Abstract
Significance: Skin wounds cause great distress and are a huge economic burden, particularly with an increasingly aging population that heals poorly. There is an urgent need for better therapies that improve repair. Intracellular signaling pathways that regulate wound repair are activated by growth factors, hormones, and cytokines released at the wound. In addition, endogenous electric fields (EFs) are generated by epithelia in response to injury and are an important cue that coordinates cell behavior at wounds. Electrical stimulation (ES), therefore, holds the potential to be effective therapeutically in treating wounds.
Recent Advances: ES of wounds is an old idea based on observations of the natural occurrence of EF at wound sites. However, it is now receiving increasing attention, because (1) the underpinning mechanisms are being clarified; (2) devices that measure skin wound currents are in place; and (3) medical devices that apply EF to poorly healing wounds are in clinical use with promising results.
Critical Issues: Several signaling proteins transduce the EF influence to cells. However, a bigger picture of the EF-proteome is needed in order to understand this complex process and target it in a controlled manner.
Future Directions: Dissecting the signaling pathways driving electrical wound healing will allow further identification of key molecular switches that control the cellular response to EFs. These findings herald the development of a new concept, the use of hydrogel electrodes impregnated with small molecules that target signaling pathways to explore the potential of dual electric-pharmacological therapies to repair wounds.

Cristina Martin-Granados

Colin D. McCaig, PhD, FRSE
Scope and Significance
Poor wound healing is an enormous clinical problem and a socioeconomic challenge. Skin wounds are frequently painful, cause scarring, and may require hospitalization, special nutrition, and medication. Annually, about 1% of the population incurs skin wounds that need medical attention.1 Surgical wound infection affects 30–40 patients per 1,000 operations, and its effects can be life threatening. Pressure ulcers have a major negative effect on patient function and quality of life and strikingly; around one in five in-patients in European hospitals has a pressure ulcer. Wound care generally, and the treatment of chronic wounds in particular, will become increasingly important in the provision of healthcare as the population ages, as chronic wounds are highly age correlated.
Individual therapies that promote wound healing include the use of biomimetic scaffolds and negative pressure, the topical administration of specific small molecules, gene-therapy approaches, and cell-based strategies such as the administration of epithelial stem cells.2 Nonhealing wounds may benefit additionally from physical approaches to achieve wound healing. Numerous animal studies and clinical trials have provided evidence and mechanistic insights into the effectiveness of Electrical stimulation (ES) for wound healing.3–8 Consequently, clinicians and scientists are becoming increasingly aware of the potential for electrical therapies, and new electrical devices have been introduced clinically.7,8 Indeed, ES is the most effective treatment for long-term, nonhealing ulcers, and this has been adopted as a policy by the European Pressure Ulcer Advisory Panel (www.epuap.org) and the National Pressure Ulcer Advisory Panel, Washington DC (www.npuap.org/resources.htm). In the United States, ES is now approved for payment by the Centers for Medicare and Medicaid Services for treating pressure ulcers and wounds that have not responded to standard wound treatment.
This clinical awareness coincides with recent advances in our understanding of the molecular mechanisms underlying electrically stimulated wound repair. The hypothesis that wound repair is guided by electric signals is supported by three main lines of evidence: (1) Strong electric currents exist at wounds and are required for effective wound healing;9–13 (2) inhibition of endogenous currents specifically impairs wound closure;13,14 and (3) applying an exogenous electric current of physiological intensity can induce faster wound closure both in vitro and in vivo.3–8,14
In this review, we have compiled the present knowledge on the effects that electric fields (EFs) have on the behavior of cells involved in the healing response, and we focus on the mechanistic understanding of how electric signals are sensed and transmitted within these cells to influence their physiological behavior. In addition, we explore the potential of interfering with EF signaling to enhance skin wound repair by highlighting the potential of dual therapies using pharmacological compounds that regulate phosphorylation signaling and ES of wounds. In vitro and Phase I preclinical studies in animal models of wound healing will be useful to assess the potential of these dual therapies to treat nonhealing wounds.14
Translational Relevance
Uncovering the mechanisms of ES wound healing has translated into new healthcare guidelines and enhanced clinical use. ES is the most effective treatment for long-term, nonhealing ulcers, and this has been adopted as policy by the European Pressure Ulcer Advisory Panel (www.epuap.org) and the National Pressure Ulcer Advisory Panel, Washington DC (www.npuap.org/resources.htm). One device, WoundEl has seen increasing clinical use. Since 2006, around 800 WoundEl devices have been manufactured to treat in excess of 6,000 patients in Europe. Further translation is likely through the development of a next generation, hydrogel-based electrical dressing that is doped with appropriate small-molecule pharmacology.
Clinical Relevance
Skin wounds cause great distress and place a huge economic burden on health care, particularly with an increasingly aging population that heals poorly. Better therapies to improve wound repair are needed. Intracellular signaling pathways regulating wound repair are activated by growth factors, hormones, and cytokines released at the wound site. EFs are also generated by epithelial layers in response to injury and are an important cue orchestrating multiple cell behaviors at wounds. Identification of the signaling proteins regulating EF-mediated cell migration and wound closure is revealing the clinical potential of dual electric-pharmacological therapies to repair wounds.
Discussion
The epidermis: a powerful healing battery
The structure of the skin
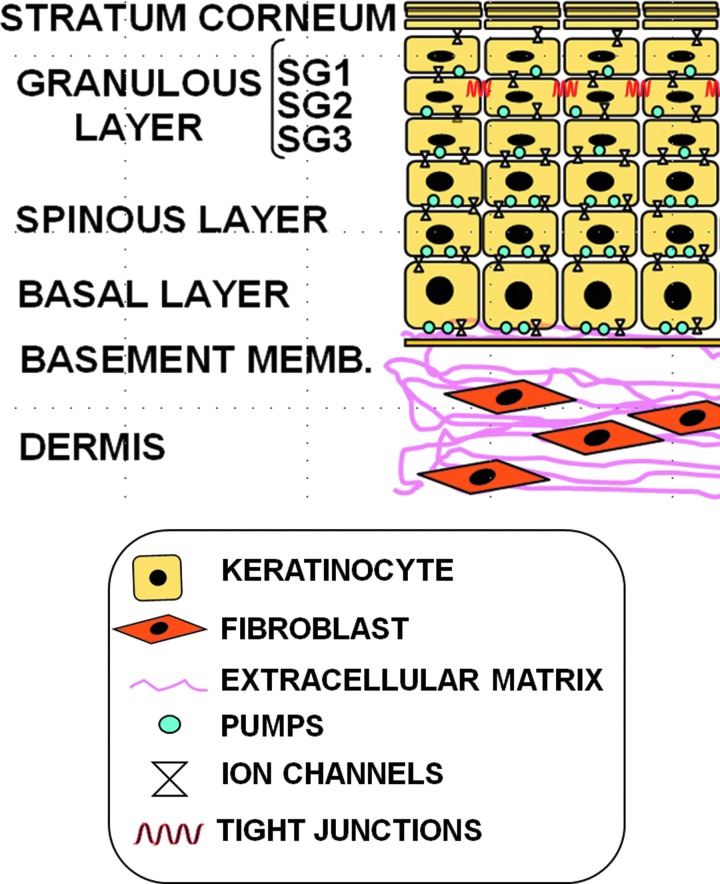
The skin is a multifunctional system that covers and protects the organism. Mammalian skin consists of two main tissue layers: a stratified squamous epithelium (epidermis), which overlies a dense connective tissue (dermis), depicted in Fig. 1.
