Abstract
Astrocyte elevated gene-1 (AEG-1), also known as metadherin (MTDH) and lysine-rich CEACAM1 coisolated (LYRIC), was initially cloned in 2002. AEG-1/MTDH/LYRIC has emerged as an important oncogene that is overexpressed in multiple types of human cancer. Expanded research on AEG-1/MTDH/LYRIC has established a functional role of this molecule in several crucial aspects of tumor progression, including transformation, proliferation, cell survival, evasion of apoptosis, migration and invasion, metastasis, angiogenesis, and chemoresistance. The multifunctional role of AEG-1/MTDH/LYRIC in tumor development and progression is associated with a number of signaling cascades, and recent studies identified several important interacting partners of AEG-1/MTDH/LYRIC in regulating cancer promotion and other biological functions. This review evaluates the current literature on AEG-1/MTDH/LYRIC function relative to signaling changes, interacting partners, and angiogenesis and highlights new perspectives of this molecule, indicating its potential as a significant target for the clinical treatment of various cancers and other diseases.
1. INTRODUCTION
Cancer progression is driven by uncontrolled cell growth and is invariably associated with genetic alterations linked to the regulation of proliferation, cell death, apoptosis, and genetic stability, such as tumor suppressor genes, oncogenes, growth factors, and cell adhesion molecules, which vary among different cancer types (Fisher, 1984; Gupta & Massague, 2006; Hanahan & Weinberg, 2000, 2011). Over the past several decades, significant advances in cancer research have led to the identification of a comprehensive list of oncogenes, tumor suppressors, and signaling pathways that are potential targets for anticancer therapeutics. Astrocyte elevated gene-1 (AEG-1), also known as metadherin (MTDH) and lysine-rich CEACAM1 coisolated (LYRIC), a novel gene that was cloned only a decade ago, is now firmly established as an oncogene in a wide array of cancer indications, reviewed in Hu, Wei, and Kang (2009) and Yoo, Emdad, et al. (2011). The increased mRNA levels of AEG-1/MTDH/LYRIC may be a direct result of the copy gain (or amplification) of the locus covering the gene, or the Ha-ras-driven activation of the PI3K/Akt pathway, which eventually leads to the recruitment of c-Myc to the AEG-1/MTDH/LYRIC promoter region (Hu, Wei, et al., 2009; Yoo, Emdad, et al., 2011).In essence, AEG-1/ MTDH/LYRIC is oncogenic because it plays important roles in the activation of diverse signaling pathways (Akt, NF-κB, and Wnt) involved in cancer proliferation, survival, and invasion (overviewed in Figs. 3.1 and 3.2) in association with turning on epithelial to mesenchymal transition (EMT) and angiogenesis. Through overexpression (by vectors) or repression (by RNAi technology) of AEG-1/MTDH/LYRIC in cancer cell lines, studies have demonstrated that the gene is involved in the increased expression of oncogenes (e.g., CCND1, CTNNB1), metastasis-promoting genes (e.g., MMP9 and MMP2), and drug resistance genes (e.g., MDR1, DPYD, ABCC11, AKR1C2, ALDH3A1), or decreased expression of tumor suppressor genes (adenomatous polyposis coli (APC), CDKN1A, FOXO1, FOXO3; overviewed in Fig. 3.1). This review summarizes the role of the AEG-1/ MTDH/LYRIC effectors as well as the crucial signaling pathways involved in AEG-1/MTDH/LYRIC-mediated transformation and tumor progression. In addition, we discuss the impact of tumor angiogenesis in AEG-1/ MTDH/LYRIC-mediated transformation and metastasis.
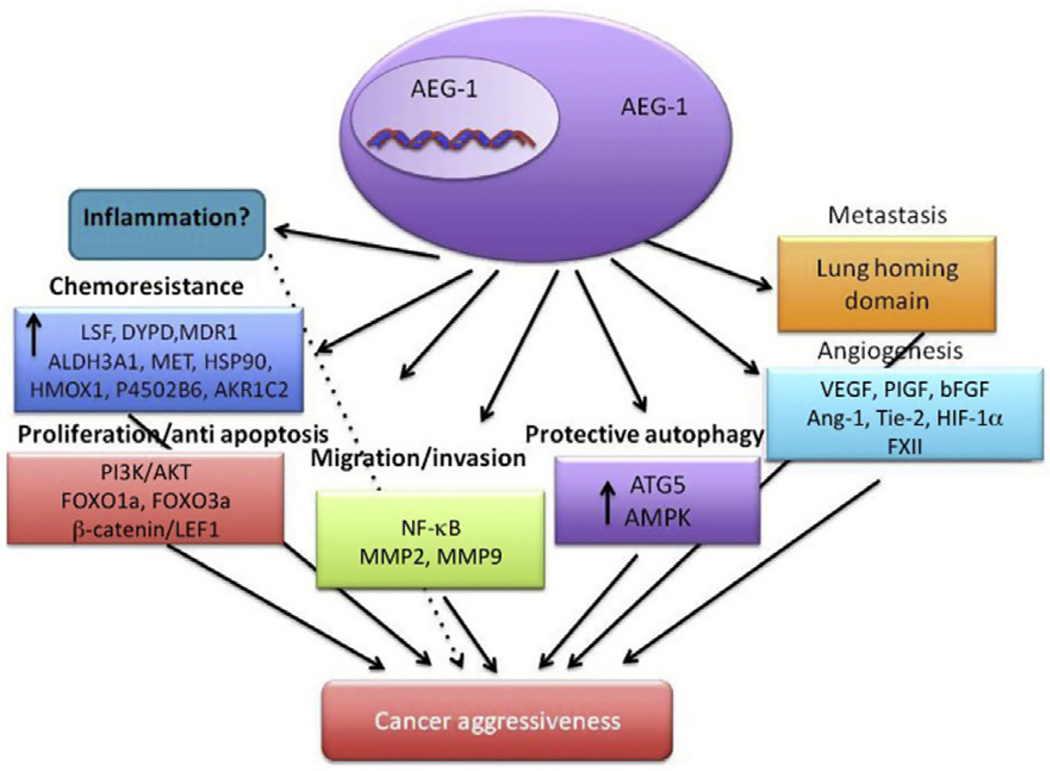
Figure 3.1.
A model illustrating diverse biological functions and possible effector molecules of AEG-1/MTDH/LYRIC. Dotted line indicates an area requiring further validation.
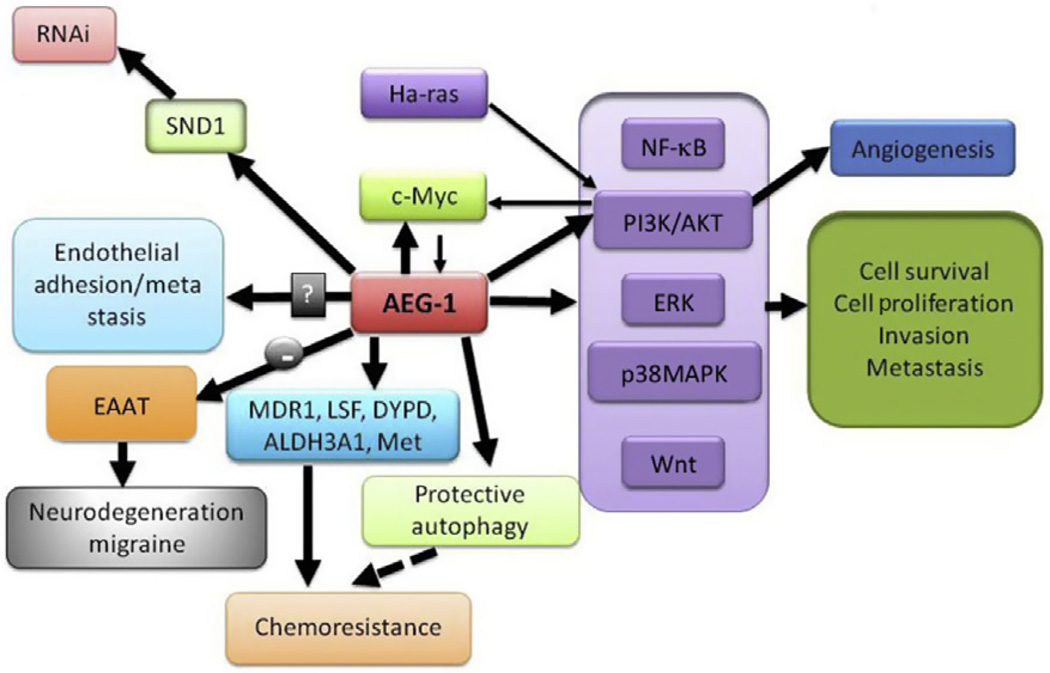
Figure 3.2.
AEG-1/MTDH/LYRIC facilitates tumor progression and other functions via the integration of multiple signaling networks. Thick arrows indicate regulation by AEG-1/MTDH/LYRIC while thin arrows indicate molecular factors that regulate AEG-1/MTDH/LYRIC. Arrow with “–” indicates negative regulation.
2. CLONING OF AEG-1/MTDH/LYRIC
AEG-1 was originally identified as a novel transcript induced in primary human fetal astrocytes (PHFAs) after human immunodeficiency virus (HIV)-1 infection or treatment with viral glycoprotein gp120 or tumor necrosis factor (TNF)-α (Su et al., 2003, 2002). Subsequently, using in vivo phage screening, Brown and Ruoslahti (2004) cloned mouse AEG-1 as aprotein that allowed the specific adhesion of mouse 4T1 mammary tumor cells to lung endothelium and named it MTDH. The mouse/rat orthologue of AEG-1 was also cloned as a tight junction (TJ) protein in polarized rat epithelial cells, named LYRIC (Britt et al., 2004), and as a novel transmembrane protein that is present in endoplasmic reticulum (ER), nuclear envelope, and nucleolus, named 3D3/LYRIC (Sutherland, Lam, Briers, Lamond, & Bickmore, 2004).
3. AEG-1/MTDH/LYRIC: HIGH EXPRESSION IS LINKED TO CANCER AGGRESSIVENESS
Numerous recent studies have demonstrated that AEG-1/MTDH/ LYRIC might play a pivotal role in the pathogenesis, progression, apoptosis regulation, angiogenesis, invasion, metastasis, and overall patient survival in diverse human cancers. Breast cancer, glioma, and prostate cancer were among the first tumor types in which AEG-1/MTDH/LYRIC upregulation was documented (Emdad et al., 2010; Kikuno et al., 2007; Li et al., 2008; Liu et al., 2010). Further evidence points to the fact that AEG-1/MTDH/LYRIC is located at chromosome 8q22, which is known to be a hot spot for genomic alterations in several cancer cells, including breast cancer, hepatocellular carcinoma (HCC), and malignant glioma (Hu, Chong, et al., 2009; Warr et al., 2001; Yoo, Emdad, et al., 2009). Several subsequent studies indicate that overexpression of AEG-1/ MTDH/LYRIC is frequently observed in a diverse array of cancer indications studied so far including colorectal cancer (CRC), gastric and gall bladder carcinoma, ovarian and endometrial carcinoma, renal cancer, non small cell lung cancer (NSCLC) and laryngeal squamous cell carcinoma, esophageal squamous cell carcinoma, neuroblastoma, oligodendroglioma, pediatric solid tumors, T-cell lymphoma, diffuse large B cell lymphoma, head and neck cancer, salivary gland tumor, carcinoma of tongue, bladder cancer, and osteosarcoma compared to normal cells and matched nonneoplastic regions (Baygi & Nikpour, 2012; Chen, Ke, Shi, Yang, & Wang, 2010; Ge et al., 2012; Gnosa et al., 2012; Hui et al., 2011; Ke et al., 2012, 2013; Li, Liu, Lu, et al., 2011; Liao et al., 2011; Liu, Liu, Han, Zhang, & Sun, 2012; Liu et al., 2013; Liu & Yang, 2013; Orentas et al., 2012; Sarkar & Fisher, 2013; Song, Li, Lu, Zhang, & Geng, 2010; Wang et al., 2011; Xia et al., 2010; Yan, Zhang, Chen, & Zhang, 2012; Yu et al., 2009; Yuan et al., 2012; Zhou, Li, Wang, Yin, & Zhang, 2012). Moreover, recent clinical studies using a large cohort of patient samples representing diverse cancer indications have convincingly linked AEG-1/MTDH/ LYRIC with tumor progression and poor clinical outcomes (Gong et al., 2012; Jiang, Zhu, Zhu, & Piao, 2012; Li et al., 2008, 2012; Sarkar & Fisher, 2013; Song, Li, Li, & Geng, 2010; Song et al., 2009; Tokunaga et al., 2012). These findings suggest that AEG-1/MTDH/LYRIC may be developed as a powerful autonomous poor-prognosis marker and a molecular target for anticancer therapeutics (Sarkar & Fisher, 2013).
