Abstract
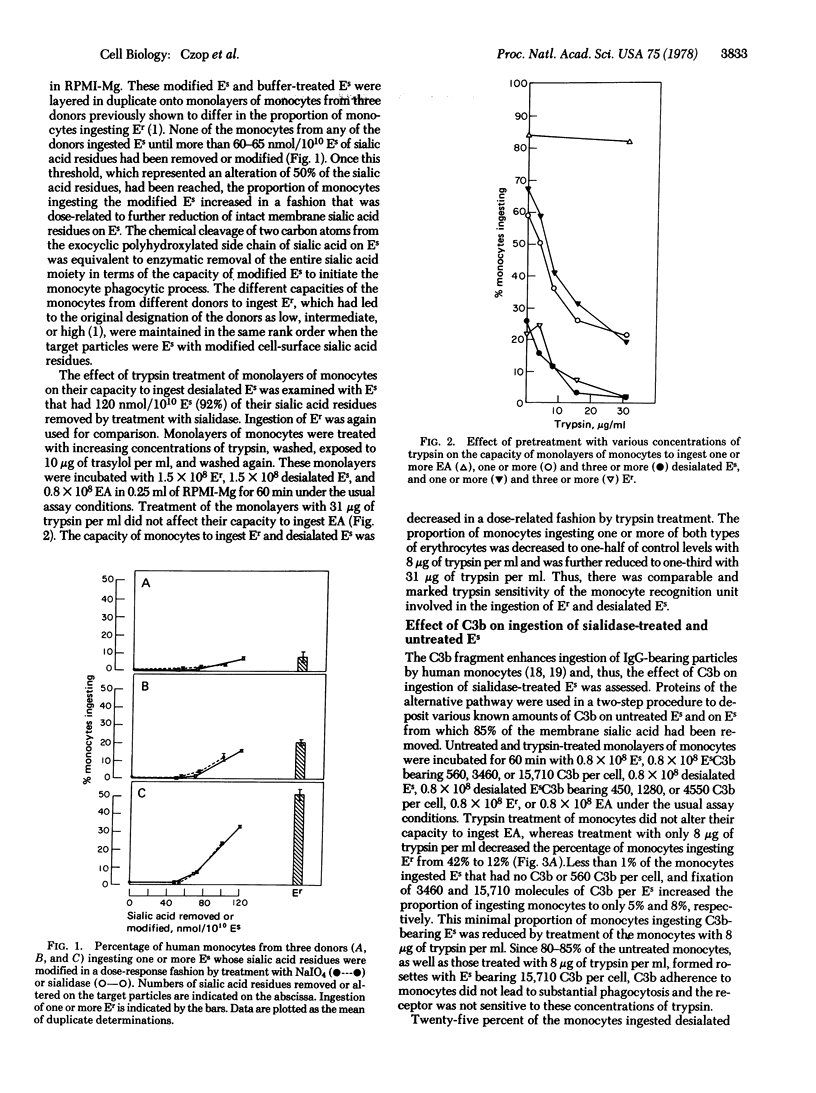
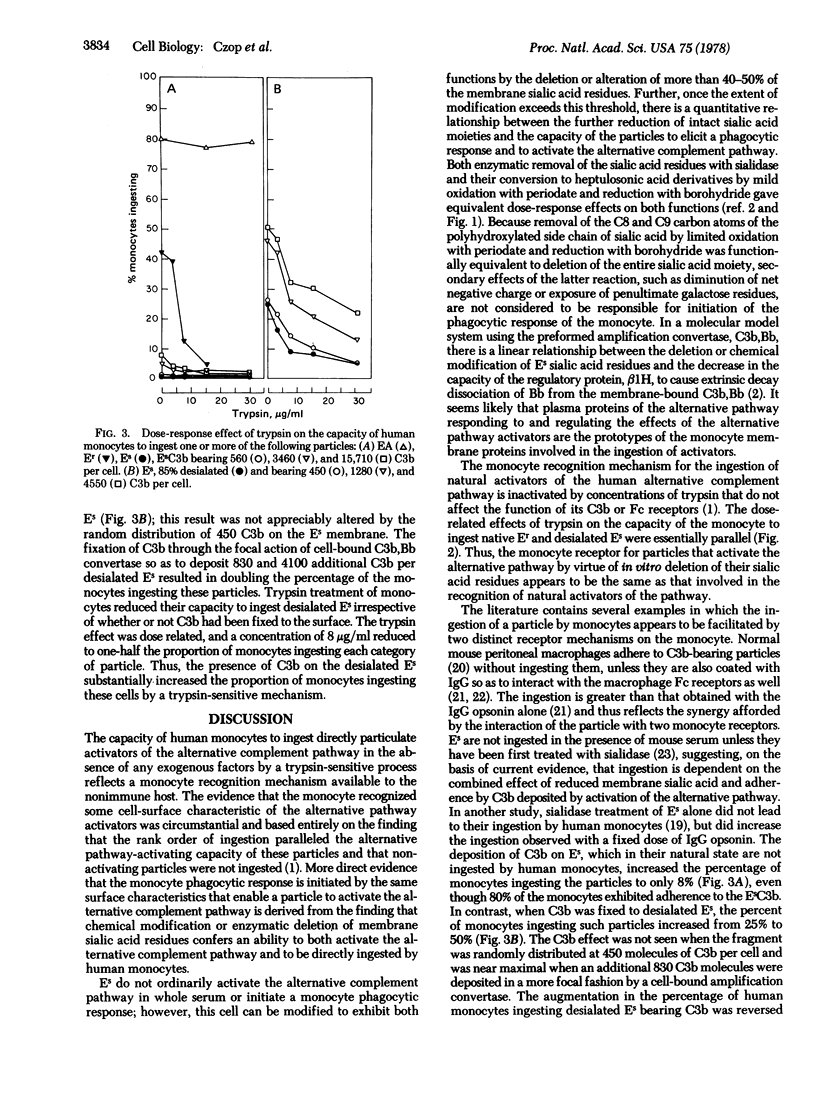
Monolayers of human peripheral blood monocytes in the absence of exogenous proteins ingest a variety of natural particulate activators of the human alternative complement pathway. Sheep erythrocytes, which do not ordinarily activate the human alternative complement pathway or initiate a direct monocyte phagocytic response, can be modified to exhibit both functions by the deletion or alteration of membrane sialic acid residues. Enzymatic removal of the sialic acid residues with sialidase or their conversion to heptulosonic acid derivatives by limited oxidation with NaIO4 and reduction with BH4- have equivalent dose-response effects on the capacity of the altered sheep erythrocytes to initiate the phagocytic response by human monocytes or to activate the alternative pathway in human serum. The deposition of C3b on native sheep erythrocytes had little effect on their ingestion by human monocytes, whereas the fixation of C3b on desialated sheep erythrocytes had a synergistic effect on the percentage of monocytes ingesting such a particle. The monocyte receptor essential for ingestion of desialated sheep erythrocytes or desialated sheep erythrocytes bearing C3b was inactivated by concentrations of trypsin that also prevented the monocytes from ingesting natural activators of the human alternative complement pathway, but did not alter the receptors for C3b or the Fc portion of IgG. The capacity of the nonimmune host to respond to desialated particles by initiating the monocyte ingestive process and by activating the alternative complement pathway to provide the synergy afforded by C3b deposition on that particle represents a primitive biochemical basis for differentiation of nonself from self.