Abstract
Sexually dimorphic vocal behavior in zebra finches (Taeniopygia guttata) is associated with a 100% larger syrinx in males and other morphological adaptations of the sound source. The songbird syrinx consists of two independent sound sources, whose specialization for different spectral ranges may be reflected in morphological properties, but the morphology of labia and syringeal skeleton have not been investigated for lateralized specializations. Similarly, little is known whether the morphology of the songbird vocal tract reflects differences in vocal behavior. Here, we tested the hypothesis that different vocal behavior and specialization is reflected in the morphology. We investigated syringeal and upper vocal tract morphology of male and female European starlings (Sturnus υulgaris). Female starlings exhibit smaller vocal repertoires and sing at lower rates than males. In males, the left syrinx produces mostly low frequencies, while the right one is used for higher notes. Macroscopic and histological techniques were used to record nineteen measurements from the syrinx and the vocal tract which were tested for sexual differences in syrinx and vocal tract and for lateral asymmetry within the syrinx. Sexually dimorphic vocal behavior is reflected in the morphology of the starling syrinx. Males have a larger syrinx with the size difference attributable to increased muscle mass and three enlarged elements of the syringeal skeleton. The upper vocal tract, however, does not differ between males and females. Distinct lateralization was found in two elements of the syringeal skeleton of females, and the labia in the left syrinx are larger than those on the right in both sexes. The sexual dimorphism of the syringeal size is smaller in starlings (35%) than in zebra finches (100%), which is consistent with the different vocal behavior of females in both species. The morphological differences between the two sound sources are discussed in relation to their vocal performance.
Keywords: labia, vocal fold, bioacoustics, vocal control
INTRODUCTION
Vocal variability among passeriform birds arises from differences in central neural control and in functional morphology of the sound source, the syrinx, the upper vocal tract and the respiratory system. Sexually dimorphic behavior in passeriformes is accompanied by differences at both levels, central nervous control (e.g., Nottebohm and Arnold, 1976; Cooke et al., 1998) and the syrinx (e.g., Tang and Wade, 2009; Riede and Goller, 2010a). The syrinx of songbirds is comprised of two sound sources, one in each bronchus. The left source tends to generate lower frequencies than the right, with a varying degree of overlap in the frequency ranges (Goller and Suthers 1995; Suthers and Zollinger, 2004; Goller and Cooper, 2004). The two sound sources are independently controlled, each allowing the generation of simultaneous independent sounds, which are combined through partial temporal overlap or alternation into complex temporal and acoustic sequences (e.g., Goller and Suthers 1996a,b, 1999; Suthers and Goller, 1997; Suthers et al., 1999; Suthers and Zollinger, 2004; Zollinger et al., 2008). Little is known to what extent morphological differences between the left and right sources contribute to the functional laterality.
Previous research on sexual dimorphism of the syrinx was conducted in the zebra finch (Taeniopygia guttata; Wade and Buhlman, 2000; Riede et al., 2010), in which only the male sings a stereotyped sequence of 3–8 acoustically different song syllables (motif; Zann, 1996). The syrinx of males is about twice as heavy as in females (Luine et al., 1980; Wade and Buhlmann, 2000; Wade et al., 2002; Veney and Wade, 2004, 2005; Riede et al., 2010). The much larger muscle mass and the more robust syringeal skeleton in male zebra finches enable generation of their greater fundamental frequency range (Riede et al., 2010). Song and call production is lateralized in zebra finches (Goller and Cooper, 2004), however the morphological asymmetry of syringeal structures is not pronounced (Riede and Goller, 2010a). Here, we contrast these findings with an investigation of the syrinx and vocal tract of European starlings (Sturnus υulgaris), a species in which both sexes sing a highly complex and variable vocal repertoire (Eens, 1997). The female song repertoire is smaller and song bouts are shorter than those of males (Pavlova et al., 2005), which is reflected in differences in the song-control nuclei (Bernard et al., 1993; Riters and Teague, 2003). It is unknown if these differences are also reflected in the morphology of the syrinx or the upper vocal tract structures. A qualitative observation suggests that labial size is laterally asymmetrical in the starling syrinx (Warner, 1972), but there is no distinct difference in muscle fiber architecture between the left and right syrinx (Uchida et al., 2010). Here, we explore lateral asymmetries of the syringeal skeleton and the labia quantitatively.
The syrinx produces the primary sound, which then must transmit through the upper vocal tract structures until it exits through the beak. The upper vocal tract is comprised of the tracheal tube, the larynx, the oropharyngeal–esophageal cavity (OEC) and the beak (Westneat et al., 1993; Hoese et al., 2000; Goller et al., 2004; Daley and Goller, 2004; Podos et al., 2004; Riede et al., 2006; Riede and Suthers, 2009; Ohm et al., 2010). Various non-passerine species possess adaptations of this space with likely acoustic effects (Fitch, 1999; Miller et al., 2007). In some species, these adaptations are sexually dimorphic (Miller et al., 2007). Although its vocal function has been investigated in passeriformes, it is unknown whether the vocal tract displays specific adaptations that parallel those in the sound source. Because songbirds adjust the volume of the OEC dynamically during song such that its primary resonance closely matches the fundamental frequency of sound (Riede et al., 2006; Fletcher et al., 2006; Riede and Suthers, 2009; Riede and Goller, 2010b), it is possible that specific morphological adaptations enable adjustment to different frequency ranges. In the current study, we therefore tested this prediction by investigating a possible sexual dimorphism of these elements of the upper vocal tract.
METHODS
Eight female and eight male adult starlings were collected near Salt Lake City, Utah, during December 2009. The birds were euthanized with an overdose of isoflurane (VetOne, Meridiane, ID). The birds were weighed (precision ± 0.1 g) and tarsus length was measured (precision ± 0.1 mm). Thirteen measurements related to vocal tract dimensions were recorded: head width (measured between left and right ear); beak length (measured from the transition between skull and maxilla and the tip of the beak), beak width (measured as the largest latero-lateral width of the mandible), beak height (measured between the transition between skull and maxilla and the lowest/most caudo-ventral point of the mandible). The maximum volume of the OEC was measured by injecting dental cast (Vinyl Polysiloxane, light body, Mydent International, Hauppauge, NY) through the beak into the oral cavity, pharynx and esophagus. The cast was dissected out of the bird and trimmed at the commissure of upper and lower beak, and 1 cm caudal from the glottis (the glottis always left a clear imprint on the cast), which is an area located in the upper third of the esophagus. The cast has a density of 1.5 g/ml which was used to calculate volume using the ratio of mass and density. We also measured tracheal length (measured from the lower edge of the cricoid cartilage to the most cranial attachment of the ventral syringeal muscles while the organ was placed on a wet glass surface), the length of the left and right epibrachiale and the left and right ceratobranchiale. The cross sectional areas of the trachea (right above the syrinx) and the left and right bronchial lumina (measured right below the 6th bronchial ring were measured from photos of trachea and bronchi. The images were opened in ImageJ (version 1.41o; NIH open source), and area measurements were taken with the ‘segmented line’ tool in reference to a known length, which had been placed next to trachea and bronchi, respectively, when the picture was taken.