4. AEG-1/MTDH/LYRIC: SIGNALING PATHWAYS
The past few years have witnessed increased interest and extensive studies on AEG-1/MTDH/LYRIC functions firmly linking major cellular signaling cascades with the ability of AEG-1/MTDH/LYRIC to implement diverse biological processes in multiple disease contexts. As a multifunctional protein, AEG-1/MTDH/LYRIC profoundly modulates a diverse array of signaling networks and effector molecules involved in tumor progression and implicated in additional disease processes such as neurodegenerative diseases (overviewed in Fig. 3.2).
4.1. Nuclear factor-κB
The nuclear factor-κB (NF-κB) transcription factor family is recognized as a fundamental mediator of the inflammatory process and a major participant in innate and adaptive immune responses (Ben-Neriah & Karin, 2011). Deregulated NF-κB signaling occurs in many pathological conditions such as autoimmune disease, chronic inflammation, and oncogenesis (Ben-Neriah & Karin, 2011). The NF-κB proteins found in most cells are p50/NF-κB1, p65/RelA, c-Rel, NF-κB2/p52, and Rel B (Ghosh, May, & Kopp, 1998). The cloning of NF-κB1/p105/p50 and p65/RelA and establishing their association with the avian viral oncoprotein v-Rel was the first evidence linking NF-κB to cancer (Gilmore, 2003; Karin, Cao, Greten, & Li, 2002; Nolan, Ghosh, Liou, Tempst, & Baltimore, 1991). Several well-established oncogenes, such as Ras, Rac, and Bcr-Abl, are inducers of NF-κB activity, and constitutive activation of NF-κB has been observed in a diverse array of human cancers, including but not restricted to pancreatic, gastric, colon, hepatocellular, breast, ovarian, and head and neck carcinomas, melanoma, leukemias, lymphomas, and Hodgkin’s disease (Karin et al., 2002). However, oncogenic mutations that provide RelA, c-Rel, or other NF-κB proteins with transforming potential were found to be relatively rare and mainly occur in malignancies of lymphoid cells (Gilmore, 2003). It has been hypothesized that NF-κB activation in cancer may be the result of either exposure to proinflammatory stimuli in the tumor microenvironment or mutational activation of upstream components of IKK–NF-κB signaling pathways. NF-κB is regulated by two main pathways: the canonical and noncanonical pathways (Hayden & Ghosh, 2008). In resting cells, NF-κB is sequestered in the cytoplasm in complex with its inhibitor, IκB. These IκB proteins include IκBα, IκBβ, IκBε, IκB ζ, and BCL-3, among others, and are defined by their ankyrin repeat domains (Hayden & Ghosh, 2004, 2011, 2012; Prasad, Ravindran, & Aggarwal, 2010). Ligand–receptor interactions lead to sequential activation of upstream signaling molecules, transmitting activation signals to the IκB kinase (IKK) complex. This multimeric protein complex comprises two catalytic subunits, IKKα and IKKβ, and one regulatory subunit, IKKγ, together with the Hsp90 chaperone complex (Salminen, Paimela, Suuronen, & Kaarniranta, 2008). Activated IKKα and IKKβ phosphorylate IκB on its N-terminal serine residues, resulting in its ubiquitination and subsequent proteasomal degradation (Kanarek & Ben-Neriah, 2012). Consequently, the released NF-κB heterodimer migrates into the nucleus. Following nuclear translocation, NF-κB binds to consensus NF-κB sequences in the promoter of diverse target genes, thereby augmenting their transcription.
Activated NF-κB increases the expression of numerous antiapoptotic genes including c-IAP1, c-IAP2, Bcl-xl, Survivin, XIAP, and Bcl-2. NF-κB activation also plays a critical role in the cell cycle by regulating certain genes such as cyclin D1, c-Myc, CDK2, and cyclin E (Ling & Kumar, 2012). Several genes involved in cancer invasion and metastasis are also controlled by NF-κB such as MMP2, VCAM-1, ICAM-1, uPA, iNOS, etc. (Ling & Kumar, 2012). Recent research has further revealed the link between NF-κB and many proinflammatory genes, such as TNF-α, COX2, MCP1, and E-selectin (Marrogi et al., 2000; Noguchi et al., 1996; van der Saag, Caldenhoven, & van de Stolpe, 1996). NF-κB is also crucially involved in tumor angiogenesis by regulation of proangiogenic molecules such as vascular endothelial growth factor (VEGF), hypoxia-inducible factor (HIF-1) α, CXCL1/8, IL-8, and TNF (Kumar, Takada, Boriek, & Aggarwal, 2004; Noguchi et al., 1996; Prasad et al., 2010; van der Saag et al., 1996).
NF-κB is the first signaling pathway showing activation by AEG-1/ MTDH/LYRIC (Emdad et al., 2006; Sarkar et al., 2008). AEG-1/ MTDH/LYRIC activates the NF-κB pathway by facilitating IκB degradation and by increasing binding of the transcriptional activator p50/p65 complex in the nucleus. NF-κB complex transcriptionally regulates several genes involved in adhesion, invasion, metastasis, and angiogenesis. A gene array analysis revealed that ectopic expression of AEG-1/MTDH/LYRIC by Ad.AEG-1 infection resulted in marked upregulation of NF-κB-responsive cell adhesion molecules (ICAM-2 and ICAM-3, selectin E, selectin L, and selectin P ligand), TLR4 and TLR5, FOS, JUN, and cytokines, such as IL-8 (Emdad et al., 2006). These proteins are important in mediating NF-κB-induced proliferation, angiogenesis, and inflammation, all required for the carcinogenic process, indicating that activation of NF-κB also mediates multiple aspects of AEG-1/MTDH/LYRIC function. In HeLa cells and human malignant glioma cells, treatment with TNF-α results in AEG-1/MTDH/LYRIC translocation into the nucleus where it interacts with the p65 subunit of NF-κB and augments NF-κB-induced gene expression (Emdad et al., 2006; Sarkar et al., 2008). Inhibition of NF-κB pathway using an IκB super-repressor (Ad.IκBα-mt32) significantly reverted AEG-1/ MTDH/LYRIC-induced agar cloning efficiency and invasion in human glioma cells (Sarkar et al., 2008). Functional analysis revealed a role of AEG-1/MTDH/LYRIC in invasion, migration, and NF-κB-activating properties that were mediated by the NH2-terminal 71 amino acids. Activation of NF-κB by AEG-1/MTDH/LYRIC has also been reported in human prostate and liver cancer cells, as well as in NSCLC (Kikuno et al., 2007; Yoo, Emdad, et al., 2009). AEG-1/MTDH/LYRIC also promotes EMT in breast cancer cells in an NF-κB-dependent manner (Li, Kong, et al., 2011).
A potential role of AEG-1/MTDH/LYRIC in inflammatory processes is suggested by the observation that this gene activates NF-κB, which is a key regulator of proinflammatory cytokine activation. A recent study demonstrated that AEG-1/MTDH/LYRIC was induced via the NF-κB pathway in U937 human promonocytic cells following lipopolysaccharide (LPS) stimulation (Khuda et al., 2009). Inhibition of AEG-1/MTDH/LYRIC expression abrogated LPS-induced TNF-α and prostaglandin E2 production. Another recent study showed that LPS also upregulates the expression of AEG-1/MTDH/LYRIC in a number of breast cancer lines (Zhao et al., 2011). Stable knockdown of AEG-1/MTDH/LYRIC by shRNA in the human breast cancer cell line MDA-MB-231 abolished LPS-induced cell migration and invasion and also diminished NF-κB activation by LPS and inhibited LPS-induced IL-8 and MMP9 production. These findings suggest that AEG-1/MTDH/LYRIC might be a target molecule for the therapy of LPS-related diseases such as septic shock and systemic inflammatory response syndrome as well as in cancer-related inflammation.
4.2. PI3K/Akt pathway
The phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway is a key regulator of physiological cell processes, which include proliferation, differentiation, apoptosis, motility, metabolism, and autophagy (Engelman, Luo, & Cantley, 2006; Hennessy, Smith, Ram, Lu, & Mills, 2005). PI3K/Akt signaling is aberrantly upregulated in many cancers where it negatively influences prognosis (Cantley, 2002). The PI3K enzymes are involved in the phosphorylation of membrane inositol lipids (Vivanco & Sawyers, 2002). The activation of PI3K generates the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3) from phosphatidylinositol 4,5-bisphosphate. This recruits proteins to the cell membrane, including the Akt/PKB kinases, resulting in their phosphorylation by phosphoinositide-dependent kinase 1 (PDK1) and PDK2 (Engelman et al., 2006; Yap et al., 2008). Activated Akt translocates to the cytoplasm and nucleus and activates downstream targets involved in survival, proliferation, cell cycle progression, growth, migration, and angiogenesis (Bellacosa, Kumar, Di Cristofano, & Testa, 2005; Yang et al., 2004). Akt is negatively regulated by the phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a tumor suppressor gene that dephosphorylates PIP3 (Bartholomeusz & Gonzalez-Angulo, 2012).
Following activation, Akt modulates the function of numerous substrates involved in the regulation of cell survival, cell cycle progression, and cellular growth (Testa & Bellacosa, 2001). Akt phosphorylates and inactivates Bad, a proapoptotic member of the Bcl-2 family, thereby promoting survival (Vivanco & Sawyers, 2002). Akt inhibits the catalytic function of caspase-9 by phosphorylation and reduces its proapoptotic activity (Datta, Brunet, & Greenberg, 1999). Forkhead family of transcription factors (FOXO) is also well-established substrates of Akt that induce the expression of proapoptotic factors such as Fas ligand (Brunet et al., 1999). Akt also phosphorylates Ikk, which in turn degrades IκB. This eventually leads to nuclear translocation of NF-κB heterodimers and activation of NF-κB-regulated target genes. Glycogen synthase kinase-3 (GSK3), mammalian target of rapamycin (mTOR), insulin receptor substrate-1, the Forkhead family member FKHR, cyclin-dependent kinase inhibitors p21Cip1/Waf-1/mda-6 and p27KIP1, and, possibly, Raf1 are all Akt targets involved in protein synthesis, glycogen metabolism, and cell cycle regulation (Blume-Jensen & Hunter, 2001). Akt phosphorylates the CDK inhibitors, p21 and p27, and inhibits their antiproliferative effects (Liang et al., 2002; Zhou et al., 2001). Additionally, phosphorylation of MDM2 by Akt promotes the degradation of p53, leading to enhanced cell cycle activity in the G1/S phase (Sherr & Weber, 2000).
The second major signaling pathway activated by AEG-1/MTDH/ LYRIC is the PI3K/Akt pathway. Lee, Su, Emdad, Sarkar, and Fisher (2006) first demonstrated that AEG-1/MTDH/LYRIC is a downstream target gene of Ha-ras, and this induction was attenuated by treatment with LY294002 or PTEN overexpression, indicating that activation of the PI3K signaling pathway regulates Ha-ras-mediated AEG-1/MTDH/LYRIC induction. Subsequent promoter mapping data indicate that Ha-ras increases binding of c-Myc to the E-box elements in the AEG-1/MTDH/LYRIC promoter through the PI3K/Akt/GSK3β/c-Myc pathway to contribute to Ha-ras-mediated oncogenesis through AEG-1/MTDH/LYRIC (Lee et al., 2006). Intriguingly, AEG-1/MTDH/LYRIC overexpression also increases phosphorylation of Akt and GSK3β, with subsequent c-Myc stabilization and MDM2 phosphorylation, leading to modulation of a number of additional Akt downstream factors that are crucial for cellular proliferation and survival (Lee et al., 2008). In neuroblastoma cells, overexpression of AEG-1/MTDH/LYRIC activates the PI3K/Akt pathways and stabilizes N-myc (Lee, Jeon, et al., 2009). Inhibition of AEG-1/MTDH/LYRIC by knockdown approach was shown to induce apoptosis through the upregulation of FOXO3a activity in prostate cancer cells (Kikuno et al., 2007) and FOXO1 activity in breast cancer cells (Li et al., 2009) via Akt signaling, respectively. In esophageal cancer cells, activation of Akt by AEG-1/ MTDH/LYRIC leads to upregulation of cyclin D1 and downregulation of p27 (Yu et al., 2009). Activation of the PI3K/Akt pathway by AEG-1/ MTDH/LYRIC led to an increase in multidrug resistance gene 1 (MDR1) levels by increased association of MDR1 mRNA to polysomes in drug-resistant human HCC cells (Yoo et al., 2010). In NSCLC, AEG-1/MTDH/LYRIC significantly increased the levels of PI3K, p110, and Akt phosphorylation and inhibited apoptosis by modulating caspase-3 and Bcl-2 (Ke et al., 2013). AEG-1/MTDH/LYRIC also mediates invasive ability of NSCLC via the NF-κB and PI3K/Akt pathways (Song et al., 2009). The activation of PI3K/Akt prosurvival pathways via upregulation of PIP3 has also been shown in endometrial cancer cells, and inhibition of AEG-1/MTDH/LYRIC reversed this signaling event and made cells more sensitive to TRAIL- and HDAC inhibitor-induced cell death (Meng et al., 2011).
