Abstract
Background:
CXCR4 is a cognitive receptor for stromal-derived factor-1 (SDF-1) and has been previously shown to be associated with tumor growth and invasion of many cancers. However, its expression and function in gastric cancer has not been well clarified.
Materials and Methods:
Herein, we studied the expression of CXCR4 on gastric samples from patients with gastric adenocarcinoma in comparison with precancerous lesions by employing qRT-PCR.
Results:
Our qRT-PCR data show that CXCR4 is highly expressed in tissue samples from patients with gastric cancer than precancerous lesions (2.4 times higher, P value < 0.05). When we correlated the level of CXCR4 with clinicopathological findings, we observed that CXCR4 level is associated with staging of the disease and lymphatic invasion.
In conclusion:
We present evidence that CXCR4 level is significantly elevated in later stages of gastric cancer. Thus, CXCR4 may play a crucial role in gastric cancer progression.
Keywords: CXCR4, gastric cancer, SDF-1
INTRODUCTION
Gastric cancer is still the second cause of cancer-related death and is considered as a major public health problem all over the world.[1] Due to its late diagnosis, identification of new diagnostic biomarkers and treatment approaches are necessary.[2]
Accumulating evidence indicates that migration and metastasis of primary tumors to distant organs and tissues is somehow similar to trafficking of leukocytes which is governed by chemokines and their receptors, growth factors, adhesion molecules, and matrix metalloproteinases.[3] It is well known that chemokines and their receptors are key players in metastasis of cancerous cells from primary site to the distant organs.[4,5] Stromal-derived factor-1 (SDF-1) through binding to its receptor, CXCR4, plays a crucial role in retention of hematopoietic stem cells (HSC) within the bone marrow.[6] Evidence from many laboratories indicates that CXCR4 is highly expressed in most of the leukocytes and HSC, whereas its expression is low or absent in most of the normal solid tissues. Interestingly, mounting evidence has demonstrated that CXCR4 is overexpressed in more over 23 human cancers including breast, ovarian, prostate, and gastric cancers.[4]
The importance of CXCR4 function in cancer is clarified by the fact that, in contrast to benign tumors, malignant tumors express a high level of CXCR4 which is correlated with metastasis to distant organs and reduced overall survival.[7,8] Consistently, gastric cancers are also shown to express a high amount of CXCR4, which is associated with tumor behavior such as deep invasion to lymph nodes, liver metastasis, and poor differentiation.[9,10] Moreover, overexpression of CXCR4 in gastric cancer has been shown to be correlated with development of malignant ascites and peritoneal carcinomatosis.[10] However, other reports have shown that CXCR4 expression was not associated with lymphatic invasion[11] and peritoneal metastasis,[12] implying that the role of CXCR4 in gastric cancer is not well-defined. In the current study, we examined the expression of CXCR4 in primary gastric cancer by employing qRT-PCR and found that CXCR4 expression is significantly higher in patients with advanced stages of the disease.
MATERIAL AND METHODS
Patients
All specimens were histologically evaluated by conventional hematoxylin and eosin staining and intestinal type adenocarcinoma diagnosis was confirmed for all of them. Gastric tissue samples from 23 patients with gastric cancer who had undergone gastrectomy at Cancer Institute, Iran National Tumor Bank (http://www.irantumorbank.com), Tehran, Iran were taken for the study. TNM stage, lymphatic invasion, and perineural invasion status of each patient were defined during clinical procedures and according to AJCC and UICC criteria. Precancerous lesions were taken from 15 patients who had undergone diagnostic endoscopic examinations at division of gastroenterology, Tohid hospital, Sanandaj, Iran.
Real-Time quantitative Reverse Transcription-PCR
Total RNA was isolated using RNA extraction kit (Bioflux, Basel, Switzerland) and mRNA was transcribed into cDNA by the use of Bioneer RT kit (Bioneer, Daejeon, South Korea). Real-Time quantitative Reverse Transcription-PCR (Real-Time qRT-PCR) was performed by Corbett rotor gene 6000 Real-Time PCR system (Corbett Research, Australia) and carried out using SYBR Green dye detection protocol. Relative quantitation of CXCR4 mRNA expression was calculated using the comparative CT method.[13] The relative quantitative value of mRNA expression for CXCR4 was normalized to endogenous housekeeping gene, β-actin, and calculated relative expression values. Primer sequences for qRT-PCR are listed in Table 1. Data are presented as mean value ± SD.