The syrinx was extracted (from the most cranial attachment of the ventral syringeal muscles to sixth bronchial ring), was weighed (±0.1 mg precision; electronic balance, AG204, Mettler, Toledo), fixed for 3 days in 10% formalin (Fisher Scientific; Fair Lawn, NJ cat. no. SF100-4) and kept for 8 h in Decalcifier 11 (Surgipath Medical Industries, Richmond, IL, cat. no. 00400) before further processing. The tissue was then embedded in paraffin. Each syrinx was embedded with identical orientation (dorsal side down). The right side was marked by a small incision in the trachea. Sections of 5 µm thickness every 50 µm were exposed to hemotoxylin and eosin (H&E) stain for a general histological evaluation (Fig. 1). Micrographs were taken with a digital camera (AxioCam HRc, Carl Zeiss, Germany) combined with an Axioplan Zeiss microscope (Axioplan, Carl Zeiss, Germany) and computer software (Axiovision 40, v. 4.6.3.0., Carl Zeiss, Germany).
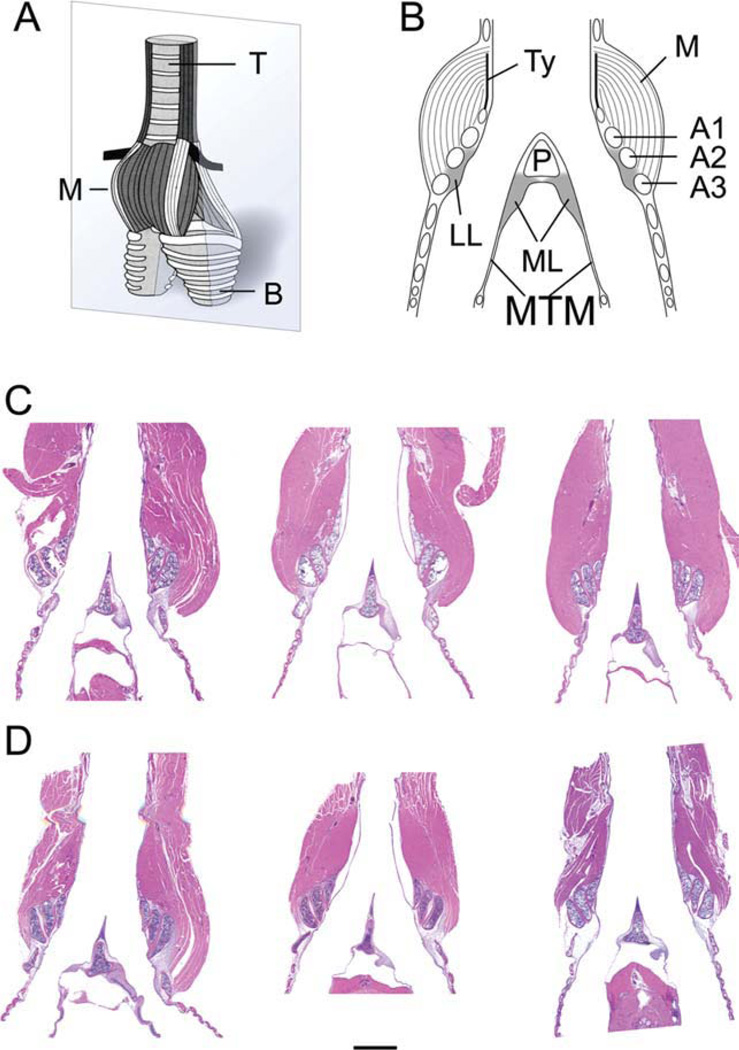
Fig. 1.
A: Schematic ventral view of a syrinx. The grey plane indicates the section level of the other images (B–D). B: Schematic of a mid-organ section through syrinx indicating all elements measured. C: Mid-organ section of three male syringes (HandE stain). D: Mid-organ section of three female syringes (HandE stain). Note the smaller muscle mass in females. The bar in D indicates a 1 mm distance and applies to C and D. M, intrinsic syringeal muscles; B, primary bronchi; T, trachea; LL, lateral labia; ML, medial labia; MTM, medial tympaniform membrane; Ty, tympanum; P, pessulus; A1, A2, A3, first, second and third bronchial half ring.
The following measurements (in reference to a known length) were made on the histological sections using ImageJ. Measurements of labial size were based on cross-sectional area and cranio-caudal length for the medial labium (ML), on cross-sectional area for the lateral labium (LL), and on the length of the medial tympaniform membrane (MTM). The sizes of bronchial half rings (A1, A2, A3) and the pessulus (P) were estimated from cross-sectional area measurements. The size of the tympanum was based on its diameter about 3 mm below its cranial end. Measurements were made with Image J. A curvilinear outline of the labia, the bronchial half rings and the pessulus was used to estimate cross-sectional area. A ‘‘segmented line’’ was drawn along the centerline of the ML starting at the pessulus and ending at the beginning of the MTM, and a second segmented line was drawn along the centerline of the MTM to estimate the cranio-caudal length of ML and MTM. The area measurements (ML, LL; P, A1, A2, A3) and both length measurements (ML and MTM) were made at 10 points equally distributed over the dorso-ventral length of the labia, starting dorsally at the point where the pessulus and ML separate from the dorso-lateral tracheal wall, and ending ventrally where the connective tissue of the ML is placed around the pessulus again. All measurements were made in reference to a known distance measured at identical magnification. Measurements (area, length) at 10 levels of syringes were tested for differences along the dorso-ventral axis using a one-way analysis of variance (ANOVA). Differences between male and female as well as left and right syrinx were tested by two-sample t-test.
RESULTS
Sexual Dimorphism
Mean body mass was significantly different between males and females, but no difference was found in the length of the tarsus (Table 1). Similar skeletal size indicates that soft tissues (muscles, fat, organs) account for the mass difference. Syringeal mass was larger in males than in females (Table 1). The 35% difference is substantially more than the difference in body mass (9%).
TABLE 1.