In addition to growth-promoting activity, the PI3K/Akt signaling pathway is also involved in the regulation of angiogenesis through the control of VEGF, HIF-1, and thrombospondin (Blancher, Moore, Robertson, & Harris, 2001; Jiang et al., 2001). Once activated by the upstream growth factor/oncogene or by the loss of PTEN function, the PI3K/Akt signaling leads to upregulation of VEGF expression either directly or indirectly through increased expression of HIF-1α. The PI3K/Akt pathway also regulates AEG-1/MTDH/LYRIC-induced angiogenesis (Emdad et al., 2009). Akt activation is essential for AEG-1/MTDH/LYRIC-mediated endothelial cell tube formation, upregulation of HIF-1α in human umbilical vein endothelial cell (HUVEC), and activation of the VEGF promoter in human glioma cells. Additionally, Noch, Bookland, and Khalili (2011) demonstrated that AEG-1/MTDH/LYRIC was induced by hypoxia via the PI3K/Akt pathway and then AEG-1/MTDH/LYRIC feeds back to activate PI3K and create a positive feedback loop. Thus, PI3K/Akt activation mediates multiple aspects of AEG-1/MTDH/LYRIC function. However, the molecular mechanism of PI3K/Akt pathway activation by AEG-1/MTDH/LYRIC remains to be determined.
4.3. Wnt and mitogen-activated protein kinase pathways
The Wnt/β-catenin signaling pathway is another significant pathway that regulates cell proliferation, migration, and differentiation, thus making it an influential regulator of embryonic development and tumorigenesis (Clevers & Nusse, 2012). The Wnt/β-catenin pathway is an important pro-tumorigenic pathway for many cancers (Clevers, 2006). Wnt proteins, which are secreted glycoproteins, bind to the low-density lipoprotein receptor-related protein5/6 (LRP5/6) and Frizzled (FZD), a seven-pass transmembrane receptor protein, to activate the Wnt/β-catenin signaling pathway (MacDonald, Tamai, & He, 2009; Polakis, 2007). In the absence of Wnts, β-catenin is sequestered in a complex that consists of the APC tumor suppressor, axin, GSK3β, and casein kinase 1 (CK1). This complex formation induces the phosphorylation of β-catenin by CK1 and GSK3β, which results in the ubiquitination and the subsequent degradation of β-catenin by the 26S proteasome. Conversely, when Wnt proteins are secreted from cells, they can form a ternary complex with FZD and LRP5/6 receptors, which results in the inhibition of GSK3β and the stabilization of cytosolic β-catenin. β-Catenin then translocates into the nucleus where it interacts with T-cell factor/lymphoid-enhancing factor (TCF/LEF). Binding of β-catenin to LEF/TCF proteins leads to transcription of a number of Wnt-responsive genes that regulate cell proliferation and migration such as c-Myc, cyclin D1, and members of the WISP family (Tanaka et al., 2003). In addition, mice demonstrating activated β-catenin signaling upregulate epidermal growth factor receptor (EGFR) (Tan et al., 2005) and genes involved in glutamine metabolism, including glutamine synthetase, ornithine aminotransferase, and glutamate transporter-1 (GLT-1) (Cadoret et al., 2002).
AEG-1/MTDH/LYRIC has also been associated with the Wnt/β-catenin pathway in several cancer indications (Yoo, Emdad, et al., 2009). In HCC cells, AEG-1/MTDH/LYRIC was found to activate Wnt/β-catenin signaling by activating ERK42/44 and upregulating LEF1/TCF1, the ultimate executor of the Wnt pathway (Yoo, Emdad, et al., 2009). The importance of Wnt signaling in mediating AEG-1/MTDH/LYRIC action was demonstrated by inhibiting LEF1 that resulted in significant attenuation of AEG-1/MTDH/LYRIC-induced invasion of HCC cells. In gastric carcinoma, inhibition of AEG-1/MTDH/LYRIC downregulates LEF1 and cyclin D1 proteins, two critical downstream effectors in Wnt/β-catenin pathway involved in cell survival (Jian-bo et al., 2011). A recent study by Zhang et al. suggests that AEG-1/MTDH/LYRIC promotes invasion and metastasis of CRC by activation of β-catenin signaling pathway. This study documented a positive correlation between high AEG-1/MTDH/LYRIC expression and β-catenin nuclear expression in CRC, and overexpression of AEG-1/MTDH/LYRIC dramatically increased nuclear β-catenin accumulation in CRC cell lines (Zhang et al., 2012). The role of AEG-1/MTDH/LYRIC in activation of the Wnt/β-catenin pathway has also been documented in diffuse B cell lymphoma (Ge et al., 2012).
Aberrant activation of mitogen-activated protein kinase (MAPK) pathway is frequently observed in cancers and usually activated by growth factors, hormones, and chemokines. Once activated, MAPK is phosphorylated by MAPK kinases, MEK1 and MEK2, that recognize and phosphorylate tyrosine and threonine residues in the Thr-X-Tyr activation loop of the MAPKs, also known as ERK1 and ERK2 (Downward, 2003; Katz, Amit, & Yarden, 2007). A recent study indicates that specific inhibitors of the MAPK pathway are able to abolish the oncogenic effect of AEG-1/MTDH/LYRIC, namely, invasion and anchorage-independent growth, indicating that AEG-1/MTDH/LYRIC also plays an oncogenic role in tumor development and progression through activation of the MAPK signal pathway (Yoo, Emdad, et al., 2009). Another recent study showed that knockdown of AEG-1/MTDH/LYRIC can enhance the sensitivity of breast cancer cells to a novel ATP-noncompetitive inhibitor of MAP/ERK kinase via regulating FOXO3 activity and expression (Kong, Moran, Zhao, & Yang, 2012).
4.4. Regulation of glutamate signaling
Glutamate is the main excitatory amino acid transmitter in the mammalian central nervous system, and excessive glutamate exposure is toxic to neurons (Choi, 1988). Accumulation of excess extracellular glutamate in the synaptic cleft results in increased production of reactive and excitotoxic oxygen/ nitrogen species, which induce oxidative stress leading to neuronal death. Five excitatory amino acid transporters have been identified and cloned, which include EAAT1 (GLAST), EAAT2 (GLT-1), EAAT3, EAAT4, and EAAT5. EAAT2 is the most abundant glutamate transporter in brain, which plays an important role in keeping the glutamate concentration low by removing the glutamate released at the synapse. Excitotoxicity caused by impaired glutamate uptake by glial cells has been implicated in various neurodegenerative disease conditions such as ischemia/stroke, epilepsy, amyotrophic lateral sclerosis, traumatic brain injury (TBI), and HIV-associated dementia, and also in psychiatric disorders such as depression and schizophrenia (Choi, 1988; Doble, 1999). AEG-1/MTDH/LYRIC was originally isolated as a novel HIV-1- and TNF-α-induced transcript from PHFA (Su et al., 2003, 2002). However, the role of AEG-1/ MTDH/LYRIC in HIV pathogenesis still remains unknown. Previously, an interesting inverse correlation between expression levels of AEG-1/ MTDH/LYRIC and EAAT2 was documented (Kang et al., 2005). AEG-1/MTDH/LYRIC expression is elevated following HIV-1 and TNF-α treatment of astrocytes, whereas EAAT2 expression is down-regulated. Additionally, a recent study by Lee et al. (2011) suggests that AEG-1/MTDH/LYRIC also contributes to glioma-induced neurodegeneration. A strong negative correlation between expression of AEG-1/MTDH/LYRIC and EAAT2 was noticed in normal brain tissues and glioma patient samples. Gain and loss of function studies in PHFA and T98G cells revealed that AEG-1/MTDH/LYRIC repressed EAAT2 expression at a transcriptional level by inducing YY1 activity to inhibit CBP function as a coactivator on the EAAT2 promoter. Moreover, AEG-1/MTDH/LYRIC overexpression in glioma impairs glutamate uptake by reducing EAAT2 expression, culminating in glioma-induced neurodegeneration (Lee et al., 2011).
Reactive astrogliosis is frequently linked to CNS insults such as ischemia, trauma, infection, neurodegeneration, and postneurosurgical healing, commonly associated with the management of brain tumors (Eddleston & Mucke, 1993; Maragakis & Rothstein, 2006). EAAT2-positive cells were shown to be significantly decreased for a prolonged survival period following traumatic injury of human brain (van Landeghem, Weiss, Oehmichen, & von Deimling, 2006). This molecular phenotype was also confirmed in a mouse model of TBI (Wei et al., 2012). Interestingly, one recent study identified a novel role of AEG-1/MTDH/LYRIC in mediating reactive astrogliosis and in regulating astrocyte responses to injury in an in vivo brain injury model (Vartak-Sharma & Ghorpade, 2012). This potential cross talk between AEG-1/MTDH/LYRIC and EAAT2 may play a significant role in TBI, which needs further validation.
Migraine is a common neurological disorder usually presented by severe attacks of headache associated with other symptoms like nausea, vomiting, and photophobia. Two main types of migraine are distinguished based on the presence of an aura that can precede the headache: migraine with aura (MA) or migraine without aura. One recently published genome-wide association study (GWAS) using data from migraine patients found evidence for a role of the AEG-1/MTDH/LYRIC gene in common migraine. In this study, a single nucleotide polymorphism (SNP) (rs1835740) was identified that was located between two interesting candidate genes: AEG-1/MTDH/ LYRIC and the plasma glutamate carboxypeptidase (PGCP) gene (Anttila, Wessman, Kallela, & Palotie, 2011). An expression quantitative locus (eQTL) analysis revealed that rs1835740 most likely affects migraine through cis-regulation of AEG-1/MTDH/LYRIC, which in turn down-regulates EAAT2. Another recent study presented GWA meta-analysis for common migraine by the Dutch Icelandic migraine genetics consortium (DICE) (Ligthart et al., 2011). In this study, they also investigated SNP rs1835740 that was found to be significantly associated with MA in the first GWAS of clinic-based populations. The SNP itself was not associated with migraine in GWA-DICE study, but the gene-based analyses provided modest support for an association of AEG-1/MTDH/LYRIC with migraine.
4.5. Autophagy signaling
Autophagy is crucial for maintaining cellular homeostasis as well as remodeling during normal development, and dysfunctions in autophagy have been associated with a variety of diseases such as cancer, inflammatory bowel diseases, and neurodegenerative diseases (Levine & Klionsky, 2004; Mathew, Karantza-Wadsworth, & White, 2007). Autophagy can be either tumor suppressive or protective. During cellular or metabolic stress, a reduced cellular ATP level is sensed by AMPK (5′-AMP-activated protein kinase). Active AMPK leads to phosphorylation and activation of the TSC1/2 complex, which inhibits mTOR activity. The inhibition of mTORC1 activity leads to the activation of a set of evolutionarily conserved autophagy-regulating proteins (Atg proteins) and starts the cascades of events of autophagy (Levine & Klionsky, 2004). Autophagy can be induced by the canonical or noncanonical pathway depending on the involvement of Beclin 1 and the class III PI3K (hVps34).
A recent study (Bhutia et al., 2010) documents that AEG-1/MTDH/ LYRIC mediates protective autophagy in various normal cell types, an important regulator of cancer survival under metabolic stress and apoptosis-deficient conditions, which may underlie its significant cancer-promoting properties. AEG-1/MTDH/LYRIC protects normal cells from serum starvation-induced death through protective autophagy, and inhibition of AEG-1/MTDH/LYRIC-induced autophagy results in serum starvation-induced cell death. AEG-1/MTDH/LYRIC induces non-canonical autophagy as evidenced by an increase in expression of ATG5, and knockdown of ATG5 significantly inhibited the autophagy-related phenotypes. AEG-1/MTDH/LYRIC-induced autophagy is activated in response to a decrease in the cellular ATP/AMP ratio. Ectopic expression of AEG-1/MTDH/LYRIC resulted in diminished cellular metabolism. AEG-1/MTDH/LYRIC causes a significant increase of AMPK phosphorylation at Thr-172, which in turn phosphorylates and activates TSC2, further inhibiting the activation of downstream targets, such as mTOR and S6 kinase. Phosphorylation of mTOR and S6 proteins is substantially decreased in AEG-1/MTDH/LYRIC-overexpressing cells. Inhibition of AMPK by siAMPK or compound C decreases expression of ATG5, ultimately attenuating AEG-1/MTDH/LYRIC-induced autophagy. Autophagy also provides resistance to therapy-mediated tumor cell death. When tumor cells induce protective autophagy, inhibition of autophagy could provide a way of sensitizing tumor cells to therapy by activating apoptosis. Indeed, protective autophagy plays a crucial role in AEG-1/MTDH/LYRIC-mediated chemoresistance in cancer cells, and inhibition of AEG-1/MTDH/LYRIC results in a decrease in protective autophagy and chemosensitization of cancer cells.