Figure 1.
Structure of the mammalian skin. The dermis contains fibroblasts, endothelial cells, and inflammatory cells that are surrounded by an organized extracellular matrix (ECM) of collagen type I, fibronectin, and elastin. The epidermis is a continuously renewing tissue composed of keratinocytes at different stages of differentiation represented by three layers: granular (composed of three cell sheets SG1–SG3), spinous, and basal. The granular layer is overlain by a stratum corneum that represents the endpoint of epidermal differentiation and cell death. The stratum corneum confers the barrier function to the skin and protects internal organs from the environment. As the differentiation process progresses, keratinocytes express proteins involved in both the scaffold function and the eventual formation of the insoluble cornified envelope (CE). The CE replaces the plasma membrane of differentiating keratinocytes and consists of keratins that are enclosed within an insoluble amalgam of proteins, which are crosslinked by transglutaminases and surrounded by a lipid envelope forming a “mortar-brick” structure. Intercellular lipids are primarily generated from exocytosis of lipid-containing granules called lamellar bodies, during the terminal differentiation of keratinocytes. Tight junctions (TJs) are intercellular junctions that are formed by various TJ transmembrane proteins; for example, claudins, occludin, tricellulin, and junctional adhesion molecule, as well as intracellular scaffold proteins, for example, ZO-1, ZO-2, ZO-3, and cingulin. These proteins regulate the passage of ions and molecules through the paracellular pathway in epithelial and endothelial cells. TJs in the skin are located in the granulous SG2 layer. TJ formation is a prerequisite for the formation of the epidermal permeability barrier and the maintenance of barrier function, in addition to the sealing of proteins into CE. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The stages of skin wound healing
Cutaneous wound healing is a complex and evolutionarily conserved process that serves to restore the skin barrier and prevent infection after injury. It involves three temporally overlapping phases: inflammation, new tissue formation, and remodeling.15 Inflammation takes place immediately after tissue damage to prevent fluid loss and infection, and to remove dead cells. This first step is orchestrated by components of the coagulation cascade, inflammatory pathways, and the immune system. After inflammation, new tissue formation occurs, involving cellular proliferation and migration of keratinocytes over the injured dermis. New blood vessels then form during the angiogenic process, and this is regulated by vascular endothelial growth factor-A and fibroblast growth factor-2. The resulting capillaries associated with fibroblasts and macrophages replace the fibrin matrix with granulation tissue, which forms a new substrate for keratinocyte migration at later stages of the repair process. The barrier function of the epithelium is restored when the opposing wound edges rejoin, and the keratinocytes behind the leading edge proliferate and mature. In the later part of this stage, fibroblasts from the edge of the wound or from the bone marrow are stimulated by macrophages, and some differentiate into myofibroblasts that close the wound edges. Fibroblasts and myofibroblasts interact and produce collagen that ultimately forms the bulk of the mature scar. Finally, remodeling takes place. This involves apoptosis or wound exit of endothelial cells, macrophages, and myofibroblasts, leaving a mass that contains a few cells and consists mostly of collagen and other proteins of the extracellular matrix (ECM). This stage completes the wound repair process when the matrix is further remodeled by metalloproteinases that are secreted by fibroblasts, macrophages, and endothelial cells.
The three stages of the repair process are activated and co-ordinated via various intercellular and intracellular signaling cascades.2,15 These trigger specific changes in gene expression and promote the proliferation, differentiation, and migration of endothelial cells, keratinocytes, and fibroblasts, which is in concert with different types of immune cells.15 As recovery progresses, these signaling cascades are gradually down-regulated in an orchestrated manner. Deregulation of the repair process can cause defects ranging from chronic, nonhealing wounds to excessive matrix deposition leading to hypertrophic scars and keloids.16 Venous insufficiency and diabetes mellitus also contribute to the formation of chronic, nonhealing wounds and ulcers.17
Electric properties of the healthy and wounded skin
Voltages measured across rodent skin free of epithelial appendages (e.g., glands, hair) range from 30 to 100 mV (surface of the skin negative), and 0 to 10 mV across hairy regions.18 In human skin, the voltages range from 10 to ∼60 mV depending on the region measured9 (Fig. 2). The ionic current in healthy epidermis moves between superficial and deep layers of the epidermis, and it does not have a significant net component in a lateral direction (i.e., parallel to the skin surface). Thus, the voltage gradient of the healthy skin battery is aligned approximately perpendicularly to the skin surface. This changes when the epidermis is injured.
Figure 2.
