Abstract
Sialic acids are common monosaccharides that are widely expressed as outer terminal units on all vertebrate cell surfaces, and play fundamental roles in cell–cell and cell–microenvironment interactions. The predominant sialic acids on most mammalian cells are N-glycolylneuraminic acid (Neu5Gc) and N-acetylneuraminic acid (Neu5Ac). Neu5Gc is notable for its deficiency in humans due to a species-specific and species-universal inactivating deletion in the CMAH gene encoding the hydroxylase that converts CMP-Neu5Ac to CMP-Neu5Gc. However, Neu5Gc is metabolically incorporated into human tissues from dietary sources (particularly red meat), and detected at even higher levels in some human cancers. Early life exposure to Neu5Gc-containing foods in the presence of certain commensal bacteria that incorporate dietary Neu5Gc into lipooligosaccharides can lead to generation of antibodies that are also cross-reactive against Neu5Gc-containing glycans in human tissues (“xeno-autoantigens”). Such anti-Neu5Gc “xeno-autoantibodies” are found in all humans, although ranging widely in levels among individuals, and displaying diverse and variable specificities for the underlying glycan. Experimental evidence in a human-like Neu5Gc-deficient Cmah−/−mouse model shows that inflammation due to “xenosialitis” caused by this antigen–antibody interaction can promote tumor progression, suggesting a likely mechanism for the well-known epidemiological link between red meat consumption and carcinoma risk. In this review, we discuss the history of this field, mechanisms of Neu5Gc incorporation into tissues, the origin and specificities of human anti-Neu5Gc antibodies, their use as possible cancer biomarkers, implications of xenosialitis in cancer initiation and progression, and current and future approaches toward immunotherapy that could take advantage of this unusual human-specific phenomenon.
Keywords: Neu5Gc, sialic acid, antibodies, inflammation, tumor antigen, red meat
Historical Background
Nearly 100 years ago, Hanganutziu and Deicher independently described human heterophile antibodies that agglutinated animal erythrocytes, as occurring in patients with serum-sickness, who had received therapeutic animal antisera (1, 2). Similar antibodies were later reported in patients with various diseases, despite no prior exposure to animal serum (3). Characterization of the antigenic determinants of these Hanganutziu–Deicher (H–D) antibodies occurred when Higashi et al. and Merrick et al. demonstrated that at least some of the major epitopes recognized were gangliosides containing a sialic acid (Sia) called N-glycolylneuraminic acid (Neu5Gc) (4, 5). This discovery spurred further research and H–D antibodies were also described in sera from patients with multiple pathological states such as rheumatoid arthritis, infectious mononucleosis, leprosy, syphilis, leukemia, Kawasaki disease, and various cancers (6–17). Expression of Neu5Gc on gangliosides and glycoproteins of human meconium and various human tumors was also detected by immunohistochemistry or thin-layer chromatography using polyclonal chicken antibodies raised against Neu5Gc-containing ganglioside GM3, or indirectly, via inhibition of bovine erythrocyte agglutination by a human H–D antiserum (10, 18–32). Given the data available at the time, it was reasonable to assume that Neu5Gc was an “oncofetal antigen,” expressed in fetal tissues, suppressed in adult life, and later up regulated during carcinogenesis. However, the evidence for an immune response raised questions about this assumption. In retrospect, the early reports of H–D antigens in cultured human cancer cell lines was probably due to animal serum used in the medium, and those in human cancer samples were likely of dietary origin (see below).
Diversity in the Sialic Acids
Classic studies of the structure, chemistry, and biosynthesis of sialic acids (Sias) occurred in parallel with the discovery and characterization of H–D antibodies. Sialic acids are a large family of nine-carbon-backbone monosaccharides primarily expressed in animals of the Deuterostome lineage (33, 34). They are most commonly found as the terminal units of the dense glycoconjugate coat covering cell surfaces, and of glycan chains attached to secreted molecules. By virtue of their position and high expression, Sias frequently act as the interface between the cell and the extracellular environment and mediate key roles such as stabilization of molecules and membranes via negative charge and hydrophilicity, transmembrane signaling, and receptor functions for self and non-self ligands (35, 36). They are also remarkably diverse owing to the various possible permutations involving different α-linkages between C-2 and the underlying glycan chain, as well as a variety of natural modifications. The predominant types of Sias in mammals include N-acetylneuraminic acid (Neu5Ac) and its hydroxylated version, Neu5Gc (Figure 1). These two molecules differ by a single oxygen atom, which is added to CMP-Neu5Ac in the cytosol, via a reaction catalyzed by the enzyme cytidine monophosphate N-acetylneuraminic acid hydroxylase (CMAH) (37–44). These activated sugars are then transported into the Golgi, where they act as donors for various sialyltransferases. (45).
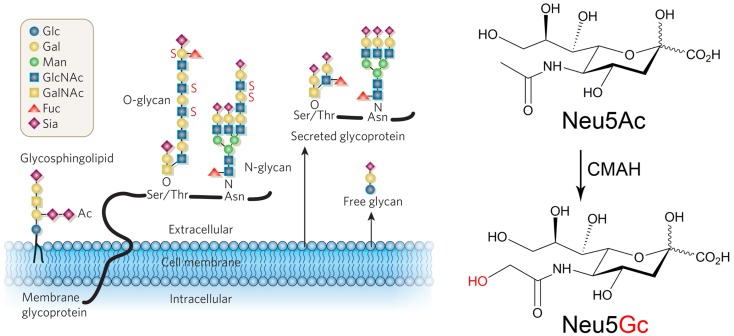
Figure 1.
Structures and predominant types of sialic acids. Left panel: sialic acids (Sia) are often terminating units of N- and O-linked glycoproteins and glycosphingolipids that can be found on the cell surface as part of the glycocalyx, as well as on secreted glycoproteins. Ac, O-acetyl ester; Fuc, fucose; Gal, galactose; GalNAc, N-acetyl galactosamine; Glc, glucose; GlcNAc, N-acetylglucosamine; Man, mannose; Sia, sialic acid, type unspecified; S, sulfate ester. Right panel: the two main mammalian sialic acids Neu5Ac (N-acetylneuraminic acid) and Neu5Gc (N-glycolylneuraminic acid) differ by one oxygen atom, which is added by the enzyme cytidine monophosphate N-acetylneuraminic acid hydroxylase (CMAH) in the cytosol. Humans lack this enzymatic activity due to an inactivating mutation of the CMAH gene. Reproduced from Varki (34).
Human Deficiency of Neu5Gc Biosynthesis
Following the reports of Neu5Gc in fetal and malignant tissues, it was found that all humans are homozygous for an Alu-mediated deletion of exon 6 in the CMAH gene, which results in a truncated, inactive enzyme (46–48). We can only speculate if positive or negative selection was involved in the fixation of this mutation in the human lineage. Negative selection by a lethal infectious pathogen that preferred Neu5Gc as a binding target is a possibility (49). Positive selection due a fertility advantage to Neu5Gc-negative females could also have been operative (50). Alternatively, the human condition may simply be the result of a random mutation that became fixed in a small population that eventually gave rise to modern humans. Regardless of these considerations, this rather drastic change in the sialic acid topology of the cell surface can be dated back to ~2–3 mya, prior to the origin of the genus Homo, and may even have been involved in the origin of the genus (50). Not only has there been a corresponding increase in the precursor Neu5Ac, but also multiple related changes in Sia biology and pathogen regimes, which are discussed elsewhere (51, 52).
Since Neu5Gc has been found in many species of the deuterostome lineage (ranging from sea urchins to fish to non-human primates), the CMAH gene is at least 500 million years old (53). Notably, Neu5Gc deficiency seems to have evolved independently in sauropsids (birds and reptiles) and possibly in monotremes such as the platypus (53). Indeed, chickens are similar to humans in recognizing Neu5Gc as a foreign antigen and mounting a strong immune response against it. A serum-sickness like condition can be induced when horse serum is injected into chickens or by the virally induced Marek’s disease, a lymphoma that expresses Neu5Gc by unknown mechanisms (54–57).
Neu5Gc Can be Metabolically Incorporated into Human Tissues from Dietary Sources
Until the end of the last century, the evidence for Neu5Gc in human tissues remained indirect, based on polyclonal antibodies raised in chickens or using H–D antibodies from human patients (26, 32). In order to confirm the findings of the classic studies that showed the oncofetal expression pattern of Neu5Gc, a chicken anti-Neu5Gc IgY with high specificity and avidity was generated by affinity purification and it indeed detected accumulation of Neu5Gc in human tumors such as breast carcinomas, in fetal epithelial cells, and in placental endothelial cells (Figure 2) (58). Surprisingly, small but definite amounts of Neu5Gc were also detected in normal human secretory epithelia and on endothelia of small- and large-blood vessels. These findings were supported by mass-spectrometry analysis of purified sialic acids (58) and of N-glycans released from human tumor samples (59). Subsequent studies of Cmah−/− mice showed a complete absence of Neu5Gc (59), indicating that there is no alternate pathway for the de novo biosynthesis of Neu5Gc. In the Absence of any other explanation, it was concluded that Neu5Gc must be entering human tissues exogenously via oral ingestion. Dietary sources that are rich in Neu5Gc include red meats such as beef, pork, lamb, and to a much lesser degree, cow’s milk products. Of significant note is the fact that plants and poultry do not contain Neu5Gc, and that fish samples studied so far contain low to trace amounts (58, 60).
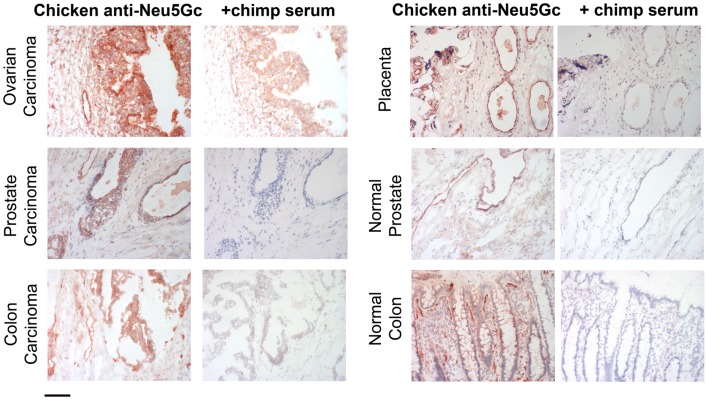
Figure 2.
