Abstract
Background:
The public conviction that ‘herbal remedies are safe’ has led to an increased consumption of these products. This study was performed in view of the wide distribution of herbal remedies, the risks posed by self-treatment with these products, and the existing reports about the toxic effects of some medicinal herbs.
Materials and Methods:
In this study the effect of some of the most used herbal drops of A, B, C, and D on the liver function of rats was examined at different doses, namely minimum dose, maximum dose, and 2.5 times the maximum dose indicated in the brochures. The rats were administered the said doses via a feeding tube for 50 days. The liver function parameters including aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), total serum protein, albumin, and urea were measured using the spectrophotometric method.
Results:
The animals’ liver tissues were examined pathologically. The A drop did not change the liver function parameters significantly. The B drop increased the LDH by 34% compared to the controls, at the maximum administered dose. The C and D drops increased the ALT, AST, and LDH significantly compared to the controls. The histological findings suggest the possible effect of C and D drops on the function of hepatocytes.
Conclusions:
We recommend that the herbal formulations available in pharmaceutical markets be more closely controlled in terms of quality, as well as toxicity, especially with regard to the possible effects on the hepatic function.
Keywords: Hepatoxicity, Medicinal herb, liver function
INTRODUCTION
Liver injury induced by drugs or chemicals, also known as toxic hepatopathy, is a major clinical challenge. It has been demonstrated by epidemiological studies that the prevalence of drug/chemical-induced hepatopathy has increased over the past decades, especially in developing countries.[1,2,3]
Based on the World Health Organization (WHO) estimates, 80% of the world's inhabitants use medicinal herbs to benefit from their primary therapeutic properties. Widespread dissatisfaction with high prices of chemical drugs toward the end of the twentieth century has been associated with recourse to natural remedies, especially medicinal herbs. Researchers found that people in different parts of the world have access to nearly 600 of 700 herbal medicines, which are prescribed by 70% of the physicians.[4,5,6,7]
Herbal products have been generally portrayed as ‘natural’ and ‘safe’ remedies; however, consumers do not seem to be fully aware of the potential side effects. The side effects reported following the use of herbal treatments has increased on par with the rising demand for herbal medicines. Hepatitis and hepatic veno-occlusive disease are two perilous side effects of some herbal medicines.[8,9]
The use of Chaparral, an antioxidant, used to slow aging, treat common colds, and some skin lesions, and Germander, a herb used to treat obesity, can lead to acute hepatitis.[8] In this study four hydro alcoholic-based herbal medicines (A, B, C, D), which are prescribed as over-the-counter drugs in Iran, are studied for their hepatotoxicity damage.
Cuminum cyminum and Tribulus terrestris are among the medicinal herbs used as ingredients of some herbal products such as A, B and C, D; there have been reports of hepatic damage resulting from the administration of these products in animals.[10,11,12,13] This study was conducted to investigate the possible hepatotoxic effect of a number of herbal products available in Iran's pharmaceutical market.
MATERIALS AND METHODS
Animals and treatments
This is a controlled empirical study using male Wistar rats. The rats were supplied from the Tehran Pasteur Institute. All animals were kept under the same laboratory conditions of temperature (25 ± 2°C) and lighting (12: 12-hour light : dark cycle) and were given free access to standard laboratory chow and tap water. All rats were allowed to acclimatize for one week prior to experimentation. All experimental procedures involving the animals were approved by the Animal Research Ethics Committee of the Isfahan Cardiovascular Research Center, Isfahan, Iran.
