Abstract
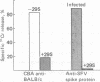
The proteins coded for by the HLA-A and HLA-B loci in man and the H-2K and H-2D loci in mice were identified as cell surface receptors for Semliki Forest virus. This conclusion is based on the following observations: (i) Water-soluble octamers of viral coat proteins inhibit the complement-dependent cytotoxicity of antibodies directed against H-2K and H-2D antigens in mouse cells. (ii) Isolated detergent-soluble HLA-A and HLA-B antigens reconstituted in lipid vesicles inhibit the binding of viral proteins to human cells (as do the water-soluble antigens to a lesser extent). (iii) Reconstituted HLA-A and HLA-B vesicles interact in solution with Semliki Forest virus (or with vesicles containing viral spike proteins), as demonstrated by coprecipitation with antisera. (iv) Complexes between viral spoke proteins and HLA-A and HLA-B antigens or H-2K and H-2D antigens can be isolated from the cell surface by utilizing affinity chromatography or immunoprecipitation.
Full text
PDF
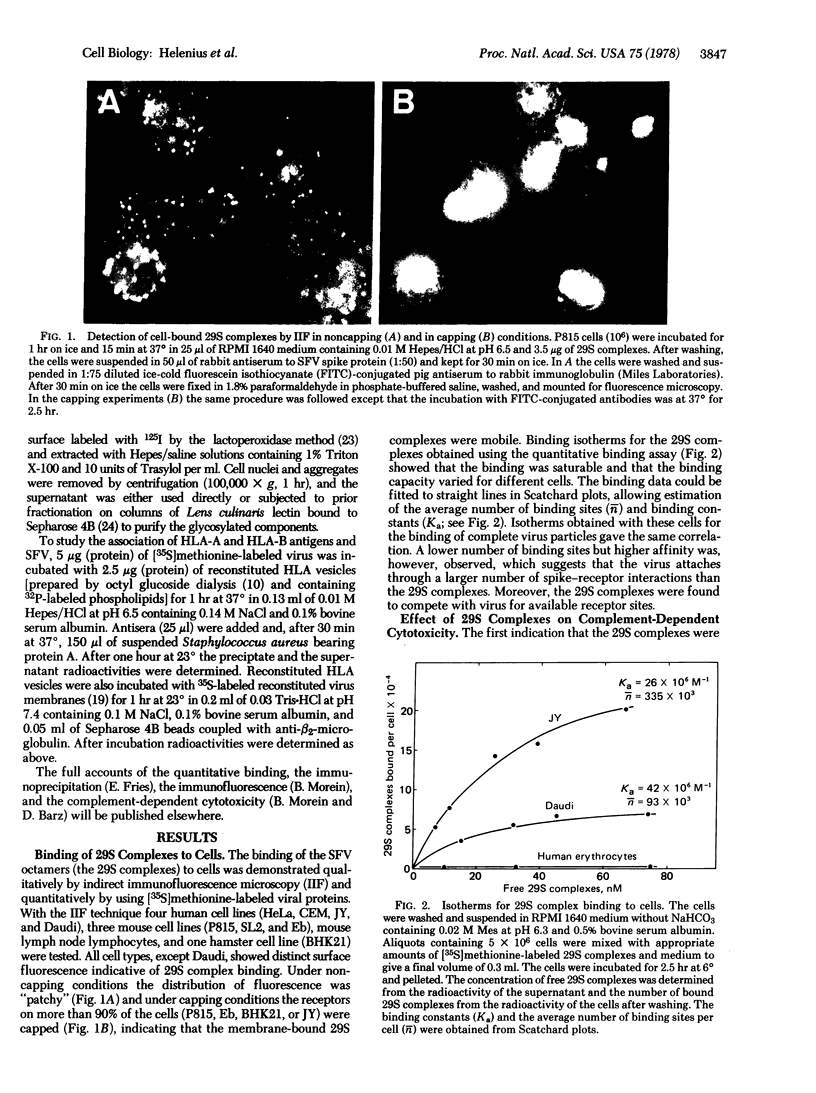
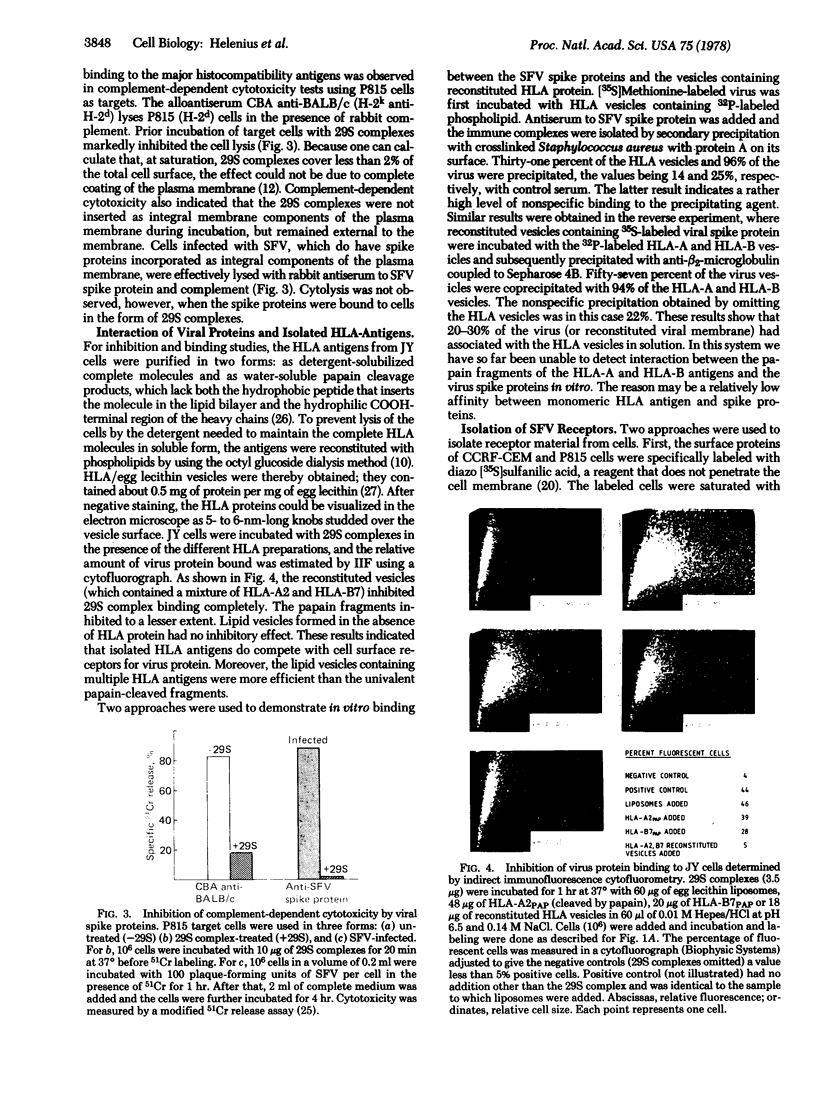
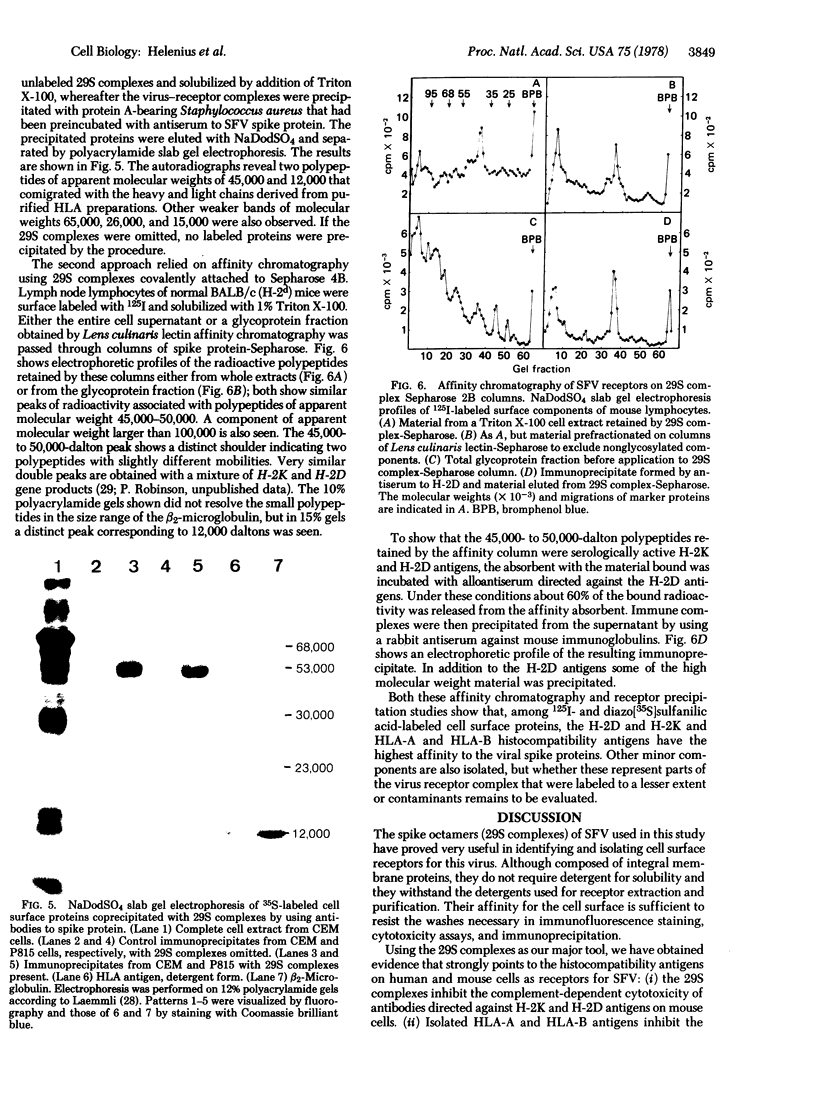

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Hirsh D. Synthesis of diazotized 35S sulfanilic acid of high specific activity: a label for the outer surface of cell membranes. Anal Biochem. 1975 Jun;66(2):629–631. doi: 10.1016/0003-2697(75)90630-2. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Strauss J. H. Distribution of the receptor sites for Sindbis virus on the surface of chicken and BHK cells. J Virol. 1974 Sep;14(3):672–678. doi: 10.1128/jvi.14.3.672-678.