Ion flows and potentials in intact skin. (A) Diagram of a typical epithelial cell in a monolayer with Na+ and Cl− channels localized on the apical plasma membrane and K+ channels localized on the basolateral membranes along with the Na+/K+-ATPase. (B) This asymmetric distribution of ion channels generates a transcellular inflow of positive current that flows back between the cells. This positive current flow coupled to Cl− movement in the opposite direction generates a transepithelial potential difference (TEP) of 10–60 mV. TJs between contiguous cells contribute to creating the TEP by providing high electrical resistance and preventing leakage of ions back down their concentration gradients between cells. Consequently, a concentration of positive charge exists behind the junctions at the deeper layers (positive sign in figure). The potential is, thus, relatively negative on the apical side of the junctions. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
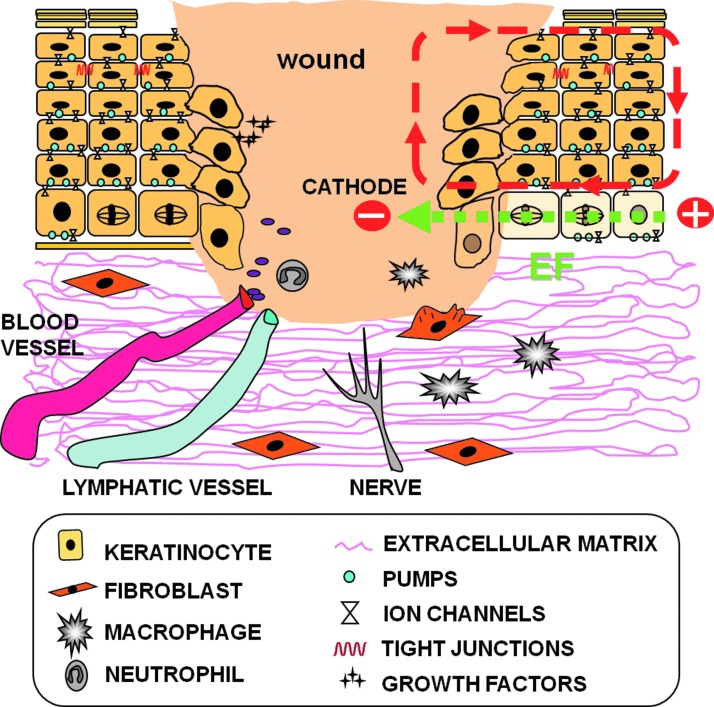
Following a wound, naturally occurring electric currents flow parallel to the epithelial layers (Fig. 3). This current arises the instant an epithelial barrier is breached due to the flow of ions down concentration gradients and out through the damaged epidermis. These wound currents generate lateral, intraepidermal voltage gradients (EFs) ranging from 100 to 200 mV/mm that decline with distance from the wound.18 Currents escaping through healing wounds and their accompanying lateral voltage gradients fall off gradually over time and ultimately become nonexistent due to the increasing resistance created by the newly regenerating epithelium. More recent work has confirmed the existence and intensity of these endogenously generated electric currents in mouse and human skin wounds.10,11,13 These epidermal electrical currents activate several major signaling cascades and promote the directional migration of many cell types involved in wound healing.
Figure 3.
Generation of wound-induced electric currents. When the skin is injured, the epithelial layer is disrupted locally, and the ionic gradient across the epithelium collapses at the wound site. A consequence of the disruption of the epithelial integrity is leakage of Na+ ions out of the wound, down their concentration gradient, and an uptake of Cl− ions. The respective ion movements give rise to a physiological injury current that flows toward the wound center (defined as the movement of positive charge) and a lateral voltage gradient oriented parallel to the epithelial sheet (EF, electric field; green discontinuous arrow at bottom). The discontinuous red arrows indicate positive ion (current) flux through the cell layers and the return path. The wound is, therefore, more negatively charged and acts as a cathode (negative sign in figure) as compared with healthy tissue (which acts as the anode, positive sign in figure). For simplification, we only show the ionic flux loop and EF on the right side of the figure, although the same applies for the left side. These endogenously generated EFs contribute to the guidance of cells, blood vessels, and nerves toward the wound site. Based on Nuccitelli et al.13 and Shaw and Martin.15 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Skin-electric currents control the behavior of cells involved in the wound healing response
Electrotaxis studies carried out in the last 40 years have shown that individual cells respond to EFs in a number of different ways. Several cell types that are implicated in wound repair increase their speed and direction of migration (electrotaxis) in response to an EF, as summarized in Table 1.14,19–30 Along with the antibacterial effect that EFs have shown in several studies,3 cells involved in the immune response such as neutrophils, lymphocytes, and monocytes show cathodal migration (toward the wound center) in an EF.14,19 Granulocytes migrate toward the anode at 2.5 mM Ca2+ and toward the cathode at 0.1 mM Ca2+.20 Macrophages also migrate in an EF,21 although the significance of their anodal response during wound closure is unclear. EFs cause macrophage migration on laminin and fibronectin by integrin-dependent cell crawling and possibly rolling.22 Intriguingly, EF stimulation of a marrow culture system increases the release of the cytokine macrophage colony stimulating factor, which is implicated in angiogenesis and accelerates wound healing.31 Vascular endothelial cell migration also determines the rate and pattern of new vessel out-growth, and this drives angiogenesis during wound repair. Endothelial cells respond to EFs by projecting broad, actin-filled lamellipodia.24 Keratinocytes that are involved in the later stages of the wound repair process migrate cathodally on several matrices.14,25,26 Fibroblasts, which are also implicated in later stages of wound repair, show voltage- and time-dependent electrotactic responses. Several studies have shown that fibroblasts can show both cathodal and anodal electrotactic responses.14,23,27–30 Additional studies on animal models and in cell cultures of dermal fibroblasts stimulated with a direct current (DC) EF demonstrated a significant increase in the ability of fibroblasts to synthesize collagen, produce DNA, and synthesize protein. In addition, the speed of epithelialization in the wound was noticeably increased.7 Fibroblasts that were exposed to EF had receptor levels of transforming growth factor-β which were six times greater than those of control fibroblasts, and this may explain the increase in collagen synthesis.3 Despite the variation in the directional response of fibroblasts reported by different studies, it seems highly probable that in the proliferative phase of wound healing, endogenous EFs activate the production of ECM by fibroblasts and induce fibroblast migration to the cathode (wound centre).7 Further studies are necessary to uncover whether differentiation of fibroblasts into myofibroblasts results in changes in the electrotactic response. Such effects could explain polarity changes in different phases of wound healing.
Table 1.
Directional responses of different cell types under a physiological electric field during the wound repair process
| Phase of Wound Repair | Cell Type | Directional Response to EF | References |
|---|---|---|---|
| Inflammatory | Macrophages | Anodal | 21,22 |
| Lymphocytes | Cathodal | 19 | |
| Monocytes | Cathodal | 14 | |
| Neutrophils | Cathodal | 14 | |
| Granulocytes | Cathodal/anodal | 20 | |
| Proliferative | Fibroblast | Cathodal/anodal | 14,23,27–30 |
| Remodeling (wound contraction and re-epithelialization)` | Keratinocytes | Cathodal | 14,25,26 |
| Myofibroblasts | ? | 24 | |
| Vascular endothelial cells | Cathodal |
EF, electric field.
How is the electric signal transduced to canonical signaling pathways in the skin?