Summary of measurements of body size, syrinx, and vocal tract in male and female starlings
| Length/width/height in mm; area in mm2; mass in g; volume in ml; Nm,f = 8 |
Coefficient of variation (%) |
Male/female difference (%) |
m/f Comparison |
Dorsoventral shape change (ANOVA, df1 = 9, df2 = 70) |
||
|---|---|---|---|---|---|---|
| Body mass | m: 76.6 ± 7.0 | 9.1 | 109.4 | T = –2.27 | ||
| f: 70.0 ± 4.2 | 6.0 | P = 0.04 | ||||
| Tarsus length | m: 29.7 ± 0.9 | 2.9 | 101.8 | T = –1.4 | ||
| f: 29.2 ± 0.6 | 2.1 | P = 0.18 | ||||
| Beak width | m: 8.5 ± 0.6 | 7.1 | 105.3 | T = –1.93 | ||
| f: 8.0 ± 0.3 | 4.3 | P = 0.07 | ||||
| Beak height | m: 8.6 ± 0.9 | 10.7 | 107.1 | T = –1.47 | ||
| f: 8.0 ± 0.6 | 7.4 | P = 0.16 | ||||
| Beak length | m: 28.0 ± 1.4 | 4.8 | 105.4 | T = –2.27 | ||
| f: 27.0 ± 1.2 | 4.3 | P = 0.04 | ||||
| Head width | m: 14.4 ± 1.5 | 10.1 | 105.5 | T = –1.28 | ||
| f: 13.7 ± 0.8 | 5.6 | P = 0.22 | ||||
| Max. OEC vol. | m: 3.7 ± 0.2 | 6.0 | 96.6 | T = 0.39 | ||
| N1 =4;N2 =8 | f: 3.8 ± 0.6 | 16.3 | P = 0.70 | |||
| Epibrachiale left | m: 13.8 ± 0.8 | 6.1 | 101.9 | T = –0.77 | ||
| f: 13.5 ± 0.4 | 3.0 | P = 0.45 | ||||
| Epibrachiale right | m: 13.4 ± 1.4 | 10.4 | 98.7 | T = 0.35 | ||
| f: 13.5 ± 0.4 | 3.3 | P = 0.73 | ||||
| Ceratobrachiale left | m: 13.6 ± 1.2 | 8.1 | 99.8 | T = 0.05 | ||
| f: 14.6 ± 1.2 | 8.6 | P = 0.95 | ||||
| Ceratobrachiale right | m: 14.6 ± 1.2 | 7.9 | 99.3 | T = 0.17 | ||
| f: 14.7 ± 1.2 | 8.2 | P = 0.87 | ||||
| Trachea length | m: 32.5 ± 1.8 | 5.5 | 103.7 | T = –1.2 | ||
| f: 31.2 ± 1.8 | 5.6 | P = 0.24 | ||||
| Trachea area | m: 0.35 ± 0.05 | 15.4 | 97.9 | T = 0.28 | ||
| f: 0.36 ± 0.05 | 13.7 | P = 0.78 | ||||
| Bronchus area left | m: 0.21 ± 0.04 | 17.0 | 100.0 | T=0 | ||
| f: 0.22 ± 0.04 | 16.8 | P = 1.0 | ||||
| Bronchus area right | m: 0.20 ± 0.03 | 16.2 | 96.9 | T = 0.34 | ||
| f: 0.20 ± 0.04 | 19.9 | P = 0.74 | ||||
| Syrinx mass | m: 0.18 ± 0.02 | 8.1 | 135.5 | T = –5.23 | ||
| f: 0.14 ± 0.02 | 15.7 | P < 0.001 | ||||
| P (area) | m: 0.21 ± 0.05 | 25.0 | 106.7 | T = –0.51 | m: F = 10.61, | P < 0.001 |
| A1-l (area) | m: 0.19 ± 0.01 | 8.1 | 115.8 | T = 23.7 | m: F = 7.3, | P < 0.001 |
| f: 0.17 ± 0.02 | 9.5 | P < 0.01 | f: F = 1.63, | P < 0.001 | ||
| A2-l (area) | m: 0.21 ± 0.05 | 23.5 | 105.8 | T = –0.51 | m: F =5.5, | P < 0.001 |
| f: 0.20 ± 0.02 | 8.3 | P = 0.62 | f: F = 16.7, | P < 0.001 | ||
| A3-l (area) | m: 0.06 ± 0.01 | 20.8 | 119.3 | T = 21.76 | m: F = 1.77, | P = 0.09 |
| f: 0.05 ± 0.01 | 16.6 | P = 0.10 | f: F = 4.3, | P < 0.001 | ||
| A1-r (area) | m: 0.20 ± 0.01 | 6.2 | 111.0 | T = –2.48 | m: F = 7.38, | P < 0.001 |
| f: 0.18 ± 0.02 | 10.7 | P = 0.026 | f: F = 16.9, | P < 0.001 | ||
| A2-r (area) | m: 0.20 ± 0.03 | 16.7 | 123.7 | T = –2.36 | m: F = 11.38, | P < 0.001 |
| f: 0.17 ± 0.02 | 13.4 | P = 0.033 | f: F = 17.3, | P < 0.001 | ||
| A3-r (area) | m: 0.06 ± 0.01 | 20.6 | 105.3 | T = –0.38 | m: F = 7.65, | P < 0.001 |
| f: 0.06 ± 0.01 | 21.5 | P = 0.71 | f: F = 1.4, | P = 0.19 | ||
| LL-area-l | m: 0.14 ± 0.03 | 21.4 | 109.0 | T = –1.09 | m: F = 3.0, | P < 0.01 |
| f: 0.13 ± 0.02 | 16.0 | P = 0.29 | f: F = 12.9, | P < 0.001 | ||
| ML-area-l | m: 0.14 ± 0.03 | 23.5 | 142.4 | T = –2.63 | m: F = 22.5, | P < 0.001 |
| f: 0.10 ± 0.03 | 34.7 | P = 0.02 | f: F = 11.4, | P < 0.001 | ||
| ML-length-l | m: 0.80 ± 0.09 | 11.8 | 100.6 | T = 21.11 | m: F = 10.0, | P < 0.001 |
| f: 0.79 ± 0.11 | 13.8 | P = 0.28 | f: F = 4.5, | P < 0.001 | ||
| MTM-length-l | m: 0.41 ± 0.19 | 48.4 | 69.1 | T = 2.86 | m: F = 16.7, | P < 0.001 |
| f: 0.59 ± 0.17 | 28.6 | P = 0.012 | f: F = 8.4, | P < 0.001 | ||
| Combined ML-MTM length-l | m: 1.21 ± 0.19 | 16.3 | 87.2 | T = 2.11 | m: F = 3.6, | P < 0.01 |
| f: 1.39 ± 0.2 | 14.7 | P = 0.052 | f: F = 5.9, | P < 0.001 | ||
| LL-area-r | m: 0.09 ± 0.02 | 18.3 | 107.8 | T = –0.48 | m: F = 3.0, | P < 0.01 |
| f: 0.09 ± 0.02 | 26.3 | P = 0.63 | f: F = 1.5, | P = 0.15 | ||
| ML-area-r | m: 0.03 ± 0.008 | 25.5 | 108.8 | T = –0.84 | m: F = 11.7, | P < 0.001 |
| f: 0.04 ± 0.008 | 21.6 | P = 0.41 | f: F = 4.4, | P < 0.001 | ||
| ML-length-r | m: 0.37 ± 0.05 | 15.4 | 92.3 | T = 1.44 | m: F = 7.2, | P < 0.001 |
| f: 0.40 ± 0.03 | 9.1 | P = 0.17 | f: F = 6.5, | P < 0.001 | ||
| MTM-length-r | m: 0.66 ± 0.19 | 29.4 | 85.5 | T = 1.25 | m: F = 23.4, | P < 0.001 |
| f: 0.77 ± 0.12 | 15.9 | P = 0.22 | f: F = 12.4, | P < 0.001 | ||
| Combined ML-MTM length-r | m: 1.02 ± 0.17 | 16.8 | 87.9 | T = 1.92 | m: F = 3.3, | P < 0.01 |
| f: 1.17 ± 0.12 | 10.6 | P = 0.07 | f: F = 6.6, | P < 0.001 | ||
Data were collected from eight males and eight females, except for OEC size which was available only from four males.