5. AEG-1/MTDH/LYRIC: DOWNSTREAM MOLECULES AND INTERACTING PROTEINS
AEG-1/MTDH/LYRIC exerts its diverse function by interacting with other proteins in multiprotein complexes. Research in the past several years has identified several intracellular interacting partners of AEG-1/MTDH/LYRIC that provide significant insights into the mechanism of action of AEG-1/ MTDH/LYRIC (reviewed in Fig. 3.3). In each intracellular location, AEG-1/MTDH/LYRIC interacts with specific protein(s) thus influencing diverse intracellular events.
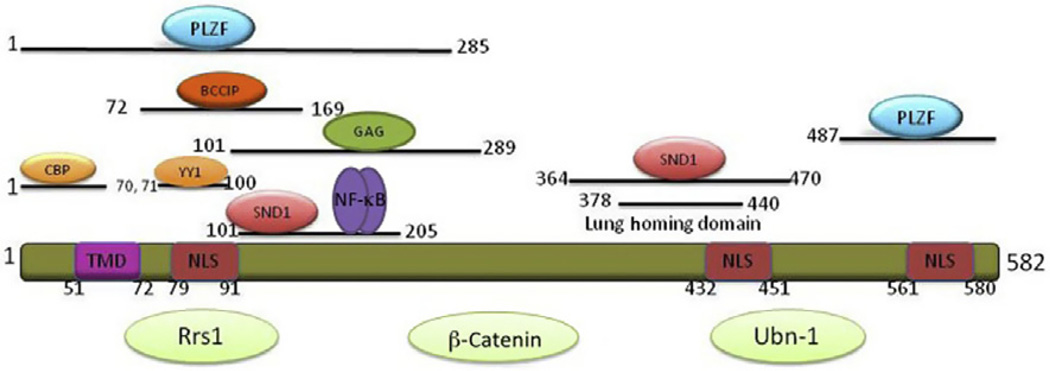
Figure 3.3.
Schematic diagram of AEG-1/MTDH/LYRIC protein and the region that interacts with various proteins. Proteins interact with AEG-1/MTDH/LYRIC, but the precise regions of interaction are not defined yet are shown in light green. NLS, nuclear localization signal; TMD, transmembrane domain.
5.1. NF-κB p65 and CBP
Activation of the NF-κB pathway by AEG-1/MTDH/LYRIC significantly contributes to AEG-1/MTDH/LYRIC-mediated oncogenic events such as invasion, migration, and anchorage-independent growth of cancer cells (Emdad et al., 2006; Sarkar et al., 2008).
In HeLa and malignant glioma cells, following infection with Ad.AEG-1 or treatment with TNF-α, AEG-1/MTDH/LYRIC was found to translocate into the nucleus where it interacted with the p65 subunit of NF-κB and enhanced NF-κB-induced gene expression. Activation of transcription by NF-κB requires transcriptional coactivator proteins, such as those possessing histone acetyltransferase (HAT) activity (Furia et al., 2002; Vanden Berghe, De Bosscher, Boone, Plaisance, & Haegeman, 1999; Zhong, May, Jimi, & Ghosh, 2002). HAT plays a key role in altering chromatin structure, allowing recruitment of the basal transcription factors and RNA polymerase II to initiate transcription (Huang, Ju, Hung, & Chen, 2007). NF-κB interacts with several HATs, such as CBP and its homologue p300, p300/CBP-associated factor, and members of the SRC/p160 family. These HATs acetylate core histone proteins as well as p50–p65 NF-κB to stimulate NF-κB-dependent gene expression (Chen & Greene, 2003). Chromatin immunoprecipitation (ChIP) assays document that AEG-1/MTDH/ LYRIC is located on the consensus NF-κB binding element in the IL-8 promoter together with p50–p65. Upon TNF-α treatment, AEG-1/MTDH/ LYRIC interacts with p65 and cyclic AMP-responsive element-binding protein-binding protein (CBP) on the IL-8 promoter and increases IL-8 transcription. Inhibition of AEG-1/MTDH/LYRIC by siRNA does not affect recruitment of NF-κB but impedes recruitment of CBP to the IL-8 promoter indicating that AEG-1/MTDH/LYRIC functions as a bridging factor among NF-κB, CBP, and the basal transcription machinery to induce IL-8 transcription (Sarkar et al., 2008). Deletion of the NH2-terminal 71 a.a. residues blocks AEG-1/MTDH/LYRIC-induced NF-κB activation, IL-8 expression, as well as invasion and anchorage-independent growth in malignant glioma cells. However, this region does not interact with NF-κB, rather a.a. 101–205 region was identified as the p65-interacting domain of AEG-1/MTDH/LYRIC. It might be possible that the proximal 71 a.a. region of AEG-1/MTDH/LYRIC interacts with CBP, thereby mediating NF-κB activation. Another recent study by Lee et al. (2011) documented that AEG-1/MTDH/LYRIC plays a critical role as a link between YY1 and CBP on the EAAT2 promoter, causing YY1 to function as a negative regulator of EAAT2 expression by inhibiting CBP. These results indicate that interactions among AEG-1/MTDH/LYRIC, YY1, and CBP are crucial for AEG-1/MTDH/LYRIC-mediated EAAT2 repression and also suggest that AEG-1/MTDH/LYRIC functions in the nucleus as an authentic transcriptional cofactor. A further domain analysis study indicated that a.a. 1–70 and 71–100 of AEG-1/MTDH/LYRIC are responsible for interaction with CBP and YY1, respectively.
5.2. Staphylococcal nuclease domain-containing 1
Staphylococcal nuclease domain-containing 1 (SND1) has been identified as an interaction partner of AEG-1/MTDH/LYRIC by two independent approaches, including yeast two-hybrid screening using a human liver cDNA library and isolation of AEG-1/MTDH/LYRIC-interacting proteins by coimmunoprecipitation followed by mass spectrometry (MS) (Yoo, Santhekadur, et al., 2011). SND1 is localized both in the nucleus and in the cytoplasm where it functions differently. In the nucleus, it facilitates transcription as a coactivator and mRNA splicing by interacting with the spliceosome machinery (Yang et al., 2007). In the cytoplasm, SND1 functions as a nuclease in the RNA-induced silencing complex (RISC) in which small RNAs (such as siRNAs or miRNAs) are complexed with ribonucleoproteins resulting in RNAi-mediated gene silencing (Caudy et al., 2003). It has been demonstrated that AEG-1/MTDH/LYRIC interacts with SND1 via the region 101–205 a.a. in the cytoplasm but not in the nucleus. Both SND1 and AEG-1/MTDH/LYRIC functionally facilitate RISC activity (Yoo, Santhekadur, et al., 2011). Studies in HCC found that both AEG-1/MTDH/LYRIC and SND1 are overexpressed in HCC and RISC activity was found to be higher in human HCC cells compared to normal immortal hepatocytes. Moreover, inhibition of either AEG-1/ MTDH/LYRIC or SND1 increased, while overexpression of AEG-1/ MTDH/LYRIC or SND1 decreased the mRNA level of the tumor suppressor PTEN, a target of miRNA-221, which is overexpressed in HCC. In another study, Blanco et al. (2011) employed MS-based screen and identified SND1 as a candidate AEG-1/MTDH/LYRIC-interacting protein in breast cancer. They further mapped the region of interaction to amino acids 364–470 of the AEG-1/MTDH/LYRIC coding sequence, which is very similar to the AEG-1/MTDH/LYRIC lung-homing domain. By “gain-of-function” and “loss-of-function” studies, a role of SND1 in breast cancer metastasis to the lungs was demonstrated (Blanco et al., 2011). Additionally, Wang et al. (2012) recently reported that the expression pattern of SND1 significantly and positively correlated with that of AEG-1/MTDH/LYRIC in CRC. AEG-1/MTDH/LYRIC+/SND1+ coexpression was more significantly associated with aggressive nodal status, high pathological stage, and poor differentiation than either AEG-1/MTDH/LYRIC expression or SND1 expression.
5.3. BRCA2- and CDKN1A–interacting protein a
Using yeast two-hybrid screening, Ash, Yang, and Britt (2008) identified BCCIPα (a BRCA2- and CDKN1A (p21Cip1/Waf-1/mda-6)-interacting protein α), a tumor suppressor gene, as an AEG-1/MTDH/LYRIC-interacting protein. Reduced BCCIPα expression has been observed in breast cancer and glioma cell lines and ectopic expression of the protein causes growth delay (Liu, Yuan, Huan, & Shen, 2001). BCCIPα binds to p21 (Cip1/ Waf-1/mda-6) and enhances p21-mediated Cdk2 kinase inhibition. Coexpression of AEG-1/MTDH/LYRIC with BCCIPα in prostate tumor cells resulted in decreased BCCIPα protein levels, relative to control, suggesting that AEG-1/MTDH/LYRIC may serve as a possible negative regulator of BCCIPα. The interaction region between AEG-1/MTDH/ LYRIC and BCCIPα was defined, and it was demonstrated that a.a. 72–169 in AEG-1/MTDH/LYRIC is the critical region for this interaction. However, the functional role of BCCIPα degradation in mediating AEG-1/ MTDH/LYRIC function remains to be determined.
5.4. Promyelocytic leukemia zinc finger protein
Thirkettle, Mills, Whitaker, and Neal (2009) identified promyelocytic leukemia zinc finger (PLZF) protein as an interacting protein of AEG-1/ MTDH/LYRIC using the yeast two-hybrid approach. PLZF is a transcriptional repressor associated with growth suppression and apoptosis (Bernardo, Yelo, Gimeno, Campillo, & Parrado, 2007). AEG-1/MTDH/LYRIC–PLZF interaction was detected in nuclear bodies and the NH2-terminal 1–285 a.a. and COOH-terminal 487–582 a.a. of AEG-1/MTDH/LYRIC interacted with PLZF as shown by coimmunoprecipitation assays. The authors further showed that a.a. 322–404 in PLZF were sufficient to interact with AEG-1/MTDH/LYRIC, which contains two lysine residues required for activation of PLZF by SUMOylation. By using a point mutation construct where the key lysine residue for PLZF SUMOylation was mutated (K242R), the authors demonstrated that AEG-1/MTDH/LYRIC was not able to interact with PLZF, suggesting that AEG-1/MTDH/LYRIC only interacts with the active form of PLZF. Coexpression of AEG-1/MTDH/ LYRIC and PLZF resulted in a significant increase in c-Myc transcript level. ChIP assay further revealed that AEG-1/MTDH/LYRIC suppressed the binding of the PLZF to the c-Myc promoter, thus causing increased transcription of c-Myc. PLZF neutralization thus might be another way, along with effects on the PI3K/Akt and Wnt signaling pathways, by which AEG-1/ MTDH/LYRIC activates c-Myc. In these contexts, AEG-1/MTDH/ LYRIC and c-Myc might have an intimate and supplementary role in mediating tumorigenesis.
5.5. HIV-1 Gag
HIV-1 Gag is the main structural protein driving assembly and release of HIV-1 virions from infected cells. Using a series of affinity purification experiments, Engeland et al. (2011) identified AEG-1/MTDH/LYRIC to be an HIV-1 Gag-interacting protein. Gag interacts with endogenous AEG-1/MTDH/LYRIC through its matrix (MA) and nucleocapsid domains. This interaction requires Gag multimerization and AEG-1/ MTDH/LYRIC amino acids 101–289. Gag–AEG-1/MTDH/LYRIC interaction was also observed for murine leukemia virus and equine infectious anemia virus, suggesting that it represents a conserved feature among retroviruses. Expression of the Gag-binding domain of AEG-1/MTDH/ LYRIC increased Gag expression levels and viral infectivity, whereas expression of an AEG-1/MTDH/LYRIC mutant lacking the Gag-binding site resulted in lower Gag expression and decreased viral infectivity. These data indicate a potential role of AEG-1/MTDH/LYRIC in regulating infectivity. Further experiments are needed to elucidate the precise purpose of this interaction.
5.6. Other interacting molecules: Rrs1, β-catenin, ubinuclein
In a knock-in mouse model of Huntington disease, regulator of ribosome synthesis 1 (Rrs1) is upregulated and might play an important role in HD pathogenesis by initiating ER stress (Carnemolla et al., 2009). In a recent study, Carnemolla et al. demonstrated that Rrs1 is localized both in the nucleolus and in the ER of neurons. By yeast two-hybrid screening, they identified AEG-1/MTDH/LYRIC as Rrs1 interactor that shares its dual subcellular localization in the ER and nucleolus (Carnemolla et al., 2009). The interaction between Rrs1 and AEG-1/MTDH/LYRIC was validated by coimmunoprecipitation experiments. These data suggest that both Rrs1 and AEG-1/MTDH/LYRIC might function as an ER stress sensor in HD and participate in transducing these signals to the nucleolus.