Mammalian skin contains an array of voltage-sensor proteins (e.g., sodium, potassium and calcium channels, and the sodium/potassium pump).32,33 In addition, G protein-coupled receptor of keratinocytes function as voltage sensors.34 Changes in the membrane potential or in extracellular currents, therefore, may affect the activity of these voltage sensors and, in turn, activate signaling cascades. Another potential way in which cells could respond to a wound-induced EF is the redistribution of charged or glycosylated (i.e., negatively charged residues) membrane proteins protruding from the cell surface. For example, epidermal growth factor receptors (EGFR) are glycosylated proteins that translocate within the plane of the lipid bilayer to accumulate at the cathodal, apical side of cells. For keratinocytes and corneal epithelial cells, this occurs within 5–10 min of EF exposure.35,36 As a consequence, EGF signaling becomes polarized, causing greater cathodal activation of extracellular signal-regulated kinases 1/2 (ERK1/2) and downstream cathodal polymerization of Filamentous actin.35,36 EF exposure also up-regulates the expression of EGF receptors in epithelial cells.35 Pharmacological inhibition of multiple tyrosine kinases, including EGFR, reduces the cell migration rate; whereas specific pharmacological inhibition of EGFR kinase activity reduces directed motility in EFs.36 EGF receptor signaling is, therefore, considered an important signaling component of the EF-mediated contribution to wound closure.
Integrins are large membrane spanning proteins that are N-glycosylated, and, hence, negatively charged in their extracellular region. Their ability to form functional heterodimers (α and β subunits) and contribute to cell migration depends on the presence of N-linked oligosaccharides. Therefore, as for EGFR, integrins may redistribute asymmetrically in EFs due to their external negative charge. Indeed, integrins α5 and α5β1 redistribute and aggregate cathodally on fibroblasts migrating cathodally, as does β1 in epithelial cells.29 Moreover, depletion of β4 integrin or an anti-integrin β1 antibody suppresses EF-directed migration.37,38 Interestingly, addition of EGF receptor recovers the electrotactic response of β4 integrin null cells, suggesting that cooperativity between EF-activated EGF and β4-integrin signaling through the small GTPase Rac might occur, possibly at focal adhesions.37 In support of the pivotal role that membrane receptors such as integrins and growth factor receptor signaling play in the directional response of cells to EF, a recent study in cancer epithelial cells demonstrates that changes in the expression of several types of these receptors and/or modulation of the activity of their downstream effectors causes robust changes in cell polarization and directional migration in DC EF.39
Other mechanisms that may contribute to the early steps of the transduction of the EF signal are electro-osmosis40 or electrophoresis of morphogens through gap junctions.41
Phosphorylation of proteins underlies the effects of EF on cell behavior
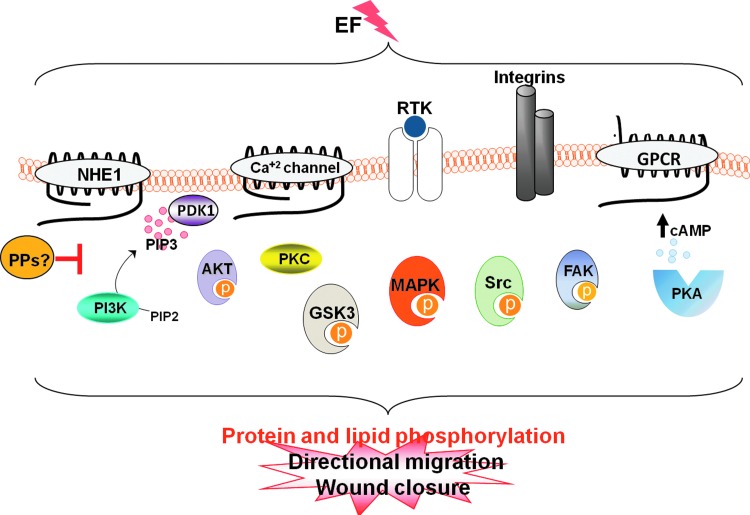
Phosphorylation can lead to conformational changes in proteins, which affect their affinity for ligands and results in functional changes. Protein (de)phosphorylation on serine/threonine/tyrosine (Ser/Thr/Tyr) is the most widespread mechanism of post-translational modification and is involved in an enormous range of cellular processes. Protein phosphorylation reactions are catalyzed by protein kinases, while the hydrolysis of phosphate esters involves protein phosphatases (PPs). Ser/Thr/Tyr dephosphorylation is involved in crucial cellular processes that are implicated in the establishment of cell polarity, cell division, and cell migration; therefore, it is not surprising that an increasing number of reports demonstrates that phosphorylation of proteins is a key signaling event underlying the effect of EFs on cells (Fig. 4).
Figure 4.
EFs activate several kinase signaling pathways. Membrane proteins transduce the electric signal by activating several intracellular signaling pathways. Eventually, transmission of the electric signal to downstream kinase effectors induces phosphorylation of proteins at Ser, Thr, and Tyr residues controlling the cell polarization and migration events that are essential for wound healing. The action of protein kinases is reversed by protein phosphatases (PPs). Strikingly, the identity of PPs involved in counteracting the action of kinases during electrotaxis remains largely unstudied. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
ES triggers the phosphorylation of focal adhesion kinase (FAK) in human epithelial cells, which is attenuated by an anti-integrin β1 subunit antibody.38 There is also evidence linking the proto-oncogenic protein tyrosine kinase Src to electrotaxis.14,42 Mouse keratinocyte EF-migration and the migration of several types of immune cells are dependent on the activity of protein kinases Akt/Protein kinase B, ERK1/2, Src, and p38.14,19 Most importantly, although EFs share signaling cascades with other growth factor receptors that also provide migrational cues, studies in neutrophils and keratinocytes have uncovered that the electric signal can induce rapid, sustained, and specific phosphorylation of protein kinases, including mitogen-activated protein kinases, ERK, Src, and Akt in serum-free medium.14 These findings uncover the importance of the electrical signal as a cue that is able to activate major signaling routes, independently of growth-factor signaling activation.
Recent studies revealed that protein kinase C (PKC) and glycogen synthase kinase-3b (GSK-3b) activities are required for optimal electrotaxis.43 Pharmacological inhibition of PKC significantly reduced EF-induced directedness of cell migration for 1–2 h in an EF. Further, pharmacological inhibition of GSK-3b completely abolished EF-induced Golgi polarization and significantly inhibited the directional cell migration, but only at 2–3 h in an EF.43
Interestingly, pharmacological inhibition of protein kinase A (PKA) does not affect cell motility in general; however, PKA activity regulates the directional migratory response to an applied EF.44
The sodium/hydrogen exchanger 1 (NHE1) is an upstream transducer of electrotaxis, as two different types of NHE1 inhibitors, cariporide and ethylisopropyl amiloride, inhibited electric-field-induced Akt activation and directed migration of fibroblasts.14 NHE1 functions as a spatially restricted integrator of migratory cues that is used to regulate early polarity signals and to coordinate events occurring at the leading and trailing edges of the cell.45 The carboxyl-terminus of NHE1 is a target for several kinases, including PI3K, RSK1, ERK1/2, p90rsk, p160ROCK, and p38. In fact, NHE1 functions as a scaffold for several signaling complexes, including MAP kinases46,47 that are activated by EFs; it is possible, therefore, that NHE1 acts as an integrator of kinase signaling which promotes the response to EFs.