To assess whether the larger syringeal mass in the male syrinx can be attributed to larger muscle mass or also to a more robust cartilaginous scaffold and vibrating tissues, we measured the size of bronchial rings and the labia in each section (Figs. 1 and 2). The first bronchial half ring (A1; see Fig. 2) on both sides of the syrinx were thicker in males (Table 1), as was the right second bronchial half ring (A2) (Table 1). The pessulus and the third bronchial half ring showed no significant differences between males and females (Table 1, Fig. 2). The left ML was larger in males, and the left MTM was longer in females (Table 1, Figs. 2 and 3). The longer left MTM in females fills the space that is occupied in males by the ML.
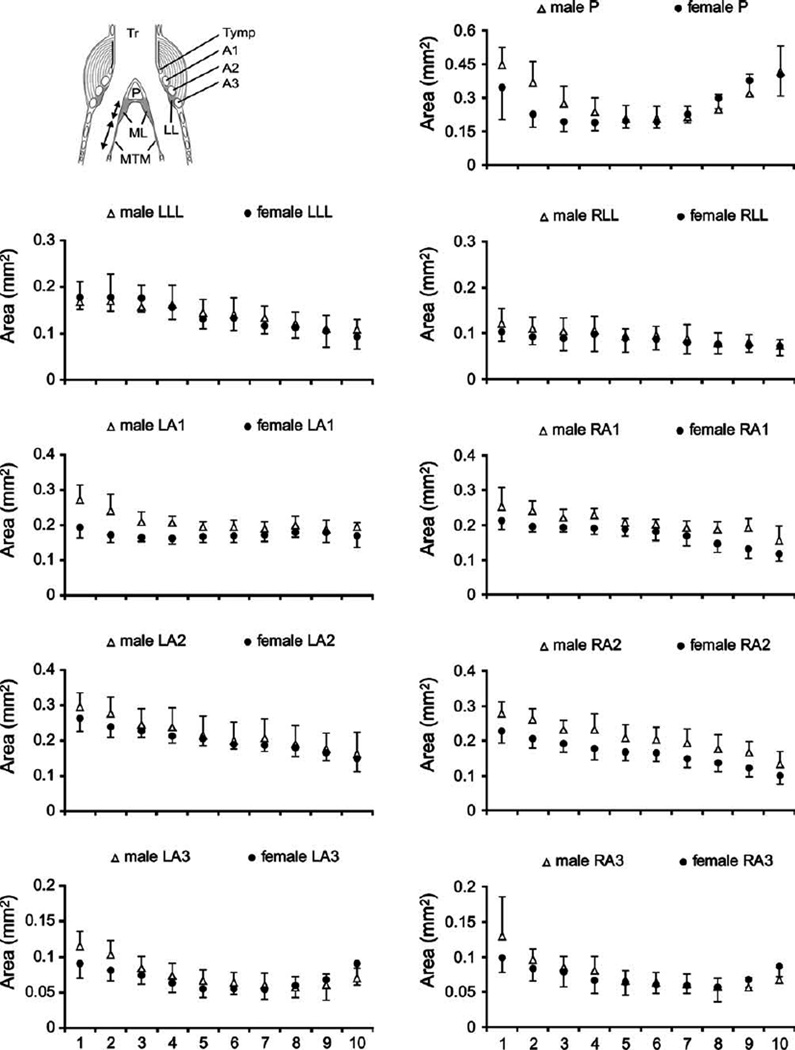
Fig. 2.
Syrinx measurements from dorsal (level 1) to ventral (level 10) in male and female starlings. Data from the left syrinx are shown in the left column and data from the right in the right column. Circles (females) and triangles (males) indicate means and error bars indicate standard deviation. LL, lateral labia; ML, medial labia; MTM, medial tympaniform membrane; Ty, tympanum; P, pessulus; A1, A2, A3, first, second and third bronchial half ring.
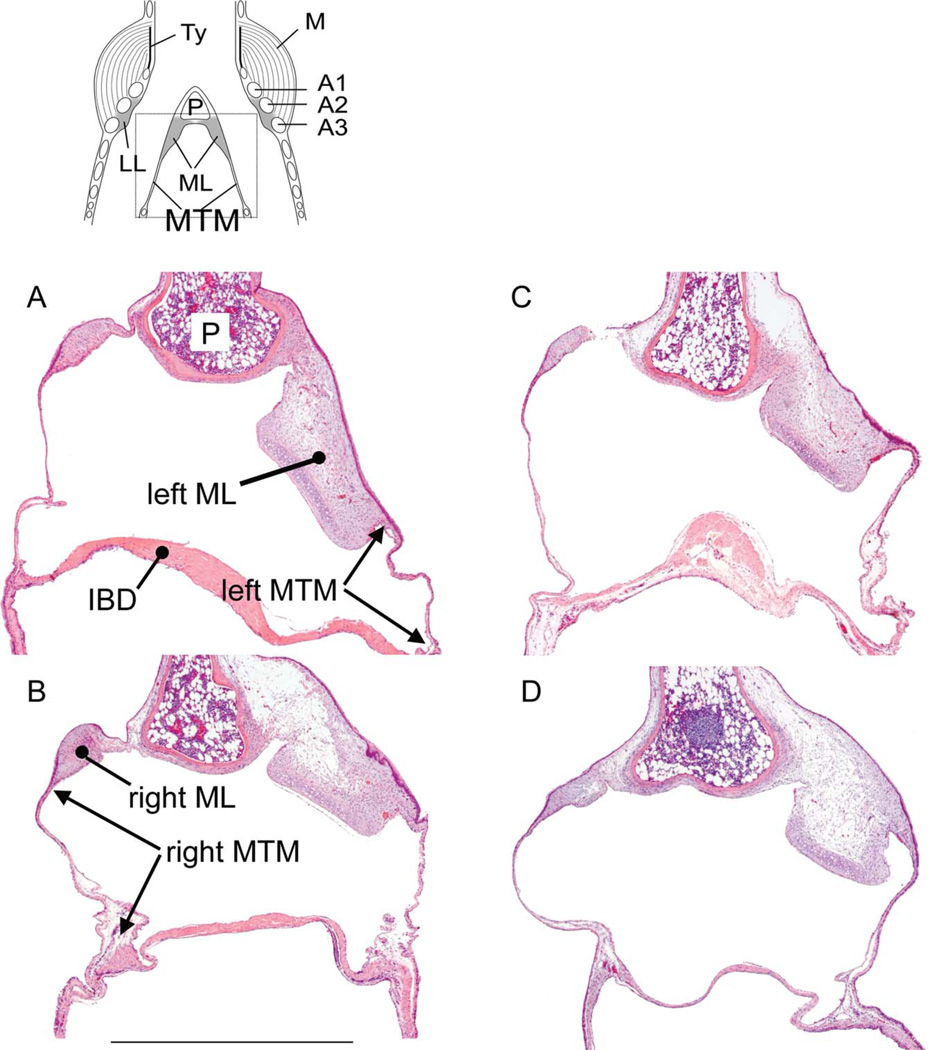
Fig. 3.
