CH | 4.23-4.26 m | 78.3, CH |
| 6″a | 4.34 d (11.1) | 62.8, CH | 4.28 d (11.1) | 62.7, CH | 4.67 dd (6.8, 11.7) | 64.9, CH |
| 6″b | 4.56 d (10.7) | 4.58 dd (2.1, 11.5) | 4.95 dd (2.2, 11.8) | |||
| 2″- Acetyl | ||||||
| CO | 170.7, C | |||||
| CH3 | 1.98 s | 21.5, CH3 | ||||
| 6″-Acetyl | ||||||
| CO | 171.2, C | |||||
| CH3 | 2.00 s | 21.0, CH3 | ||||
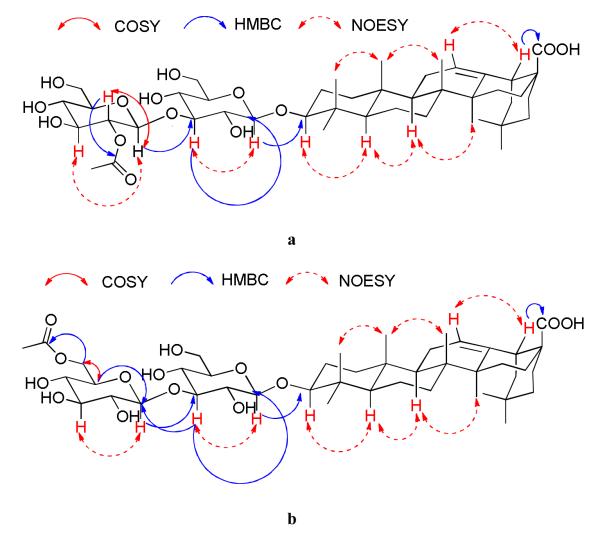
Inspection of the 1H and 13C NMR spectra of compound 2 indicated that it had the same oleanolic acid aglycone as compound 1. The HMBC correlation between H(18) (dd, J = 4.1, 14.0 Hz) and C(28) confirmed that the carboxylic carbon was connected to C(17) [12]. HMBC correlations between the H(1′) anomeric proton and C(3), as well as between H(3) and the anomeric carbon C(1′), confirmed that the disaccharide moiety was connected to C(3) (Figure 2a).
Figure 2.
a. HMBC, COSY and NOESY correlations of 2.
b. HMBC, COSY and NOESY correlations of 3.
Both sugar molecules, which were represented by the of two sets of anomeric signals at δ(H) 4.83/δ(C) 107.1 ppm and δ(H) 5.43/δ(C) 103.7 ppm, respectively, were identified as glucose, based on the similarity of their 13C NMR chemical shifts with those of the sugar moiety of 1. The linkage between the two glucopyranosyl units was concluded to be 1→3 by the observation of HMBC correlations between H(3′) and two anomeric carbons (C(1′) and C(1″)), as well as the cross-peak between H(3′) and H(2′) in the COSY spectrum (Figure 2a). The coupling constants between H(1′) and H(2′), and H(1″) and H(2″) (J= 7.8 and 8.1 Hz, respectively) indicated their axial-axial conformation and thus the β-configuration of the two sugar units. The location of the acetyl group was deduced to be at the hydroxyl group of C(2″) of a glucopyranosyl residue, based on the comparison of the 1H and 13C NMR spectra of 2 with those of 1. Due to this acetylation, the chemical shift of H(2″) of compound 2 was δH 5.66 as compared to δH 4.04 for compound 1, while other protons in the distal glucose had chemical shifts similar to those of compound 1. The position of the acetyl group was confirmed by the COSY cross-peak between the downfield H(2″) (δH 5.66) and the corresponding anomeric proton H(1″), and a three-bond HMBC correlation between H(2″) and the carbonyl carbon of the acetyl group (Figure 2a).
In order to determine the absolute configuration of the two glucoses and to confirm the overall structure assignment, compound 2 was hydrolyzed with 6 M ammonium hydroxide to yield a product identified as randianin (1) by its 1H and 13C-NMR spectra. Further hydrolysis of 1 with 3 M hydrochloric acid yielded oleanolic acid, identified by its 1H and 13C-NMR spectra, and a single monosaccharide, identified as d-glucose by observation of a single TLC spot with the same Rf value as a d-glucose standard. Its absolute configuration was determined to be d based on its positive optical rotation.