The animals were randomly divided into four groups, with five rats in each group. Each herbal drop was tested on three groups of five rats. Based on the information printed in the products’ brochures, the minimum and maximum doses, as well as 2.5 times the maximum dose of each product were administered to the rats orally by using feeding tubes (or by oral gavage). The administered doses were as follows (all doses in ml/kg.b.w):
A is made from Cucurbita pepo, Urtica dioica, Matricaria chamomilla, Tribulus terrestris, Pimpinella anisum: 4.5, 6, and 15
B is made from Foeniculum vulgare, Cuminum cyminum, Trigonella foenum-graecum, Anethum graveolens: 3, 4.5, and 11.5
C is made from Foeniculum vulgare, Cuminum cyminum, Laurus nobilis, Cerasus avium, Zea mays, Tribulus terrestris, Cucumis melo: 7.5, 9, and 22.5
D is made from Hypericum perforatum: 2, 4.5, and 11.5
The herbal drops were hydroalcoholic-based; hence, the control group was administered only with alcohol (70%).
Sample collection and hepatic function tests
At the end of the experiment, all the animals were anesthetized and blood samples were obtained from their hearts. After coagulation, the serum was separated by centrifuge at 3000 × g for 15 minutes to determine the biochemical parameters. The serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), total protein, albumin, and urea, as markers of hepatic function, were determined with an automated Hitachi Analyzer Model 902 (Hitachi).
Histological examination
The animals were sacrificed and their liver tissues were removed immediately and fixed overnight in 10% neutral formalin solution, embedded in paraffin wax, and sectioned for histological evaluation. Paraffin sections (6 μm thickness) were stained with hematoxylin and eosin (H and E) using the standard protocol, and then analyzed by light microscopy.
Statistical analysis
The data were expressed as mean ± SD and were analyzed with the SPSS, Version 17.0 software. Differences between the mean values corresponding to the groups under study were calculated by one-way analysis of variance (ANOVA), and the Bonferroni was used post hoc. The results were considered statistically significant when the P-value was below 0.05.
RESULTS
Drug A caused a significant change in total protein and urea of the measured parameters in the case group, compared to the controls, for all the three doses used [Table 1]. The drug B drops increased the LDH significantly, by 34%, at a dose of 11.5 ml/kg.b.w [Table 2]. Pathological examination of the liver in this group revealed dilated portal spaces, arteries, and veins. The biliary ducts were also dilated, with some monocytic infiltration in the spaces between the venous, arterial, and biliary ductal spaces [Figure 1].
Table 1.
Biochemical parameters showing liver function of rats following administration of the A drop with different doses (Mean ± SD)
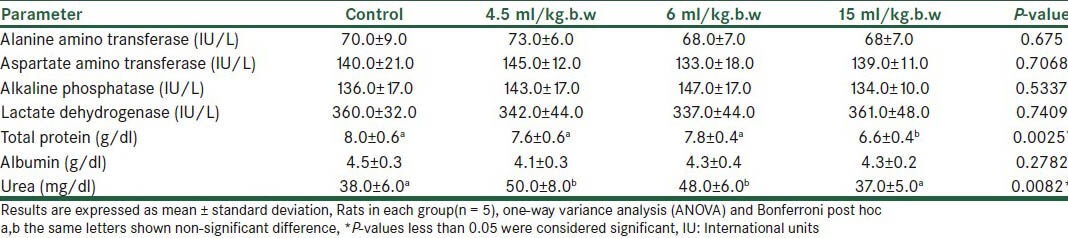
Table 2.
Biochemical parameters showing liver function of rats following administration of B drop with different doses (Mean ± SD)
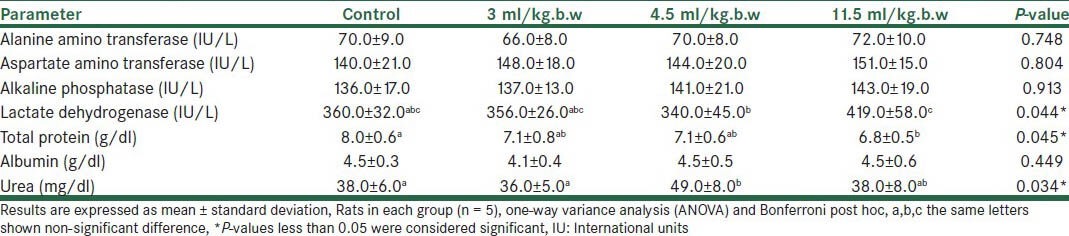
Figure 1.