Table 1.
Primer sequences for qRT-PCR

Statistical analysis
The SPSS package (SPSS, Inc, Chicago, IL) was employed for statistical analysis. One-way Analysis of Variance was used for comparison of CXCR4 expression within clinicopathological features. Due to lack of normality of the CXCR4 expression, log transformation was employed. Given the assumption of equal variances in clinicopathological features is well established (from Levene's test), Tukey method was used for multiple comparisons. Finally, we also used a Mean ± SD, Boxplot, and Error Bar to represent the results. P values < 0.05 considered significant.
RESULTS
CXCR4 expression in precancerous and cancer samples
As the gastric cancer is a multistep process staring from precancerous lesions, we first examined the levels of CXCR4 expression in tumor samples and precancerous lesions by employing qRT-PCR and found that CXCR4 is significantly (P = 0.024) higher in tumors than precancerous lesion [Figure 1].
Figure 1.
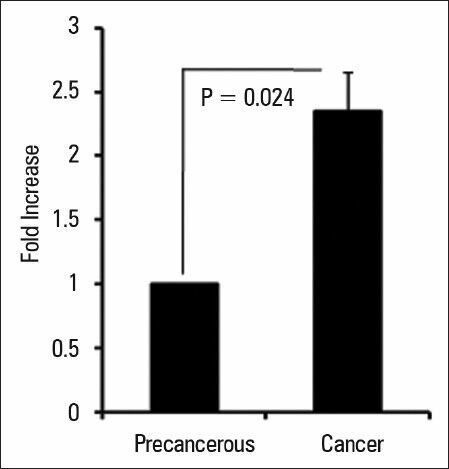
CXCR4 expression in gastric cancers and precancerous lesions. The expression of CXCR4 was analyzed by qRT-PCR in 23 patients with gastric cancers and 15 precancerous lesions. CXCR4 expression was determined by qRT-PCR and normalized to endogenous β-actin. Data are expressed as mean ± SD; P < 0.05
CXCR4 expression in different stages of gastric cancer
Next, we evaluated the level of CXCR4 in patients and observed that CXCR4 expression is significantly higher in tumor samples from patients with stage IV in comparison to the early stages of I, II, and III (P = 0.001) [Figure 2]. Our data clearly show that CXCR4 expression in patients (n = 7) with stage IV is 5 times higher than those patients (n = 7) with stages I and II (7.72 ± 6.31 vs 1.51 ± 0.56, P = 0.001). Accordingly, patients with stage IV showed a significant higher expression of CXCR4 compared to patients (n = 9) with stage III (7.72 ± 6.31 vs 3.42 ± 3.47, P = 0.045). Overall, our data indicate that CXCR4 expression is elevated in latter stages of gastric cancer.
Figure 2.
