Abstract
Bites associated with wild and domestic Norway and black rats (Rattus norvegicus and Rattus rattus) may have a variety of health consequences in people. Bite-related infections are among the most significant of these consequences; however, there is little data on the infectious agents that can be transmitted from rats to people through biting. This is problematic because without an accurate understanding of bite-related infection risks, it is difficult for health professionals to evaluate the adequacy of existing guidelines for empirical therapy. The objectives of this study were to increase our knowledge of the bacterial species associated with rat bites by studying bite wounds that wild rats inflict upon one another and to review the literature regarding rat bites and bite wound management. Wild Norway and black rats (n=725) were trapped in Vancouver, Canada, and examined for bite wounds in the skin. All apparently infected wounds underwent aerobic and anaerobic culture, and isolated bacteria were identified. Thirty-six rats had bite wound–related infections, and approximately 22 different species of bacteria belonging to 18 genera were identified. Staphylococcus aureus was the most common isolate; however, the majority of infections (72.5%) were polymicrobial. Rat bites can result in infection with a number of aerobic and anaerobic Gram-positive and Gram-negative bacteria. In humans, these wounds are best managed through early recognition and cleansing. The benefit of prophylactic antimicrobial treatment is debatable, but given the deep puncturing nature of rodent bites, we suggest that they should be considered a high risk for infection. Antibiotics selected should include coverage for a broad range of bacterial species.
Key Words: : Animal bite, Bacterial infections, Rats, Rattus, Wound infection
Introduction
Animal bites are a significant public health issue in countries around the world. Although dog and cat bites have been studied extensively, there is relatively little information available about the consequences or management of bites from rodents, specifically rats (Rattus spp.).
Norway and black rats (Rattus norvegicus and Rattus rattus) have an almost worldwide distribution (Feng and Himsworth 2013) and are found in both wild and domesticated settings. In the United States, it has been estimated that there are 1.39–10 rat bites per 100,000 people per year (Hirschhorn and Hodge 1999), and approximately 3696 emergency department visits related to rat bites annually (Langley 2012). The true incidence of rat bites is very difficult to estimate because rat bites are generally not reported to health authorities, and people may not seek health care subsequent to a bite.
Studies have, however, been able to identify risk factors for rat bites. Bites associated with wild rats occur most commonly in urban areas, in children, in those with a mental or physical disability, and in impoverished communities (Ordog et al. 1985, Hirschhorn and Hodge 1999, Abbas et al. 2006, Langley 2012). Most wild rat bites that are reported occur at night when an individual is asleep and are therefore most likely to affect the uncovered face and upper extremities (Ordog et al. 1985, Hirschhorn and Hodge 1999, Abbas et al. 2006). In our experience, homelessness and scavenging in garbage containers may also be risk factors. It should be noted that rats are also found in domestic and laboratory settings, and in these environments, laboratory workers, pet store workers, and pet owners (particularly children) may be bitten (Elliott 2007).
Rat bites can result in a number of different health issues, including physical injury (Wykes 1989, Street et al. 2001, Haldar et al. 2011, Sethi et al. 2011), diabetic ulcers (Abbas et al. 2006, Kalra et al. 2006), anaphylactic shock (Hesford et al. 1995, Rankin et al. 2007), and even hypovolemic shock in infants (Donoso et al. 2004). Rat bites can also cause infections (usually bacterial), ranging from cellulitis (Diwan et al. 1970) and fasciitis (Anyanwu and Yakubu 2012) to life-threatening systemic disease (Abbas et al. 2006, Elliott 2007). Many sources suggest that 10% of rat bites become infected (Elliott 2007, Dendle and Looke 2008); however, this figure appears to originate from a series of 22 “rodent” bites for which neither the species of rodent involved nor the circumstances surrounding the bite were disclosed (Kizer 1979). For this reason, the true incidence of infection subsequent to a rat bite is not clear.