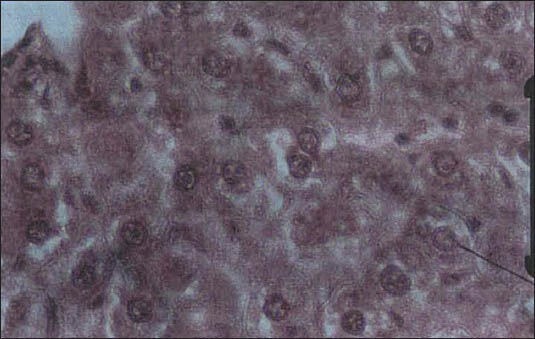
Liver tissue in the group receiving the B drop with a dose of 11.5 ml/kg: Dilation and congestion of portal spaces of arteries and veins, dilation of biliary ducts, monocytic infiltration in spaces between the arteries, veins, and biliary ducts
Administration of the C drops at a dose of 22.5 ml/kg.b.w significantly increased AST, ALT, and LDH (19, 35, and 59%, respectively) [Table 3] but there were no significant changes for the other doses and liver tests. Examination of the liver tissue in this group revealed dilation of some central lobular veins and edematous fluid accumulation [Figure 2].
Table 3.
Biochemical parameters showing liver function of rats following administration of C drop with different doses(Mean ± SD)
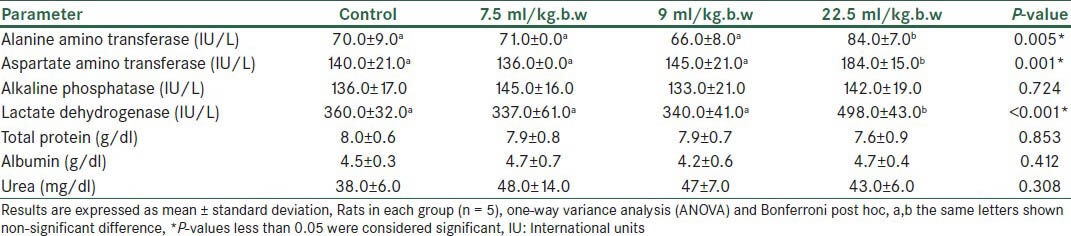
Figure 2.
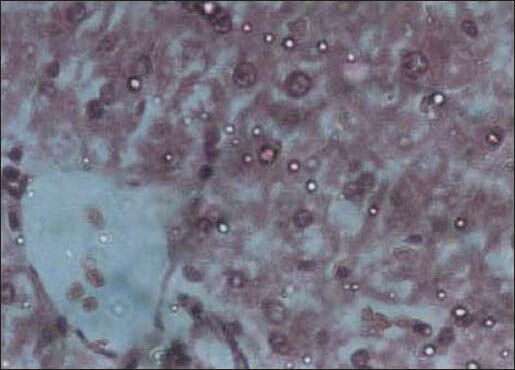
Liver tissue in the group receiving the C drop with a dose of 22.5 ml/kg: Dilation of some central lobular veins and edematous fluid accumulation
Administration of D drops at a dose of 11.5 ml/kg.b.w significantly increased the AST, ALT, and LDH by 25, 33, and 286%, respectively [Table 4]. Histological examination of the livers of rats in this group showed dilation and congestion of sinusoid and central lobular veins, with considerable monocytic infiltration [Figure 3]. The D drops at doses of 4.5 and 2 ml/kg.b.w did not have any significant effect on the measured biochemical parameters.
Table 4.
Biochemical parameters showing liver function of rats following administration of D drop with different doses (Mean ± SD)
Figure 3.