Partial mid-organ sections of two male (A,B) and two female syringes (C,D). The dotted square in the schematic on top indicates the location of the images in A–D. The cranio-caudal length of the medial tympaniform membrane (MTM) was measured between the transition between labium and MTM and the point where the interbronchial desmus (IBD), a band of connective tissue and muscle, connects to the bronchus (indicated by arrows). Bar in C: 100 lm, applies to all images. LL, lateral labia; Ty, tympanum; P, pessulus; A1, A2, A3, first, second and third bronchial half ring.
As a morphological correlate of upper vocal tract filtering we assessed the maximal OEC volume and skeletal elements involved in its regulation. No significant differences were found in either OEC volume or in the long elements of the hyoid skeleton (epibrachiale and ceratobrachiale; Table 1). Tracheal and bronchial lengths and cross sectional areas were also not significantly different (Table 1). Male beaks were longer by a small but significant amount.
Lateral Asymmetry Between Left and Right Sound Source
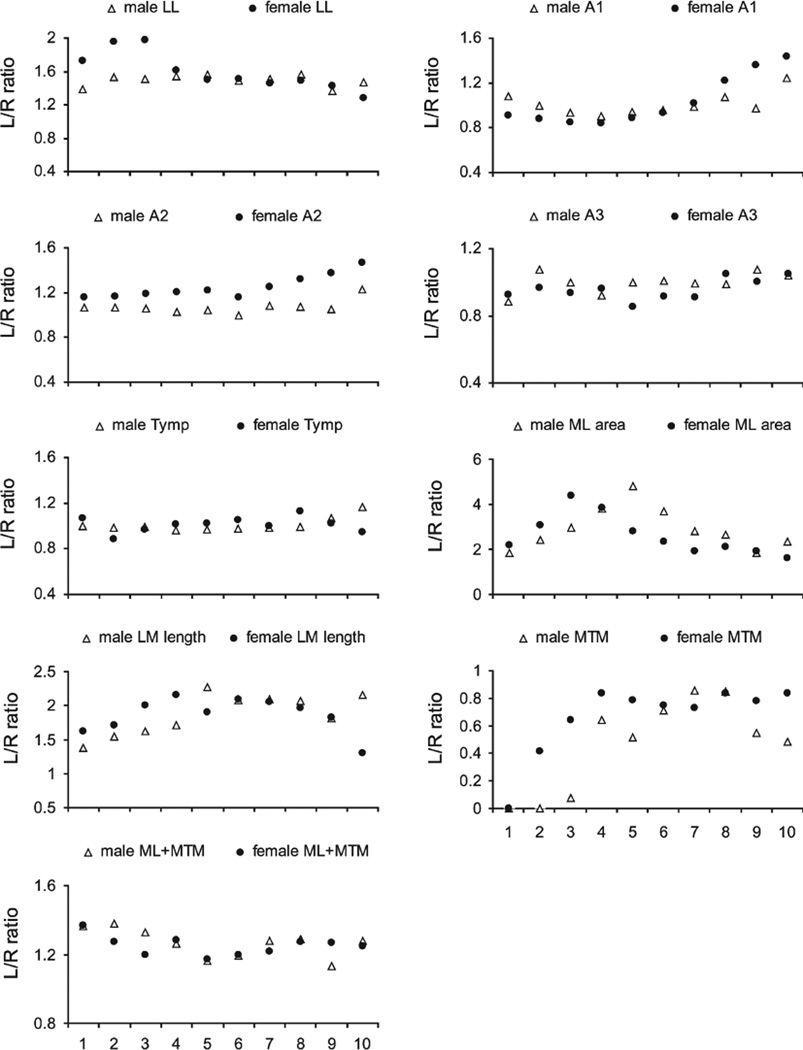
There were large lateral asymmetries in both sexes. Most dramatic was the up to five times larger cross sectional area of the left compared to the right ML (Table 2, Fig. 4). The left LL was two-fold larger (Tables 1 and 2). Significant asymmetry of cartilaginous components (A1, A2) occurred only in females.
TABLE 2.
Summary statistics of left-right asymmetry
| Males | Females | |
|---|---|---|
| Bronchus area | T = –1.56 | T = –1.45 |
| P = 0.16 | P = 0.19 | |
| A1 (area) | T = 1.59 | T = –3.55 |
| P = 0.15 | P < 0.01 | |
| A2 (area) | T = 0.59 | T = 5.81 |
| P = 0.57 | P < 0.001 | |
| A3 (area) | T = –0.04 | T = –1.63 |
| P = 0.96 | P = 0.14 | |
| LL-area | T = 6.42 | T = 5.49 |
| P < 0.001 | P < 0.001 | |
| ML-area | T = 14.5 | T = 5.57 |
| P < 0.001 | P < 0.001 | |
| ML-length | T = 8.01 | T = 13.8 |
| P < 0.001 | P < 0.001 | |
| MTM-length | T = –3.78 | T = –2.0 |
| P < 0.01 | P = 0.08 | |
| Combined ML-MTM length | T = –2.41 | T = –2.37 |
| P = 0.04 | P = 0.049 |
The comparison was performed for measurements at level 5, which is about mid-organ level along the dorsoventral axis.