The infectious agent most commonly associated with rat bites is Streptobacillus moniliformis, the causative agent of “rat bite fever” (Elliott 2007). Infection with S. moniliformis is somewhat distinct in the arthralgia/arthritis and skin rash that it produces (Elliott 2007). Other infectious agents associated with rat bites include: Corynebacterium kutscheri (Holmes and Korman 2007), Staphylococcus aureus (Postma et al. 1991), Staphylococcus epidermidis (Ordog et al. 1985), Streptococcus spp. (Ordog et al. 1985, Postma et al. 1991), Leptospira interrogans (Luzzi et al. 1987, Roczek et al. 2008), and Bacillus subtilis (Ordog et al. 1985).
Information on rat bite–associated infections has largely been gleaned through case studies and small case series; therefore, the true range of infectious agents associated with rat bites remains unknown. This knowledge gap stems from the fact that rat bites in humans, being relatively rare and/or rarely reported, are difficult to study in a comprehensive way. However, rat bites are very common among rats, as biting is a relatively frequent occurrence during a variety of social interactions among conspecifics (Barnett 1976). For this reason it might be possible to learn more about bite-related infectious risks by studying bite-induced infections in rats themselves.
The objectives of this study were to: (1) Identify the bacteria causing bite-related skin and soft tissue infections in urban Norway and black rats (R. norvegicus and R. rattus), and thereby to supplement the existing knowledge base regarding bacteria that could be transmitted to people through rat bites, and (2) review the literature regarding rat bites and bite wound management.
Materials and Methods
Sample collection
The study area (Fig. 1) was comprised of 43 contiguous city blocks (0.82 km2) in an inner-city area of Vancouver, British Columbia (N49°17′/W123°6′). Also included was a property (0.03 km2) within the port terminal (exact location not disclosed here to preserve anonymity), which is a center for international shipping that forms the northern border the study area. Each block (and the port site) was assigned to a randomly selected 3-week study period over the course of 1 year (September, 2011–August, 2012). Within each block, approximately 20 Tomahawk Rigid Traps for rats (Tomohawk Live Trap llc., Hazlelhurst, WI) were set out along each side of the back alley that bisected the block. Traps were evenly spaced where possible, but had to be placed in a location where they did not obstruct traffic and could be secured to outdoor public property to prevent theft. Traps were prebaited (filled with bait but fixed open) for 1 week, prior to 2 weeks of active trapping. Baits used included peanut butter, bacon fat, flour, and oats. At the port, traps were placed in areas where port staff had observed rats. Trapped rats were anesthetized with isoflurane prior to intracardiac pentobarbital euthanasia. Rats trapped at the port by a collaborating pest control professional using snap-type traps were also collected. After euthanasia, sex, weight, and species were determined, and each rat underwent a full physical examination, which included examination for bite wounds and bite wound–related skin infections, i.e., draining abscesses and open sores (Fig. 2).
FIG. 1.
Map of the study area in Vancouver, Canada. City blocks included in the study are highlighted in black.
FIG. 2.
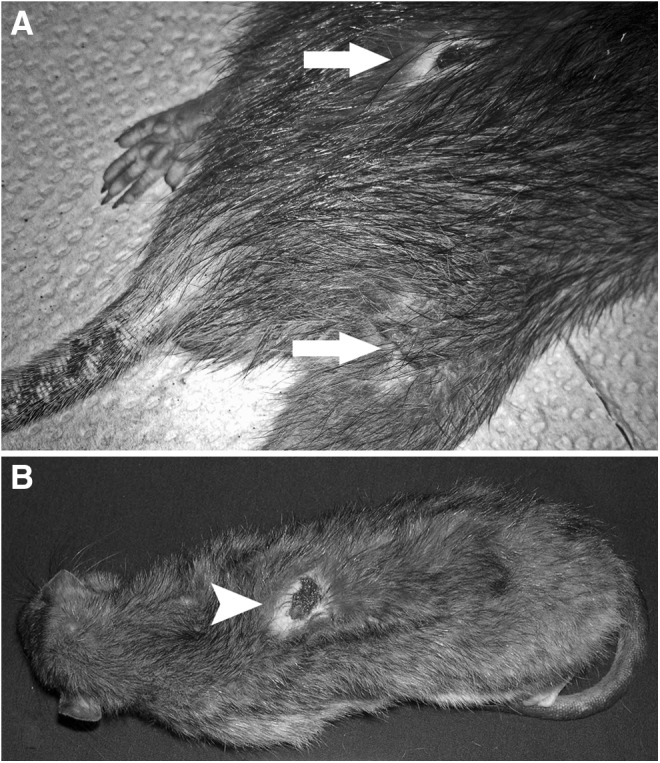
(A) Rat with healing (noninfected) bite wounds (arrows) on rump and thigh. (B) Rat with infected bite wound (scab removed, arrowhead) on back.