Known and potential “molecular switches” controlling the directional response of cells
Identification of molecules that control the direction of cell migration by turning it on/off or by acting as switches controlling attraction/repulsion of migration holds the potential to find new targets for combined electrical and pharmacological therapies to treat wounds. Here, we have compiled information on known and potential molecular switches, the modulation of which may have a beneficial effect in ES wounds by “sensitizing” cells to the effect of exogenously applied EFs.
The PI3K/PTEN axis
The molecular signaling cascades controlling the process of skin wound healing have gained new significant insights in recent years. The emerging picture of the field is that a diversity of molecular signaling cascades involved in the process of wound healing ultimately converges on the activation of the phosphoinositide 3 kinase (PI3K) pathway. Consistent with this new notion, phosphatidylinositol-3,4,5-triphosphate (PIP3), the end product of Class I PI3K reactions, appears polarized in the direction of cell migration as a response to both chemotactic and electrical signals present at wound sites. In fact, the electrotactic “healing” response of keratinocytes and fibroblasts has been found to be PI3K dependent.14,23 PI3K inhibition is, therefore, likely to be detrimental in wound repair strategies. Nonetheless, chronic inflammation may cause a delay in wound healing.48 Since PI3Kγ has recently emerged as a new anti-inflammatory drug target,49 it would be interesting to test whether there is a window during which topical application of PI3Kγ inhibitors can improve delayed wound healing by suppressing chronic inflammation, without blocking EF-driven epidermal migration.
Phosphatase and tensin homolog (PTEN) is a lipid phosphatase that functions by hydrolyzing phosphates in position 3′ from phosphoinositides, and, therefore, the major function of PTEN is the buffering of PI3K signaling.50 PTEN has been proposed as a pharmacological target to enhance wound healing in epithelia.51–53 Unsurprisingly, PTEN deletion enhances the rate and directionality of cathodal migration of keratinocytes in single-cell assays and wounded monolayers.14 These findings highlight that PI3K/PTEN act as a cellular compass during both chemotaxis and electrotaxis and, therefore, are critical signaling molecules at wound sites where both electric and chemical signals arise. Interestingly, there are a number of useful side-effects for inhibiting PTEN in the context of wound healing. Ulcers that fail to heal are a common vascular complication of diabetes, and it is well documented that tissue vascularization enhances wound healing. Therapies that increase tissue vascularization in such patients may, therefore, prove effective in the reduction of ulcers. Although the mechanisms of vascular remodeling are as yet incompletely understood, initial evidence indicates that physiological EFs induce angiogenic responses in endothelial cells which are mediated by both the VEGF receptor and PI3K.54 PTEN has been identified as a negative regulator of angiogenesis.55 Therefore, PTEN inhibition would potentially be advantageous to enhance EF-mediated angiogenesis and wound repair responses in diabetic patients. Interestingly, PTEN has also emerged as an attractive target to treat diabetes, because it counteracts insulin-stimulated glucose uptake.56 Hence, all evidence thus far indicates that PTEN inhibition may be a good strategy to aid wound healing and, in particular, in the context of defective healing in diabetic patients.
Cyclic AMP/GMP ratio
Cyclic AMP (cAMP) levels influence whether scratch wounds in epithelial monolayers close or open in response to a DC EF, probably through modulation of PKA activity. Indeed, cAMP-dependent-PKA has a role in the regulation of the directional migratory response to applied EFs.44 In parallel with these observations, studies in Dictyostelium revealed that the catalytic domains of soluble guanylate cyclase and cyclic guanosine monophosphate (cGMP)-binding protein C also mediate the cathodal response via cGMP. By contrast, the N-terminal domain of soluble guanylate cyclase is responsible for the anode-directed signaling in conjunction with both the inhibition of PI3Ks and cGMP production.57
Protein kinase/phosphatase switches
Ser/Thr/Tyr kinases and PPs regulate human physiology, and dysregulation of their signaling is linked to an array of human diseases. Therefore, kinases and phosphatase enzymes are used as drug targets to treat several human pathologies underlying protein phosphorylation dysregulation such as cancer, diabetes, Alzheimer's, liver fibrosis, or viral infections.58,59
PPs have essential roles during cell migration, orchestrating the formation and maintenance of the actin cytoskeleton, regulating small GTPase molecular switches, and modulating the dynamics of matrix–adhesion interaction, actin contraction, and cell detachment at the trailing edge,60 all of which are required during wound repair. Despite all reports highlighting the involvement of protein kinases in electrotaxis, the potential role of PPs in regulating the directional migration of cells in response to an EF has been largely ignored. The activity of most transmitters of the electric signal recruited and activated by EFs, such as membrane receptors, integrins, and ion exchangers, is modulated by phosphorylation; therefore, it seems likely that a balance between kinases and their counteracting phosphatases is required for electrotaxis and ES wound healing. For example, FAK activity, integrins, and growth factor receptors are modulated by several phosphatases.60 Moreover, NHE1 is phosphorylated by several kinases which are activated by EFs, and a number of reports demonstrate that NHE1 is also a target of several phosphatases.61,62 Interestingly, phosphorylation of NHE1 by these kinases is correlated with an increase in NHE1 activity, whereas dephosphorylation of NHE1 by protein phosphatase 1 (PP1) down-regulates NHE1 activity.62 It is, therefore, interesting to suggest that by modulating the balance between kinase and phosphatase activity, one could, in turn, modify the transmission of the electric signal and, therefore, cell behavior during ES-wound closure. Hence, studies to decipher the individual roles of phosphatases in electrotaxis are much required to gain a full mechanistic understanding of this complex process.