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard V. H., Guild B. C., Helenius A., Terhorst C., Strominger J. L. Reconstitution of purified detergent-soluble HLA-A and HLA-B antigens into phospholipid vesicles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3230–3234. doi: 10.1073/pnas.75.7.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- Haywood A. M. Characteristics of Sendai virus receptors in a model membrane. J Mol Biol. 1974 Mar 15;83(4):427–436. doi: 10.1016/0022-2836(74)90504-x. [DOI] [PubMed] [Google Scholar]
- Helenius A., Fries E., Kartenbeck J. Reconstitution of Semliki forest virus membrane. J Cell Biol. 1977 Dec;75(3):866–880. doi: 10.1083/jcb.75.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., von Bonsdorff C. H. Semlike Forest virus membrane proteins. Preparation and characterization of spike complexes soluble in detergent-free medium. Biochim Biophys Acta. 1976 Jul 15;436(4):895–899. doi: 10.1016/0005-2736(76)90421-1. [DOI] [PubMed] [Google Scholar]
- Hennache B., Boulanger P. Biochemical study of KB-cell receptor for adenovirus. Biochem J. 1977 Aug 15;166(2):237–247. doi: 10.1042/bj1660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfenhaus J. Propagation of Semliki Forest virus in various human lymphoblastoid cell lines. J Gen Virol. 1976 Dec;33(3):539–542. doi: 10.1099/0022-1317-33-3-539. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Käriäinen L., Simons K., von Bonsdorff C. H. Studies in subviral components of Semliki Forest virus. Ann Med Exp Biol Fenn. 1969;47(4):235–248. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Humphreys R. E., Yocum R. R., Strominger J. L. Enhanced representation of HL-A antigens on human lymphocytes after mitogenesis induced by phytohemagglutinin or Epstein-Barr virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3206–3209. doi: 10.1073/pnas.72.8.