Open sores and draining abscesses were sampled in the field using a sterile BD CultureSwab™ with Liquid Amies medium (BD, Franklin Lakes, NJ). Rats were subsequently frozen and sent to the Animal Health Centre, British Columbia Ministry of Agriculture for full postmortem examination, including sterile sampling of nondraining skin and superficial soft tissue infections.
It should be noted that conspecific bite wounds are common in rats and can occur all over the body subsequent to a variety of different types of social interactions (Tackahashi and Blanchard 1982, Glass et al. 1988). They are usually sharply demarcated incision- or puncture-type wounds (Dyring-Andersen et al. 2012), although some can become secondarily infected and develop into open sores or abscesses (Glass et al. 1988). For this reason, any incision/puncture-type wound in the skin was considered a bite wound, and any open cutaneous sore or abscess associated with the skin was considered a bite wound-related infection. Ultimately, it was not possible to definitively determine that the aforementioned skin wounds and infections were due to rat bites. However, other causes of these types of skin lesions (e.g., injury from inert objects or other animal species) could not be identified in the literature.
This study was approved by the University of British Columbia's Animal Care Committee (A11-0087).
Bacterial culture and identification
Using aseptic technique, all specimens were inoculated onto Columbia Blood agar and MacConkey agar (Oxoid, Canada) and incubated at 35°C in 5–10% CO2 and aerobic conditions, respectively, for 48 h. Additionally, each specimen was set up for anaerobic culture on Columbia Blood agar and incubated at 35°C for 48 h. Aerobic cultures were observed at 24 and 48 h, and subcultures of all bacterial growth were made for further diagnostic evaluation. After 48 h, all anaerobic cultures were observed, and all suspect anaerobic organisms were subsequently subcultured aerobically and anaerobically to confirm the organism as a suspect anaerobic organism and to obtain a pure culture before proceeding with identification.
All subcultured organisms were observed for their growth characteristics and colony morphology on Columbia Blood agar and MacConkey agar, and microscopic morphology was determined using Gram stain. On the basis of these analyses, organisms were further screened by individual biochemical reactions, including the indole, oxidase, catalase, and coagulase tests. Where necessary, further biochemical testing was performed using the API 20E, API Coryne, API Strep (Biomerieux, Canada) and Biolog MicroLog system (Biolog Inc., USA).
Organisms that could not be identified using the aforementioned techniques (e.g., S. moniliformis, Pasteurella pneumotropica, C. kutscheri, etc.) underwent DNA extraction (QIAamp DNA Mini Kit, Qiagen, Canada) with PCR amplification and sequencing of the V1–V3 regions (492 bp) of the 16S rRNA gene (Lane 1991). Sequenced products were classified using BLAST against the NCBI GenBank database (http://blast.ncbi.nlm.nih.gov/), as well as using the Bio Informatic Bacteria Identification (leBIBI V5) system (http://umr5558-sud-str1.univ-lyon1.fr/lebibi/lebibi.cgi). It should be noted that because samples were set up for routine aerobic and anaerobic cultures only, some fastidious organisms requiring enhanced culture techniques (e.g., chocolate agar, Yersina spp.–selective agars, etc.) may have been missed.