Based on this evidence, the structure of 2 was assigned as oleanolic acid-3-O-β-d-glucopyranosyl-(1→3)-(2″-O-acetyl)-β-d-glucopyranoside, or 2″-O-acetylrandianin.
Identification of Compound 3
Compound 3, isolated as light yellowish solid, [α]21D +17° (c 1.2, MeOH), had the same molecular formula as compound 2 as determined by HRESIMS (m/z 845.4643 [M+Na]+ and 861.4569 [M+K]+), corresponding to a molecular formula of C44H70O14. Due to the similarity of its NMR spectroscopic data with those of compounds 1 and 2, the aglycone portion of 3 was also assigned as oleanolic acid, with the disaccharide moiety connected to C-3 of the aglycone.
As in compound 2, the presence of two sugar molecules was suggested by the NMR spectra, which showed two sets of anomeric signals at δH 4.91/δC 106.7 and δH 5.25/δC 106.3, respectively. The two sugars were determined to be glucose, as corroborated by the similarity of the 13C NMR chemical shifts of all carbons compared to those of compound 1. The linkage between the two glucopyranosyl units was determined to be 1→3 by the observation of the HMBC correlations between H(3′) and two anomeric carbons (C(1′) and C(1″)), as well as the cross-peak between H(3′) and H(2′) in the COSY spectrum (Figure 2b). The coupling constants between H(1′) and H(2′), and H(1″) and H(2″) (J = 7.8 and 7.8 Hz, respectively) indicated their axial-axial orientation and thus the β-configuration of the two sugar units. The presence of an acetyl group was indicated by a carbonyl absorption at 1727 cm−1 in its IR spectrum, 13C NMR resonances at δC 171.2 ppm, and a singlet signal at 2.00 ppm in its 1H NMR spectrum. The C(6″) hydroxyl group of the outer glucose of 3 was acetylated, as opposed to the C(2″) of compound 2. This was determined by comparing the NMR spectral data of the outer glucose of 3 with those of 1. The chemical shift of the two diastereotopic protons H(6″) of 1 were shifted from δH 4.54 and 4.58 ppm to δH 4.67 and 4.95 ppm in 3, while the resonances of the other protons of the outer glucose are similar to those of compound 1. Furthermore, the location of the acetyl group at C(6″) was confirmed by the COSY cross-peak between H(6″) and H(5″), and a three-bond HMBC correlation between H(6″) and the acetyl carbonyl carbon at 171.2 ppm, and between H(5″) and the anomeric carbon C(1″) (Figure 2b).
As with compound 2, the absolute configuration of the two glucose units and the overall structure assignment were confirmed by successive basic hydrolysis of 3 to randianin followed by acidic hydrolysis to oleanolic acid and d-glucose. Based on this evidence, the structure of 3 was elucidated as oleanolic acid-3-O-β-d-glucopyranosyl-(1→3)-(6″-O-acetyl)- β-d-glucopyranoside, or 6″-O-acetylrandianin.
Biological Evaluation
Compounds 1 – 3 were tested for antiproliferative activity against the A2780 ovarian cancer, the A2058 melanoma, and the H522 lung cancer cell lines. All three compounds showed modest inhibition of the proliferation of A2780 ovarian cancer cells, with IC50 values in the low micromolar range. However, they showed only weak inhibition of the proliferation of A2058 melanoma and the H522 lung cancer cell lines (Table 2). Several hundred cytotoxic triterpene saponins have been identified from plants, but only a few of them showed selective antiproliferative activity [13]. 2″-O-Acetylrandianin (2) and 6″-O-acetylrandianin (3) are examples of compounds that selectively inhibit the proliferation of A2780 ovarian cancer cells. Furthermore, in the A2780 assay, the cytotoxicity of the two acetylated saponins is stronger than that of randianin (1), which has no acetyl group in its structure. This suggests that the increase in activity on acetylation may be due to an increase in lipophilicity, facilitating cellular uptake [14].
Table 2.