The PP1/NIPP1 phosphatase complex
PP1 is a ubiquitous enzyme that regulates diverse essential cellular processes by catalyzing most dephosphorylation reactions of ser/thr residues on proteins. Substrate specificity by PP1 requires its binding to a myriad of regulatory proteins. Nuclear inhibitor of PP1 (NIPP1) was first identified as an inhibitory protein of PP1, although more recent work indicates that NIPP1 directs PP1 activity to the nucleus to dephosphorylate a number of substrates.63 A recent report has identified PP1 and NIPP1 as essential for directional cell migration and activation of the PP1/NIPP1 complex as a cellular mechanism that controls the cathodal/anodal polarisation and directed migration of epithelial cells in a physiological DC EF. Interestingly, the PP1/NIPP1 complex mediates this effect via up-regulation of growth factor and integrin expression and Cdc42 activity.39 These findings provide the identification of two genes that are required for directional switching in electrotaxis and suggest that activation of PP1/NIPP1 before ES or during ES (anode at wound side in this last case) could induce changes in gene expression which would make cells more prone to the healing effects of exogenously applied EFs.
Future Directions
Trends for wound repair therapies
Clinicians and scientists are increasingly aware of the potential for electrical therapies, and new electrical devices are now available that measure wound-induced electric currents (Dermacorder™).21 Moreover, the WoundEL®-therapy (GerroMed now owned by Molnlycke), which produces pulsed DC ES, has been used with some success to treat more than 6,000 patients in Germany since 2006. Most of these were patients with nonhealing wounds that had shown no response to any alternative treatment over many months.8 Both the WoundEL-therapy8 and a former similar device called Dermapulse®7 were CE marked and are currently being used in both European and U.S. hospitals.
Take-Home Messages.
• Intracellular signaling pathways that regulate wound repair are activated by growth factors, hormones, and cytokines that are released at the wound. In addition, endogenous EFs of 100–200 mV/mm intensity arise when the epidermis is injured. These EFs play a pivotal role in wound healing.
• Oriented and increased cell division of epithelial cells and migration of several cell types toward the cathode (wound centre) underlie the “healing” effects of these EFs.
• EFs polarize important membrane receptors such as integrins and growth factor receptors and activate canonical phosphorylation signaling pathways.
• The role of PPs in reversing the effects of protein kinases in electrotaxis remains largely unexplored. A bigger picture of the EF proteome is needed in order to understand this complex process and to target it in a controlled manner.
• EF-based therapies may represent a powerful approach and a direction of future wound management. Demonstration of the effect of EFs in directing cell migration has energized the development of devices for ES of wound healing by medical bioengineering companies.
• These findings will pave the way for the development of new concepts, that is, dual electric-pharmacological therapies to repair wounds.
The dissection of the various signaling pathways that regulate electrotaxis and EF-mediated wound closure is underway and paves the way for the identification of protein targets for electrically driven tissue repair therapies. Since phosphorylation of lipids and proteins seems to be a hallmark of the effect of EFs on cells, we propose that the use of molecules which modulate the activity of kinases and phosphatases in in vitro and Phase I preclinical studies in combination with exogenously applied EFs may be a promising tool useful for treating nonhealing wounds. Although the individual roles of PPs in electrotaxis and ES-wound healing remain elusive, recent evidence indicates that studies to identify these roles hold the promise to find molecular switches which can be used to modulate the directional response of cells to EF.39 An array of compounds that modulate (activate or inhibit) phosphatase activities is available59 and can be used to identify the therapeutic potential of individual phosphatases in electrotaxis and ES-wound healing.
To our knowledge, there have been no clinical attempts as yet to use electrical and chemical therapies together to target skin wound repair. A full mechanistic understanding of the signaling events underpinning electrically mediated wound closure aided by the use of pharmacological molecules will allow the manipulation of these signals to achieve rational control over skin repair.
Abbreviations and Acronymns
- cAMP
cyclic AMP or 3′-5′-cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- CE
cornified envelope
- DC
direct current
- ECM
extracellular matrix
- EF
electric field
- EGFR
epidermal growth factor receptor
- EIPA
ethylisopropyl amilorido
- ERK1/2
extracellular signal-regulated kinases 1/2
- ES
electrical stimulation
- FAK
focal adhesion kinase
- GPCR
G protein-coupled receptor
- GSK-3b
glycogen synthase kinase-3b
- MAPK
mitogen-activated protein kinases
- NHE1
sodium/hydrogen exchanger 1
- NIPP1
nuclear inhibitor of phosphatase 1
- PKA
protein kinase A
- PKC
protein kinase C
- PI3K
phosphoinositide 3 kinase
- PIP3
phosphatidylinositol-3,4,5-triphosphate
- PP1
protein phosphatase 1
- PP
protein phosphatase
- PTEN
phosphatase and tensin homolog
- Ser
Serine
- TEP
transepithelial potential difference
- Thr
Threonine
- TJs
tight junctions
- Tyr
Tyrosine
Acknowledgments and Funding Sources
The authors are grateful to Dr. Tanya Shaw for her critical comments and scientific discussion.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Professor Colin D. McCaig, FRSE, Regius Professor of Physiology and Head of School of Medical Sciences, University of Aberdeen. After completing his PhD with Professor Otto Hutter at Glasgow University, he undertook NIH-funded postdoctoral training in the United States. This introduced him to electrical control of cell behavior and his first scientific paper, J Physiol 314, 121–135 (1981) remains seminal in reopening an entire area of biology: the issue of electrical guidance of nerve growth and of wound healing. He won a prestigious Beit Memorial Research Fellowship in 1983 and established his own lab. He has concentrated on issues of cell polarity and cell migration and leads one of only a few groups in the world that studies the electrical controls of cell behaviors. His group established that electrical signals control wound healing, nerve guidance, and epithelial cell guidance in vivo and also regulate proliferation and guidance of neurons, vascular endothelial cells, tumor cells, and lens epithelial cells. Collectively, these wide-ranging events have major clinical relevance in a tissue engineering and regenerative medicine context. Clinical collaborators are using ES to treat human spinal cord injuries with protocols designed from our experiments, and electrical devices are also marketed to treat skin wounds that are also based on his seminal wound healing studies. Dr. Cristina Martin-Granados, Research Fellow, Division of Applied Medicine, University of Aberdeen. After completing her PhD at the Technischen Universität Berlin, Germany, Cristina Martin-Granados undertook an MRC Career Development Fellowship post in Prof. Patricia Cohen's lab at the Protein Phosphorylation Unit in Dundee, where she studied the potential for pharmacological inhibition of two different PPs, Ppp4 and PP2C, to treat cancer and metabolic disease, respectively (Martin-Granados et al. 2008 J Biochem Cell Biol 40:2315–2332; Voss et al. 2011 Cell Signal 23:114–124). In her current position working with Colin McCaig and John V. Forrester, she has united the field of phosphatase signaling with that of the electrical control of cell behavior, providing the first evidence of the involvement of PPs in electrotaxis. She has uncovered the PP-based mechanism that drives a cathodal/anodal directional switch in human epithelial cells.39 She is also investigating how dysregulation of phosphatase signalling contributes to altered migration of epithelial and immune cells and how this impinges on inflammation and wound healing.