Results
A total of 725 rats were trapped, including 685 (94.5%) Norway rats and 40 (5.5%) black rats. Four hundred of 725 rats (55.2%) were male and 317 (43.7%) were female (sex could not be determined for eight rats). The average weight was 162.9 grams (range=20.0–446.2 grams).
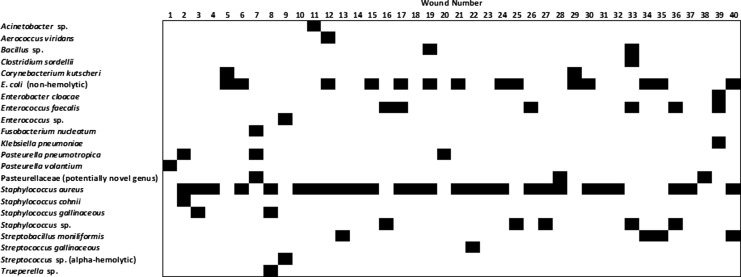
Visible bite wounds were present in 177 rats (24.1%). Among the rats with bite wounds, the average number of wounds was 2.26 (range=1–15) and there were 309 bite wounds in total. Thirty-six of 725 rats (5.0%) had active bite wound–related skin and soft tissue infections; therefore, wound infections were present in 20.3% (36/177) of rats with bite wounds. Among these 36 rats there were a total of 40 bite wound–associated infections; therefore, 12.9% (40/309) of all bite wounds were infected. The majority of bite wounds were polymicrobial, with 19/40 (47.5%) yielding two species of bacteria, 9/40 (22.5%) yielding three species, and 1/40 (2.5%) yielding four species; the remainder (11/40 or 27.5%) were monomicrobial (Fig. 3).
FIG. 3.
Bacterial species isolated from 40 bite wound–related skin and soft tissue infections in Norway and black rats (R. norvegicus and R. rattus).
A total of 80 isolates were obtained, which included approximately 22 different species of bacteria (Table 1, Fig. 3). Three isolates had identical 16S rRNA sequences and did not match to any known bacterial genera. On the basis of its 16S rRNA sequence, this bite wound–associated bacterium fell within the Pasteurellaceae family and was most closely related to Haemophilus spp. and Aggregatibacter spp.
Table 1.
Bacteria Isolated from 40 Bite Wound–Related Skin and Soft Tissue Infections in Norway and Black Rats (Rattus rattus and Rattus norvegicus)
| Bacterial species | Number of isolates |
|---|---|
| Acinetobacter spp. | 1 |
| Aerococcus viridans | 1 |
| Bacillus spp. | 2 |
| Clostridium sordellii | 1 |
| Corynebacterium kutscheri | 2 |
| E. coli (nonhemolytic) | 14 |
| Enterobacter cloacae | 1 |
| Enterococcus faecalis | 6 |
| Enterococcus spp. | 1 |
| Fusobacterium nucleatum | 1 |
| Klebsiella pneumoniae | 1 |
| Pasteurellaceae (potentially novel genus) | 3 |
| Pasteurella pneumotropica | 3 |
| Pasteurella volantium | 1 |
| Staphylococcus aureus | 27 |
| Staphylococcus cohnii | 1 |
| Staphylococcus gallinaceous | 1 |
| Staphylococcus spp. | 5 |
| Streptobacillus moniliformis | 4 |
| Streptococcus gallinaceous | 2 |
| Streptococcus spp. (alpha hemolytic) | 1 |
| Truperella spp. | 1 |
Of the 36 rats with bite wound–associated infections, only one was a black rat. This rat had one wound from which S. aureus, and the aforementioned unidentified bacterial species were isolated. Although we were unable to find a significant association between species and the presence of bite wounds (p>0.05), whether or not this is reflective of reality may be related to the low number of black rats in the study as a whole. However, we did find that odds of having bite wounds was greater in males (odds ratio [OR]=1.76, 95% confidence interval [CI]=1.24–2.51) and heavier rats (OR=1.11, 95% CI=1.09–1.13 per 10-gram increase in weight). The odds of having an infected bite wounds was also greater in heavier rats (OR=1.08, 95% CI=1.05–1.11 per 10-gram increase in weight), but was not associated with sex or species (p>0.05). Note that weight is used as a proxy for age in rats (Feng and Himsworth 2013).