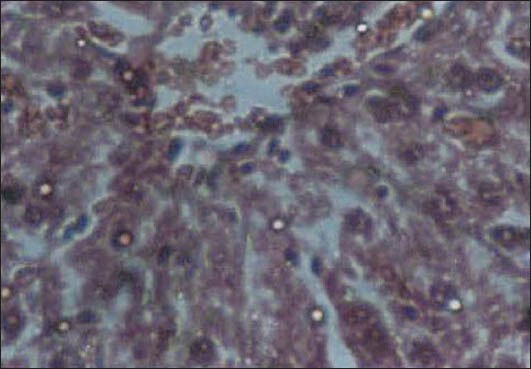
Liver tissue in the group receiving the D drop with a dose of 11.5 ml/kg: Dilation and congestion of the sinusoid and central lobular veins, with considerable inflammatory monocytic infiltration
DISCUSSION
The A drop is used to treat acute and chronic inflammation of the prostate, as well as urinary urgency and frequency. This herbal product contains 20% Tribulus terrestris.
A studied show specifies the hepatoprotective and antioxidant properties of T. terrestris against acetaminophen-induced toxicity in freshwater fish, O. mossambicus[10], and reports suggest liver damage in animals feeding on this herb.[11,12,13] In this study, however, administration of the A drop did not affect the liver function of any of the treated rats [Table 1].
The B drop is mainly used to increase milk in breastfeeding mothers. This herb has been reported to increase AST and serum urea in rats.[14] In this study, oral administration of the B drop at low doses has not affected the liver function, however, it has increased the serum LDH by 34% compared to the controls, at a dose of 11.5 ml/kg.b.w [Table 2]. Nonetheless, LDH has different isoenzymes and measurement of the total LDH is not sufficient for the assessment of liver function. Histological examination of rat livers in this group have shown dilation and congestion of the portal spaces of arteries and veins, as well as dilation of the biliary ducts and some monocytic infiltration in spaces between the arteries, veins, and biliary ducts. The B drop also contains 50% Foeniculum vulgare; reports are suggestive of this herb's protective effects against hepatotoxicity induced by carbon tetrachloride in rats.[15] Thus, the presence of Foeniculum vulgare in this herbal product may have offset the hepatotoxic effects of Cuminum cyminum in rats.
The C drop contains 12.5% Cuminum cyminum and 12.5% Foeniculum vulgare. At a low dose, the C drop does not affect the liver function in rats; however, at 2.5 times the maximum dose printed in the brochures (22.5 ml/kg.b.w), the herb significantly increases the ALT, AST, and LDH when compared with the controls [Table 3]. Histological examination of rat livers has also shown dilation of some central lobular veins and accumulation of edematous fluid, as well as limited monocytic infiltration in the biliary ducts.
The D drop contains the hydroalcoholic extract of Hypericum perforatum and is used in the treatment of depression, insomnia, anxiety, neurological headaches, and migraine. This herb causes sensitivity to ultraviolet radiation (UV) and may promote allergic reactions, as well as gastric discomfort. Some studies have shown that Hypericum perforatum interferes with some drugs, including warfarin and contraceptives.[16] Thus far, there have been no reports of the hepatotoxicity of this herb, or at least we have not found them. However, administration of drop D at a dose of 11.5 ml/kg.b.w significantly increases ALT, AST, and LDH, when compared to the controls. Histological examination of the liver tissue in this group confirms its effect on the liver function of the treated rats.
It is in certain situations and in the use higher doses that herbal products may be just as harmful as conventional drugs. Although findings on the hepatotoxicity of drugs in rats cannot be reliably generalized to humans, they are of high predictive value. In the present study, administration of medicinal herbs at doses indicated in the brochures has not been associated with hepatotoxicity. At higher doses, however, B, C, and D affect the liver function; this has been evidenced by changes in the concentration of hepatic enzymes in rats. Further studies are warranted to assess the hepatotoxic properties of these herbs. We recommend that herbal formulations available in pharmaceutical markets be more closely controlled in terms of quality, as well as toxicity, especially with regard to the possible effects on the hepatic function.