Antiproliferative activities of compounds 1-3.
| Compound | IC50 (μM) | ||
|---|---|---|---|
| A2780a,b) | A2058b) | H522b) | |
| 1 | 2.2 ± 0.2 | 7.63 | 7.32 |
| 2 | 1.7 ± 0.1 | >3.3, <10 | >10 |
| 3 | 1.2 ± 0.3 | >3.3, <10 | >10 |
| Paclitaxel | 0.028± 0.003 | ND | ND |
| Vinblastine | ND | 0.004 | 0.009 |
Mean of three replicates
ND = not determined
Experimental Part
General
Optical rotations were recorded on a JASCO P-2000 polarimeter. IR spectroscopic data were measured on a MIDAC M-series FTIR spectrophotometer. NMR spectra were recorded in pyridine-d5 on a Bruker Avance 500 spectrometer. The chemical shifts are given in δ (ppm), and coupling constants (J) are reported in Hz. Mass spectra were obtained on an Agilent 6220 LC-TOF-MS in the positive ion mode.
Antiproliferative Bioassays
Antiproliferative activities were obtained at Virginia Polytechnic Institute and State University against the drug-sensitive A2780 human ovarian cancer cell line as previously described [15]. The values reported are the mean of three replicates. Antiproliferative activities against the A2058 melanoma and the H522 lung cancer cell lines were determined at Eisai Inc. by similar procedures to those used for the H460 cell line [16].
Plant Materials
A sample of the roots of Nematostylis anthophylla (A. Rich.) Baill. was collected in March 2011. The sample was a shrub of 60 cm with red flowers and succulent leaves, growing in rocky habitat in the Vakinakaratra region of the Antsirabe II district, Madagascar at an elevation of 1650 m., and coordinates 20°03′59″S 047°00′01”E (−20.0663889, 47.0002778). Duplicate voucher specimens (Richard Randrianaivo et al. 1803) have been deposited at the Parc Botanique et Zoologique de Tsimbazaza (TAN), at the Centre National d’Application des Recherches Pharmaceutiques in Antananarivo, Madagascar (CNARP), the Missouri Botanical Garden in St. Louis, Missouri (MO), and the Muséum National d’Histoire Naturelle in Paris, France (P).
Extraction and Isolation
Dried root parts of N. anthophylla (273 g) were ground in a hammer mill, then extracted with EtOH by percolation for 24 h at room temperature to give the crude extract MG 4657 (12.4 g), of which 3.2 g was shipped to Virginia Tech for bioassay-guided isolation. A 1.1 g sample of MG 4657 (IC50 6.9 μg/ml) was suspended in aqueous MeOH (MeOH-H2O 9:1, 100 ml), and extracted with hexane (3 × 100 ml portions). The aqueous layer was then diluted to 60% MeOH (v/v) with H2O and extracted with dichloromethane (DCM) (3 × 150 ml portions). The remaining aqueous layer was further extracted with BuOH (3 × 100 ml portions). The hexane fraction was evaporated in vacuo to leave 131.2 mg of material with IC50 > 20 μg/ml. The residue from the DCM fraction (166.1 mg) had an IC50 of 7.7 μg/ml, the residue from the BuOH fraction (248.6 mg) had an IC50 of 2.5 μg/ml and the remaining aqueous MeOH fraction had an IC50 > 20 μg/ml. Chromatography of the DCM fraction over a Sephadex® LH-20 size exclusion column with elution by DCM: MeOH 1:1 was used to obtain six fractions, of which the most active fraction (40.3 mg) had an IC50 of 2.0 μg/ml. This fraction was then applied to a silica gel column with elution by chloroform:MeOH 9:1 to give fourteen fractions, of which fraction 11 (4.8 mg) was the most active (IC50 1.0 μg/ml) and yielded compound 3. The BuOH fraction was applied to an open column of Diaion HP-20 resin and eluted with a step MeOH:H2O gradient of 40%, 70% and 100% MeOH. The 100% MeOH fraction was the most active fraction (100 mg) with an IC50 of 2.2 μg/ml. This fraction was applied to a silica gel column and eluted with chloroform:MeOH 6:1 to give thirteen fractions, of which fraction 4 (1.8 mg) yielded compound 2, with an IC50 of 1.5 μg/ml, and fraction 7 (6.3 mg) yielded compound 1, with an IC50 of 1.9 μg/ml.