Discussion
Bacteria associated with rat bites
This study demonstrates the spectrum of bacterial species that may be associated with rat bite–related infections in the studied area. Many of these isolates clearly represent inoculation of rat oral flora, because they are commonly found colonizing mucous membranes in rats. These include C. kutscheri, Klebsiella pneumonia, P. pneumotropica, as well as S. moniliformis (Percy and Barthold 2007). The isolation of Bacillus spp. and Clostridium sordellii, on the other hand, might suggest inoculation of environmental bacteria (Quinn et al. 2005). Similarly, although S. aureus can be found colonizing the mucous membranes of rats (Percy and Barthold 2007), it is also found on the skin, and could have been translocated to deeper tissues through the act of biting.
Most interesting is the relative prevalence of these organisms. Within the literature, S. moniliformis is represented as the bacterial species most commonly associated with rat bites (Glaser et al. 2000, Morgan 2005, Dendle and Looke 2008). In our study, however, S. moniliformis was found in only 5% (4/80) of bite-related infections. By far, the most common isolate was S. aureus, which represented 33.8% of all isolates. This raises the question as to whether S. moniliformis infection is really more common subsequent to rat bites in people (vs. rat bites in rats), or whether there is bias toward the recognition and/or reporting of S. moniliformis infections compared to other bacteria—perhaps related to the fact that S. moniliformis is associated with a distinct septicemic disease (Elliott 2007). Additionally, while anaerobes are commonly associated with dog and cat bites (Ball et al. 2007), very few anaerobes were isolated in this study. This could, however, be a result of sampling technique (i.e., use of transport media not specific for anaerobe recovery and/or prolonged time between sample collection and anaerobic culture).
Although we were able to identify a number of bacteria not previously associated with rat bites, we did not isolate several species that have been associated with rat bites in the past. For example, one study of rat bites in humans found that S. epidermidis represented 43% of bacteria cultured from noninfected wounds at presentation (Ordog et al. 1985). However, because S. epidermidis is commonly found on human skin, it may be the case that these isolates represent human skin flora inoculated into the wounds. Indeed, previous researchers have suggested that animal bite wound cultures obtained from noninfected wounds soon after the bite has occurred are rarely useful (Freer 2004, Morgan 2005), likely because of the scant number and deep location of inoculated bacteria in an early bite wound. Morgan (2005) goes as far as to suggest that cultures are only useful at the onset of clinical infection.
This is also worth noting that although P. multocida is the most likely organism to cause infection subsequent to cat bite (Abrahamian and Goldstein 2011), we did not isolate this bacterium in our study and rat bite–related Pasteurella multocida infections appear to be exceedingly rare (Hubbert and Rosen 1970).
Risk of infection subsequent to a rat bite
In our study, approximately 12% of bite wounds were associated with infection. This number is similar to the 10% figure often quoted by other sources (Elliott 2007, Dendle and Looke 2008). However, we suggest that this likely underestimates the true prevalence of infection in this rat population because we could not account for infections that had resolved, caused systemic infections not visible to the naked eye (e.g., infection with Leptospira spp.), or resulted in significant morbidity or mortality. We suggest that the nature of the rat bite wound, i.e., deep punctures with small openings, could put these wounds at an elevated risk of infection similar to those of cats, where up to 80% of bites may become infected (Ball and Younggren 2007).
