Abstract
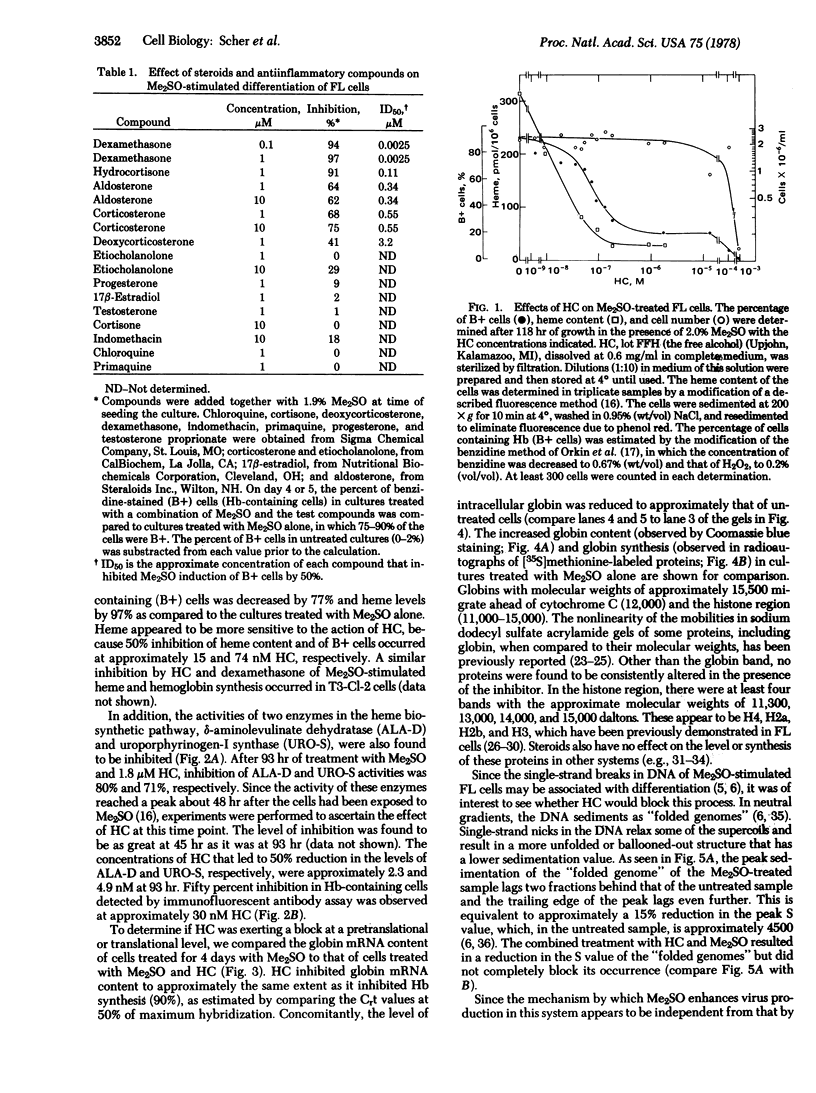
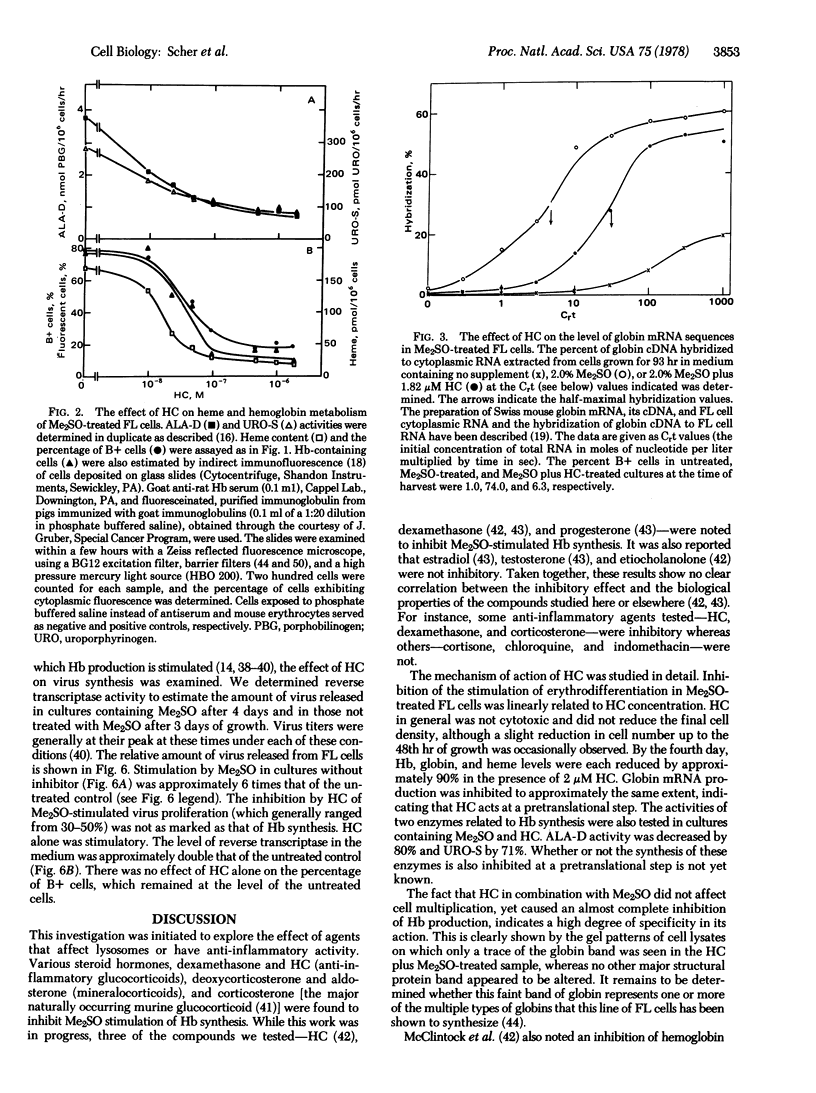
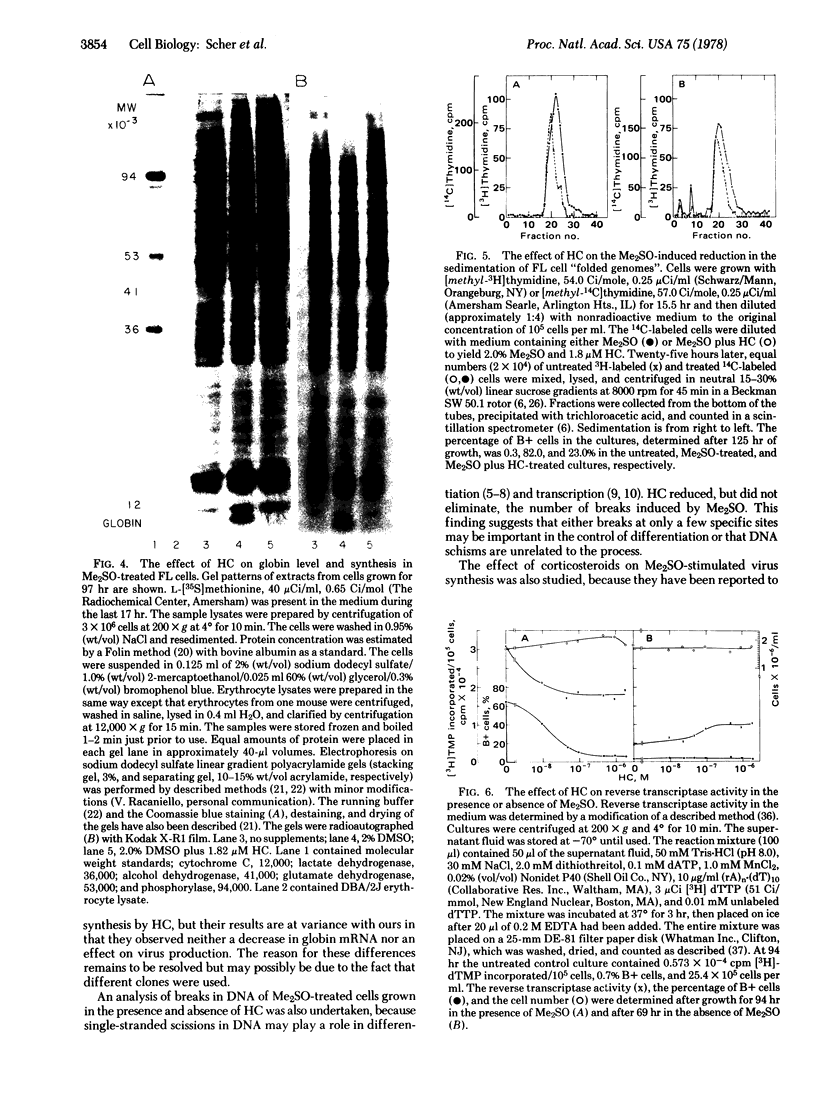
Erythrodifferentiation and hemoglobin synthesis in dimethyl sulfoxide-stimulated Friend erythroleukemia cells were inhibited by hydrocortisone (HC) and four other steroids: dexamethasone, deoxycorticosterone, corticosterone, and aldosterone. The effect was specific, because no significant cytotoxicity occurred with any of these compounds at the concentrations that were inhibitory. The mechanism of action of HC was studied in detail. In the absence of dimethyl sulfoxide, it had no effect on hemoglobin levels; but, in the presence of this inducer, the synthesis of heme and globin were each inhibited by approximately 90%. There was no alteration in the synthesis of any major protein other than globin, as determined by gel electrophoresis of cell lysates. The activities of two enzymes in the heme biosynthetic pathway, delta-aminolevulinate dehydratase and uroporphyrinogen-I synthase, were inhibited by 80% and 70%, respectively. Globin mRNA induction was reduced by approximately 90%. This demonstrated that the HC inhibition of globin synthesis occurred at a pretranslational step. The dimethyl sulfoxide-induced single-stranded breaks in DNA, which have been suggested to play a role in Friend leukemia cell differentiation, were reduced in number but not eliminated. HC reduced the dimethyl sulfoxide-stimulation of virus release into the medium by approximately 50%. HC treatment in the absence of dimethyl sulfoxide doubled the production of virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Appleby D. W., Modak S. P. DNA degradation in terminally differentiating lens fiber cells from chick embryos. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5579–5583. doi: 10.1073/pnas.74.12.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976 Nov;9(3):393–407. doi: 10.1016/0092-8674(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Bick M. D. Bromodeoxyuridine inhibition of Friend leukemia cell induction. Mechanism of reversal by deoxycytidine. Biochim Biophys Acta. 1977 Jun 17;476(4):279–286. doi: 10.1016/0005-2787(77)90292-1. [DOI] [PubMed] [Google Scholar]