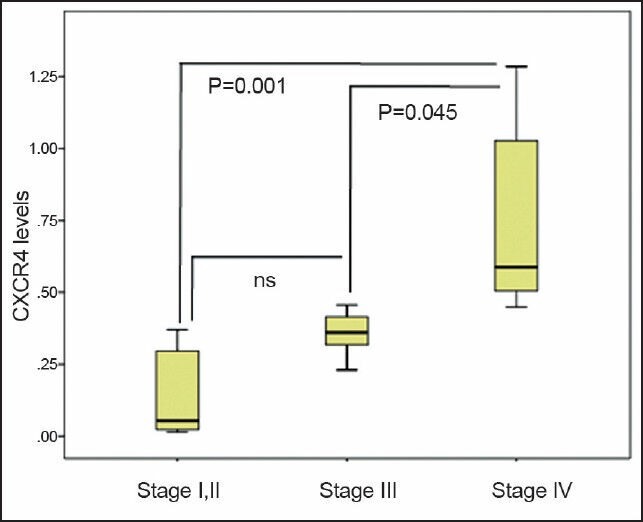
CXCR4 expression is higher in later stages of gastric patients. CXCR4 mRNA levels were detected by qRT-PCR in primary gastric cancers and its levels were correlated with the clinical staging of gastric cancer. CXCR4 expression was normalized to endogenous β-actin and its relative expression was determined by comparative CT method as described in materials and methods. Data are expressed as mean ± SD; P < 0.05; ns: non-significant
CXCR4 expression and lymph node metastasis
Furthermore, we observed that CXCR4 is tended to be higher in patient with lymph node invasion (n = 11) in comparison with patients without lymph node invasion (n = 12) [Figure 3]. However, other parameters such as age, gender, tumor size, and location of lesions had no relationship with the CXCR4 expression at mRNA level. Collectively, our data indicate that CXCR4 expression at mRNA level is associated with clinical findings of gastric cancer.
Figure 3.
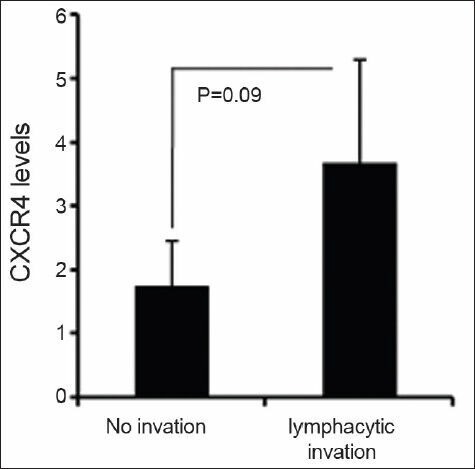
Expression of CXCR4 in primary gastric tumors with or without lymph node invasion. CXCR4 mRNA level was detected by qRT-PCR in primary gastric cancers and its expression was analyzed in patients with or without lymph node invasion. CXCR4 expression was normalized to endogenous β-actin and its relative expression was determined by comparative CT method as described in materials and methods. Data are expressed as mean ± SD
DISCUSSION
It is well known that CXCR4 expression is associated with more aggressive behavior of many tumors and CXCR4-expressing tumors may show an organ-specific migration toward the SDF-1-producing tissues/organs.[14] In the current study, we demonstrate that CXCR4 expression in significantly higher in tumor samples compared to precancerous lesions. In addition, CXCR4 expression levels are associated the later stage of gastric cancer.
H. pylori is the most important etiological factor associated with gastric cancer. The complex interplay between H. pylori and host immune system may lead to inducing a cascade of morphological events for example atrophy, intestinal metaplasia and dysplasia (known as precancerous lesion) and eventually genetic changes causing gastric cancer[15,16]. Herein, we demonstrate for the first time that the levels of CXCR4 expression is significantly increased than precancerous lesions implying that CXCR4 might play a role in cancer progression.
The clinical significance of CXCR4 expression in gastric cancer has not been clarified and is controversial. For example, Yasumoto K et al. have shown that CXCR4 expression in primary gastric tumors is associated with the development of peritoneal carcinomatosis. Interestingly, they have shown that the level of SDF-1 is significantly higher in malignant ascitic fluids from patients with gastric cancer than non-malignant peritoneal exudates, indicating that SDF-1/CXCR4 axis plays a crucial role in peritoneal carcinomatosis and could be a suitable therapeutic target for gastric cancer.[10] On the other hand, other studies have reported that there is no significant clinical implication of CXCR4 expression in gastric cancer.[11,12] However, in the current study, we have found that CXCR4 expression is significantly associated with staging of the disease. Importantly, we observed that expression of CXCR4 is significantly higher in stage III and IV compared to early stages I and II, indicating that CXCR4 could be used as a new factor for staging of gastric cancer. Further supporting of previous studies showing that CXCR4 expression is higher in metastatic gastric cancer to lymph nodes, we observed that high CXCR4 expression is tended to be associated with invasion of lymph node. We consider, however, that our study has involved a limited number of patients and more studies with larger number of gastric patients are required to determine correlation between CXCR4 expression and clinicopathological features of gastric cancer.