Medical management of rat bites
There is no literature specifically regarding the medical management of rat bites. However, a review of protocols for dealing with animal bites in general shows that there is consensus regarding the importance of early recognition as well as thorough examination, debridement, irrigation, and disinfection (Freer 2004, Morgan 2005, Ball and Younggren 2007, Dendle and Looke 2008). Given the deep, puncture-type wounds inflicted by rats, assessing the depth of the wound and involvement of underlying structures, and providing adequate cleaning may be particularly challenging.
With regard to diagnostics, it has been suggested, as mentioned above, that early wound cultures are rarely useful (Morgan 2005). Rather, cultures should be performed if there is evidence of clinical infection (Morgan 2005, Dendle and Looke 2008). Local wound cultures are most appropriate for cellulitis and localized infections, whereas blood cultures are needed where there are signs of systemic disease or septicemia (Morgan 2005). Because infection may not be apparent at the outset, the literature suggests that the patient should be re-examined in 24–48 h and taught to monitor themselves for signs of infection in the interim (Freer 2004, Morgan 2005, Dendle and Looke 2008). Given that rat bites are usually associated with small punctures, rather than large lacerations, the need for primary wound closure is probably minimal and should be avoided where possible to avoid infection (Freer 2004, Morgan 2005).
There is considerable debate regarding the need for prophylactic antibiotic treatment in bite wound management. One study withheld prophylactic antibiotics from 50 patients with rat bite wounds that were not infected at presentation and found that only one patient developed an infection (although these wounds were generally recognized early and appropriately cleansed and debrided) (Ordog et al. 1985). Similarly, a Cochrane review of antibiotic prophylaxis for mammalian bites found that antibiotic prophylaxis was not effective at preventing most infections (Medeiros and Saconato 2001). This review, however, consisted almost entirely of dog-bite studies and included only one series of cat bites with puncture-type wounds that are more prone to infection (Medeiros and Saconato 2001). Antimicrobial prophylaxis did appear to be effective for bites involving the hands, where underlying structures, such as bones and joints, are more likely to be affected (Medeiros and Saconato 2001). Other than the location and nature of the wound, the need for antimicrobial prophylaxis may be influenced by the time lapse between the bite and medical intervention and patient factors that may increase susceptibility to infection (e.g., immunodeficiency, prosthetic heart valves or joints, old age, etc.) (Freer 2004).
With regard to the choice of antimicrobial, a number of different drugs have been used and/or recommended. However, given the polymicrobial nature of animal bites, there is some consensus that antimicrobials should be selected in a manner that ensures coverage for a variety of Gram-positive and Gram-negative aerobic and anaerobic bacteria (Morgan 2005, Dendle and Looke 2008). Amoxicillin/clavulanic acid combinations have been suggested as a good first choice for this reason (Morgan 2005). Our findings suggest that this would remain a valid choice for empirical therapy of rat bite–related infections pending culture results.
An additional factor to consider is the need for rabies and/or tetanus prophylaxis. The spores of Clostridium tetani are ubiquitous in the environment and therefore have the potential to be inoculated into an animal bite, as with any other penetrating injury (Freer 2004). For this reason, individuals with unknown or noncurrent vaccination status should be managed with tetanus human immunoglobulin and tetanus toxoid booster vaccination (Freer 2004).
Although some sources have suggested that rabies prophylaxis should be considered for all rodent bites (Freer 2004), to our knowledge there has never been a confirmed case of rabies in a Norway or black rat, or a case of human rabies associated with these species. For this reason, we suggest that rabies prophylaxis is not warranted subsequent to most bites associated with apparently healthy rats. Similarly, although tularemia (infection with Francisella tularensis) has been suggested as a consequence of rodent exposure (Freer 2004), we could not find a confirmed case of tularemia in a Norway or black rat.
However, if the rat is ill or behaving abnormally, then diagnostics should be undertaken to rule out any unusual or unforeseen pathogen. It is important to note that biting can be a response to illness in an animal (Freer 2004); therefore, due consideration of the health or behavioral status of the rat, if possible, would be prudent.