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney J. J., Dalrymple J. M., Alving C. R., Russell P. K. Interaction of Sindbis virus with liposomal model membranes. J Virol. 1975 Feb;15(2):225–231. doi: 10.1128/jvi.15.2.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neauport-Sautes C., Bismuth A., Kourilsky F. M., Manuel Y. Relationship between HL-A antigens and beta-2-microglobulin as studied by immunofluorescence on the lymphocyte membrane. J Exp Med. 1974 Apr 1;139(4):957–968. doi: 10.1084/jem.139.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Evrin P. E., Welsh K. I. Production of beta 2-microglobulin by normal and malignant human cell lines and peripheral lymphocytes. Transplant Rev. 1974;21(0):53–84. doi: 10.1111/j.1600-065x.1974.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Ostberg L., Rask L., Nilsson K., Peterson P. A. Independent expression of the two HL-A antigen polypeptide chains. Eur J Immunol. 1976 Jul;5(7):462–468. doi: 10.1002/eji.1830050707. [DOI] [PubMed] [Google Scholar]
- Osterrieth P. M., Calberg-Bacq C. M. Changes in morphology, infectivity and haemagglutinating activity of Semliki Forest virus produced by the treatment with caseinase C from Streptomyces albus G. J Gen Microbiol. 1966 Apr;43(1):19–30. doi: 10.1099/00221287-43-1-19. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Strominger J. L. Rapid purification of detergent-solubilized HLA antigen by affinity chromatography employing anti-beta2-microglobulin serum. J Biol Chem. 1976 Sep 10;251(17):5427–5428. [PubMed] [Google Scholar]