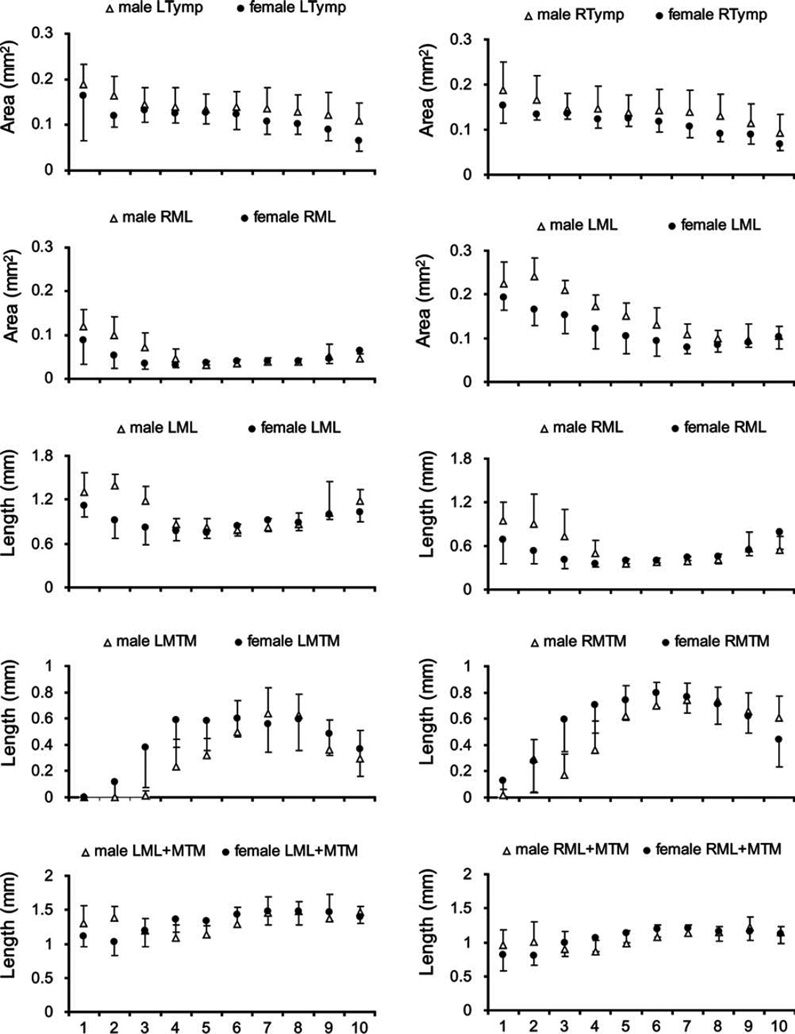
Fig. 4.
Left-right ratio of syringeal measurements. Circles are females and triangles are males. Levels 1 to 10 represent dorsal to ventral. LL, lateral labia; ML, medial labia; MTM, medial tympaniform membrane; Ty, tympanum; P, pessulus; A1, A2, A3, first, second and third bronchial half ring.
Most syringeal structures demonstrated shape changes from dorsal to ventral (Fig. 2), except for the left third bronchial halfring (A3) in males, the first left and third right bronchial halfring as well as the right LL in females (Table 1).
DISCUSSION
In the starling syrinx, we found sexual dimorphisms in skeletal structures and labial size as well as clear left-right asymmetries in labial size. The morphological differences are consistent with differences in the vocal behavior between males and females (Pavlova et al., 2005) and with the functional lateralization in the acoustic properties of left and right-side generated sounds. In addition, we discuss these results in comparison with the sexual dimorphism and lateralization in the zebra finch for which comparative data are available.
The larger size of the male syrinx (35%) cannot simply be explained by their larger body mass (9.4%). This size dimorphism is to a large degree the result of increased muscle mass, which was not quantified separately here. Some skeletal elements and the left labia are also larger in males. Higher circulating testosterone levels in males (levels are higher in male starlings by a factor of 2 to 4; Pinxten et al., 2007; Lo´pez-Rull and Gil, 2009) probably lead to proliferation of syringeal muscles, as has been shown in other species (Veney and Wade, 2004, 2005). The syrinx of male zebra finches is much larger (100%) than that of females (Luine et al., 1980; Wade and Buhlmann, 2000; Wade et al., 2002; Veney and Wade, 2004, 2005; Riede et al., 2010). Our birds were collected out of the breeding season and had probably lower testosterone levels than breeding males. Zebra finch males are not seasonal breeders and males in our study may have had high testosterone titers. However, the sexual dimorphisms found in starlings in this study are certainly highly robust since they are carried even through the non-breeding season. The effect of testosterone is mediated via absolute levels of circulating hormone and the number and availability of androgen receptors. We have no information on number or availability of androgen receptors. Absolute testosterone levels and the sexual differences in testosterone levels in zebra finches are comparable to those in starlings (Adkins-Reagan et al., 1990). Alternatively to a testosterone mediated size difference, the vocal behavior itself could contribute. How do the size differences relate to vocal behavior? We will discuss the relation of morphological differences to two aspects, vocal activity and fundamental frequency range.
Whether muscle mass might be related to vocal activity is not clear, but information from denervation experiments (we found that male syrinx mass decreases by 20–40% within a week after bilateral nerve transection) and from other motor systems (e.g., MacDougall et al., 1980) suggest strongly that muscle mass also depends on use. Female starlings sing less frequently than males. A much larger difference in vocal behavior occurs in zebra finches, in which females do not sing. The larger muscle mass in male starlings is supported by a larger skeletal framework. Because bronchial half rings are the main attachment sites for syringeal muscles, significant enlargement of some bronchial half-rings and tendencies towards larger areas in others in the male syrinx may provide a functional parallel to the enlarged muscles. Larger cartilages present increased surface area for muscle attachment and thicker cartilages can withstand greater muscle force. In male zebra finches, bronchial half rings (A1 on both sides and A2 on the right side) were also found to be larger than in females (Riede et al., 2010).
In addition to rate of use, the sexually dimorphic syringeal structures must also reflect differences in acoustic parameters of the respective vocal repertoires of males and females. The range of fundamental frequencies, which a syrinx can produce, is limited and characterizes boundaries of a vocal organ in birds (Zollinger and Suthers, 2004). In starlings and zebra finches, males vocalize with a wider range of fundamental frequencies than females, but the difference is much more dramatic in zebra finches. Male starlings produce fundamental frequencies between 300 and 10,000 Hz, whereas in females frequencies range between 500 and 8,000 Hz (Eens, 1997; Pavlova et al., 2005; Jensen et al., 2007). Male zebra finches sing and call between 500 and 7000 Hz and female zebra finches only call at frequencies between 500 and 700 Hz (Zann, 1996). The generation of a wider frequency range is consistent with more muscle mass and a more robust skeletal framework. Larger muscles can exert more force and therefore indirectly generate more tension of the labia, leading to higher sound frequencies.
Extending the frequency range of a sound source appears to be a major aspect of the evolution of sexual dimorphism in the songbird syrinx. The frequency range of vocal signals may be under strong sexual and natural selection, either driven by female choice for diverse acoustic repertoires (e.g., Gentner and Hulse, 2000), or by pressures for improving signal to noise ratio in environments with frequency-specific background noise (Bermúdez-Cuamatzin et al., 2011; Luther and Baptista, 2010).
All investigated songbirds demonstrate a functional lateralization of the two syringeal sound sources (Suthers, 1990). Typically, the right side generates higher frequencies than the left side (e.g., Suthers and Zollinger, 2004; exception: Suthers et al., 2011). Although, a physiological study in starlings and zebra finches showed that specific very low frequencies can be produced by both sound sources (Jensen et al., 2007). Could morphological/size differences between the two sound sources enhance the frequency range of each? There are no differences in the muscle fiber type composition on the two sides of the starling syrinx (Uchida et al., 2010). There is an asymmetry in the skeletal framework in female starlings but not in male starlings or male zebra finches or male white-crown sparrows (Zonotrichia leu-cophrys oriantha) (Riede and Goller, 2010a). The left ML was larger in males, and the left MTM was longer in females. The longer left MTM in females presumably fills the space that is occupied in males by the ML. A similar phenomenon has been observed in zebra finches (Riede and Goller, 2010a). Most strikingly, the labia in male and female starlings are substantially larger in the left syrinx, like in Northern cardinals (Cardinalis car-dinalis) (Jensen et al., 2008), but unlike in male zebra finches or male white-crowned sparrows (Riede and Goller, 2010a). We therefore hypothesize that size differences alone do not contribute to a specialization of a sound source to a fundamental frequency range. If labia are elongated and stiffened, the effective mass that is drawn into oscillation depends on viscoelastic properties of the tissue, and a variable portion of the labium may be recruited into vibration. Viscoelastic properties of vocal folds and labia are determined by the composition and organization of cells and extracellular matrix (Gray et al., 2000). In ongoing research, we found that the matrix composition of the starling labia demonstrates a rather complex layer structure compared to that in zebra finches (Riede and Goller, 2010a).