Conclusions
Overall, this study demonstrates that rat bites could result in the transmission of a wide variety of bacteria. S. aureus was the most common isolate; however, the majority of infections (72.5%) were polymicrobial. In humans, these wounds are best managed through early recognition and cleansing. The benefit of prophylactic antimicrobial treatment is debatable, but given the deep puncturing nature of rodent bites, we suggest that they should be considered a high risk for infection. Antibiotics selected should include coverage for a broad range of bacterial species.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (MOP–119530 and CGV–104833).
Author Disclosure Statement
No competing financial interests exist
References
- Abbas ZG, Lutale J, Archibald LK. Comment on: Kalra B, Kalra S, Chatley G, Singh H. Rat bite as a cause of diabetic foot ulcer: A series of eight cases. Diabetologia 2006; 49:1452–1453Diabetologia 2006; 49:2811–2812. [DOI] [PubMed] [Google Scholar]
- Abrahamian FM, Goldstein EJC. Microbiology of animal bite wound infections. Clin Microbiol Rev 2011; 24:231–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyanwu LJ, Yakubu AA. Necrotizing fasciitis of the lower limb following rat bites. Int J Low Extrem Wounds 2012; 11:169–170 [DOI] [PubMed] [Google Scholar]
- Ball V, Younggren BN. Emergency management of difficult wounds: Part I. Emerg Med Clin North Am 2007; 25:101–121 [DOI] [PubMed] [Google Scholar]
- Ball V, Younggren BN. Emergency management of difficult wounds: Part I. Emerg Med Clin N Am 2007;25:101–121 [DOI] [PubMed] [Google Scholar]
- Barnett SA. The rat: A study in behaviour. Chicago: The University of Chicago Press, 1976:318 [Google Scholar]
- Curtis PE, Ollerhead GE, Ellis CE. Pasteurella multocida infection of poultry farm rats. Vet Rec 1980; 107:326–327 [DOI] [PubMed] [Google Scholar]
- Dendle C, Looke D. Animal bites: An update for management with a focus on infections. Emerg Med Australas 2008; 20:458–467 [DOI] [PubMed] [Google Scholar]
- Diwan R, Sen D, Sood G. Rat bite orbital cellulitis. Br J Ophthalmol 1970; 54:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso A, Leon J, Rojas G, Ramirez M, et al. Hypovolaemic shock by rat bites. A paradigmatic case of social deprivation. Emerg Med J 2004; 21:640–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyring-Andersen B, Menne T, Skov L. Sharply demarcated incisions caused by rat bites. Arch Dermatol 2012; 148:1209–1210 [DOI] [PubMed] [Google Scholar]
- Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev 2007; 20:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng AYT, Himsworth C. The secret life of the city rat: A review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosystems 2013; 16:341–350 [Google Scholar]
- Freer L. North American wild mammalian injuries. Emerg Med Clin North Am 2004; 22: 445–473 [DOI] [PubMed] [Google Scholar]
- Glaser C, Lewis P, Wong S. Pet-, animal-, and vector-borne infections. Pediatr Rev 2000; 21:219–232 [DOI] [PubMed] [Google Scholar]
- Glass GE, Childs JE, Korch GW, Leduc JW. Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvegicus). Epidemiol Infect 1988; 101:459–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar P, Mukherjee PP, Ghosh TJ, Shukla RM, et al. Animal bite of penis in a neonate and macroscopic repair. J Indian Assoc Pediatr Surg 2011; 16:163–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesford JD, Platts-Mills TA, Edlich RF. Anaphylaxis after laboratory rat bite: An occupational hazard. J Emerg Med 1995; 13:765–768 [DOI] [PubMed] [Google Scholar]