2″-O-Acetylrandianin
(2). Light yellow solid; [α]D21 +12 ( c 1.2, MeOH); IR νmax cm−1: 3453, 2935, 1734, 1689, 1027 cm−1. 1H NMR (500 MHz, pyridine-d5), and 13C NMR (125 MHz, pyridine-d5), see Table 1; HRESIMS m/z 845.4692 [M+Na]+ (calc for C44H70NaO14, 845.4663).
6″-O-Acetylrandianin
(3). Light yellow solid; [α]D21 +17 ( c 1.2, MeOH); IR νmax cm−1: 3439, 2935, 1727, 1689, 1027 cm−1. 1H NMR (500 MHz, pyridine-d5), and 13C NMR (125 MHz, pyridine-d5), see Table 1; HRESIMS m/z 845.4643 [M+Na]+ (calc for C44H70NaO14, 845.4663).
Hydrolysis of compounds 2 and 3
Compound 3 (3.0 mg) was hydrolyzed with 6 M NH4OH for 2 h at 110 °C. The solution was evaporated to dryness under reduced pressure, and then dissolved in water and extracted 3-times with BuOH [17, 18]. The BuOH extract was evaporated to dryness and yielded a light-yellow power (2.6 mg) identified as compound 1 by its 1H and 13C-NMR spectra. The light-yellow power was further hydrolyzed with 3 M HCl for 4 h at 100 °C. The solution was extracted 3 times with EtOAc, and both the organic and the water layers were evaporated to dryness under reduced pressure. The structure of the white powder (1.4 mg) derived from the organic layer was determined to be oleanolic acid by 1H and 13C-NMR spectroscopy. The semi-solid carbohydrate mixture from the water layer (0.9 mg) was dissolved in 2 mL of water and kept overnight before TLC analysis and determination of its optical rotation. The same procedure was also applied to compound 2. The sugar from both 2 and 3 had an identical Rf value to glucose by TLC on a silica gel plate with CHCl3:MeOH:H2O = 15:6:1, and had [α]D21 +13.9 and +14.2, respectively, (c 0.1, H2O).
Supplementary Material
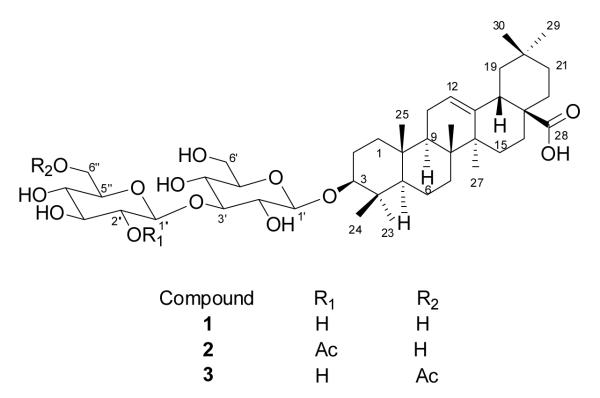
Figure 1.
The structures of compounds 1 – 3.
Acknowledgement
This project was supported by the Fogarty International Center, the National Cancer Institute, the National Science Foundation, the National Heart, Lung and Blood Institute, the National Institute of Mental Health, the Office of Dietary Supplements, and the Office of the Director of NIH, under Cooperative Agreement U01 TW000313 with the International Cooperative Biodiversity Groups. This project was also supported by the National Research Initiative of the Cooperative State Research, Education and Extension Service, USDA, Grant #2008-35621-04732. Funds for the plant collection and extraction were provided by Dow AgroSciences and Eisai Inc. This support is gratefully acknowledged. This work was also supported by the National Science Foundation under Grant no. CHE-0619382 for purchase of the Bruker Avance 500 NMR spectrometer and Grant no. CHE-0722638 for the purchase of the Agilent 6220 mass spectrometer. We thank Mr. Bill Bebout for obtaining the mass spectra, Dr. Hugo Azurmendi for assistance with the NMR spectra, and Drs. Donald Hahn (Dow AgroSciences) and Edward Suh (Eisai Inc.) for their encouragement and support. Field work essential for this project was conducted under a collaborative agreement between the Missouri Botanical Garden and the Parc Botanique et Zoologique de Tsimbazaza and a multilateral agreement between the ICBG partners, including the Centre National d’Applications des Recherches Pharmaceutiques. We gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts).
Footnotes
‘Biodiversity Conservation and Drug Discovery in Madagascar’, Part 52. For Part 51, see [1].