Examples of incorporation of Neu5Gc in malignant and healthy human tissue. Expression of Neu5Gc is observed to be enhanced in malignant epithelia as seen here in carcinomas of the ovary, prostate and colon (left panel). In contrast, expression of Neu5Gc in normal tissue is seen in the ducts of the prostate gland and in the epithelial lining of the colon (Right panel). Endothelial cells of the normal placenta is used here as a positive control for Neu5Gc immunostaining. As a negative control, the binding is blocked competitively with Neu5Gc-containing chimpanzee serum. Magnification used was 200× and scale bar is 100 μm.
Given that Neu5Gc incorporation is practically significant, there is a need for the sensitive and specific detection of Neu5Gc in human and Cmah−/−mouse tissues, as well as in biotherapeutic products (see below). There are several immunological and chemical methods to detect such trace amounts of Neu5Gc. Table 1 summarizes the different methods with description of advantages and disadvantages of each. Immunodetection with a polyclonal anti-Neu5Gc IgY was among the first methods employed for the detection of Neu5Gc in tissues. Polyclonal antibodies are able to broadly detect epitopes with a terminating Neu5Gcα2-R unit, whereas the various monoclonal antibodies are very specific for different Neu5Gc-containing gangliosides. While such antibodies (and newer versions that are more specific) are commonly applied to immunohistochemical techniques now, certain caveats apply. Gangliosides can be lost in the paraffin-embedding process, therefore, frozen section analysis is preferred. Strong binding to endothelial Neu5Gc maybe used as a positive control and negative control can include competitive inhibition with a Neu5Gc-containing reagent such as 10% chimpanzee serum, mild periodate oxidation of the sialic acid chain, or sialidase treatment of tissue sections. Each approach has pros and cons and must be properly controlled.
Table 1.
Overview of methods to detect small amounts of Neu5Gc.
| Method | Methodological features | Commentsa |
|---|---|---|
| Polyclonal anti-Neu5Gc chicken IgY | Ganglioside GM3(Neu5Gc) as immunogen (54) | Recognizes gangliosides with Neu5Gcα2-R terminus. Applied to immunohistochemistry and TLC overlays |
| Affinity-purified polyclonal anti-Neu5Gc chicken IgY | Above polyclonal affinity purified on octyl-sepharose immobilized GM3(Neu5Gc) (157) | Specificity as above, likely cleaner background |
| Improved affinity-purified polyclonal anti-Neu5Gc chicken IgY | Above polyclonal antibody affinity purified on sequential columns of immobilized human and chimpanzee serum sialoglycoproteins, then eluted with Neu5Gc (158). Original immunogen noted to include bovine serum albumin as stabilizer – actually contaminated with Neu5Gc-rich bovine serum sialoglycoproteins, which acted as additional immunogens | Recognizes terminal Neu5Gc irrespective of linkage or underlying glycan chain (128). Applicable to ELISA, western blot, immunohistochemistry, and flow cytometry. Still contains IgY against animal albumin, removable by adsorption with column of mild periodate-borohydride-treated BSA (unpublished observations) |
| Monoclonal anti-Neu5Gc chicken IgYs | Monoclonal chicken anti-Neu5Gc IgYs (mChGc6-1, mChGc2-7) (74, 159) | mChGc2-7 has relatively broad specificity, similar to above polyclonal (128). Not yet studied for most applications |
| Monoclonal anti-Neu5Gc mouse/human IgM or IgG | MK2-34 murine IgM anti-GM2(Neu5Gc) (160), Y-2-HD-1, GMR14 murine IgG anti-GM2 (Neu5Gc) (81), 14F7 murine IgG anti-GM3(Neu5Gc) (140), GMR8 murine IgG anti-GM3(Neu5Gc) (161), GMR3 murine IgG anti-GD3(Neu5Gc-Neu5Gc-) (162), YK-3 human IgM against Neu5Gc disialogangliosides (163) | Each specific for different Neu5Gc gangliosides, but will miss many other Neu5Gc epitopes. Gangliosides get extracted from tissues during paraffin-embedding process and can be missed |
| Detection of sialidase or acid-released free Neu5Gc by HPAE-PAD | High-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD) (164–166) | Purification of released Neu5Gc improves background. Alkaline HPAEC conditions destroy O-acetyl groups |
| Detection of sialidase or acid-released free Neu5Gc by mass spectrometry | RP-HPLC with tandem MS interfaced with an electrospray ionization source (ESI) (167, 168) | Purification of released Neu5Gc and selective ion monitoring in mass spectrometry improves signal to noise |
| Detection of derivatized free Neu5Gc (sialidase or acid-released) | Fluorescent labeling with 1,2-diamino-4,5-methylenedioxybenzene (DMB) followed by RP-HPLC (164, 169, 170) | Sensitivity of fluorescence labeling can detect in the pmol range and ultra HPLC (UHPLC) allows run times as short as 10 min. DMB derivatization less likely to change Sia O-acetylation |
| Mass-spectrometry of glycans carrying Neu5Gc | Released N- or O-Glycan mixtures permethylated to facilitate purification and enhance signal in MALDI-TOF mass spectrometry (59) | Requires specific release of glycans. Alkaline permethylation destroys O-acetyl groups |
| Neu5Gc specific aptamers | Aptamers screened from a chemically synthesized nucleic acid library by “systematic evolution of ligands by exponential enrichment” (SELEX) (171) | Novel method with high affinity and apparent specificity Not tested on biological samples yet |
aSee text for further discussion.
Mechanisms for Metabolic Incorporation of Neu5Gc into Cmah Null Cells and Tissues
In vitro evidence shows that cultured human epithelial cells can incorporate Neu5Gc into endogenous glycoproteins from animal products (such as fetal calf serum) in the medium. Incorporation of Neu5Gc involves fluid-phase pinocytosis to enter the lysosome, where a sialidase releases Neu5Gc from glycoconjugates, and a sialic acid transporter then delivers the foreign sialic acid to the cytosol. Free Neu5Gc in the extracellular fluid can follow the same pathway (61). Cytosolic Neu5Gc now becomes available for activation to CMP-Neu5Gc in the nucleus and is subsequently transported into the Golgi. Evidently, the single oxygen atom difference between the foreign Neu5Gc and the native Neu5Ac remains permissive for the human sialic biosynthetic machinery, which utilizes Neu5Gc as if it were a “self” molecule (61, 62).
With regard to the intact organism, few studies had investigated the fate of orally ingested Neu5Gc in mammals. Nöhle and Schauer first showed that radioactive free sialic acid fed to mice and rats appeared in urine and was metabolized, as a portion of the radioactivity was recovered as expired CO2 (63–65). Radioactivity from labeled sialic acids in mucins was also detected in murine tissues after oral gavage (65). Oral ingestion studies in normal adult humans showed that free Neu5Gc can be absorbed and excreted within 4–6 h and detected only in trace amounts in salivary mucins and facial hair (58). The generation of a Cmah−/−mouse in 2007 paved the way to further investigate the consequences of Neu5Gc deficiency (66). Using this human-like Neu5Gc-deficient mouse, it was shown that this foreign Sia can indeed masquerade as a self-molecule and be incorporated in normal healthy tissues, into the fetus, and into cancers (67). In these mice, free Neu5Gc (i.e., the monosaccharide) can be rapidly absorbed into circulation with peak levels seen at 1–2 h post ingestion and is efficiently excreted by the kidneys. In contrast, oral ingestion of glycosidically bound Neu5Gc (i.e., Neu5Gc-glycoconjugates such as porcine submaxillary mucins) led to incorporation into the small intestinal wall, liver, kidney, and was detectable in the peripheral circulation for several hours (67). Further investigation is necessary to understand the factors affecting the bioavailability of ingested Neu5Gc, the mechanisms behind the selective incorporation of glycosidically bound Neu5Gc, the preferential incorporation into endothelial and epithelial cells, and the eventual metabolic fate of this foreign molecule in human cells. With regard to the latter issue, recent work has revealed a degradative pathway involving sequential conversion to N-glycolylmannosamine (ManNGc), N-glycolylglucosamine (GlcNGc), and GlcNGc 6-phosphate, with eventual release of the glycolyl group into cellular metabolic pathways (68). With the exception of the last step, all other reactions are reversible, and little is known about rates of turnover and any other metabolic pathway that may be involved. The same studies also practically ruled out prior suggestions that Neu5Gc expression in humans might originate from an alternate pathway involving glycolyl-CoA (69, 70).
Origins and Implications of the Human Immune Response Against Neu5Gc
Until the end of the 1990s, anti-Neu5Gc antibodies were thought to be present only in humans who had received therapeutic animal sera injections or those diagnosed with diseases like cancer, but not in normal healthy individuals. However, reports from the xenotransplantation field identified non-Gal antibodies specific for Neu5Gc that were detectable by flow cytometry in the majority of normal healthy humans (71). Parallel studies in our lab showed that the immune response against Neu5Gc was actually universal in humans (58), but of widely ranging levels. The antibodies were also diverse and of broad polyclonal specificities, arising from selective recognition of the underlying glycan as well (72, 73). The discrepancy with the earlier studies was likely due to the different methods of detection and/or antigens used in the assays. One of the cancer-associated H–D antigens used as the ELISA detection target in earlier studies was the ganglioside GM3(Neu5Gc) (4, 74, 75), an antigen against which antibodies in healthy humans tend to be low. Also, in some studies the antibody binding signal in normal subjects was simply assumed to be non-specific and subtracted as background (30, 76). Further, it was assumed that H–D antibodies were directed against Neu5Gc alone. However, a monosaccharide by itself cannot fill the binding pocket or paratope of the antibody, which can accommodate four to five sugars (77). Thus, the wide range of naturally occurring Neu5Gc-glycoconjugates would warrant a diverse, polyclonal immune response not just against the Sia, but also the underlying glycan (72). Indeed, chemo-enzymatically synthesized α2-3 or α2-6 linked sialoside pairs (i.e., identical glycans with either Neu5Gc or Neu5Ac) (78, 79) were used to analyze anti-Neu5Gc antibodies, revealing complex patterns of IgG, IgM, and IgA reactivities with marked variations in the antibody profiles within the normal human population (73). In retrospect, this also explains prior successes in obtaining monoclonal antibodies with specificities for particular Neu5Gc-containing molecules (74, 80–84).