- Sanderson A. R., Welsh K. I. Properties of histocompatibility (HL-A) determinants. I. Site density of antigens of the two HL-A segregant series on peripheral human lymphocytes. Transplantation. 1974 Mar;17(3):281–289. [PubMed] [Google Scholar]
- Simons K., Helenius A., Garoff H. Solubilization of the membrane proteins from Semliki Forest virus with Triton X100. J Mol Biol. 1973 Oct 15;80(1):119–133. doi: 10.1016/0022-2836(73)90236-2. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Cresswell P., Grey H., Humphreys R. E., Mann D., McCuneJ, Parham P., Robb R., Sanderson A. R., Springer T. A. The immunoglobulin-like structure of human histocompatibility antigens. Transplant Rev. 1974;21(0):126–143. doi: 10.1111/j.1600-065x.1974.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Parham P., Mann D. L., Strominger J. L. Structure of HLA antigens: amino-acid and carbohydrate compositions and NH2-terminal sequences of four antigen preparations. Proc Natl Acad Sci U S A. 1976 Mar;73(3):910–914. doi: 10.1073/pnas.73.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Simons K. Studies on the amphipathic nature of the membrane proteins in Semliki Forest virus. J Mol Biol. 1974 Jan 5;85(4):569–587. doi: 10.1016/0022-2836(74)90316-7. [DOI] [PubMed] [Google Scholar]
- WIGZELL H. QUANTITATIVE TITRATIONS OF MOUSE H-2 ANTIBODIES USING CR-51-LABELLED TARGET CELLS. Transplantation. 1965 May;3:423–431. doi: 10.1097/00007890-196505000-00011. [DOI] [PubMed] [Google Scholar]
- Yefenof E., Klein G., Kvarnung K. Relationships between complement activation, complement binding, and EBV absorption by human hematopoietic cell lines. Cell Immunol. 1977 Jun 15;31(2):225–233. doi: 10.1016/0008-8749(77)90024-7. [DOI] [PubMed] [Google Scholar]