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, and Longaker MT: Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009; 17:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurtner GC, Werner S, Barrandon Y, and Longaker MT: Wound repair and regeneration. Nature 2008; 453:314. [DOI] [PubMed] [Google Scholar]
- 3.Kloth LC: Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 2005; 4:23. [DOI] [PubMed] [Google Scholar]
- 4.Balakatounis KC. and Angoules AG: Low-intensity electrical stimulation in wound healing: review of the efficacy of externally applied currents resembling the current of injury. Eplasty 2008; 8:e28. [PMC free article] [PubMed] [Google Scholar]
- 5.Ramadan A, Elsaidy M, and Zyada R: Effect of low-intensity direct current on the healing of chronic wounds: a literature review. J Wound Care 2008; 17:292. [DOI] [PubMed] [Google Scholar]
- 6.Regan MA, Teasell RW, Wolfe DL, Keast D, Mortenson WB, and Aubut JA: A systematic review of therapeutic interventions for pressure ulcers after spinal cord injury. Arch Phys Med Rehabil 2009; 90:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Junger M, Arnold A, Zuder D, Stahl HW, and Heising S: Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse): a prospective, placebo controlled, double blind trial. Wound Repair Regen 2008; 16:480. [DOI] [PubMed] [Google Scholar]
- 8.Herberger K, Debus E, Larena-Avellaneda A, Blome C, and Augustin M: Effectiveness, tolerability, and safety of electrical stimulation of wounds with an electrical stimulation device: results of a retrospective register study. Wounds 2012; 24:76. [PubMed] [Google Scholar]
- 9.Foulds IS. and Barker AT: Human skin battery potentials and their possible role in wound healing. Br J Dermatol 1983; 109:515. [DOI] [PubMed] [Google Scholar]
- 10.Reid B, Nuccitelli R, and Zhao M: Non-invasive measurement of bioelectric currents with a vibrating probe. Nat Protoc 2007; 2:661. [DOI] [PubMed] [Google Scholar]
- 11.Nuccitelli R: A role for endogenous electric fields in wound healing. Curr Top Dev Biol 2003; 58:1. [DOI] [PubMed] [Google Scholar]
- 12.Reid B. and Zhao M: Measurement of bioelectric current with a vibrating probe. J Vis Exp 2011; 4: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuccitelli R, Nuccitelli P, Ramlatchan S, Sanger R, and Smith PJ: Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen 2008; 16:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, and Penninger JM: Electrical signals control wound healing through phosphatidylinositol-3-oh kinase-gamma and Pten. Nature 2006; 442:457. [DOI] [PubMed] [Google Scholar]
- 15.Shaw TJ. and Martin P: Wound repair at a glance. J Cell Sci 2009; 122:3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw TJ, Kishi K, and Mori R: Wound-associated skin fibrosis: mechanisms and treatments based on modulating the inflammatory response. Endocr Metab Immune Disord Drug Targets 2010; 10:320. [DOI] [PubMed] [Google Scholar]
- 17.Chen WY. and Rogers AA: Recent insights into the causes of chronic leg ulceration in venous diseases and implications on other types of chronic wounds. Wound Repair Regen 2007; 15:434. [DOI] [PubMed] [Google Scholar]
- 18.Barker AT, Jaffe LF, and Vanable JW: The glabrous epidermis of cavies contains a powerful battery. Am J Physiol 1982; 242:358. [DOI] [PubMed] [Google Scholar]
- 19.Lin F, Baldessari F, Gyenge CC, Sato T, Chambers RD, Santiago JG, and Butcher EC: Lymphocyte electrotaxis in vitro and in vivo. J Immunol 2008; 181:2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke K. and Gruler H: Galvanotaxis of human granulocytes: electric field jump studies. Eur Biophys J 1990; 18:335. [DOI] [PubMed] [Google Scholar]
- 21.Orida N. and Feldman JD: Directional protrusive pseudopodial activity and motility in macrophages induced by extracellular electric fields. Cell Motil 1982; 2:243. [DOI] [PubMed] [Google Scholar]
- 22.Cho MR, Thatte HS, Lee RC, and Golan DE: Integrin-dependent human macrophage migration induced by oscillatory electrical stimulation. Ann Biomed Eng 2000; 28:234. [DOI] [PubMed] [Google Scholar]
- 23.Guo A, Song B, Reid B, Gu Y, Forrester JV, Jahoda CA, and Zhao M: Effects of physiological electric fields on migration of human dermal fibroblasts. J Invest Dermatol 2010; 130:2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X and Kolega J: Effects of direct current electric fields on cell migration and actin filament distribution in bovine vascular endothelial cells. J Vasc Res 2002; 39:391. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan DM, Isseroff RR, and Nuccitelli R: Imposition of a physiologic dc electric field alters the migratory response of human keratinocytes on extracellular matrix molecules. J Invest Dermatol 1996; 106:642. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura KY, Isseroff RR, and Nuccitelli R: Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J Cell Sci 1996; 109:199. [DOI] [PubMed] [Google Scholar]
- 27.Erickson CA. and Nuccitelli R: Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J Cell Biol 1984; 98:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang WP, Onuma EK, and Hui SW: Response of C3H/10T1/2 fibroblasts to an external steady electric field stimulation. Reorientation, shape change, cona receptor and intramembranous particle distribution and cytoskeleton reorganization. Exp Cell Res 1984; 155:92. [DOI] [PubMed] [Google Scholar]
- 29.Brown MJ. and Loew LM: Electric field-directed fibroblast locomotion involves cell surface molecular reorganization and is calcium independent. J Cell Biol 1994; 127:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkelstein E, Chang W, Chao PH, Gruber D, Minden A, Hung CT, and Bulinski JC: Roles of microtubules, cell polarity and adhesion in electric-field-mediated motility of 3T3 fibroblasts. J Cell Sci 2004; 117:1533. [DOI] [PubMed] [Google Scholar]
- 31.Chang K, Chang WH, Huang S, Huang S, and Shih C: Pulsed electromagnetic fields stimulation affects osteoclast formation by modulation of osteoprotegerin, rank ligand and macrophage colony-stimulating factor. J Orthop Res 2005; 23:1308. [DOI] [PubMed] [Google Scholar]
- 32.Denda M, Ashida Y, Inoue K, and Kumazawa N: Skin surface electric potential induced by ion-flux through epidermal cell layers. Biochem Biophys Res Commun 2001; 284:112. [DOI] [PubMed] [Google Scholar]