The levels of these naturally occurring polyclonal antibodies can be quite high in some humans, approaching those of the major anti-glycan antibodies in normal human serum: anti-ABO blood group and anti-α-Gal (anti-Galα1-3Galβ1-4GlcNAc) (72). Such antibodies arise from antigenic stimulation by gastrointestinal bacteria and the latter are well known as an immunological barrier for xenotransplantation. In contrast to α-Gal, which is not expressed on human cells, the “xenogenic” Neu5Gc is enriched in cancer cells and can be incorporated into normal vertebrate cells as it is recognized by the biochemical pathways as a self or “autogenic” entity. Thus, Neu5Gc is the first example of a “xeno-autoantigen” (59, 62). Given this incorporation, circulating anti-Neu5Gc antibodies become potentially relevant in the pathogenesis of diseases associated with chronic inflammation such as cancer, atherosclerosis, and autoimmune disease (73, 85).
It was recently shown that anti-Neu5Gc antibodies in humans emerge during the first year of life and are likely not germ-line encoded “natural” antibodies (86). They appear at 6 months and steadily rise to adult levels by 1 year of age. This occurs coincident with the introduction of Neu5Gc via cow’s milk in baby formula. Cmah−/−mice were used to study this temporal effect further. A variety of Neu5Gc-feeding paradigms failed to generate anti-Neu5Gc antibodies in these mice. Instead, it was found that commensal non-typeable Haemophilus influenza is able to scavenge free dietary Neu5Gc and express it as an immunogenic epitope, thereby effectively “vaccinating” the host to generate anti-Neu5Gc IgM and IgG antibodies (86). It is possible that other commensal or pathogenic bacteria can also carry out this “xeno-autoimmunization,” and it is likely that there are other routes of immunization as well, considering the variable response that is seen across normal individuals. Further investigation is necessary to define the cellular and molecular pathways required for antibody selection, generation, and secretion, the specific B cell populations responsible, the levels of IgA antibodies in secretions, whether the anti-Neu5Gc response is T cell independent, and whether affinity maturation of the antibody binding site occurs following class-switching.
Red Meat Consumption Increases Carcinoma Risk
Diet remains one of the most modifiable risk factors for many chronic diseases including cancer. Although meat is a valuable source of essential amino acids, iron, key vitamins and minerals, numerous epidemiological studies agree that consumption of red meat (meat from mammalian sources such as lamb, pork, and beef), is associated with not only increased risk of certain cancers but also cardiovascular disease (87, 88). Prominent among the large prospective cohorts are the Health Professionals Follow-up Study and the Nurses’ Health Study that recently confirmed that a higher intake of red meat was associated with a significantly elevated risk of cancer, cardiovascular disease, and total mortality (89).
The cancer type that has been most prominently associated with red meat consumption is colorectal cancer (CRC), one of the leading causes of cancer-related deaths in the United States (90). A statistically significant increase in CRC risk of 1.35-fold is seen with a high intake (>160 g/day) of red meat (91). This association is stronger for processed meat than for unprocessed meat. Esophageal, gastric, prostate, and endometrial cancer risk is also significantly elevated by high meat consumption, although conflicting reports exist (92–95). These associations are understandably variable, as diet is difficult to measure accurately, especially given the multiple correlations between different components of food.
There have been several mechanisms proposed by which red meat consumption increases the risk of cancer including: (1) high-fat intake (96); (2) the production of heterocyclic amines and polycyclic aromatic hydrocarbons formed by high temperatures during grilling (97); (3) the presence of mutagenic N-nitroso compounds (98); and, (4) the higher levels of heme iron as a promoter of carcinogenesis through increased cellular proliferation, increased oxidative stress, and iron-induced hypoxia signaling (99). The first three mechanisms have been effectively ruled out by epidemiological data, and by the fact that the risk is exclusive for red meat, and not for poultry or fish. More recently, a new mechanism for the link between cardiovascular disease and red meat was proposed to involve the intestinal microbiota. Metabolism of l-carnitine that is abundant in red meat by the microbiota generates trimethylamine-N-oxide (TMAO), which is proatherogenic in mice (100) and is associated with increased cardiovascular risk in humans (101). However, no connection of TMAO to cancer risk was reported.
Overall, there is no theory that has been conclusively proven. We propose that “xenosialitis” or the interaction between the non-human Neu5Gc and circulating anti-Neu5Gc results in chronic inflammation that promotes carcinogenesis and atherogenesis. In this regard, red meat consumption and the “western diet” have been associated with increased circulating markers of inflammation in human population studies (102). Thus, Neu5Gc may be the missing link between red meat consumption and risk of cancer and cardiovascular disease, an association that so far appears unique to humans. Evidence supporting our hypothesis is presented in the further sections.
Role of Inflammation in Cancer
The mammalian immune system recognizes and eliminates cells with non-native DNA, including transformed cells (103–108). During cancer progression, however, tumor cells can escape elimination by the immune system by being selected for low immunogenicity or by being able to inhibit immune cell activation and induce an immunosuppressive microenvironment (104, 109–111). Some of these pathways can be targeted for cancer immunotherapies (108, 112–114).
In contrast to this immunosurveillance function, inflammation and associated activation of the immune system can also promote cancer progression (115–117). Chronic inflammation due to infectious and non-infectious agents such as auto-inflammatory diseases and diet-induced metabolic syndrome is an important etiology for the development of cancer (116–118). In this regard, epidemiological analyses have confirmed that interference with inflammation using non-steroidal anti-inflammatory drugs including aspirin are protective for the development of inflammation-induced cancers such as colorectal carcinomas (119, 120). Aspirin use has also been associated with reduced incidence of other cancers including those of the esophagus and stomach (121).
Inflammation not only works as promoter during carcinogenesis (inflammation-induced cancer), but growing tumors that escaped immunosurveillance also induce an inflammatory response that can support cancer progression (cancer-related inflammation) (115, 122). In particular, cells from the myeloid lineage such as neutrophils and monocytes/macrophages support cancer progression (110, 123–125). Thus, while the immune system exerts considerable immunosurveillance to eliminate tumor cells, inflammatory pathways can be co-opted by tumor cells to promote cancer progression. Moreover, inflammation can also act as an oncogenic promoter during tumorigenesis, inducing DNA damage via reactive oxygen species.
Experimental Studies of Neu5Gc-Induced Xenosialitis in Cancer
Aberrant cell surface glycosylation is a well-known characteristic of cancer and can be altered by the loss or gain of certain structures, the presence of truncated structures, accumulation of precursors, and the synthesis of novel structures (126). Increased cell surface sialylation is one such example. Not only is there an increase in the total sialic acid content, but also significant changes in their modifications such as the accumulation of Neu5Gc. This molecule preferentially accumulates in malignant tissue due to multiple mechanisms, including increased macropinocytosis (61), rapid growth rates, and up-regulation of the sialin transporter in response to hypoxia (127). In keeping with this, subcutaneous syngeneic tumors showed high levels of Neu5Gc incorporation when Cmah−/−mice drank water containing Neu5Gc (67), conditions under which normal tissues were not easily loaded.
This accumulation occurs in the presence of circulating xeno-autoantibodies against Neu5Gc-glycans. Different experimental models were used to ask if inflammation mediated by the anti-Neu5Gc immune response (xenosialitis) could influence tumor growth by affecting cancer-related inflammation (59, 73). Cmah−/− mice subcutaneously injected with the low metastatic B16F1 murine melanoma cell line that expresses Neu5Gc on its surface developed antibodies against Neu5Gc and the tumors then grew larger over time (59). Subcutaneously injected murine MC38 tumor cells also formed larger tumors in Cmah−/−mice when polyclonal mono-specific murine anti-Neu5Gc antibodies were transferred passively via intraperitoneal injection (59). The increase in tumor growth was associated with enhanced infiltration of cells of the innate immune system, suggesting a role of these cells in promoting effects.
Blunting cancer-related inflammation in the subcutaneous MC38 model with a cyclooxygenase-2 inhibitor repressed the effect of anti-Neu5Gc antibodies (59). However, when higher doses of human anti-Neu5Gc antibodies purified from commercially available human intravenous immunoglobulins (IVIG) were transferred passively into mice by intraperitoneal injection, the growth of subcutaneous syngeneic MC38 tumors was significantly inhibited (73). This finding indicates that Neu5Gc-containing glycans could potentially serve as targets for immunotherapy. Indeed, when Neu5Gc production in MC38 cells was silenced via siRNA targeting the Cmah transcript, inhibition by transferred Neu5Gc antibodies was blunted (73). The experimental evidence discussed here indicates that low levels of anti-Neu5Gc antibodies can support cancer progression by enhancing tumor-related inflammation via induction of “xenosialitis,” and that higher doses might possibly be used to target tumors that have high levels of Neu5Gc in their glycocalyx. Further studies of this issue are underway, including models where tumors in mice accumulate Neu5Gc from dietary sources, in a manner similar to the human condition.
Circulating Anti-Neu5Gc Antibodies as Tumor Markers
Earlier studies discussed above had reported an increased incidence of H–D antibodies in patients with cancer. Sera of patients with cancer were therefore analyzed for the presence of anti-Neu5Gc antibodies with specificity toward different Neu5Gc-glycan epitopes (73). A sialoglycan microarray of over 70 chemo-enzymatically synthesized sialoglycans including unique pairs of Neu5Gc- and Neu5Ac-containing glycans were used to analyze the different binding properties of antibodies from patients with breast cancer versus controls in order to identify a classifier of 20 Neu5Gc-containing glycans (73). Using these 20 Neu5Gc-containing glycan targets, analysis of binding properties of antibodies in sera were further tested and validated in more breast cancer patients. The four most significant glycans were also able to differentiate between controls and patients with various carcinomas including prostate, ovary, endometrium, colon, lung, and pancreas (73). These findings suggest that anti-Neu5Gc antibodies can function as tumor markers. Notably one of the most significant cancer-associated epitopes was very similar to the known sialyl-Tn tumor antigen, except that Neu5Gc replaced Neu5Ac (73). A caveat to the use of glycan microarrays is that interpretation of the results should take into consideration the differences in producing, presenting, coupling, and detecting the glycans (128). Anti-Neu5Gc antibodies can also be analyzed by using Neu5Gc-rich natural glycoproteins (such as wild type mouse serum) or by coupling glycans to polymers such as polyacrylamide and probing with antibodies on an ELISA plate (72, 86). This less expensive, simpler method could be used as a screening tool, perhaps to monitor disease progression and/or therapeutic response. However, dilution effects on antibody binding and competition between different antibody classes also complicate such studies, and caution must be taken to avoid reagents of animal origin that might introduce Neu5Gc contamination. Population studies are necessary to identify correlations between anti-Neu5Gc antibody levels and carcinoma risk and progression.