Another study showed that there was a positive correlation between AEG-1/MTDH/LYRIC high expression and β-catenin nuclear expression in CRC (Zhang et al., 2012). AEG-1/MTDH/LYRIC overexpression dramatically increased nuclear β-catenin accumulation in CRC cell lines. Furthermore, AEG-1/MTDH/LYRIC interacted with β-catenin in SW480 cells by immunoprecipitation assay (Zhang et al., 2012).
Ubinuclein (Ubn-1) is a cellular protein described as a nuclear protein interacting with the EBV transcription factor EB1 (also called ZEBRA or Zta) and other cellular transcription factor of the leucine-zipper family such as C/EBP or Jun (Lupo et al., 2012). One recent study identified AEG-1/MTDH/LYRIC as a new binding partner of Ubn-1 in epithelial cells. The authors used MS-based identifications of proteins eluted from pull-down assay and AEG-1/MTDH/LYRIC was validated as true partners of Ubn-1 through coimmunoprecipitation and confocal microscopy analyses (Lupo et al., 2012). In HT29 cells, the colocalization of AEG-1/ MTDH/LYRIC and Ubn-1 was shown at the TJ level. It would be interesting to further identify the domain of this interaction and potential role of this interaction in tumorigenesis and/or other processes.
5.7. Chemoresistance-related genes
Recent studies discovered a crucial role of AEG-1/MTDH/LYRIC in chemoresistance in various cancer indications (Hu, Chong, et al., 2009; Liu et al., 2009; Yoo et al., 2009; Yoo, Gredler, et al., 2009). Pharmacogenomic analysis of the NCI-60 panel of cancer cell lines revealed a significant correlation of AEG-1/MTDH/LYRIC overexpression and resistance of cancer cells to a broad spectrum of chemotherapeutics (Hu, Wei, et al., 2009). Gene expression profiles comparing AEG-1/MTDH/LYRIC overexpressed HCC cells versus control cells identified a cluster of genes associated with chemoresistance, including drug-metabolizing enzymes for various chemotherapeutic agents, such as dihydropyrimidine dehydrogenase (DPYD), cytochrome P4502B6 (CYP2B6), and dihydrodiol dehydrogenase (AKR1C2), ATP-binding cassette transporter ABCC11 for drug efflux, and the transcription factor LSF (Yoo, Gredler, et al., 2009). LSF regulates the expression of thymidylate synthase, a target of 5-fluorouracil (5-FU). In addition, AEG-1/MTDH/LYRIC enhanced the expression of DPYD, which catalyzes the initial and rate-limiting step in the catabolism of 5-FU. The increase in LSF and DPYD confers AEG-1/MTDH/LYRIC-induced 5-FU resistance. A recent study in HCC reported another novel mechanism through which AEG-1/MTDH/LYRIC plays a role in chemoresistance (Yoo et al., 2010). In this study, AEG-1/MTDH/LYRIC was shown to increase the expression of MDR1 protein, resulting in increased efflux and decreased accumulation of doxorubicin, thereby promoting doxorubicin resistance (Yoo et al., 2010). In breast cancer, inhibition of AEG-1/ MTDH/LYRIC sensitizes different cancer cell lines to paclitaxel, cisplatin, doxorubicin, 4-hydroxycylcophosphamide, hydrogen peroxide, and UV radiation (Hu, Chong, et al., 2009). Microarray analysis in breast cancer cells revealed that knockdown of AEG-1/MTDH/LYRIC led to decreased expression of chemoresistance genes ALDH3A1, MET, HSP90, and HMOX1 and increased expression of proapoptotic genes BNIP3 and TRAIL (Hu, Chong, et al., 2009). In neuroblastoma cells, a significant enhancement in chemosensitivity to cisplatin and doxorubicin was observed following knockdown of AEG-1/MTDH/LYRIC; however, no molecular characterization of downstream effectors was delineated (Liu et al., 2009). AEG-1/ MTDH/LYRIC does not affect the uptake or retention of chemotherapy drugs. Instead, AEG-1/MTDH/LYRIC may increase chemoresistance by promoting cell survival after chemotherapeutic stress. This could be mediated by the prosurvival pathways suchas PI3K and NF-κB or through other downstream genes affected by AEG-1/MTDH/LYRIC that directly regulate chemoresistance.
5.8. Invasion-associated molecules
The invasion-promoting ability of AEG-1/MTDH/LYRIC has been confirmed in diverse cancers including glioma, prostate cancer, HCC, neuroblastoma, osteosarcoma, CRC, and NSCLC (Emdad et al., 2010; Kikuno et al., 2007; Lee, Jeon, et al., 2009; Wang et al., 2011; Yoo, Emdad, et al., 2009; Zhang et al., 2012). Matrix metalloproteinases (MMPs) play a critical role in cancer invasion and metastasis. The MMPs are a family of zinc-dependent endopeptidases that remodel and degrade extracellular matrix, which are considered to play important roles in matrix degradation for tumor growth, invasion, and tumor-induced angiogenesis. AEG-1/ MTDH/LYRIC has previously been shown to increase expression of adhesion molecules by activating NF-κB. Meanwhile, the promoter of MMP2 and MMP9 contains multiple functional elements, including NF-κB and AP-1. Among the MMPs, MMP2 has been shown to be modulated by AEG-1/MTDH/LYRIC in glioma and osteosarcoma (Emdad et al., 2010; Wang et al., 2011), while MMP9 was shown in glioma, prostate cancer, and NSCLC (Emdad et al., 2010; Kikuno et al., 2007; Liu et al., 2010; Sun et al., 2012).
5.9. Other Downstream Molecules
A recent study indicates that AEG-1/MTDH/LYRIC plays a seminal role in hepatocarcinogenesis and profoundly downregulates insulin-like growth factor-binding protein-7 (IGFBP7) (Chen et al., 2011). This study further suggested that IGFBP7 expression is significantly downregulated in human HCC samples and cell lines compared with normal liver and hepatocytes, respectively, and inversely correlates with the stages and grades of HCC. Forced overexpression of IGFBP7 in AEG-1/MTDH/LYRIC-overexpressing HCC cells inhibited in vitro growth and induced senescence and strongly suppressed in vivo growth in nude mice that might be an end result of inhibition of angiogenesis by IGFBP7. This study by Chen et al. provides evidence that IGFBP7 functions as a novel putative tumor suppressor for HCC and establishes the corollary that IGFBP7 downregulation can effectively modify AEG-1/MTDH/LYRIC function.
To further understand the role of AEG-1/MTDH/LYRIC in HCC, Srivastava et al. (2012) developed a transgenic mouse with hepatocyte-specific expression of AEG-1/MTDH/LYRIC (Alb/AEG-1). Treating Alb/AEG-1, but not wild-type (WT) mice, with N-nitrosodiethylamine (DEN) resulted in multinodular HCC with steatotic features. Using oligonucleotide microarray, the authors investigated the global gene expression changes in Alb/AEG-1 mice by comparing DEN-treated WT and Alb/ AEG-1 livers. Apart from already known AEG-1/MTDH/LYRIC downstream effector molecules, a large number of novel molecules regulated by AEG-1/MTDH/LYRIC were discovered, including HCC marker alphafetoprotein; invasion and metastasis-associated genes tetraspanin 8 and lipocalin 2; several genes associated with fat metabolism, such as stearoyl coenzyme A desaturase-2, lipoprotein lipase, apolipoprotein A-IV, and apolipoprotein C-II; and genes regulating angiogenesis, such as trefoil factor 3 and mesenchyme homeobox 2.
6. AEG-1/MTDH/LYRIC AND ANGIOGENESIS
Cancer development is a multifactorial and multistep process and angiogenesis is a crucial component in this process. Angiogenesis, the process of forming neovascularization from existing vascular networks, is a fundamental event in the development and maintenance of solid tumors and their metastases. Increased intratumoral microvascular density relative to normal tissue is observed in tumors of different tissues including the brain, colon, and breast (Emdad et al., 2009). The process of angiogenesis is regulated by a continuous interplay of proangiogenic factors such as VEGF, basic fibroblast growth factor, epidermal growth factor, interleukins, nitric oxide synthase, transforming growth factor beta, TNF-α, platelet-derived growth factor (PDGF), and MMPs and angiogenic inhibitors such as endostatin, platelet factor-4, tumastin, thrombospondin-1, plasminogen activator inhibitor-1, and angiostatin (Carmeliet & Jain, 2011; Folkman, 2007; Weis & Cheresh, 2011). In many disorders including cancer, the balance between pro- and antiangiogenic factors is tilted to favor proangiogenic one, resulting in an “angiogenic switch.”
Tumor angiogenesis is induced by hypoxic conditions through the regulation of several proangiogenic factors. Hypoxia leads to stabilization of VEGF mRNA and induces expression of HIF-1, which upregulates expression of VEGF through binding to a HIF-1 consensus sequence in the 5′ flanking region of the VEGF gene. VEGF is an established potent endothelial-specific mitogen and has a central role in tumor angiogenesis (An, Matsuda, Fujii, & Matsumoto, 2000; Dvorak, 2002; Semenza, 2010). Increased expression of VEGF has been known to be associated with a wide range of solid tumors and is considered as negative predictor of tumor prognosis.
Another important component of angiogenesis in normal and tumor tissue is the angiopoietin (Ang) pathway. Two Ang family members have been identified, Ang-1 and 2. Ang1 is the ligand for the Ang receptor Tie2. Recent studies demonstrated that Ang1 and Tie2 are expressed in human glioma cell lines and a transgenic mouse astrocytoma model (Davis et al., 1996; Stratmann, Risau, & Plate, 1998).
Another important regulatory molecule of tumor angiogenesis is HIF induced by hypoxia as indicated above. HIF-1 is a transcription factor that regulates the expression of several genes involved in various cellular functions including angiogenesis (Majmundar, Wong, & Simon, 2010), invasion, and metastasis, resistance to chemotherapy, metabolic reprogramming, EMT, and stem cell maintenance (Semenza, 2012). HIF-1 tran-scriptional activity requires formation of a heterodimer consisting of HIF-1α and HIF-1β. The heterodimer binds to hypoxia response elements in the promoter regions of its target genes, where it activates transcription. HIF-1β is ubiquitously expressed, whereas, HIF-1α expression is tightly regulated by oxygen tension (Majmundar et al., 2010). Among the growth/survival factors that are encoded by HIF-regulated genes are transforming growth factor-α, insulin-like growth factor-2, VEGF, endothelin 1, adrenomedullin, and erythropoietin. HIF-1 controls the expression of multiple proangiogenic molecules, including VEGF, stromal-derived factor 1, placental growth factor, PDGF, and Ang 1 and 2 (Semenza, 2012). In mouse models, inhibition of HIF-1 activity dramatically inhibits tumor vasculari-zation (Lee, Zhang, et al., 2009). Additionally, HIF-1 activates transcription of genes involved in invasion and metastasis such as MMP2, MMP9, MMP14, MET, VEGF, and ANGPT2. Interestingly, one recent study demonstrated that AEG-1/MTDH/LYRIC is induced by hypoxia and glucose deprivation in glioblastoma and that hypoxic induction of AEG-1/MTDH/ LYRIC depends on the stabilization of HIF-1α (Noch et al., 2011). AEG-1/MTDH/LYRIC was induced by hypoxia via the PI3K/Akt pathway and then AEG-1/MTDH/LYRIC feeds back to activate PI3K, thereby creating a positive feedback loop and consequently might facilitate angiogenesis.
AEG-1/MTDH/LYRIC has also been shown to be proangiogenic both in vitro and in vivo and can also augment expression of key angiogenesis molecules, such as Ang1, MMP2, and HIF-1α. Tumors formed by AEG-1/ MTDH/LYRIC-overexpressing cloned rat embryo fibroblasts (CREF-AEG-1) are highly aggressive and angiogenic. In vitro angiogenesis studies also demonstrated that AEG-1/MTDH/LYRIC promotes tube formation in Matrigel and increases invasion of HUVECs via the PI3K/Akt signaling pathway (Emdad et al., 2009). The proangiogenic property of AEG-1/ MTDH/LYRIC was also reinforced by the chicken chorioallantoic membrane assay. The angiogenesis-promoting function of AEG-1/MTDH/ LYRIC was also noted in human HCC (Yoo, Emdad, et al., 2009). AEG-1/MTDH/LYRIC overexpression in human HCC cells resulted in increased production of angiogenic factors, such as VEGF, PIGF, and fibroblast growth factor-α (FGF-α), and AEG-1/MTDH/LYRIC overexpression in human HCC cells resulted in highly aggressive, angiogenic, and metastatic tumors. Recent study (Srivastava et al., 2012) using a transgenic mouse with hepatocyte-specific expression of AEG-1/MTDH/ LYRIC (Alb/AEG-1) has further supported the proangiogenic role of AEG-1/MTDH/LYRIC. Conditioned media from Alb/AEG-1 hepatocytes induced marked angiogenesis with elevation in several coagulation factors. Among these factors, AEG-1/MTDH/LYRIC facilitated the association of Factor XII (FXII) messenger RNA with polysomes, resulting in increased translation. FXII displays angiogenic activity, which is independent of its function in coagulation. FXII binds to urokinase plasminogen activator receptor or cross talks with EGFR on HUVEC membranes, leading to activation of MAPK and Akt with subsequent proliferation and differentiation. siRNA-mediated knockdown of FXII resulted in a profound inhibition of AEG-1/MTDH/LYRIC-induced angiogenesis, indicating a central role of FXII in this process.