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aminoff D., Bell W. C., Fulton I., Ibgebrigtsen N. Effect of sialidase on the viability of erythrocytes in circulation. Am J Hematol. 1976;1(4):419–432. doi: 10.1002/ajh.2830010407. [DOI] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Opsonin-independent phagocytosis of activators of the alternative complement pathway by human monocytes. J Immunol. 1978 Apr;120(4):1132–1138. [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975 Oct 1;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Austen K. F. Alternate complement pathway: factors involved in cobra venom factor (CoVF) activation of the third component of complement (C3). J Immunol. 1973 Jan;110(1):128–138. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. Effect of neuraminidase on the phagocytosis of heterologous red cells by mouse peritoneal macrophages. Proc Soc Exp Biol Med. 1968 Jul;128(3):891–894. doi: 10.3181/00379727-128-33150. [DOI] [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- PHAFF H. J. CELL WALL OF YEASTS. Annu Rev Microbiol. 1963;17:15–30. doi: 10.1146/annurev.mi.17.100163.000311. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., ROSS O. A., TODD E. W., WARDLAW A. C. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954 Aug 20;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Larsson I. Lactoperoxidase coupled to polyacrylamide for radio-iodination of proteins to high specific activity. Immunochemistry. 1974 Apr;11(4):203–206. doi: 10.1016/0019-2791(74)90329-2. [DOI] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