Implications for Cancer Prevention and Treatment
Given the evidence for Neu5Gc in human tissues along with circulating anti-Neu5Gc antibodies, the observed xenosialitis in mouse models could potentially play an important role in the development of cancer (59). Since the source of this foreign sialic acid is the diet, avoidance of Neu5Gc-rich food could possibly reduce cancer risk. Indeed, as discussed above, Neu5Gc-rich red meat increases the risk of inflammation-induced cancers including CRC (89) and our proposed xenosialitis model could be replicating this increased risk. Furthermore, the data predict that the reduction of Neu5Gc consumption could also be important during therapy of already established malignancies in order to interfere with cancer progression. Indeed, high intake of a “western” dietary pattern, rich in red meat, was associated with a higher risk of recurrence and mortality among patients with stage III colon cancer treated with surgery and adjuvant chemotherapy (129).
It is well described that tumor cells are aberrantly sialylated and that content of sialic acid on the surface of tumor cells significantly increase as compared to cells in healthy tissues (130, 131). The up-regulation of sialylation might also explain why ingested Neu5Gc preferentially accumulates in cancer tissues (58, 67). Aberrant sialylation includes an increase of the tumor antigen sialyl-Tn (132–136), which is uncommon in normal tissues (132, 137, 138) or cryptic due to O-acetylation of the sialic acid (133) in healthy tissue. As discussed before, analysis of anti-Neu5Gc antibodies by a sialoglycan microarray showed significant up-regulation of antibodies targeting Neu5Gc-sialyl-Tn suggesting that such antibodies might function as specific tumor biomarkers (73). If this epitope is indeed a relatively cancer-specific antigen, it could be potentially useful as a target for imaging as well as a therapeutic tool for drug delivery. As in vitro assays have shown that human antibodies purified from IVIG preparations against Neu5Gc-Tn antigen can activate antibody-dependent cellular and complement-dependent cytotoxicity (ADCC and CDC) (73), the epitope could potentially be directly targeted by anti-Neu5Gc-Tn antibodies.
Neu5Gc-containing gangliosides including GM3 were originally described as an epitope for H–D antibodies (4, 5) and (Neu5Gc)GM3 was found to be a tumor-associated antigen particularly in skin and breast cancer (139, 140). Vaccination with (Neu5Gc)GM3 along with the outer membrane protein complex of Neisseria meningitidis in proteoliposomes lead to antibody production in advanced stage breast cancer patients in a phase I study (141). Immunization of mice with (Neu5Gc)GM3 led to the isolation of specific antibody 14F7 (140), which was recently humanized and named racotumumab (142, 143). This monoclonal antibody is able to bind several malignant tissues including skin cancers, neuroectodermal tumors, genitourinary cancer, non-small cell lung cancer, and tumors of the gastrointestinal tract (144–148). Trials testing the efficacy of racotumumab are currently recruiting (e.g., NCT01598454, NCT01460472). But there is no attempt to control for dietary Neu5Gc intake by patients.
Similar approaches might be taken toward other Neu5Gc-glycan epitopes that are enriched in tumors. For example, vaccination with proteins containing Neu5Gc as terminal sugars could potentially boost an immune response against established tumors. For instance, self-assembling MUC1 with Neu5Gc-Tn antigen could be used to immunize patients with carcinomas (149, 150).
Notably, Neu5Gc is present as a component of some cancer therapeutic agents. Immunotherapy with monoclonal antibodies against other tumor epitopes such as targeting HER2 with trastuzumab, EGF receptor 1 with cetuximab, or CD20 with rituximab are well integrated in today’s cancer therapies (151). Antibodies are glycosylated and biotechnological production of such glycoproteins can involve Neu5Gc-rich media and/or non-human cells expressing Neu5Gc (152). Thus, it was previously shown that incorporation of Neu5Gc in cetuximab enhanced the formation of immune complexes and promoted drug clearance (153). Moreover, Cmah−/−mice injected with cetuximab reacted with an anti-Neu5Gc immune response (153). Thus, avoidance of Neu5Gc during production of glycoproteins might improve half-life of therapeutics and also reduce the immunogenicity and therefore has important implications in cancer therapy.
Conclusions and Future Directions
We have discussed the incorporation of the non-human sialic acid Neu5Gc into human tissues, and its potential impact on cancer initiation and progression. We particularly emphasized the resulting inflammatory activation or xenosialitis induced by xeno-autoantibodies against Neu5Gc-containing epitopes (Figure 3). Notably, Neu5Gc can also be incorporated into glycans of endothelial cells in healthy individuals and the subsequent xenosialitis has been potentially implicated in the pathogenesis and progression of atherosclerosis (85). Additionally, screening of antibody specificity by Neu5Ac/Neu5Gc specific sialoglycan microarray revealed a Neu5Gc antibody response in children with Kawasaki disease suggesting a possible role of these antibodies in disease progression (154). All these findings support the hypothesis that xenosialitis may be involved in various inflammatory diseases. Thus, avoidance of the Neu5Gc by reducing the consumption of Neu5Gc-containing food could not only have a major impact on the prevention of malignant diseases, but also potentially reduce the risk of cardiovascular disorders. Furthermore, examination and cloning of human anti-Neu5Gc xeno-autoantibodies can provide information about the nature of the anti-Neu5Gc immune response and offer ways to manipulate this in order to produce more efficient vaccines against Neu5Gc-containing tumor-associated epitopes.
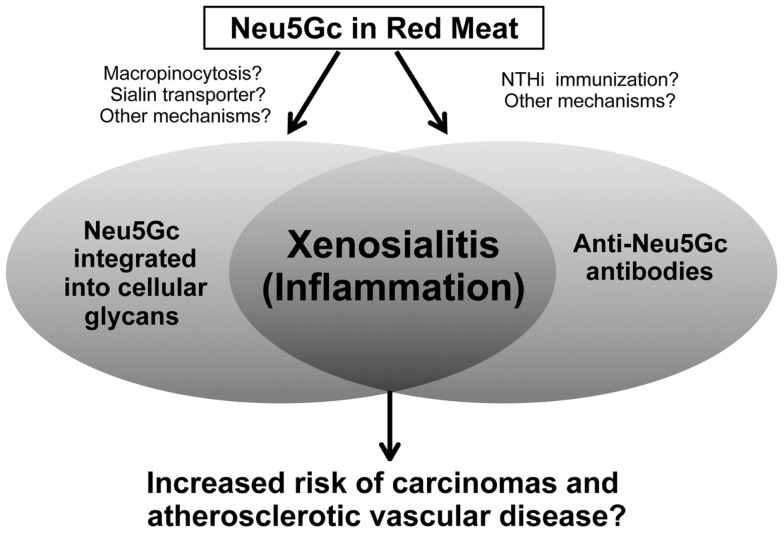
Figure 3.
Xenosialitis hypothesis. Neu5Gc is metabolically incorporated from the diet (primarily red meats) into cellular glycans to form xeno-autoantigens. Anti-Neu5Gc antibodies or xeno-autoantibodies are induced by immunization via commensal bacteria (such as non-typeable Haemophilus influenza or NTHi) that scavenge and express Neu5Gc. The resulting antigen–antibody interaction is hypothesized to lead to chronic inflammation termed xenosialitis that potentially promote cancer and/or atherosclerotic vascular disease.
Further analysis is needed to determine the pathways by which Neu5Gc is absorbed and transported to healthy and cancerous tissues, in order to be able to interfere with pathological consequences of Neu5Gc incorporation. Since Neu5Gc uses the same biochemical pathways as Neu5Ac, Neu5Gc could be potentially “flushed” out of the body by high amounts of Neu5Ac. The relatively specific accumulation of Neu5Gc on glycans of tumor cells, in particular as Neu5Gc-Tn antigen and (Neu5Gc)GM3 ganglioside is particularly intriguing and should be further studied to harness tumor cells for therapeutic and diagnostic purposes. However, it must be carefully determined if anti-Neu5Gc antibodies are well suited for immunotherapy of tumors or if there are “off-target” effects due to the presence of Neu5Gc-containing epitopes in other tissues. The accumulation of Neu5Gc-containing glycans in tumors in Cmah−/−mice fed with the monosaccharide Neu5Gc (67) suggests another strategy of deliberately loading tumors in a selective fashion in order to subsequently target them. Such “loading” of tumor tissue with Neu5Gc might be optimally combined with monoclonal antibody immunotherapies. Finally, it remains to be seen if the increase of Neu5Gc-containing sialoglycans on the surface of cancer cells might directly influence the cell intrinsic properties such as response to hypoxia or signaling through glycosylated surface receptors.
While much more work is needed to prove or disprove the overall hypothesis, it is encouraging that a prominent textbook of cancer biology has designated this as an “area to watch” (155). But major milestones of progress in the field are likely to be slow. While we are not assuming a similar level of importance to disease pathogenesis, it is instructive to compare the progress to date (Table 2) with those underlying the seminal discovery that cholesterol played a key role in atherosclerosis progression and risk (156). Cholesterol was found in atherosclerotic plaques in 1910, familial hypercholesterolemia was associated with early heart attacks in 1949, and population studies showed an association of heart disease with cholesterol levels by the early 1960s. However two more decades followed before cholesterol lowering by statins was shown to reduce heart attacks and another decade passed before a large scale double-blinded statin trial showed a positive effect in 1994 (156). While studies have clearly shown that Neu5Gc is a tumor-associated antigen with diagnostic and therapeutic potential and mouse models have established the role of xenosialitis in tumor progression, fundamental questions remain unanswered. Can anti-Neu5Gc antibodies and/or Neu5Gc tissue load predict carcinoma risk in human population studies? Will lowering Neu5Gc load reduce carcinoma risk? Is it possible to eliminate the foreign Neu5Gc from tissues or perhaps interfere with the metabolic incorporation process? Considering the potential impact on the prevention and treatment of human disease, it is evident that Neu5Gc and its interactions warrant further experimental and translational investigation.
Table 2.