In one recent study, analysis of clinical samples taken from large cohorts of patients of breast cancer showed that AEG-1/MTDH/LYRIC correlated positively with VEGF levels and microvascular density (Li, Li, Song, et al., 2011). VEGF and VEGFR play very important roles in angiogenesis (Carmeliet & Jain, 2011). IL-8 and COX2 are also suggested as proangiogenic factors regulated by NF-κB signaling pathway. IL-8 and COX2 are overexpressed in breast cancer (Lerebours et al., 2008), and the expression of IL-8 can be regulated by AEG-1/MTDH/LYRIC (Emdad et al., 2006), indicating that AEG-1/MTDH/LYRIC may promote the angiogenesis of breast cancer by activating the NF-κB and/or VEGF signaling pathway. Further experiments should be performed to clarify the mechanism.
Angiogenesis is classically triggered by hypoxia and inflammation, conditions which can also induce EMT (Sanchez-Tillo et al., 2012). EMT-activating transcription factors ZEB, Snail, and Twist are often overexpressed by endothelial cells in the peritumoral stroma and are shown to promote tumor angiogenesis in vivo. EMT and hypoxic conditions also increase the population of cancer cells with stem-like phenotype (Bao et al., 2012). Two recent studies, one in breast cancer and another in HCC, documented a pivotal role of AEG-1/MTDH/LYRIC in inducing EMT. In breast cancer, overexpression of AEG-1/MTDH/LYRIC led to upregulation of the mesenchymal marker fibronectin and downregulation of the epithelial marker E-cadherin, and also, transcription factors Snail and Slug were upregulated in AEG-1/MTDH/LYRIC-overexpressing breast cancer cells. Interestingly, overexpression of AEG-1/MTDH/LYRIC also led to increased acquisition of CD44+/CD24−/low markers that are characteristic of breast cancer stem cells (Li, Kong, et al., 2011). Similarly, knockdown of AEG-1/MTDH/LYRIC expression in HCC cell lines resulted in downregulation of N-cadherin and snail, upregulation of E-cadherin, and translocation of β-catenin (Zhu et al., 2011). An overview of the role of AEG-1/MTDH/LYRIC in modulating angiogenesis is presented in Fig. 3.4. The present findings indicating a potential cross talk between AEG-1/MTDH/LYRIC-induced EMT and AEG-1/MTDH/ LYRIC-induced enhanced tumor angiogenesis deserve further in-depth exploration.
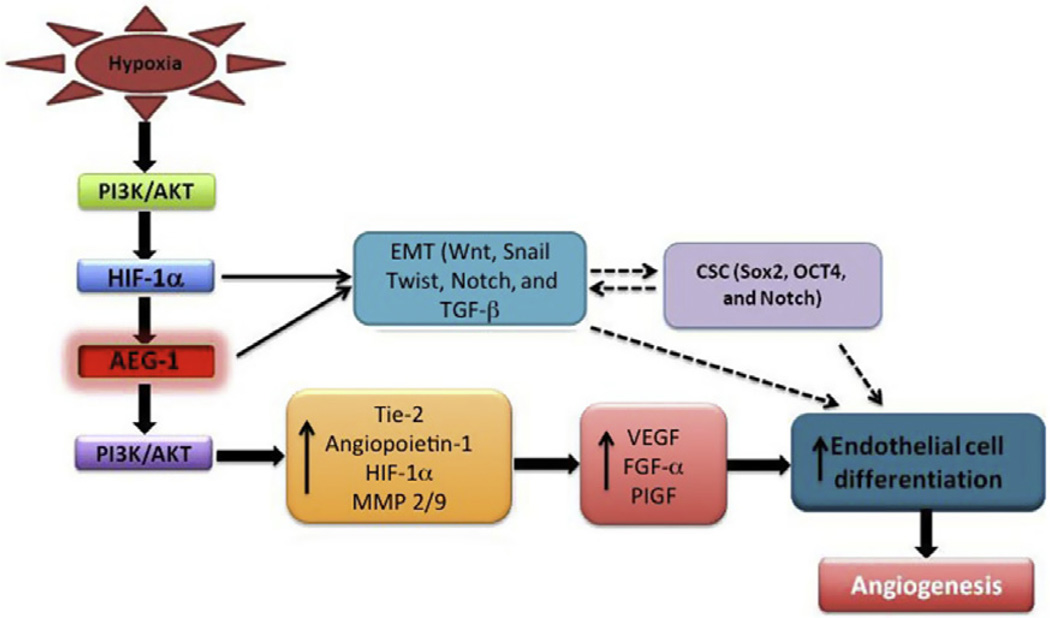
Figure 3.4.
Potential pathways and molecules linking AEG-1/MTDH/LYRIC to angiogenesis. Dotted lines indicate potential pathways involved in AEG-1/MTDH/LYRIC-regulated angiogenesis that require further validation.
7. CONCLUSION AND FUTURE PERSPECTIVES
In recent years, AEG-1/MTDH/LYRIC has emerged as an important mediator of cancer development and progression through regulation of essential processes of tumorigenesis such as transformation, invasion, metastasis, angiogenesis, and chemoresistance. Although numerous contributions to our literature base since its discovery in 2002 by Su et al. through subtraction hybridization have documented a diverse array of AEG-1/ MTDH/LYRIC functions, much work is still required to unravel the fundamental roles of AEG-1/MTDH/LYRIC in physiological and pathological conditions. Evolutionally, AEG-1/MTDH/LYRIC is only found in vertebrates that have developed complete immune systems, and AEG-1/ MTDH/LYRIC has recently been shown to be associated with the regulation of inflammation and immune responses. It would be worthwhile investigating further the significance of dysregulated AEG-1/MTDH/LYRIC expression in pathogenesis of cancer-related inflammation, autoimmune diseases, and other immunopathological conditions. Additionally, the intriguing novel role of AEG-1/MTDH/LYRIC in neurodegeneration and trauma also requires further exploration.
The multiple functions of AEG-1/MTDH/LYRIC highlight several important clinical ramifications. AEG-1/MTDH/LYRIC might be a ubiquitous biomarker for aggressive cancers with potential for routine screening of patients. However, to establish AEG-1/MTDH/LYRIC as a viable candidate biomarker, a larger cohort of patient studies and correlative investigations quantifying and comparing AEG-1/MTDH/LYRIC mRNA or protein in blood, urine, and biopsy samples with clinical characteristics are mandatory. AEG-1/MTDH/LYRIC expression might also help stratify patients receiving chemotherapeutic regimens based on the broad-spectrum chemoresistance conferred by AEG-1/MTDH/LYRIC. Cell surface expression of AEG-1/MTDH/LYRIC might also be useful for diagnostic and therapeutic purposes. Similarly, therapeutic toxins or radioactive isotopes might be conjugated with anti-AEG-1/MTDH/LYRIC antibody for targeted destruction of AEG-1/MTDH/LYRIC overexpressing cells. Qian et al. (2011) recently used an AEG-1/MTDH/LYRIC-based DNA vaccine, delivered by attenuated Salmonella typhimurium, and showed that this AEG-1/MTDH/LYRIC vaccine increased chemosensitivity to doxorubicin and inhibited breast cancer lung metastasis. The potential of AEG-1/ MTDH/LYRIC-based vaccine requires further evaluation in other cancers.
The observation that AEG-1/MTDH/LYRIC is able to interact with a plethora of proteins and assist in the formation of multiprotein complexes indicates that AEG-1/MTDH/LYRIC might be a new member of scaffolding proteins. Moreover, the biochemical modifications occurring to AEG-1/MTDH/LYRIC itself are also of great interest and worthy of further investigation, as such modification(s), if proved to be functionally important for AEG-1/MTDH/LYRIC, would represent potentially promising targets for optimal inhibitors or therapeutic drugs against diseases potentially mediated by aberrant AEG-1/MTDH/LYRIC expression, including cancer. Solving the crystal structure of the novel protein–protein interaction domains might also help in the design of small molecule inhibitors of AEG-1/MTDH/LYRIC. High-throughput screening combined with NMR with small molecules that disrupt interactions of AEG-1/MTDH/ LYRIC with its known interacting proteins might be yet another approach to identify AEG-1/MTDH/LYRIC inhibitors.
Experimental evidence derived from AEG-1/MTDH/LYRIC knockout or transgenic animal models would be important for further understanding the functional significance of AEG-1/MTDH/LYRIC in embryonic development and disease progression. As a positive regulator of cancer progression, transgenic mice expressing AEG-1/MTDH/LYRIC as well as conditional targeted AEG-1/MTDH/LYRIC knockout mice would prove extremely valuable for analyzing tumor progression in vivo. In this context, the transgenic mouse with hepatocyte-specific expression of AEG-1/ MTDH/LYRIC explored several novel aspects of AEG-1/MTDH/ LYRIC function that might not have been possible using in vitro models and nude mice xenograft studies. This mouse model might also be valuable in evaluating novel therapeutic approaches targeted toward nonalcoholic fatty liver disease and HCC. Additionally, developing more conditional transgenic animal model to express AEG-1/MTDH/LYRIC specifically in target tissue using a tissue-specific promoter would be beneficial in more precisely defining AEG-1/MTDH/LYRIC functions in additional cancer contexts. Moreover, crossing these animals with other tumor models to determine potential cross talk between AEG-1/MTDH/LYRIC and other tumor-promoting pathways, as well as with other disease models, would also prove very informative.
In summary, this review has tried to capture the varied and important functions of AEG-1/MTDH/LYRIC in diverse disease contexts. This is a continuing story and we are optimistic that new seminal information will be uncovered in the future as more investigators work on this intriguing molecule. Understanding the role of AEG-1/MTDH/LYRIC in normal physiological functions is also a worthwhile endeavor that clearly requires further research (Jeon et al., 2010). The observation that AEG-1/ MTDH/LYRIC may be an important contributor to many diverse cancers highlights the significance of developing approaches for selectively inhibiting its expression. These efforts are extremely valuable and hold potential for heralding in a new way of developing rationale target-based therapies for cancer, inflammatory diseases, and neurodegeneration.
ACKNOWLEDGMENTS
The research that served as the basis of this review were provided in part by the National Institutes of Health, National Cancer Institute Grants CA134721 (P. B. F.) and CA138540 (D. S.), the Trauma Fund, Virginia Commonwealth University, School of Medicine (L. E. and P. B. F.), and the VCU Massey Cancer Center (MCC). D. S. is a Harrison Scholar in the VCU MCC, and P. B. F. holds the Thelma Newmeyer Chair in Cancer Research in the VCU MCC.