References and Notes
- [1].Harinantenaina L, Brodie PJ, Callmander MW, Razafitsalama LJ, Rasamison VE, Rakotobe E, Kingston DGI. Nat. Prod. Commun. 2012;7:705. [PMC free article] [PubMed] [Google Scholar]
- [2].Kingston DGI. J. Nat. Prod. 2011;74:496. doi: 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pan E, Cao S, Brodie PJ, Callmander M, Rakotonandrasana S, Rakotobe E, Rasamison V, Tendyke K, Shen Y, Suh EM, Kingston DGI. J. Nat. Prod. 2011;74:1169. doi: 10.1021/np200093n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Karou SD, Tchacondo T, Ilboudo DP, Simpore J. Pak. J. Biol. Sci. 2011;14:149. doi: 10.3923/pjbs.2011.149.169. [DOI] [PubMed] [Google Scholar]
- [5].Comini LR, Fernandez IM, Vittar NBR, Montoya SCN, Cabrera JL, Rivarola VA. Phytomedicine. 2011;18:1093. doi: 10.1016/j.phymed.2011.05.008. [DOI] [PubMed] [Google Scholar]
- [6].Mukhtar MR, Osman N, Awang K, Hazni H, Qureshi AK, Hadi AHA, Zaima K, Morita H, Litaudon M. Molecules. 2012;17:267. doi: 10.3390/molecules17010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nazari AS, Dias SA, Costa WFD, Bersani-Amado CA, Vidotti GJ, Souza MCD, Sarragiotto MH. Pharm. Biol. 2006;44:7. [Google Scholar]
- [8].Rasoarivelo STR, Grougnet R, Vérité P, Lecsö M, Butel M-J, Tillequin F, Guillou CR, Deguin B. Chem. Biodiversity. 2011;8:145. doi: 10.1002/cbdv.201000208. [DOI] [PubMed] [Google Scholar]
- [9].He Z-D, Ma C-Y, Zhang H-J, Tan GT, Tamez P, Sydara K, Bouamanivong S, Southavong B, Soejarto DD, Pezzuto JM, Fong HHS. Chem. Biodiversity. 2005;2:1378. doi: 10.1002/cbdv.200590110. [DOI] [PubMed] [Google Scholar]
- [10].Abdel-Kader MS, Bahler BD, Malone S, Werkhoven MCM, Wisse JH, Neddermann K, Bursuker I, Kingston DGI. J. Nat. Prod. 2000;63:1461. doi: 10.1021/np0000926. [DOI] [PubMed] [Google Scholar]
- [11].Sotheeswaran S, Bokel M, Kraus W. Phytochemistry. 1989;28:1544. [Google Scholar]
- [12].Tori K, Seo S, Shimaoka A, Tomita Y. Tetrahedron Lett. 1974;48:4227. [Google Scholar]
- [13].Arai M, Hayashi A, Sobou M, Ishida S, Kawachi T, Kotoku N, Kobayashi M. J. Nat. Med. 2011;65:149. doi: 10.1007/s11418-010-0477-7. [DOI] [PubMed] [Google Scholar]
- [14].Colin D, Gimazane A, Lizard G, Izard J-C, Solary E, Latruffe N, Delmas D. Int. J. Cancer. 2009;124:2780. doi: 10.1002/ijc.24264. [DOI] [PubMed] [Google Scholar]
- [15].Cao S, Brodie PJ, Miller JS, Randrianaivo R, Ratovoson F, Birkinshaw C, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2007;70:679. doi: 10.1021/np060627g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pan E, Harinantenaina L, Brodie PJ, Callmander M, Rakotonandrasana S, Rakotobe E, Rasamison VE, Tendyke K, Shen Y, Suh EM, Kingston DGI. Bioorg. Med. Chem. 2011;19:1169. doi: 10.1016/j.bmc.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang LL, Li ZL, Song DD, Sun L, Pei YH, Jing YK, Hua HM. Planta Med. 2008;74:1735. doi: 10.1055/s-2008-1081355. [DOI] [PubMed] [Google Scholar]
- [18].Seo Y, Berger JM, Hoch J, Neddermann KM, Bursuker I, Mamber SW, Kingston DGI. J. Nat. Prod. 2001;65:65. doi: 10.1021/np010327t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.