Timeline of discoveries/studies concerning Neu5Gc and anti-Neu5Gc antibodies.
| Year(s) | Discoveries/studies | Reference |
|---|---|---|
| 1924 | Discovery of H–D antibodies in patients with serum-sickness | (1, 2) |
| 1970–1980s | Association of H–D antibodies with multiple pathological states, including cancer | (3, 6–9) |
| 1977–1978 | Definition of Neu5Gc as key component of H–D antigen | (5) |
| 1980s | Neu5Gc detected in human meconium and carcinomas by immunohistochemistry or TLC; assumed to be an “oncofetal” antigen | (10, 18–22) |
| 1998 | Human deficiency of Neu5Gc synthesis due to CMAH inactivation | (46–48) |
| 2003 | First results of trial with vaccine containing GM3 (Neu5Gc) | (141) |
| 2003 | Metabolic incorporation of Neu5Gc in human tissues from dietary sources | (58) |
| 2003 | Neu5Gc is enriched in red meats | (58) |
| 2003 | Anti-Neu5Gc response is universal to humans | (58, 71, 72) |
| 2005 | Molecular mechanisms of uptake and incorporation of Neu5Gc into cells elucidated – role of macropinocytosis and lysosomal sialin transporter noted | (61) |
| 2006 | Up-regulation of the sialin transporter in response to hypoxia in cancer increases Neu5gc accumulation | (127) |
| 2007 | Development of Cmah−/−mouse model. No alternate pathway for Neu5Gc synthesis | (66) |
| 2008 | Passively transferred anti-Neu5Gc antibodies enhance Cmah-positive carcinoma progression in Cmah−/−mice | (59) |
| 2010 | Origin of anti-Neu5Gc antibodies via “xeno-autoimmunization” by commensal bacteria that incorporate diet-derived Neu5Gc | (86) |
| 2011 | Humanized anti-Neu5Gc-GM3 antibody racotumumab, first trials using this antibody start recruiting | (143) |
| 2011 | Neu5Gc-sialyl Tn as potential biomarker cancer biomarker | (73) |
| 2012 | Oral glycosidically linked bound Neu5Gc preferentially incorporated into Cmah−/−mouse tissues, fetuses, and orthotopic tumors | (67) |
| 2012 | Intracellular degradative pathway for Neu5Gc discovered | (68) |
| 2013 | Simple method for assessment of human anti-Neu5Gc antibodies | (154) |
See text for discussion.
Conflict of Interest Statement
Ajit Varki and Nissi Varki are co-founders of and advisors to Sialix, Inc., which has licensed UCSD technologies related to anti-Neu5Gc antibodies in cancer. The other co-authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Frederico Alisson Silva, Anne Bergfeld, and Yuko Naito-Matsui for critical reading of the manuscript and helpful suggestions. Supported by NIH grant CA38701.
References
- 1.Hanganutziu M. Hémagglutinines hétérogénétiques après injection de sérum de cheval. CR Séances Soc Biol (1924) 91:1457–9 [Google Scholar]
- 2.Deicher H. Über die Erzeugung heterospezifischer Hämagglutinine durch Injektion artfremden. Serums Z Hyg (1926) 106:561–79 10.1007/BF02176298 [DOI] [Google Scholar]
- 3.Kasukawa R, Kano K, Bloom ML, Milgrom F. Heterophile antibodies in pathologic human sera resembling antibodies stimulated by foreign species sera. Clin Exp Immunol (1976) 25:122–32 [PMC free article] [PubMed] [Google Scholar]
- 4.Higashi H, Naiki M, Matuo S, Okouchi K. Antigen of “serum sickness” type of heterophile antibodies in human sera: identification as gangliosides with N-glycolylneuraminic acid. Biochem Biophys Res Commun (1977) 79:388–95 10.1016/0006-291X(77)90169-3 [DOI] [PubMed] [Google Scholar]
- 5.Merrick JM, Zadarlik K, Milgrom F. Characterization of the Hanganutziu-Deicher (serum-sickness) antigen as gangliosides containing N-glycolylneuraminic acid. Int Arch Allergy Appl Immunol (1978) 57:477–80 10.1159/000232140 [DOI] [PubMed] [Google Scholar]
- 6.Nishimaki T, Kano K, Milgrom F. Studies on heterophile antibodies in rheumatoid arthritis. Arthritis Rheum (1978) 21:634–8 10.1002/art.1780210604 [DOI] [PubMed] [Google Scholar]
- 7.Nishimaki T, Kano K, Milgrom F. Studies on immune complexes in rheumatoid arthritis. Arthritis Rheum (1978) 21:639–44 10.1002/art.1780210605 [DOI] [PubMed] [Google Scholar]
- 8.Nishimaki T, Kano K, Milgrom F. Hanganutziu-Deicher antigen and antibody in pathologic sera and tissues. J Immunol (1979) 122:2314–8 [PubMed] [Google Scholar]
- 9.Morito T, Kano K, Milgrom F. Hanganutziu-Deicher antibodies in infectious mononucleosis and other diseases. J Immunol (1982) 129:2524–8 [PubMed] [Google Scholar]
- 10.Ikuta K, Nishi Y, Shimizu Y, Higashi H, Kitamoto N, Kato S, et al. Hanganutziu-Deicher type-heterophile antigen-positive cells in human cancer tissues demonstrated by membrane immunofluorescence. Biken J (1982) 25:47–50 [PubMed] [Google Scholar]
- 11.Arita K, Ikuta K, Nishi Y, Kato S, Yamauchi E, Maki S, et al. Heterophile Hanganutziu-Deicher antibodies in sera of patients with Kawasaki diseases. Biken J (1982) 25:157–62 [PubMed] [Google Scholar]
- 12.Takiguchi M, Tamura T, Goto M, Kusakawa S, Milgrom F, Kano K. Immunological studies on Kawasaki disease. I. Appearance of Hanganutziu-Deicher antibodies. Clin Exp Immunol (1984) 56:345–52 [PMC free article] [PubMed] [Google Scholar]
- 13.Mukuria JC, Naiki M, Hashimoto M, Kato S. A specific enzyme-linked immunosorbent assay (ELISA) procedure for detection of heterophile Hanganutziu and Deicher (HD) antibodies. J Immunol Methods (1986) 86:179–85 10.1016/0022-1759(86)90450-3 [DOI] [PubMed] [Google Scholar]
- 14.Mukuria CJ, Fujii Y, Kato S, Naiki M. Specificities of human heterophile Hanganutziu and Deicher (HD) antibodies to glycosphingolipids and a glycoprotein. J Biochem (1986) 100:469–75 [DOI] [PubMed] [Google Scholar]
- 15.Morito T, Nishimaki T, Masaki M, Yoshida H, Kasukawa R, Nakarai H, et al. Studies on Hanganutziu-Deicher antigens-antibodies. I Hanganutziu-Deicher antibodies of IgG class in liver diseases. Int Arch Allergy Appl Immunol (1986) 81:204–8 10.1159/000234135 [DOI] [PubMed] [Google Scholar]
- 16.Nakarai H, Saida T, Shibata Y, Irie RF, Kano K. Expression of heterophile, Paul-Bunnell and Hanganutziu-Deicher antigens on human melanoma cell lines. Int Arch Allergy Appl Immunol (1987) 83:160–6 10.1159/000234349 [DOI] [PubMed] [Google Scholar]
- 17.Higashihara T, Takeshima T, Anzai M, Tomioka M, Matsumoto K, Nishida K, et al. Survey of Hanganutziu and Deicher antibodies in operated patients. Int Arch Allergy Appl Immunol (1991) 95:231–5 10.1159/000235434 [DOI] [PubMed] [Google Scholar]
- 18.Ohashi Y, Sasabe T, Nishida T, Nishi Y, Higashi H. Hanganutziu-Deicher heterophile antigen in human retinoblastoma cells. Am J Ophthalmol (1983) 96:321–5 [DOI] [PubMed] [Google Scholar]
- 19.Higashi H, Fukui Y, Ueda S, Kato S, Hirabayashi Y, Matsumoto M, et al. Sensitive enzyme-immunostaining and densitometric determination on thin-layer chromatography of N-glycolylneuraminic acid-containing glycosphingolipids, Hanganutziu-Deicher antigens. J Biochem (1984) 95:1517–20 [DOI] [PubMed] [Google Scholar]
- 20.Higashi H, Nishi Y, Fukui Y, Ikuta K, Ueda S, Kato S, et al. Tumor-associated expression of glycosphingolipid Hanganutziu-Deicher antigen in human cancers. Gann (1984) 75:1025–9 [PubMed] [Google Scholar]
- 21.Mukuria JC, Naiki M, Hashimoto M, Nishiura K, Okabe M, Kato S. A potential radioimmunoassay system for detection of Hanganutziu-Deicher type heterophile antigen(s) and antibodies in tissues and fluids. J Immunol Methods (1985) 80:97–106 10.1016/0022-1759(85)90168-1 [DOI] [PubMed] [Google Scholar]
- 22.Higashi H, Hirabayashi Y, Fukui Y, Naiki M, Matsumoto M, Ueda S, et al. Characterization of N-glycolylneuraminic acid-containing gangliosides as tumor-associated Hanganutziu-Deicher antigen in human colon cancer. Cancer Res (1985) 45:3796–802 [PubMed] [Google Scholar]
- 23.Nowak JA, Jain NK, Stinson MW, Merrick JM. Interaction of bovine erythrocyte N-glycolylneuraminic acid-containing gangliosides and glycoproteins with a human Hanganutziu-Deicher serum. Mol Immunol (1986) 23:693–700 10.1016/0161-5890(86)90079-9 [DOI] [PubMed] [Google Scholar]
- 24.Mukuria JC, Naiki M, Kato S. Microstructure of the sialic acid moiety of N-glycolylneuraminyllactosylceramide and the elucidation of Hanganutziu and Deicher (HD) antigenicity. Immunol Lett (1986) 12:165–9 [DOI] [PubMed] [Google Scholar]
- 25.Hirabayashi Y, Higashi H, Kato S, Taniguchi M, Matsumoto M. Occurrence of tumor-associated ganglioside antigens with Hanganutziu-Deicher antigenic activity on human melanomas. Jpn J Cancer Res (1987) 78:614–20 [PubMed] [Google Scholar]
- 26.Hirabayashi Y, Kasakura H, Matsumoto M, Higashi H, Kato S, Kasai N, et al. Specific expression of unusual GM2 ganglioside with Hanganutziu-Deicher antigen activity on human colon cancers. Jpn J Cancer Res (1987) 78:251–60 [PubMed] [Google Scholar]
- 27.Higashi H, Sasabe T, Fukui Y, Maru M, Kato S. Detection of gangliosides as N-glycolylneuraminic acid-specific tumor-associated Hanganutziu-Deicher antigen in human retinoblastoma cells. Jpn J Cancer Res (1988) 79:952–6 10.1111/j.1349-7006.1988.tb00060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawachi S, Saida T, Uhara H, Uemura K, Taketomi T, Kano K. Heterophile Hanganutziu-Deicher antigen in ganglioside fractions of human melanoma tissues. Int Arch Allergy Appl Immunol (1988) 85:381–3 10.1159/000234536 [DOI] [PubMed] [Google Scholar]
- 29.Fukui Y, Maru M, Ohkawara K, Miyake T, Osada Y, Wang DQ, et al. Detection of glycoproteins as tumor-associated Hanganutziu-Deicher antigen in human gastric cancer cell line, NUGC4. Biochem Biophys Res Commun (1989) 160:1149–54 10.1016/S0006-291X(89)80123-8 [DOI] [PubMed] [Google Scholar]