In conclusion, we demonstrated that CXCR4 appears to be involved in lymph node metastasis, and is significantly higher in advanced stages of gastric cancer, implying that CXCR4 could play a role in tumor progression in patients with gastric cancer.
List of abbreviations: Stromal-derived factor-1 (SDF-1), Hematopoietic stem cells (HSC), Real Time quantitative Reverse Transcription-PCR (Real-Time qRT-PCR).
ACKNOWLEDGMENT
All authors thank Kurdistan University of Medical Sciences for the financial supports.
Footnotes
Source of Support: This work was supported by a grant from Kurdistan University of Medical Sciences to AJ
Conflict of Interest: None declared.
REFERENCES
- 1.Correia M, Machado JC, Ristimaki A. Basic aspects of gastric cancer. Helicobacter. 2009;14(Suppl 1):36–40. doi: 10.1111/j.1523-5378.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- 2.Leja M, Wex T, Malfertheiner P. Markers for gastric cancer premalignant lesions: Where do we go? Dig Dis. 2012;30:268–76. doi: 10.1159/000336990. [DOI] [PubMed] [Google Scholar]
- 3.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 5.Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R, Tang XJ. Interleukin-8 associates with adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. World J Gastroenterol. 2012;18:979–85. doi: 10.3748/wjg.v18.i9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liekens S, Schols D, Hatse S. CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Curr Pharm Des. 2010;16:3903–20. doi: 10.2174/138161210794455003. [DOI] [PubMed] [Google Scholar]
- 7.Sekiya R, Kajiyama H, Sakai K, Umezu T, Mizuno M, Shibata K, et al. Expression of CXCR4 indicates poor prognosis in patients with clear cell carcinoma of the ovary. Hum Pathol. 2012;43:904–10. doi: 10.1016/j.humpath.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhao C, Lu X, Bu X, Zhang N, Wang W. Involvement of tumor necrosis factor-alpha in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC cancer. 2010;10:419. doi: 10.1186/1471-2407-10-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pituch-Noworolska A, Drabik G, Szatanek R, Bialas M, Kolodziejczyk P, Szczepanik A, et al. Immunophenotype of isolated tumour cells in the blood, bone marrow and lymph nodes of patients with gastric cancer. Pol J Pathol. 2007;58:93–7. [PubMed] [Google Scholar]
- 10.Yasumoto K, Koizumi K, Kawashima A, Saitoh Y, Arita Y, Shinohara K, et al. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006;66:2181–7. doi: 10.1158/0008-5472.CAN-05-3393. [DOI] [PubMed] [Google Scholar]
- 11.Kwak MK, Hur K, Park DJ, Lee HJ, Lee HS, Kim WH, et al. Expression of chemokine receptors in human gastric cancer. Tumour Biol. 2005;26:65–70. doi: 10.1159/000085587. [DOI] [PubMed] [Google Scholar]
- 12.Tsuboi K, Kodera Y, Nakanishi H, Ito S, Mochizuki Y, Nakayama G, et al. Expression of CXCL12 and CXCR4 in pT3-stage gastric cancer does not correlate with peritoneal metastasis. Oncol Rep. 2008;20:1117–23. [PubMed] [Google Scholar]
- 13.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 14.Burger JA, Kipps TJ. CXCR4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 15.Rugge M, Capelle LG, Cappellesso R, Nitti D, Kuipers EJ. Precancerous lesions in the stomach: from biology to clinical patient management. Best Pract Res Clin Gastroenterol. 2013;27:205–23. doi: 10.1016/j.bpg.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Kapadia CR. Gastric atrophy, metaplasia, and dysplasia: a clinical perspective. J Clin Gastroenterol. 2003;36(5 Suppl):S29–36. doi: 10.1097/00004836-200305001-00006. [DOI] [PubMed] [Google Scholar]