- Blankstein L. A., Levy S. B. Changes in histone f2a2 associated with proliferation of Friend leukaemic cells. Nature. 1976 Apr 15;260(5552):638–640. doi: 10.1038/260638a0. [DOI] [PubMed] [Google Scholar]
- Blankstein L. A., Stollar B. D., Franklin S. G., Zweidler A., Levy S. B. Biochemical and immunological characterization of two distinct variants of histone H2A in Friend leukemia. Biochemistry. 1977 Oct 18;16(21):4557–4562. doi: 10.1021/bi00640a003. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Price P., Pedrinan L., Acs G. Correlation between hypomethylation of DNA and expression of globin genes in Friend erythroleukemia cells. Eur J Biochem. 1977 Nov 15;81(1):53–61. doi: 10.1111/j.1432-1033.1977.tb11926.x. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Tragnos F., Sharpless T., Friend C., Melamed M. R. Nuclear chromatin changes during erythroid differentiation of friend virus induced leukemic cells. Exp Cell Res. 1976 May;99(2):301–309. doi: 10.1016/0014-4827(76)90587-5. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Plowman J. K., Kelley S. P., Arthur L. O., Hillman E. A. Enhanced production of mouse mammary tumor virus in dexamethasone-treated, 5-iododeoxyuridine-stimulated mammary tumor cell cultures. J Natl Cancer Inst. 1974 Jun;52(6):1881–1886. doi: 10.1093/jnci/52.6.1881. [DOI] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch J. W., Bedigian H. G., Meier H. Steroid hormones increase the growth of MuLV in rat tumor XC cells. Virology. 1977 Dec;83(2):462–466. doi: 10.1016/0042-6822(77)90196-9. [DOI] [PubMed] [Google Scholar]
- Grossi G., Macchiato M., Gialanella G., Cascino A. Endonucleolytic cleavage of parental DNA and T4 late-gene expression: distribution analysis of single-strand and double-strand breaks. Eur J Biochem. 1977 Oct 17;80(1):73–77. doi: 10.1111/j.1432-1033.1977.tb11857.x. [DOI] [PubMed] [Google Scholar]
- HUGGINS C., YANG N. C. Induction and extinction of mammary cancer. A striking effect of hydrocarbons permits analysis of mechanisms of causes and cure of breast cancer. Science. 1962 Jul 27;137(3526):257–262. doi: 10.1126/science.137.3526.257. [DOI] [PubMed] [Google Scholar]
- Hanoune J., Feigelson P. The absence of cortisone effect on the synthesis of specific histones and ribosomal protein subunits in liver. Biochem Biophys Res Commun. 1969 Jan 27;34(2):215–220. doi: 10.1016/0006-291x(69)90634-2. [DOI] [PubMed] [Google Scholar]