- 30.Nakarai H, Chandler PJ, Kano K, Morton DL, Irie RF. Hanganutziu-Deicher antigen as a possible target for immunotherapy of melanoma. Int Arch Allergy Appl Immunol (1990) 91:323–8 10.1159/000235135 [DOI] [PubMed] [Google Scholar]
- 31.Saida T, Ikegawa S, Takizawa Y, Kawachi S. Immunohistochemical detection of heterophile Hanganutziu-Deicher antigen in human malignant melanoma. Arch Dermatol Res (1990) 282:179–82 10.1007/BF00372619 [DOI] [PubMed] [Google Scholar]
- 32.Kawachi S, Saida T. Analysis of the expression of Hanganutziu-Deicher (HD) antigen in human malignant melanoma. J Dermatol (1992) 19:827–30 [DOI] [PubMed] [Google Scholar]
- 33.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol (2009) 19:507–14 10.1016/j.sbi.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature (2007) 446:1023–9 10.1038/nature05816 [DOI] [PubMed] [Google Scholar]
- 35.Varki A, Schauer R. Sialic acids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; (2009). p. 199–218 [Google Scholar]
- 36.Chen X, Varki A. Advances in the biology and chemistry of sialic acids. ACS Chem Biol (2010) 5:163–76 10.1021/cb900266r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schauer R, Schoop HJ, Faillard H. On biosynthesis of the glycolyl groups of N-glycolylneuraminic acid oxidative conversion of N-acetyl groups to glycolyl groups. Hoppe Seylers Z Physiol Chem (1968) 349:645–52 10.1515/bchm2.1968.349.1.645 [DOI] [PubMed] [Google Scholar]
- 38.Buscher HP, Casals SJ, Schauer R, Mestres VP. Biosynthesis of N-glycolylneuraminic acid in porcine submandibular glands. Subcellular site of hydroxylation of N-acetylneuraminic acid in the course of glycoprotein biosynthesis. Eur J Biochem (1977) 77:297–310 10.1111/j.1432-1033.1977.tb11668.x [DOI] [PubMed] [Google Scholar]
- 39.Shaw L, Schauer R. The biosynthesis of N-glycoloylneuraminic acid occurs by hydroxylation of the CMP-glycoside of N-acetylneuraminic acid. Biol Chem Hoppe Seyler (1988) 369:477–86 10.1515/bchm3.1988.369.1.477 [DOI] [PubMed] [Google Scholar]
- 40.Muchmore EA, Milewski M, Varki A, Diaz S. Biosynthesis of N-glycoly neuraminic acid. The primary site of hydroxylation of N-acetylneuraminic acid is the cytosolic sugar nucleotide pool. J Biol Chem (1989) 264:20216–23 [PubMed] [Google Scholar]
- 41.Kozutsumi Y, Kawano T, Kawasaki H, Suzuki K, Yamakawa T, Suzuki A. Reconstitution of CMP-N-acetylneuraminic acid hydroxylation activity using a mouse liver cytosol fraction and soluble cytochrome b5 purified from horse erythrocytes. J Biochem (1991) 110:429–35 [DOI] [PubMed] [Google Scholar]
- 42.Shaw L, Schneckenburger P, Carlsen J, Christiansen K, Schauer R. Mouse liver cytidine-5’-monophosphate-N-acetylneuraminic acid hydroxylase – catalytic function and regulation. Eur J Biochem (1992) 206:269–77 10.1111/j.1432-1033.1992.tb16925.x [DOI] [PubMed] [Google Scholar]
- 43.Kawano T, Kozutsumi Y, Takematsu H, Kawasaki T, Suzuki A. Regulation of biosynthesis of N-glycolylneuraminic acid-containing glycoconjugates: characterization of factors required for NADH-dependent cytidine 5’monophosphate-N-acetylneuraminic acid hydroxylation. Glycoconj J (1993) 10:109–15 10.1007/BF00731194 [DOI] [PubMed] [Google Scholar]
- 44.Kawano T, Koyama S, Takematsu H, Kozutsumi Y, Kawasaki H, Kawashima S, et al. Molecular cloning of cytidine monophospho-N-acetylneuraminic acid hydroxylase. Regulation of species- and tissue-specific expression of N-glycolylneuraminic acid. J Biol Chem (1995) 270:16458–63 10.1074/jbc.270.27.16458 [DOI] [PubMed] [Google Scholar]
- 45.Shaw L, Schauer R. Detection of CMP-N-acetylneuraminic acid hydroxylase activity in fractionated mouse liver. Biochem J (1989) 263:355–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A (1998) 95:11751–6 10.1073/pnas.95.20.11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem (1998) 273:15866–71 10.1074/jbc.273.25.15866 [DOI] [PubMed] [Google Scholar]
- 48.Hayakawa T, Satta Y, Gagneux P, Varki A, Takahata N. Alu-mediated inactivation of the human CMP-N-acetylneuraminic acid hydroxylase gene. Proc Natl Acad Sci U S A (2001) 98:11399–404 10.1073/pnas.191268198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varki A, Gagneux P. Human-specific evolution of sialic acid targets: explaining the malignant malaria mystery? Proc Natl Acad Sci U S A (2009) 106:14739–40 10.1073/pnas.0908196106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghaderi D, Springer SA, Ma F, Cohen M, Secrest P, Taylor RE, et al. Sexual selection by female immunity against paternal antigens can fix loss of function alleles. Proc Natl Acad Sci U S A (2011) 108:17743–8 10.1073/pnas.1102302108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci U S A (2010) 107(Suppl 2):8939–46 10.1073/pnas.0914634107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Mitra N, Cruz P, Deng L, NISC Comparative Sequencing Program. Varki N, et al. Evolution of siglec-11 and siglec-16 genes in hominins. Mol Biol Evol (2012) 29(8):2073–86 10.1093/molbev/mss077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schauer R, Srinivasan GV, Coddeville B, Zanetta JP, Guerardel Y. Low incidence of N-glycolylneuraminic acid in birds and reptiles and its absence in the platypus. Carbohydr Res (2009) 344:1494–500 10.1016/j.carres.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 54.Fujii Y, Higashi H, Ikuta K, Kato S, Naiki M. Specificities of human heterophilic Hanganutziu and Deicher (H-D) antibodies and avian antisera against H-D antigen-active glycosphingolipids. Mol Immunol (1982) 19:87–94 [DOI] [PubMed] [Google Scholar]
- 55.Miyoshi I, Fujii Y, Naiki M. Avian antisera to various gangliosides: detection by enzyme immunoassay. J Biochem (1982) 92:89–94 [DOI] [PubMed] [Google Scholar]
- 56.Naiki M, Fujii Y, Ikuta K, Higashi H, Kato S. Expression of Hanganutziu and Deicher type heterophile antigen on the cell surface of Marek’s disease lymphoma. Adv Exp Med Biol (1982) 152:445–56 [PubMed] [Google Scholar]
- 57.Higashi H, Ikuta K, Ueda S, Kato S, Hirabayashi Y, Matsumoto M, et al. Characterization of N-glycolyneuraminic acid-containing glycosphingolipids from a Marek’s disease lymphoma-derived chicken cell line, MSB1, as tumor-associated heterophile Hanganutziu-Deicher antigens. J Biochem (1984) 95:785–94 [DOI] [PubMed] [Google Scholar]
- 58.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A (2003) 100:12045–50 10.1073/pnas.2131556100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci U S A (2008) 105:18936–41 10.1073/pnas.0803943105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Pan L, Liu N, Troy FA, Wang B. LC-MS/MS quantification of N-acetylneuraminic acid, N-glycolylneuraminic acid and ketodeoxynonulosonic acid levels in the urine and potential relationship with dietary sialic acid intake and disease in 3- to 5-year-old children. Br J Nutr (2014) 111(2):332–41 10.1017/S0007114513002468 [DOI] [PubMed] [Google Scholar]
- 61.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem (2005) 280:4228–37 10.1074/jbc.M412040200 [DOI] [PubMed] [Google Scholar]
- 62.Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol (2005) 175:228–36 [DOI] [PubMed] [Google Scholar]
- 63.Nohle U, Beau JM, Schauer R. Uptake, metabolism and excretion of orally and intravenously administered, double-labeled N-glycoloylneuraminic acid and single-labeled 2-deoxy-2,3-dehydro-N-acetylneuraminic acid in mouse and rat. Eur J Biochem (1982) 126:543–8 10.1111/j.1432-1033.1982.tb06815.x [DOI] [PubMed] [Google Scholar]
- 64.Nohle U, Schauer R. Uptake, metabolism and excretion of orally and intravenously administered, 14C- and 3H-labeled N-acetylneuraminic acid mixture in the mouse and rat. Hoppe Seylers Z Physiol Chem (1981) 362:1495–506 10.1515/bchm2.1981.362.2.1495 [DOI] [PubMed] [Google Scholar]
- 65.Nohle U, Schauer R. Metabolism of sialic acids from exogenously administered sialyllactose and mucin in mouse and rat. Hoppe Seylers Z Physiol Chem (1984) 365:1457–67 10.1515/bchm2.1984.365.2.1457 [DOI] [PubMed] [Google Scholar]
- 66.Hedlund M, Tangvoranuntakul P, Takematsu H, Long JM, Housley GD, Kozutsumi Y, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol (2007) 27:4340–6 10.1128/MCB.00379-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banda K, Gregg CJ, Chow R, Varki NM, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: mechanisms underlying gastrointestinal incorporation of the non-human sialic acid xeno-autoantigen N-glycolylneuraminic acid. J Biol Chem (2012) 287:28852–64 10.1074/jbc.M112.364182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bergfeld AK, Pearce OM, Diaz SL, Pham T, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: elucidating the intracellular fate of the non-human sialic acid N-glycolylneuraminic acid. J Biol Chem (2012) 287:28865–81 10.1074/jbc.M112.363549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vamecq J, Poupaert JH. Studies on the metabolism of glycolyl-CoA. Biochem Cell Biol (1990) 68:846–51 10.1139/o90-125 [DOI] [PubMed] [Google Scholar]
- 70.Vamecq J, Mestdagh N, Henichart J-P, Poupaert J. Subcellular distribution of glycolyltransferases in rodent liver and their significance in special reference to the synthesis of N-glycolylneuraminic acid. J Biochem (1992) 111:579–83 [DOI] [PubMed] [Google Scholar]
- 71.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation (2002) 9:376–81 10.1034/j.1399-3089.2002.02138.x [DOI] [PubMed] [Google Scholar]