REFERENCES
- An FQ, Matsuda M, Fujii H, Matsumoto Y. Expression of vascular endothelial growth factor in surgical specimens of hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology. 2000;126:153–160. doi: 10.1007/s004320050025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila V, Wessman M, Kallela M, Palotie A. Towards an understanding of genetic predisposition to migraine. Genome Medicine. 2011;3:17. doi: 10.1186/gm231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash SC, Yang DQ, Britt DE. LYRIC/AEG-1 overexpression modulates BCCIPalpha protein levels in prostate tumor cells. Biochemical and Biophysical Research Communications. 2008;371:333–338. doi: 10.1016/j.bbrc.2008.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao B, Azmi AS, Ali S, Ahmad A, Li Y, Banerjee S, et al. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochimica et Biophysica Acta. 2012;1826:272–296. doi: 10.1016/j.bbcan.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeusz C, Gonzalez-Angulo AM. Targeting the PI3K signaling pathway in cancer therapy. Expert Opinion on Therapeutic Targets. 2012;16:121–130. doi: 10.1517/14728222.2011.644788. [DOI] [PubMed] [Google Scholar]
- Baygi ME, Nikpour P. Deregulation of MTDH gene expression in gastric cancer. Asian Pacific Journal of Cancer Prevention. 2012;13:2833–2836. doi: 10.7314/apjcp.2012.13.6.2833. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Advances in Cancer Research. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nature Immunology. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- Bernardo MV, Yelo E, Gimeno L, Campillo JA, Parrado A. Identification of apoptosis-related PLZF target genes. Biochemical and Biophysical Research Communications. 2007;359:317–322. doi: 10.1016/j.bbrc.2007.05.085. [DOI] [PubMed] [Google Scholar]
- Bhutia SK, Kegelman TP, Das SK, Azab B, Su ZZ, Lee SG, et al. Astrocyte elevated gene-1 induces protective autophagy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22243–22248. doi: 10.1073/pnas.1009479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Research. 2001;61:7349–7355. [PubMed] [Google Scholar]
- Blanco MA, Aleckovic M, Hua Y, Li T, Wei Y, Xu Z, et al. Identification of staphylococcal nuclease domain-containing 1 (SND1) as a Metadherin-interacting protein with metastasis-promoting functions. The Journal of Biological Chemistry. 2011;286:19982–19992. doi: 10.1074/jbc.M111.240077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- Britt DE, Yang DF, Yang DQ, Flanagan D, Callanan H, Lim YP, et al. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Experimental Cell Research. 2004;300:134–148. doi: 10.1016/j.yexcr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH, et al. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnemolla A, Fossale E, Agostoni E, Michelazzi S, Calligaris R, De Maso L, et al. Rrs1 is involved in endoplasmic reticulum stress response in Huntington disease. The Journal of Biological Chemistry. 2009;284:18167–18173. doi: 10.1074/jbc.M109.018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy AA, Ketting RF, Hammond SM, Denli AM, Bathoorn AM, Tops BB, et al. A micrococcal nuclease homologue in RNAi effector complexes. Nature. 2003;425:411–414. doi: 10.1038/nature01956. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. Journal of Molecular Medicine (Berlin) 2003;81:549–557. doi: 10.1007/s00109-003-0469-0. [DOI] [PubMed] [Google Scholar]
- Chen W, Ke Z, Shi H, Yang S, Wang L. Overexpression of AEG-1 in renal cell carcinoma and its correlation with tumor nuclear grade and progression. Neoplasma. 2010;57:522–529. doi: 10.4149/neo_2010_06_522. [DOI] [PubMed] [Google Scholar]
- Chen D, Yoo BK, Santhekadur PK, Gredler R, Bhutia SK, Das SK, et al. Insulin-like growth factor-binding protein-7 functions as a potential tumor suppressor in hepatocellular carcinoma. Clinical Cancer Research. 2011;17:6693–6701. doi: 10.1158/1078-0432.CCR-10-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three Akts. Genes & Development. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: Implications for therapy. Pharmacology & Therapeutics. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nature Reviews. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. Journal of Clinical Oncology. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Mucke L. Molecular profile of reactive astrocytes—Implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche H, Sarkar D, et al. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21300–21305. doi: 10.1073/pnas.0910936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK, Dash R, et al. Astrocyte elevated gene-1: A novel target for human glioma therapy. Molecular Cancer Therapeutics. 2010;9:79–88. doi: 10.1158/1535-7163.MCT-09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, et al. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: Implications for tumor progression and metastasis. Cancer Research. 2006;66:1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- Engeland CE, Oberwinkler H, Schumann M, Krause E, Muller GA, Krausslich HG. The cellular protein lyric interacts with HIV-1 Gag. Journal of Virology. 2011;85:13322–13332. doi: 10.1128/JVI.00174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature Reviews. Genetics. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Fisher PB. Enhancement of viral transformation and expression of the transformed phenotype by tumor promoters. In: Slaga TJ, editor. Tumor promotion and cocarcinogenesis in vitro, mechanisms of tumor promotion. Florida: CRC Press, Inc.; 1984. pp. 57–123. [Google Scholar]
- Folkman J. Angiogenesis: An organizing principle for drug discovery? Nature Reviews. Drug Discovery. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Furia B, Deng L, Wu K, Baylor S, Kehn K, Li H, et al. Enhancement of nuclear factor-kappa B acetylation by coactivator p300 and HIV-1 Tat proteins. The Journal of Biological Chemistry. 2002;277:4973–4980. doi: 10.1074/jbc.M107848200. [DOI] [PubMed] [Google Scholar]
- Ge X, Lv X, Feng L, Liu X, Gao J, Chen N, et al. Metadherin contributes to the pathogenesis of diffuse large B-cell lymphoma. PLoS One. 2012;7:e39449. doi: 10.1371/journal.pone.0039449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annual Review of Immunology. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. The Re1/NF-kappa B/I kappa B signal transduction pathway and cancer. Cancer Treatment and Research. 2003;115:241–265. [PubMed] [Google Scholar]
- Gnosa S, Shen YM, Wang CJ, Zhang H, Stratmann J, Arbman G, et al. Expression of AEG-1 mRNA and protein in colorectal cancer patients and colon cancer cell lines. Journal of Translational Medicine. 2012;10:109. doi: 10.1186/1479-5876-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Liu W, You N, Wang T, Wang X, Lu P, et al. Prognostic significance of metadherin overexpression in hepatitis B virus-related hepatocellular carcinoma. Oncology Reports. 2012;27:2073–2079. doi: 10.3892/or.2012.1749. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes & Development. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Research. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes & Development. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nature Reviews. Drug Discovery. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, et al. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15:9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clinical Cancer Research. 2009;15:5615–5620. doi: 10.1158/1078-0432.CCR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Molecular Cell. 2007;26:75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui AB, Bruce JP, Alajez NM, Shi W, Yue S, Perez-Ordonez B, et al. Significance of dysregulated metadherin and microRNA-375 in head and neck cancer. Clinical Cancer Research. 2011;17:7539–7550. doi: 10.1158/1078-0432.CCR-11-2102. [DOI] [PubMed] [Google Scholar]
- Jeon HY, Choi M, Howlett EL, Vozhilla N, Yoo BK, Lloyd JA, et al. Expression patterns of astrocyte elevated gene-1 (AEG-1) during development of the mouse embryo. Gene Expression Patterns. 2010;10:361–367. doi: 10.1016/j.gep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian-bo X, Hui W, Yu-long H, Chang-hua Z, Long-juan Z, Shi-rong C, et al. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Medical Oncology. 2011;28:455–462. doi: 10.1007/s12032-010-9475-6. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth & Differentiation. 2001;12:363–369. [PubMed] [Google Scholar]
- Jiang T, Zhu A, Zhu Y, Piao D. Clinical implications of AEG-1 in liver metastasis of colorectal cancer. Medical Oncology. 2012;29:2858–2863. doi: 10.1007/s12032-012-0186-z. [DOI] [PubMed] [Google Scholar]
- Kanarek N, Ben-Neriah Y. Regulation of NF-kappaB by ubiquitination and degradation of the IkappaBs. Immunological Reviews. 2012;246:77–94. doi: 10.1111/j.1600-065X.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: From innocent bystander to major culprit. Nature Reviews. Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochimica et Biophysica Acta. 2007;1773:1161–1176. doi: 10.1016/j.bbamcr.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke ZF, He S, Li S, Luo D, Feng C, Zhou W. Expression characteristics of astrocyte elevated gene-1 (AEG-1) in tongue carcinoma and its correlation with poor prognosis. Cancer Epidemiology. 2012;37(2):179–185. doi: 10.1016/j.canep.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Ke ZF, Mao X, Zeng C, He S, Li S, Wang LT. AEG-1 expression characteristics in human non-small cell lung cancer and its relationship with apoptosis. Medical Oncology. 2013;30:383. doi: 10.1007/s12032-012-0383-9. [DOI] [PubMed] [Google Scholar]
- Khuda II, Koide N, Noman AS, Dagvadorj J, Tumurkhuu G, Naiki Y, et al. Astrocyte elevated gene-1 (AEG-1) is induced by lipopolysaccharide as toll-like receptor 4 (TLR4) ligand and regulates TLR4 signalling. Immunology. 2009;128:e700–e706. doi: 10.1111/j.1365-2567.2009.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–7655. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- Kong X, Moran MS, Zhao Y, Yang Q. Inhibition of metadherin sensitizes breast cancer cells to AZD6244. Cancer Biology & Therapy. 2012;13:43–49. doi: 10.4161/cbt.13.1.18868. [DOI] [PubMed] [Google Scholar]
- Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: Its role in health and disease. Journal of Molecular Medicine (Berlin) 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- Lee SG, Jeon HY, Su ZZ, Richards JE, Vozhilla N, Sarkar D, et al. Astrocyte elevated gene-1 contributes to the pathogenesis of neuroblastoma. Oncogene. 2009;28:2476–2484. doi: 10.1038/onc.2009.93. [DOI] [PubMed] [Google Scholar]
- Lee SG, Kim K, Kegelman TP, Dash R, Das SK, Choi JK, et al. Oncogene AEG-1 promotes glioma-induced neurodegeneration by increasing glutamate excitotoxicity. Cancer Research. 2011;71:6514–6523. doi: 10.1158/0008-5472.CAN-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K–Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lerebours F, Vacher S, Andrieu C, Espie M, Marty M, Lidereau R, et al. NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer. 2008;8:41. doi: 10.1186/1471-2407-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Developmental Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Li X, Kong X, Huo Q, Guo H, Yan S, Yuan C, et al. Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Science. 2011;102:1151–1157. doi: 10.1111/j.1349-7006.2011.01919.x. [DOI] [PubMed] [Google Scholar]
- Li C, Li R, Song H, Wang D, Feng T, Yu X, et al. Significance of AEG-1 expression in correlation with VEGF, microvessel density and clinicopathological characteristics in triple-negative breast cancer. Journal of Surgical Oncology. 2011;103:184–192. doi: 10.1002/jso.21788. [DOI] [PubMed] [Google Scholar]
- Li C, Li Y, Wang X, Wang Z, Cai J, Wang L, et al. Elevated expression of astrocyte elevated gene-1 (AEG-1) is correlated with cisplatin-based chemoresistance and shortened outcome in patients with stages III-IV serous ovarian carcinoma. Histopathology. 2012;60:953–963. doi: 10.1111/j.1365-2559.2012.04182.x. [DOI] [PubMed] [Google Scholar]
- Li C, Liu J, Lu R, Yu G, Wang X, Zhao Y, et al. AEG-1 overexpression: A novel indicator for peritoneal dissemination and lymph node metastasis in epithelial ovarian cancers. International Journal of Gynecological Cancer. 2011;21:602–608. doi: 10.1097/IGC.0b013e3182145561. [DOI] [PubMed] [Google Scholar]
- Li J, Yang L, Song L, Xiong H, Wang L, Yan X, et al. Astrocyte elevated gene-1 is a proliferation promoter in breast cancer via suppressing transcriptional factor FOXO1. Oncogene. 2009;28:3188–3196. doi: 10.1038/onc.2009.171. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang N, Song LB, Liao WT, Jiang LL, Gong LY, et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clinical Cancer Research. 2008;14:3319–3326. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nature Medicine. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- Liao WT, Guo L, Zhong Y, Wu YH, Li J, Song LB. Astrocyte elevated gene-1 (AEG-1) is a marker for aggressive salivary gland carcinoma. Journal of Translational Medicine. 2011;9:205. doi: 10.1186/1479-5876-9-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligthart L, de Vries B, Smith AV, Ikram MA, Amin N, Hottenga JJ, et al. Meta-analysis of genome-wide association for migraine in six population-based European cohorts. European Journal of Human Genetics. 2011;19:901–907. doi: 10.1038/ejhg.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Kumar R. Crosstalk between NFkB and glucocorticoid signaling: A potential target of breast cancer therapy. Cancer Letters. 2012;322:119–126. doi: 10.1016/j.canlet.2012.02.033. [DOI] [PubMed] [Google Scholar]
- Liu HY, Liu CX, Han B, Zhang XY, Sun RP. AEG-1 is associated with clinical outcome in neuroblastoma patients. Cancer Biomarkers: Section A of Disease Markers. 2012;11:115–121. doi: 10.3233/CBM-2012-0268. [DOI] [PubMed] [Google Scholar]