Sexual dimorphism of vocal organs is not limited to the sound source (syrinx or larynx) as examples from non-passerine species (Miller et al., 2007) or from other vertebrate taxa illustrate (Reby and McComb, 2003; Frey et al., 2007; Riede et al., 2008). However, the only sexually dimorphic structure in the upper vocal tract of starlings was beak length. Width and height were not significantly different. It is unlikely that the longer tip contributes to the acoustic filter function of the beak because the vocal tract opens at the base of the beak and sound is radiated there.
Male vocal display requires the smooth interaction of sound source and vocal tract. Why are there sexual differences only in the syrinx but not in the vocal tract anatomy? In this study, we have only looked at passive components of the vocal tract. It is possible that there are sexual differences in the musculature which operates the hyoid skeleton. Riede et al. (2006) suggested that the vocal tract resonance follows the fundamental frequency by variable OEC size. The motor pattern of hyoid skeleton and musculature adjusts the OEC resonance to the syrinx-generated source signal which is sexually dimorphic. Sexual differences and seasonal changes have been reported for the muscles of the hyoid apparatus of anole lizards (Anolis carolinensis; O’Bryant and Wade, 1999). Male anoles court females with a series of head-bobbing movements and the extension of a bright red throat fan called a dewlap. Dewlap extension is induced by the contraction of the ceratohyoideus muscle, which causes the ceratobranchial cartilage to bow out unfolding the dewlap skin for a visual signal, much the same way as songbirds increase OEC volume during vocal production to modulate their vocal resonance.
Acknowledgments
Contract grant sponsor: NIH; Contract grant numbers: DC 04390, DC 06876.
LITERATURE CITED
- Bermúdez-Cuamatzin E, Ríos-Chelén AA, Gil D, Macías Garcia C. Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol Lett. 2011;7:36–38. doi: 10.1098/rsbl.2010.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard DJ, Castro JM, Ball GF. Sexual dimorphism in the volume of song control nuclei in European Starlings: Assessment by a Nissl stain and autoradiography for musca-rinic cholinergic receptors. J Comp Neurol. 1993;334:559–570. doi: 10.1002/cne.903340405. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: Principles and mechanisms. Front Neuroendocrin. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Daley M, Goller F. Tracheal length changes during zebra finch song and their possible role in upper vocal tract filtering. J Neurobiol. 2004;59:319–330. doi: 10.1002/neu.10332. [DOI] [PubMed] [Google Scholar]
- Eens M. Understanding the complex song of the European starling: An integrated ethological approach. Adv Study Beh. 1997;26:355–434. [Google Scholar]
- Fitch T. Acoustic exaggeration of size in birds via tracheal elongation: Comparative and theoretical analysis. J Zool. 1999;248:31–48. [Google Scholar]
- Fletcher N, Riede T, Suthers RA. Model for vocalization by a bird with distensible vocal cavity and open beak. J Acoust Soc Am. 2006;119:1005–1011. doi: 10.1121/1.2159434. [DOI] [PubMed] [Google Scholar]
- Frey R, Volodin I, Volodina E. A nose that roars: Anatomical specializations and behavioural features of rutting male saiga. J Anat. 2007;211:717–736. doi: 10.1111/j.1469-7580.2007.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH. Female European starling preference and choice for variation in conspecific male song. Anim Behav. 2000;59:443–458. doi: 10.1006/anbe.1999.1313. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Implications for lateralization of bird song from unilateral gating of bilateral motor patterns. Nature. 1995;373:63–66. [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles on gating airflow and sound production in singing brown thrashers. J Neurophysiol. 1996a;75:867–876. doi: 10.1152/jn.1996.75.2.867. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. J Neurophysiol. 1996b;76:287–300. doi: 10.1152/jn.1996.76.1.287. [DOI] [PubMed] [Google Scholar]
- Goller F, Larsen ON. A new mechanism of sound generation in songbirds. Proc Nat Acad Sci USA. 1997;94:14787–14791. doi: 10.1073/pnas.94.26.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Bilaterally symmetrical respiratory acitivity during lateralized birdsong. J Neurobiol. 1999;41:513–523. doi: 10.1002/(sici)1097-4695(199912)41:4<513::aid-neu7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Goller F, Cooper B. Peripheral motor dynamics of song production in the zebra finch. Annal New York Acad Sci. 2004;1016:130–152. doi: 10.1196/annals.1298.009. [DOI] [PubMed] [Google Scholar]
- Goller F, Mallinckrodt MJ, Torti SD. Beak gape dynamics during song in the zebra Wnch. J Neurobiol. 2004;59:289–303. doi: 10.1002/neu.10327. [DOI] [PubMed] [Google Scholar]
- Gray SD, Titze IR, Alipour F, Hammond TH. Biomechani-cal and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- Hoese WJ, Podos J, Boetticher NC, Nowicki S. Vocal tract function in birdsong production: Experimental manipulation of beak movements. J Exp Biol. 2000;203:1845–1855. doi: 10.1242/jeb.203.12.1845. [DOI] [PubMed] [Google Scholar]
- Jensen KK, Cooper BG, Larsen ON, Goller F. Songbirds use pulse tone register in two voices to generate low-frequency sound. Proc R Soc B. 2007;274:2703–2710. doi: 10.1098/rspb.2007.0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KK, Zollinger SA, Childress S, Suthers RA. Second International Conference. Corvallis, Oregon: Oregon State University; 2008. Anatomy and vibration dynamics of the sound-producing medial labia in songbird syrinxes; p. 106. Conference abstract, Acoustic Communication by Animals. [Google Scholar]
- Luine V, Nottebohm F, Harding C, McEwen BS. Androgen affects cholinergic enzymes in syringeal motor neurons and muscle. Brain Res. 1980;192:89–107. doi: 10.1016/0006-8993(80)91011-2. [DOI] [PubMed] [Google Scholar]
- Lo´pez-Rull I, Gil D. Elevated testosterone levels affect female breeding success and yolk androgen deposition in a passerine bird. Behav Proc. 2009;82:312–318. doi: 10.1016/j.beproc.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Luther D, Baptista L. Urban noise and cultural evolution of bird songs. Proc Royal Soc B. 2010;277:469–473. doi: 10.1098/rspb.2009.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall JD, Elder GCB, Sale DG, Moroz JR, Sutton JR. Effects of strength training and immobilization on human muscle fibres. Eur J Appl Physiol Occup Physiol. 1980;43:25–34. doi: 10.1007/BF00421352. [DOI] [PubMed] [Google Scholar]