- 72.Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology (2008) 18:818–30 10.1093/glycob/cwn072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Padler-Karavani V, Hurtado-Ziola N, Pu M, Yu H, Huang S, Muthana S, et al. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res (2011) 71:3352–63 10.1158/0008-5472.CAN-10-4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asaoka H, Nishinaka S, Wakamiya N, Matsuda H, Murata M. Two chicken monoclonal antibodies specific for heterophil Hanganutziu-Deicher antigens. Immunol Lett (1992) 32:91–6 [DOI] [PubMed] [Google Scholar]
- 75.Malykh YN, Schauer R, Shaw L. N-glycolylneuraminic acid in human tumours. Biochimie (2001) 83:623–34 10.1016/S0300-9084(01)01303-7 [DOI] [PubMed] [Google Scholar]
- 76.Iznaga N, Carr A, Fernández LE, Solozabal J, Núñez G, Perdomo Y, et al. Amplified ELISA to detect autoantibodies to N-glycolyl-GM3 ganglioside. J Clin Lab Immunol (1996) 48:75–85 [PubMed] [Google Scholar]
- 77.Padlan EA, Kabat EA. Model-building study of the combining sites of two antibodies to alpha(1–6)dextran. Proc Natl Acad Sci U S A (1988) 85:6885–9 10.1073/pnas.85.18.6885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang WW, Yu CY, Lin TW, Wang PH, Tsai YC. Soyasaponin I decreases the expression of alpha2,3-linked sialic acid on the cell surface and suppresses the metastatic potential of B16F10 melanoma cells. Biochem Biophys Res Commun (2006) 341:614–9 10.1016/j.bbrc.2005.12.216 [DOI] [PubMed] [Google Scholar]
- 79.Yu H, Chokhawala HA, Varki A, Chen X. Efficient chemoenzymatic synthesis of biotinylated human serum albumin-sialoglycoside conjugates containing O-acetylated sialic acids. Org Biomol Chem (2007) 5:2458–63 10.1039/b706507h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Usuba O, Fujii Y, Miyoshi I, Naiki M, Sendo F. Establishment of a human monoclonal antibody to Hanganutziu-Deicher antigen as a tumor-associated carbohydrate antigen. Jpn J Cancer Res (1988) 79:1340–8 10.1111/j.1349-7006.1988.tb01565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanai Y, Yamasaki M, Nagai Y. Monoclonal antibody directed to a Hanganutziu-Deicher active ganglioside, GM2 (NeuGc). Biochim Biophys Acta (1988) 958:368–74 10.1016/0005-2760(88)90222-6 [DOI] [PubMed] [Google Scholar]
- 82.Asaoka H, Matsuda H. Detection of N-glycolylneuraminic acid-containing glycoproteins from various animal erythrocytes by chicken monoclonal antibody against Hanganutziu-Deicher antigens. J Vet Med Sci (1994) 56:375–7 10.1292/jvms.56.375 [DOI] [PubMed] [Google Scholar]
- 83.Koda T, Shimosakoda T, Nishinaka S, Asaoka H, Nakaba H, Tamura I, et al. Diagnosis of hepatocellular carcinoma using chicken anti-monoclonal antibody – as tumor marker on the heterophilic Hanganutziu-Deicher antigen. Gan To Kagaku Ryoho (1994) 21:2771–7 [PubMed] [Google Scholar]
- 84.Watarai S, Kushi Y, Shigeto R, Misawa N, Eishi Y, Handa S, et al. Production of monoclonal antibodies directed to Hanganutziu-Deicher active gangliosides, N-glycolylneuraminic acid-containing gangliosides. J Biochem (1995) 117:1062–9 [DOI] [PubMed] [Google Scholar]
- 85.Pham T, Gregg CJ, Karp F, Chow R, Padler-Karavani V, Cao H, et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood (2009) 114:5225–35 10.1182/blood-2009-05-220400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med (2010) 207:1637–46 10.1084/jem.20100575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr (2011) 94:1088–96 10.3945/ajcn.111.018978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation (2010) 121:2271–83 10.1161/CIRCULATIONAHA.109.924977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med (2012) 172:555–63 10.1001/archinternmed.2011.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.World Cancer Research Fund International Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; (2007). [Google Scholar]
- 91.Gonzalez CA, Riboli E. Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Eur J Cancer (2010) 46:2555–62 10.1016/j.ejca.2010.07.025 [DOI] [PubMed] [Google Scholar]
- 92.Arem H, Gunter MJ, Cross AJ, Hollenbeck AR, Sinha R. A prospective investigation of fish, meat and cooking-related carcinogens with endometrial cancer incidence. Br J Cancer (2013) 109:756–60 10.1038/bjc.2013.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salehi M, Moradi-Lakeh M, Salehi MH, Nojomi M, Kolahdooz F. Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev (2013) 71:257–67 10.1111/nure.12028 [DOI] [PubMed] [Google Scholar]
- 94.Zhu H, Yang X, Zhang C, Zhu C, Tao G, Zhao L, et al. Red and processed meat intake is associated with higher gastric cancer risk: a meta-analysis of epidemiological observational studies. PLoS One (2013) 8:e70955. 10.1371/journal.pone.0070955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hori S, Butler E, McLoughlin J. Prostate cancer and diet: food for thought? BJU Int (2011) 107:1348–59 10.1111/j.1464-410X.2010.09897.x [DOI] [PubMed] [Google Scholar]
- 96.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res (1994) 54:2390–7 [PubMed] [Google Scholar]
- 97.Stavric B. Biological significance of trace levels of mutagenic heterocyclic aromatic amines in human diet: a critical review. Food Chem Toxicol (1994) 32:977–94 10.1016/0278-6915(94)90093-0 [DOI] [PubMed] [Google Scholar]
- 98.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer (2008) 60:131–44 10.1080/01635580701684872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila) (2011) 4:177–84 10.1158/1940-6207.CAPR-10-0113 [DOI] [PubMed] [Google Scholar]
- 100.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med (2013) 19:576–85 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med (2013) 368:1575–84 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr (2004) 80:1029–35 [DOI] [PubMed] [Google Scholar]
- 103.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet (2008) 371:771–83 10.1016/S0140-6736(08)60241-X [DOI] [PubMed] [Google Scholar]
- 104.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (2011) 331:1565–70 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 105.Boon T, Cerottini J-C, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol (1994) 12:337–65 [DOI] [PubMed] [Google Scholar]
- 106.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature (2011) 480:480–9 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol (2003) 21:807–39 [DOI] [PubMed] [Google Scholar]
- 108.Finn OJ. Cancer immunology. N Engl J Med (2008) 358:2704–15 10.1056/NEJMra072739 [DOI] [PubMed] [Google Scholar]
- 109.Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420:860–7 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell (2012) 21:309–22 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 111.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol (2009) 9:162–74 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med (2013) 369(2):122–33 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol (2012) 12:269–81 10.1038/nri3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A (2004) 101(Suppl 2):14639–45 10.1073/pnas.0405730101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science (2013) 339:286–91 10.1126/science.1232227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (2001) 357:539–45 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 117.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140:883–99 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang D, DuBois RN. Inflammatory mediators and nuclear receptor signaling in colorectal cancer. Cell Cycle (2007) 6:682–5 10.4161/cc.6.6.4030 [DOI] [PubMed] [Google Scholar]
- 119.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet (2011) 377:31–41 10.1016/S0140-6736(10)62110-1 [DOI] [PubMed] [Google Scholar]
- 120.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer (2001) 1:11–21 10.1038/35094017 [DOI] [PubMed] [Google Scholar]
- 121.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet (2009) 373:1301–9 10.1016/S0140-6736(09)60243-9 [DOI] [PubMed] [Google Scholar]
- 122.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454:436–44 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 123.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer (2009) 9:239–52 10.1038/nrc2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer (2009) 9:285–93 10.1038/nrc2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell (2013) 23:277–86 10.1016/j.ccr.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 126.Varki A, Kannagi R, Toole BP. Glycosylation changes in cancer. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; (2009). p. 617–32 [PubMed] [Google Scholar]
- 127.Yin J, Hashimoto A, Izawa M, Miyazaki K, Chen GY, Takematsu H, et al. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res (2006) 66:2937–45 10.1158/0008-5472.CAN-05-2615 [DOI] [PubMed] [Google Scholar]
- 128.Padler-Karavani V, Song X, Yu H, Hurtado-Ziola N, Huang S, Muthana S, et al. Cross-comparison of protein recognition of sialic acid diversity on two novel sialoglycan microarrays. J Biol Chem (2012) 287:22593–608 10.1074/jbc.M112.359323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA (2007) 298:754–64 10.1001/jama.298.7.754 [DOI] [PubMed] [Google Scholar]
- 130.Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity (2012) 36:322–35 10.1016/j.immuni.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 131.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer (2005) 5:526–42 10.1038/nrc1649 [DOI] [PubMed] [Google Scholar]
- 132.Yonezawa S, Tachikawa T, Shin S, Sato E. Sialosyl-Tn antigen. Its distribution in normal human tissues and expression in adenocarcinomas. Am J Clin Pathol (1992) 98:167–74 [DOI] [PubMed] [Google Scholar]