- 33.Denda M, Fujiwara S, and Hibino T: Expression of voltage-gated calcium channel subunit alpha1c in epidermal keratinocytes and effects of agonist and antagonists of the channel on skin barrier homeostasis. Exp Dermatol 2006; 15:455. [DOI] [PubMed] [Google Scholar]
- 34.Bezanilla F: How membrane proteins sense voltage. Nat Rev Mol Cell Biol 2008; 9:323. [DOI] [PubMed] [Google Scholar]
- 35.Zhao M, Dick A, Forrester JV, and McCaig CD: Electric field-directed cell motility involves up-regulated expression and asymmetric redistribution of the epidermal growth factor receptors and is enhanced by fibronectin and laminin. Mol Biol Cell 1999; 10:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang KS, Ionides E, Oster G, Nuccitelli R, and Isseroff RR: Epidermal growth factor receptor relocalization and kinase activity are necessary for directional migration of keratinocytes in dc electric fields. J Cell Sci 1999; 112:1967. [DOI] [PubMed] [Google Scholar]
- 37.Pullar CE, Baier BS, Kariya Y, Russell AJ, Horst BA, Marinkovich MP, and Isseroff RR: Beta4 integrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol Biol Cell 2006; 17:4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han J, Yan XL, Han QH, Li YJ, Du ZJ, and Hui YN: Integrin beta 1 subunit signaling is involved in the directed migration of human retinal pigment epithelial cells following electric field stimulation. Ophthalmic Res 2011; 45:15. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Granados C, Prescott AR, Van Dessel N, Van Eynde A, Arocena M, Klaska IP, Gornemann J, Beullens M, Bollen M, Forrester JV, and McCaig CD: A role for PP1/NIPP1 in steering migration of human cancer cells. PLoS One 2012; 7:e40769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaughlin S. and Poo MM: The role of electro-osmosis in the electric-field-induced movement of charged macromolecules on the surfaces of cells. Biophys J 1981; 34:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levin M: Large-scale biophysics: ion flows and regeneration. Trends Cell Biol 2007; 17:261. [DOI] [PubMed] [Google Scholar]
- 42.Pu J. and Zhao M: Golgi polarization in a strong electric field. J Cell Sci 2005; 118:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao L, Graue-Hernandez EO, Tran V, Reid B, Pu J, Mannis MJ, and Zhao M: Downregulation of pten at corneal wound sites accelerates wound healing through increased cell migration. Invest Ophth Vis Sci 2011; 52:2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pullar CE, Isseroff RR, and Nuccitelli R: Cyclic AMP-dependent protein kinase a plays a role in the directed migration of human keratinocytes in a dc electric field. Cell Motil Cytoskeleton 2001; 50:207. [DOI] [PubMed] [Google Scholar]
- 45.Denker SP. and Barber DL: Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol 2002; 159:1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumgartner M, Patel H, and Barber DL: Na(+)/H(+) exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Cell Physyol 2004; 287:C844. [DOI] [PubMed] [Google Scholar]
- 47.Bandyopadhyay S, Chiang CY, Srivastava J, Gersten M, White S, Bell R, Kurschner C, Martin CH, Smoot M, Sahasrabudhe S, Barber DL, Chanda SK, and Ideker T: A human MAP kinase interactome. Nat Methods 2010; 7:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eming SA, Krieg T, and Davidson JM: Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermtol 2007; 127:514. [DOI] [PubMed] [Google Scholar]
- 49.Williams O, Houseman BT, Kunkel EJ, Aizenstein B, Hoffman R, Knight ZA, and Shokat KM: Discovery of dual inhibitors of the immune cell PI3Ks p110delta and p110gamma: a prototype for new anti-inflammatory drugs. Chem Biol 2010; 17:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leslie NR. and Downes CP: PTEN: the down side of PI 3-kinase signalling. Cell Signal 2002; 14:285. [DOI] [PubMed] [Google Scholar]
- 51.Lai JP, Dalton JT, and Knoell DL: Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair. Br J Pharmacol 2007; 152:1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao M: PTEN: a promising pharmacological target to enhance epithelial wound healing. Br J Pharmacol 2007; 152:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castilho R, Squarize C, and Gutkind J: Exploiting PI3k/mTOR signaling to accelerate epithelial wound healing. Oral Dis 2013; 19:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao M, Bai H, Wang E, Forrester JV, and McCaig CD: Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci 2004; 117:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez S. and Huynh-Do U: The role of PTEN in tumor angiogenesis. J Oncol 2012; 2012:141236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong JT, Kim PT, Peacock JW, Yau TY, Mui AL, Chung SW, Sossi V, Doudet D, Green D, Ruth TJ, Parsons R, Verchere CB, and Ong CJ: Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity. Diabetologia 2007; 50:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato MJ, Kuwayama H, van Egmond WN, Takayama AL, Takagi H, van Haastert PJ, Yanagida T, and Ueda M: Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling. Proc Natl Acad Sci USA 2009; 106:6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen P: The role of protein phosphorylation in human health and disease. The sir hans krebs medal lecture. Eur J Biochem 2001; 268:5001. [DOI] [PubMed] [Google Scholar]
- 59.De Munter S, Kohn M, and Bollen M: Challenges and opportunities in the development of protein phosphatase-directed therapeutics. ACS Chem Biol 2013; 8:36. [DOI] [PubMed] [Google Scholar]
- 60.Larsen M, Tremblay ML, and Yamada KM: Phosphatases in cell-matrix adhesion and migration. Nat Rev Mol Cell Biol 2003; 4:700. [DOI] [PubMed] [Google Scholar]
- 61.Snabaitis AK, D'Mello R, Dashnyam S, and Avkiran M: A novel role for protein phosphatase 2A in receptor-mediated regulation of the cardiac sarcolemmal Na+/H+ exchanger NHE1. J Biol Chem 2006; 281:20252. [DOI] [PubMed] [Google Scholar]
- 62.Misik AJ, Perreault K, Holmes CF, and Fliegel L: Protein phosphatase regulation of Na+/H+ exchanger isoform I. Biochemistry 2005; 44:5842. [DOI] [PubMed] [Google Scholar]
- 63.O'Connell N, Nichols SR, Heroes E, Beullens M, Bollen M, Peti W, and Page R: The molecular basis for substrate specificity of the nuclear NIPP11:PP1 holoenzyme. Structure 2012; 20:1746. [DOI] [PMC free article] [PubMed] [Google Scholar]