- Miller EH, Williams J, Jamieson SE, Gilchrist HG, Mallory ML. Allometry, bilateral asymmetry and sexual differences in the vocal tract of common eiders Somateria mollissima and king eiders S. spectabilis . J Avian Biol. 2007;38:224–233. [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- O’Bryant EL, Wade J. Sexual dimorphisms in a neuromuscu-lar system regulating courtship in the green anole lizard: Effects of season and androgen treatment. J Neurobiol. 1999;40:202–213. doi: 10.1002/(sici)1097-4695(199908)40:2<202::aid-neu6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Ohm V, Snelderwaard PC, ten Cate C, Beckers GJL. Vocal tract articulation in zebra finches. PLoS ONE. 2010;5:e11923. doi: 10.1371/journal.pone.0011923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova E, Pinxten R, Eens M. Female song in European starlings: Sex differences, complexity, and composition. The Condor. 2005;107:559–569. [Google Scholar]
- Pinxten R, de Ridder E, Arckens L, Darras VM, Eens M. Plasma testosterone levels of male European starlings (Sturnus υulgaris) during the breeding cycle and in relation to song and paternal care. Behavior. 2007;144:393–410. [Google Scholar]
- Podos J, Southall JA, Rossi-Santos MR. Vocal mechanics in Darwin’s finches: Correlation of beak gape and song frequency. J Exp Biol. 2004;207:607–619. doi: 10.1242/jeb.00770. [DOI] [PubMed] [Google Scholar]
- Reby D, McComb K. Anatomical constraints generate honesty: Acoustic cues to age and weight in the roars of red deer stags. Anim Beh. 2003;65:519–530. [Google Scholar]
- Riede T, Suthers RA, Fletcher NH, Blevins WE. Songbirds tune their vocal tract to the fundamental frequency of their song. Proc Nat Acad Sci USA. 2006;103:5543–5548. doi: 10.1073/pnas.0601262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Tokuda IT, Munger JB, Thompson SL. Mammalian laryngeal air sacs add variability to the vocal tract impedance: Physical and computational modeling. J Acoust Soc Am. 2008;124:634–647. doi: 10.1121/1.2924125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Suthers RA. Vocal tract motor patterns and resonance during constant frequency song: The white-throated sparrow. J Comp Physiol A. 2009;195:183–192. doi: 10.1007/s00359-008-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Goller F. Functional morphology of the sound generating labia in the syrinx of two songbird species. J Anatomy. 2010a;216:23–36. doi: 10.1111/j.1469-7580.2009.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Goller F. Peripheral mechanisms for vocal production in birds - differences and similarities to human speech and singing. Brain Language. 2010b;115:69–80. doi: 10.1016/j.bandl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Fisher J, Goller F. Sexual dimorphism of the zebra finch syrinx indicates adaptation for high fundamental frequencies in males. PLoS One. 2010;5:e11368. doi: 10.1371/journal.pone.0011368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Teague DP. The volumes of song control nuclei, HVC and lMAN, relate to differential behavioral responses of female European starlings to male songs produced within and outside of the breeding season. Brain Research. 2003;978:91–98. doi: 10.1016/s0006-8993(03)02771-9. [DOI] [PubMed] [Google Scholar]
- Suthers RA. Contributions to birdsong from the left and right sides of the intact syrinx. Nature. 1990;347:473–477. [Google Scholar]
- Suthers RA, Goller F. Motor correlates of vocal diversity in songbirds. In: Nolan V Jr, Ketterson E, Thompson CF, editors. Current Ornithology. New York: Plenum Press; 1997. pp. 235–288. [Google Scholar]
- Suthers RA, Goller F, Pytte C. The neuromuscular control of birdsong. Phil Trans Royal Soc London B. 1999;354:927–939. doi: 10.1098/rstb.1999.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers RA, Zollinger SA. Producing song: The vocal apparatus. Ann NY Acad Sci. 2004;1016:109–129. doi: 10.1196/annals.1298.041. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Wild JM, Kaplan G. Mechanisms of song production in the Australian magpie. J Comp Physiol A. 2011;197:45–59. doi: 10.1007/s00359-010-0585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Wade J. Sexual differentiation of the zebra finch song system: Potential roles for estradiol and ribosomal proteins L17 and L37. Dev Neurobiol. 2009;69:462–75. doi: 10.1002/dneu.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida AM, Meyers RA, Cooper BG, Goller F. Fibre architecture and song activation rates of syringeal muscles are not lateralized in the European starling. J Exp Biol. 2010;213:1069–1078. doi: 10.1242/jeb.038885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veney SL, Wade J. Steroid receptors in the adult zebra finch syrinx: A sex difference in androgen receptor mRNA, minimal expression of estrogen receptor a and aromatase. Gen Comp Endocrinol. 2004;136:192–199. doi: 10.1016/j.ygcen.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Veney SL, Wade J. Post-hatching syrinx development in the zebra finch: An analysis of androgen receptor, aromatase, estrogen receptor a and estrogen receptor β mRNAs. J Comp Physiol A. 2005;191:97–104. doi: 10.1007/s00359-004-0577-5. [DOI] [PubMed] [Google Scholar]
- Wade J, Buhlman L. Lateralization and effects of adult androgen in a sexually dimorphic neuromuscular system controlling song in zebra finches. J Comp Neurol. 2000;426:154–164. [PubMed] [Google Scholar]
- Wade J, Buhlman L, Swender D. Post-hatching hormonal modulation of a sexually dimorphic neuromuscular system controlling song in zebra finches. Brain Res. 2002;929:191–201. doi: 10.1016/s0006-8993(01)03389-3. [DOI] [PubMed] [Google Scholar]
- Warner RW. The anatomy of the syrinx in passerine birds. J Zool Lond. 1972;168:381–393. [Google Scholar]
- Westneat MW, Long J, John H, Hoese W, Nowicki S. Kinematics of birdsong: Functional correlation of cranial movements and acoustic featuresinsparrows. JExp Biol. 1993;182:147–171. doi: 10.1242/jeb.182.1.147. [DOI] [PubMed] [Google Scholar]
- Zann RA. Zebra Finch: A Synthesis of Field and Laboratory Studies. New York: Oxford University Press; 1996. [Google Scholar]
- Zollinger SA, Riede T, Suthers RA. Two-voice complexity from a single side of the syrinx in northern mockingbird mimus polyglottos vocalizations. J Exp Biol. 2008;211:1978–1991. doi: 10.1242/jeb.014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger SA, Suthers RA. Motor mechanisms of a vocal mimic: Implications for birdsong production. Proc R Soc Lond B. 2004;271:483–491. doi: 10.1098/rspb.2003.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]