- Hendry L. B., Witham F. H., Chapman O. L. Gene regulation: the involvement of stereochemical recognition in DNA-small molecule interactions. Perspect Biol Med. 1977 Autumn;21(1):120–130. doi: 10.1353/pbm.1977.0018. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau A. F., Ruddon R. W. Proteins of transcriptionally active and inactive chromatin from Friend erythroleukemia cells. Exp Cell Res. 1977 Jun;107(1):35–46. doi: 10.1016/0014-4827(77)90383-4. [DOI] [PubMed] [Google Scholar]
- Leibovitch S. A., Tapiero H., Harel J. Single-stranded DNA from oncornavirus-infected cells enriched in virus-specific DNA sequences. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3720–3724. doi: 10.1073/pnas.74.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. R. Histone acetylation and hormone action. Early effects of oestradiol-17beta on histone acetylation in rat uterus. Biochem J. 1972 Dec;130(3):663–669. doi: 10.1042/bj1300663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Giotta G., Nork E., Nicolson G. L. Characterization of the inhibitory effects of retinoids on the in vitro growth of two malignant murine melanomas. J Natl Cancer Inst. 1978 May;60(5):1035–1041. doi: 10.1093/jnci/60.5.1035. [DOI] [PubMed] [Google Scholar]
- Mayer R. J., Smith R. G., Gallo R. C. Reverse transcriptase in normal rhesus monkey placenta. Science. 1974 Sep 6;185(4154):864–867. doi: 10.1126/science.185.4154.864. [DOI] [PubMed] [Google Scholar]
- Misch D. W., Misch M. S. Histochemical activation of rat-liver lysosomes by dimethyl sulfoxide. Histochemie. 1973 Oct 26;37(2):131–140. doi: 10.1007/BF00305584. [DOI] [PubMed] [Google Scholar]
- Modak S. P., Bollum F. J. Terminal lens cell differentiation. 3. Initiator activity of DNA during nuclear degeneration. Exp Cell Res. 1970 Oct;62(2):421–432. doi: 10.1016/0014-4827(70)90573-2. [DOI] [PubMed] [Google Scholar]