- Liu H, Song X, Liu C, Xie L, Wei L, Sun R. Knockdown of astrocyte elevated gene-1 inhibits proliferation and enhancing chemo-sensitivity to cisplatin or doxorubicin in neuroblastoma cells. Journal of Experimental & Clinical Cancer Research. 2009;28:19. doi: 10.1186/1756-9966-28-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Su Z, Li G, Yu C, Ren S, Huang D, Fan S, Tian Y, Zhang X, Qiu Y. Increased expression of metadherin protein predicts worse disease-free and overall survival in laryngeal squamous cell carcinoma. International Journal of Cancer. 2013;133(3):671–679. doi: 10.1002/ijc.28071. [DOI] [PubMed] [Google Scholar]
- Liu L, Wu J, Ying Z, Chen B, Han A, Liang Y, et al. Astrocyte elevated gene-1 upregulates matrix metalloproteinase-9 and induces human glioma invasion. Cancer Research. 2010;70:3750–3759. doi: 10.1158/0008-5472.CAN-09-3838. [DOI] [PubMed] [Google Scholar]
- Liu DC, Yang ZL. MTDH and EphA7 are markers for metastasis and poor prognosis of gallbladder adenocarcinoma. Diagnostic Cytopathology. 2013;41(3):199–205. doi: 10.1002/dc.21821. [DOI] [PubMed] [Google Scholar]
- Liu J, Yuan Y, Huan J, Shen Z. Inhibition of breast and brain cancer cell growth by BCCIPalpha, an evolutionarily conserved nuclear protein that interacts with BRCA2. Oncogene. 2001;20:336–345. doi: 10.1038/sj.onc.1204098. [DOI] [PubMed] [Google Scholar]
- Lupo J, Conti A, Sueur C, Coly PA, Coute Y, Hunziker W, et al. Identification of new interacting partners of the shuttling protein ubinuclein (Ubn-1) Experimental Cell Research. 2012;318:509–520. doi: 10.1016/j.yexcr.2011.12.020. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Developmental Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Molecular Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of disease: Astrocytes in neurodegenerative disease. Nature Clinical Practice. Neurology. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Marrogi A, Pass HI, Khan M, Metheny-Barlow LJ, Harris CC, Gerwin BI. Human mesothelioma samples overexpress both cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (NOS2): In vitro antiproliferative effects of a COX-2 inhibitor. Cancer Research. 2000;60:3696–3700. [PubMed] [Google Scholar]
- Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nature Reviews. Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Brachova P, Yang S, Xiong Z, Zhang Y, Thiel KW, et al. Knockdown of MTDH sensitizes endometrial cancer cells to cell death induction by death receptor ligand TRAIL and HDAC inhibitor LBH589 co-treatment. PLoS One. 2011;6:e20920. doi: 10.1371/journal.pone.0020920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noch E, Bookland M, Khalili K. Astrocyte-elevated gene-1 (AEG-1) induction by hypoxia and glucose deprivation in glioblastoma. Cancer Biology & Therapy. 2011;11:32–39. doi: 10.4161/cbt.11.1.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi Y, Makino T, Yoshikawa T, Nomura K, Fukuzawa K, Matsumoto A, et al. The possible role of TNF-αlpha and IL-2 in inducing tumor-associated metabolic alterations. Surgery Today. 1996;26:36–41. doi: 10.1007/BF00311989. [DOI] [PubMed] [Google Scholar]
- Nolan GP, Ghosh S, Liou HC, Tempst P, Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991;64:961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- Orentas RJ, Yang JJ, Wen X, Wei JS, Mackall CL, Khan J. Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Frontiers in Oncology. 2012;2:194. doi: 10.3389/fonc.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Current Opinion in Genetics & Development. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and cancer: How intimate is this relationship. Molecular and Cellular Biochemistry. 2010;336:25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BJ, Yan F, Li N, Liu QL, Lin YH, Liu CM, et al. MTDH/AEG-1-based DNA vaccine suppresses lung metastasis and enhances chemosensitivity to doxorubicin in breast cancer. Cancer Immunology, Immunotherapy. 2011;60:883–893. doi: 10.1007/s00262-011-0997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Paimela T, Suuronen T, Kaarniranta K. Innate immunity meets with cellular stress at the IKK complex: Regulation of the IKK complex by HSP70 and HSP90. Immunology Letters. 2008;117:9–15. doi: 10.1016/j.imlet.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Sanchez-Tillo E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, et al. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cellular and Molecular Life Sciences. 2012;69:3429–3456. doi: 10.1007/s00018-012-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: Clinical significance. Advances in Cancer Research. 2013 doi: 10.1016/B978-0-12-401676-7.00002-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Research. 2008;68:1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends in Pharmacological Sciences. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Weber JD. The ARF/p53 pathway. Current Opinion in Genetics & Development. 2000;10:94–99. doi: 10.1016/s0959-437x(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Song H, Li C, Li R, Geng J. Prognostic significance of AEG-1 expression in colorectal carcinoma. International Journal of Colorectal Disease. 2010;25:1201–1209. doi: 10.1007/s00384-010-1009-3. [DOI] [PubMed] [Google Scholar]
- Song H, Li C, Lu R, Zhang Y, Geng J. Expression of astrocyte elevated gene-1: A novel marker of the pathogenesis, progression, and poor prognosis for endometrial cancer. International Journal of Gynecological Cancer. 2010;20:1188–1196. doi: 10.1111/igc.0b013e3181ef8e21. [DOI] [PubMed] [Google Scholar]
- Song L, Li W, Zhang H, Liao W, Dai T, Yu C, et al. Over-expression of AEG-1 significantly associates with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. The Journal of Pathology. 2009;219:317–326. doi: 10.1002/path.2595. [DOI] [PubMed] [Google Scholar]
- Srivastava J, Siddiq A, Emdad L, Santhekadur PK, Chen D, Gredler R, et al. Astrocyte elevated gene-1 promotes hepatocarcinogenesis: Novel insights from a mouse model. Hepatology. 2012;56:1782–1791. doi: 10.1002/hep.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. The American Journal of Pathology. 1998;153:1459–1466. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Chen Y, Kang DC, Chao W, Simm M, Volsky DJ, et al. Customized rapid subtraction hybridization (RaSH) gene microarrays identify overlapping expression changes in human fetal astrocytes resulting from human immunodeficiency virus-1 infection or tumor necrosis factor-alpha treatment. Gene. 2003;306:67–78. doi: 10.1016/s0378-1119(03)00404-9. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- Sun S, Ke Z, Wang F, Li S, Chen W, Han A, et al. Overexpression of astrocyte-elevated gene-1 is closely correlated with poor prognosis in human non-small cell lung cancer and mediates its metastasis through up-regulation of matrix metalloproteinase-9 expression. Human Pathology. 2012;43:1051–1060. doi: 10.1016/j.humpath.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Sutherland HG, Lam YW, Briers S, Lamond AI, Bickmore WA. 3D3/lyric: A novel transmembrane protein of the endoplasmic reticulum and nuclear envelope, which is also present in the nucleolus. Experimental Cell Research. 2004;294:94–105. doi: 10.1016/j.yexcr.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, et al. Epidermal growth factor receptor: A novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129:285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Sugimachi K, Kameyama T, Maehara S, Shirabe K, Shimada M, et al. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003;37:1122–1129. doi: 10.1053/jhep.2003.50187. [DOI] [PubMed] [Google Scholar]
- Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirkettle HJ, Mills IG, Whitaker HC, Neal DE. Nuclear LYRIC/ AEG-1 interacts with PLZF and relieves PLZF-mediated repression. Oncogene. 2009;28:3663–3670. doi: 10.1038/onc.2009.223. [DOI] [PubMed] [Google Scholar]
- Tokunaga E, Nakashima Y, Yamashita N, Hisamatsu Y, Okada S, Akiyoshi S, et al. Overexpression of metadherin/MTDH is associated with an aggressive phenotype and a poor prognosis in invasive breast cancer. Breast Cancer. 2012 doi: 10.1007/s12282-012-0398-2. http://dx.doi.org/10.1007/s12282-012-0398-2. [DOI] [PubMed]
- Vanden Berghe W, De Bosscher K, Boone E, Plaisance S, Haegeman G. The nuclear factor-kappaB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. The Journal of Biological Chemistry. 1999;274:32091–32098. doi: 10.1074/jbc.274.45.32091. [DOI] [PubMed] [Google Scholar]
- van der Saag PT, Caldenhoven E, van de Stolpe A. Molecular mechanisms of steroid action: A novel type of cross-talk between glucocorticoids and NF-kappa B transcription factors. The European Respiratory Journal. Supplement. 1996;22:146s–153s. [PubMed] [Google Scholar]
- van Landeghem FK, Weiss T, Oehmichen M, von Deimling A. Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. Journal of Neurotrauma. 2006;23:1518–1528. doi: 10.1089/neu.2006.23.1518. [DOI] [PubMed] [Google Scholar]
- Vartak-Sharma N, Ghorpade A. Astrocyte elevated gene-1 regulates astrocyte responses to neural injury: Implications for reactive astrogliosis and neurodegeneration. Journal of Neuroinflammation. 2012;9:195. doi: 10.1186/1742-2094-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Reviews. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wang N, Du X, Zang L, Song N, Yang T, Dong R, et al. Prognostic impact of Metadherin-SND1 interaction in colon cancer. Molecular Biology Reports. 2012;39:10497–10504. doi: 10.1007/s11033-012-1933-0. [DOI] [PubMed] [Google Scholar]
- Wang F, Ke ZF, Sun SJ, Chen WF, Yang SC, Li SH, et al. Oncogenic roles of astrocyte elevated gene-1 (AEG-1) in osteosarcoma progression and prognosis. Cancer Biology & Therapy. 2011;12:539–548. doi: 10.4161/cbt.12.6.16301. [DOI] [PubMed] [Google Scholar]
- Warr T, Ward S, Burrows J, Harding B, Wilkins P, Harkness W, et al. Identification of extensive genomic loss and gain by comparative genomic hybridisation in malignant astrocytoma in children and young adults. Genes, Chromosomes & Cancer. 2001;31:15–22. doi: 10.1002/gcc.1113. [DOI] [PubMed] [Google Scholar]
- Wei J, Pan X, Pei Z, Wang W, Qiu W, Shi Z, et al. The beta-lactam antibiotic, ceftriaxone, provides neuroprotective potential via anti-excitotoxicity and anti-inflammation response in a rat model of traumatic brain injury. The Journal of Trauma and Acute Care Surgery. 2012;73:654–660. doi: 10.1097/TA.0b013e31825133c0. [DOI] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nature Medicine. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Xia Z, Zhang N, Jin H, Yu Z, Xu G, Huang Z. Clinical significance of astrocyte elevated gene-1 expression in human oligodendrogliomas. Clinical Neurology and Neurosurgery. 2010;112:413–419. doi: 10.1016/j.clineuro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang M, Chen Q, Zhang X. Expression of AEG-1 in human T-cell lymphoma enhances the risk of progression. Oncology Reports. 2012;28:2107–2114. doi: 10.3892/or.2012.2055. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. Physiological functions of protein kinase B/Akt. Biochemical Society Transactions. 2004;32:350–354. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- Yang J, Valineva T, Hong J, Bu T, Yao Z, Jensen ON, et al. Transcriptional co-activator protein p100 interacts with snRNP proteins and facilitates the assembly of the spliceosome. Nucleic Acids Research. 2007;35:4485–4494. doi: 10.1093/nar/gkm470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K–AKT-mTOR pathway: Progress, pitfalls, and promises. Current Opinion in Pharmacology. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Yoo BK, Chen D, Su ZZ, Gredler R, Yoo J, Shah K, et al. Molecular mechanism of chemoresistance by astrocyte elevated gene-1. Cancer Research. 2010;70:3249–3258. doi: 10.1158/0008-5472.CAN-09-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Emdad L, Lee SG, Su ZZ, Santhekadur P, Chen D, et al. Astro-cyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacology & Therapeutics. 2011;130:1–8. doi: 10.1016/j.pharmthera.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. The Journal of Clinical Investigation. 2009;119:465–477. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen D, Forcier T, et al. Identification of genes conferring resistance to 5-fluorouracil. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12938–12943. doi: 10.1073/pnas.0901451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BK, Santhekadur PK, Gredler R, Chen D, Emdad L, Bhutia S, et al. Increased RNA-induced silencing complex (RISC) activity contributes to hepatocellular carcinoma. Hepatology. 2011;53:1538–1548. doi: 10.1002/hep.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, et al. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30:894–901. doi: 10.1093/carcin/bgp064. [DOI] [PubMed] [Google Scholar]
- Yuan C, Li X, Yan S, Yang Q, Liu X, Kong B. The MTDH (-470G > A) polymorphism is associated with ovarian cancer susceptibility. PLoS One. 2012;7:e51561. doi: 10.1371/journal.pone.0051561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Yang Q, Meng F, Shi H, Li H, Liang Y, et al. Astrocyte elevated gene-1 interacts with beta-catenin and increases migration and invasion of colorectal carcinoma. Molecular Carcinogenesis. 2012 doi: 10.1002/mc.21894. http://dx.doi.org/10.1002/mc.21894. [DOI] [PubMed]
- Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W, et al. Metadherin mediates lipopolysaccharide-induced migration and invasion of breast cancer cells. PLoS One. 2011;6:e29363. doi: 10.1371/journal.pone.0029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Molecular Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Li J, Wang Z, Yin C, Zhang W. Metadherin is a novel prognostic marker for bladder cancer progression and overall patient survival. Asia-Pacific Journal of Clinical Oncology. 2012;8:e42–e48. doi: 10.1111/j.1743-7563.2012.01541.x. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nature Cell Biology. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- Zhu K, Dai Z, Pan Q, Wang Z, Yang GH, Yu L, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clinical Cancer Research. 2011;17:7294–7302. doi: 10.1158/1078-0432.CCR-11-1327. [DOI] [PubMed] [Google Scholar]