- Hirschhorn R, Hodge R. Identification of risk factors in rat bite incidents involving humans. Pediatrics 1999; 104:e35. [DOI] [PubMed] [Google Scholar]
- Holmes NE, Korman TM. Corynebacterium kutscheri infection of skin and soft tissue following rat bite. J Clin Microbiol 2007; 45:3468–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert WT, Rosen MN. Pasteurella multocida infection due to animal bite. Am J Public Health 1970; 60:1103–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra B, Kalra S, Chatley G, Singh H. Rat bite as a cause of diabetic foot ulcer: A series of eight cases. Diabetologia 2006; 49:1452–1453 [DOI] [PubMed] [Google Scholar]
- Kizer KW. Epidemiologic and clinical aspects of animal bite injuries. J Am Coll Emer 1979; 8:134–141 [DOI] [PubMed] [Google Scholar]
- Lane DJ. 16S/23S rRNA sequencing In: Stackebrandt E, Goodfellow M. eds. Nucleic Acid Techniques in Bacterial Systematics. New York: Wiley, 1991:115–175 [Google Scholar]
- Langley RL. Animal-related injuries resulting in emergency department visits and hospitalizations in the United States, 2006–2008. Human-Wildlife Interactions 2012; 6:123–136 [Google Scholar]
- Luzzi G, Milne L, Watikins S. Rat-bite acquired leptospirosis. J Infect 1987; 15:57–60 [DOI] [PubMed] [Google Scholar]
- Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev 2001:CD001738. [DOI] [PubMed] [Google Scholar]
- Morgan M. Hospital management of animal and human bites. J Hosp Infect 2005; 61:1–10 [DOI] [PubMed] [Google Scholar]
- Ordog GJ, Balasubramanium S, Wasserberger J. Rat bites: Fifty cases. Ann Emerg Med 1985; 14:126–130 [DOI] [PubMed] [Google Scholar]
- Percy DH, Barthold W. Pathology of Laboratory Rodents and Rabbits. Ames, Iowa: Blackwell, 2007:356 [Google Scholar]
- Postma BH, Diepersloot RJ, Niessen GJ, Droog RP. Cowpox-virus-like infection associated with rat bite. Lancet 1991;337:733–734 [DOI] [PubMed] [Google Scholar]
- Quinn PJ, Markey BK.Carter ME, Donnelly WJ, et al. Bacillus species and Clostridium species. In Veterinary Microbiology and Microbial Disease. Oxford: Blackwell Science, 2005:80–96 [Google Scholar]
- Rankin TJ, Hill RJ, Overton D. Anaphylactic reaction after a laboratory rat bite. Am J Emerg Med 2007; 25:985.el–e2 [DOI] [PubMed] [Google Scholar]
- Roczek A, Forster C, Raschel H, Hoermansdorfer S, et al.. Severe course of rat bite-associated Weil's disease in a patient diagnosed with a new Leptospira-specific real-time quantitative LUX-PCR. J Med Microbiol 2008; 57:658–663 [DOI] [PubMed] [Google Scholar]
- Roczek A, Forster C, Raschel H, Hoermansdorfer S, et al. Severe course of rat bite-associated Weil's disease in a patient diagnosed with a new Leptospira-specific real-time quantitative LUX-PCR. J Med Microbiol 2008; 57:658–663 [DOI] [PubMed] [Google Scholar]
- Sethi SK, Saha A, Karela M, Dubey NK. Infantile trauma due to a rat bite. J Emerg Trauma Shock 2011; 4:409–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackahashi LK, Blanchard RJ. Attack and defense in laboratory and wild Norway and black rats. Behav Processes 1982; 7:49–62 [DOI] [PubMed] [Google Scholar]
- Street K, Kinder S, Perkins CS. An unusual cause of injury to an infant. Arch Dis Child 2001; 85:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes WN. Rat bite injury to the eyelid in a 3-month-old child. Br J Ophthalmol 1989; 73:202–204 [DOI] [PMC free article] [PubMed] [Google Scholar]