LIMITATIONS
Important intrinsic limitations include the fact that histological studies are relatively less on the hepatotoxic plants and often the unknown nature of induced hepatic damage plants. On the other hand, plants include various combinations, so it is difficult to accurately identify a toxic compound in the plant. Hence, further research is needed to assess the prevalence, efficacy, and safety of commonly used herbs.
ACKNOWLEDGEMENT
This article is the result of a research that was funded by the Research Council of the Isfahan University of Medical Sciences and the Isfahan Pharmaceutical Sciences Research Center (research project No. 81389).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH. Drug-induced liver injury: Mechanisms and test systems. Hepatology. 2001;33:1009–13. doi: 10.1053/jhep.2001.23505. [DOI] [PubMed] [Google Scholar]
- 2.Larrey D. Drug-induced liver diseases. J Hepathol. 2000;32:77–88. doi: 10.1016/s0168-8278(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–85. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 4.Herbal medicines and perioperative care. JAMA. 2001;286:208–16. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- 5.Barrett B, Kiefer D, Rabago D. Assessing the risks and benefits of herbal medicine: An overview of scientific evidence. Altern Ther Health Med. 1999;5:40–9. [PubMed] [Google Scholar]
- 6.Egan CD. Addressing the use of herbal medicine in the primary care setting. J Am Acad Nurse Pract. 2002;14:166–71. doi: 10.1111/j.1745-7599.2002.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 7.Fungh-Berman A. Herbal supplements: Indications, clinical concerns, and safety. Nutr Today. 2002;37:122–4. doi: 10.1097/00017285-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Shad JA, Chinn CG, Brann OS. Acute hepatitis after ingestion of herbs. South Med J. 1999;92:1095–7. doi: 10.1097/00007611-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Bach N, Thung SN, Schaffner F. Comfrey herb tea-induced hepatic veno-occlusive disease. Am J Med. 1989;87:97–9. doi: 10.1016/s0002-9343(89)80492-9. [DOI] [PubMed] [Google Scholar]
- 10.Kavitha P, Ramesh R, Bupesh G, Stalin A, Subramanian P. Hepatoprotective activity of Tribulus terrestris extract against acetaminophen-induced toxicity in a freshwater fish (Oreochromis mossambicus) In Vitro Cell Dev Biol Anim. 2011;47:698–706. doi: 10.1007/s11626-011-9457-9. [DOI] [PubMed] [Google Scholar]
- 11.Tapia MO, Giordano MA, Gueper HG. An outbreak of hepatogenous photosensitization in sheep grazing Tribulus terrestris in Argentina. Vet Hum Toxicol. 1994;36:311–3. [PubMed] [Google Scholar]
- 12.Glostonbury JR, Doughty FR, Whitaker SJ, Sergeant E. A syndrome of hepatogenous photosensitisation, resembling geeldikkop, in sheep grazing Tribulus terrestris. Aust Vet J. 1984;61:314–6. doi: 10.1111/j.1751-0813.1984.tb07135.x. [DOI] [PubMed] [Google Scholar]
- 13.Sangeeta D, Sidhu H, Thind SK, Nath R. Effect of Tribulus terrestris on oxalate metabolism in rats. J Ethnopharmacol. 1994;44:61–6. doi: 10.1016/0378-8741(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 14.Haroun ME, Mohmoud OM, Adam SE. Effect of feeding Cuminum cyminum fruits, Thymus vulgaris leaves or their mixture to rats. Vet Hum Toxicol. 2002;44:67–9. [PubMed] [Google Scholar]
- 15.Ozbek H, Uğraş S, Dülger H, Bayram I, Tuncer I, Oztürk G, et al. Hepatoprotective effects of Foeniculum vulgare essential oil. Fitoterapia. 2003;74:317–9. doi: 10.1016/s0367-326x(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 16.Mcintyre M. A review of the benefits, adverse events, drug interactions, and safety of St. John's Wort (Hypericum perforatum): The implications with regard to the regulation of herbal medicines. J Altern Complement Med. 2000;6:115–24. doi: 10.1089/acm.2000.6.115. [DOI] [PubMed] [Google Scholar]