- 133.Ogata S, Koganty R, Reddish M, Longenecker BM, Chen A, Perez C, et al. Different modes of sialyl-Tn expression during malignant transformation of human colonic mucosa. Glycoconj J (1998) 15:29–35 10.1023/A:1006935331756 [DOI] [PubMed] [Google Scholar]
- 134.Kobayashi H, Terao T, Kawashima Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J Clin Oncol (1992) 10:95–101 [DOI] [PubMed] [Google Scholar]
- 135.Imai J, Ghazizadeh M, Naito Z, Asano G. Immunohistochemical expression of T, Tn and sialyl-Tn antigens and clinical outcome in human breast carcinoma. Anticancer Res (2001) 21:1327–34 [PubMed] [Google Scholar]
- 136.Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology (2002) 123:1052–60 10.1053/gast.2002.36018 [DOI] [PubMed] [Google Scholar]
- 137.Conze T, Carvalho AS, Landegren U, Almeida R, Reis CA, David L, et al. MUC2 mucin is a major carrier of the cancer-associated sialyl-Tn antigen in intestinal metaplasia and gastric carcinomas. Glycobiology (2010) 20:199–206 10.1093/glycob/cwp161 [DOI] [PubMed] [Google Scholar]
- 138.Cao Y, Stosiek P, Springer GF, Karsten U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem Cell Biol (1996) 106:197–207 10.1007/BF02484401 [DOI] [PubMed] [Google Scholar]
- 139.Marquina G, Waki H, Fernandez LE, Kon K, Carr A, Valiente O, et al. Gangliosides expressed in human breast cancer. Cancer Res (1996) 56:5165–71 [PubMed] [Google Scholar]
- 140.Carr A, Mullet A, Mazorra Z, Vázquez AM, Alfonso M, Mesa C, et al. A mouse IgG1 monoclonal antibody specific for N-glycolyl GM3 ganglioside recognized breast and melanoma tumors. Hybridoma (2000) 19:241–7 10.1089/02724570050109639 [DOI] [PubMed] [Google Scholar]
- 141.Carr A, Rodríguez E, Arango Mdel C, Camacho R, Osorio M, Gabri M, et al. Immunotherapy of advanced breast cancer with a heterophilic ganglioside (NeuGcGM3) cancer vaccine. J Clin Oncol (2003) 21:1015–21 10.1200/JCO.2003.02.124 [DOI] [PubMed] [Google Scholar]
- 142.Fernández-Marrero Y, Hernández T, Roque-Navarro L, Talavera A, Moreno E, Griñán T, et al. Switching on cytotoxicity by a single mutation at the heavy chain variable region of an anti-ganglioside antibody. Mol Immunol (2011) 48:1059–67 10.1016/j.molimm.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 143.Fernández-Marrero Y, Roque-Navarro L, Hernández T, Dorvignit D, Molina-Pérez M, González A, et al. A cytotoxic humanized anti-ganglioside antibody produced in a murine cell line defective of N-glycolylated-glycoconjugates. Immunobiology (2011) 216:1239–47 10.1016/j.imbio.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 144.Scursoni AM, Galluzzo L, Camarero S, Lopez J, Lubieniecki F, Sampor C, et al. Detection of N-glycolyl GM3 ganglioside in neuroectodermal tumors by immunohistochemistry: an attractive vaccine target for aggressive pediatric cancer. Clin Dev Immunol (2011) 2011:245181. 10.1155/2011/245181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scursoni AM, Galluzzo L, Camarero S, Pozzo N, Gabri MR, de Acosta CM, et al. Detection and characterization of N-glycolyated gangliosides in Wilms tumor by immunohistochemistry. Pediatr Dev Pathol (2010) 13:18–23 10.2350/08-10-0544.1 [DOI] [PubMed] [Google Scholar]
- 146.Blanco R, Rengifo E, Cedeno M, Rengifo CE, Alonso DF, Carr A. Immunoreactivity of the 14F7 Mab raised against N-Glycolyl GM3 ganglioside in epithelial malignant tumors from digestive system. ISRN Gastroenterol (2011) 2011:645641. 10.5402/2011/645641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Blanco R, Rengifo E, Rengifo CE, Cedeno M, Frometa M, Carr A. Immunohistochemical reactivity of the 14F7 monoclonal antibody raised against N-glycolyl GM3 ganglioside in some benign and malignant skin neoplasms. ISRN Dermatol (2011) 2011:848909. 10.5402/2011/848909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Blanco R, Rengifo CE, Cedeno M, Frometa M, Rengifo E, Carr A. Immunoreactivity of the 14F7 Mab (raised against N-Glycolyl GM3 ganglioside) as a positive prognostic factor in non-small-cell lung cancer. Patholog Res Int (2012) 2012:235418. 10.1155/2012/235418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.von Mensdorff-Pouilly S, Petrakou E, Kenemans P, van Uffelen K, Verstraeten AA, Snijdewint FG, et al. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and N-acetylgalactosamine (GalNAc) peptides. Int J Cancer (2000) 86:702–12 10.1002/(SICI)1097-0215(20000601)86:53.3.CO;2-T [DOI] [PubMed] [Google Scholar]
- 150.Huang ZH, Shi L, Ma JW, Sun ZY, Cai H, Chen YX, et al. A totally synthetic, self-assembling, adjuvant-free MUC1 glycopeptide vaccine for cancer therapy. J Am Chem Soc (2012) 134:8730–3 10.1021/ja211725s [DOI] [PubMed] [Google Scholar]
- 151.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science (2013) 341:1192–8 10.1126/science.1241145 [DOI] [PubMed] [Google Scholar]
- 152.Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev (2012) 28:147–75 10.5661/bger-28-147 [DOI] [PubMed] [Google Scholar]
- 153.Ghaderi D, Taylor RE, Padler-Karavani V, Diaz S, Varki A. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol (2010) 28:863–7 10.1038/nbt.1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Padler-Karavani V, Tremoulet AH, Yu H, Chen X, Burns JC, Varki A. A simple method for assessment of human anti-Neu5Gc antibodies applied to Kawasaki disease. PLoS One (2013) 8:e58443. 10.1371/journal.pone.0058443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weinberg RA. Supplement 11.7 How Does Diet Affect Colon Cancer Incidence? The Biology of Cancer. New York: Garland Publishing; (2014). [Google Scholar]
- 156.Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res (2006) 47:1339–51 10.1194/jlr.R600009-JLR200 [DOI] [PubMed] [Google Scholar]
- 157.Hirabayashi Y, Suzuki T, Suzuki Y, Taki T, Matsumoto M, Higashi H, et al. A new method for purification of anti-glycosphingolipid antibody. Avian anti-hematoside (NeuGc) antibody. J Biochem (1983) 94:327–30 [DOI] [PubMed] [Google Scholar]
- 158.Diaz SL, Padler-Karavani V, Ghaderi D, Hurtado-Ziola N, Yu H, Chen X, et al. Sensitive and specific detection of the non-human sialic acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS One (2009) 4:e4241. 10.1371/journal.pone.0004241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koda T, Aosasa M, Asaoka H, Nakaba H, Matsuda H. Application of tyramide signal amplification for detection of N-glycolylneuraminic acid in human hepatocellular carcinoma. Int J Clin Oncol (2003) 8:317–21 10.1007/s10147-003-0346-4 [DOI] [PubMed] [Google Scholar]
- 160.Miyake M, Ito M, Hitomi S, Ikeda S, Taki T, Kurata M, et al. Generation of two murine monoclonal antibodies that can discriminate N-glycolyl and N-acetyl neuraminic acid residues of GM2 gangliosides. Cancer Res (1988) 48:6154–60 [PubMed] [Google Scholar]
- 161.Ozawa H, Kawashima I, Tai T. Generation of murine monoclonal antibodies specific for N-glycolylneuraminic acid-containing gangliosides. Arch Biochem Biophys (1992) 294:427–33 10.1016/0003-9861(92)90707-4 [DOI] [PubMed] [Google Scholar]
- 162.Kawashima I, Ozawa H, Kotani M, Suzuki M, Kawano T, Gomibuchi M, et al. Characterization of ganglioside expression in human melanoma cells: immunological and biochemical analysis. J Biochem (1993) 114:186–93 [DOI] [PubMed] [Google Scholar]
- 163.Nakamura K, Suzuki H, Hirabayashi Y, Suzuki A. IV3_(NeuGcalpha2-8NeuGc)-Gg4Cer is restricted to CD4+ T cells producing interleukin-2 and a small population of mature thymocytes in mice. J Biol Chem (1995) 270:3876–81 10.1074/jbc.270.8.3876 [DOI] [PubMed] [Google Scholar]
- 164.Manzi AE, Diaz S, Varki A. High-pressure liquid chromatography of sialic acids on a pellicular resin anion-exchange column with pulsed amperometric detection: a comparison with six other systems. Anal Biochem (1990) 188:20–32 10.1016/0003-2697(90)90523-C [DOI] [PubMed] [Google Scholar]
- 165.Rohrer JS. Analyzing sialic acids using high-performance anion-exchange chromatography with pulsed amperometric detection. Anal Biochem (2000) 283:3–9 10.1006/abio.2000.4643 [DOI] [PubMed] [Google Scholar]
- 166.Hurum DC, Rohrer JS. Five-minute glycoprotein sialic acid determination by high-performance anion exchange chromatography with pulsed amperometric detection. Anal Biochem (2011) 419:67–9 10.1016/j.ab.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 167.van der Ham M, Prinsen BH, Huijmans JG, Abeling NG, Dorland B, Berger R, et al. Quantification of free and total sialic acid excretion by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci (2007) 848(2):251–7 10.1016/j.jchromb.2006.10.066 [DOI] [PubMed] [Google Scholar]
- 168.Allevi P, Femia EA, Costa ML, Cazzola R, Anastasia M. Quantification of N-acetyl- and N-glycolylneuraminic acids by a stable isotope dilution assay using high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A (2008) 1212:98–105 10.1016/j.chroma.2008.10.039 [DOI] [PubMed] [Google Scholar]
- 169.Hara S, Yamaguchi M, Takemori Y, Furuhata K, Ogura H, Nakamura M. Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal Biochem (1989) 179:162–6 10.1016/0003-2697(89)90218-2 [DOI] [PubMed] [Google Scholar]
- 170.Lacomba R, Salcedo J, Alegria A, Jesus Lagarda M, Barbera R, Matencio E. Determination of sialic acid and gangliosides in biological samples and dairy products: a review. J Pharm Biomed Anal (2010) 51:346–57 10.1016/j.jpba.2009.04.023 [DOI] [PubMed] [Google Scholar]
- 171.Gong S, Ren HL, Tian RY, Lin C, Hu P, Li YS, et al. A novel analytical probe binding to a potential carcinogenic factor of N-glycolylneuraminic acid by SELEX. Biosens Bioelectron (2013) 49:547–54 10.1016/j.bios.2013.05.024 [DOI] [PubMed] [Google Scholar]