- Neumann J. R., Housman D., Ingram V. M. Nuclear protein synthesis and phosphorylation in Friend erythroleukemia cells stimulated with DMSO. Exp Cell Res. 1978 Feb;111(2):277–284. doi: 10.1016/0014-4827(78)90171-4. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Hays E. F., Doyle T., Linkhart S., Medeiros E., Pickering R. Oncornaviruses produced by murine leukemia cells in culture. Virology. 1977 Sep;81(2):363–370. doi: 10.1016/0042-6822(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Ono T., Terayama H., Takaku F., Nakao K. Hydrocortisone effect upon the phytohemagglutin-stimulated acetylation of histones in human lymphocytes. Biochim Biophys Acta. 1969 Mar 18;179(1):214–220. doi: 10.1016/0005-2787(69)90138-5. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Preisler H. S., Housman D., Scher W., Friend C. Effects of 5-bromo-2' -deoxyuridine on production of globin messenger RNA in dimethyl sulfoxide-stimulated Friend leukemia cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2956–2959. doi: 10.1073/pnas.70.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., Magarian C., Borun T. W. Resolution of hemoglobin subunits by electrophoresis in acid urea polyacrylamide gels containing Triton X-100. Anal Biochem. 1978 Apr;85(2):506–518. doi: 10.1016/0003-2697(78)90248-8. [DOI] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher W., Friend C. Breakage of DNA and alterations in folded genomes by inducers of differentiation in Friend erythroleukemic cells. Cancer Res. 1978 Mar;38(3):841–849. [PubMed] [Google Scholar]
- Scher W., Preisler H. D., Friend C. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro. 3. Effects of 5-bromo-2'-deoxyuridine, dimethylformamide and dimethylsulfoxide. J Cell Physiol. 1973 Feb;81(1):63–70. doi: 10.1002/jcp.1040810108. [DOI] [PubMed] [Google Scholar]
- Sherton C. C., Evans L. H., Polonoff E., Kabat D. Relationship of Friend murine leukemia virus production to growth and hemoglobin synthesis in cultured erythroleukemia cells. J Virol. 1976 Jul;19(1):118–125. doi: 10.1128/jvi.19.1.118-125.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman D. H., Riley V. Corticosterone concentrations in the mouse. Science. 1978 Apr 7;200(4337):87–87. doi: 10.1126/science.635580. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Hamilton T. H. Role of chromatin in estrogen action in the uterus. II. Hormone-induced synthesis of nonhistone acidic proteins which restore histone-inhibited DNA-dependent RNA synthesis. Proc Natl Acad Sci U S A. 1969 Jun;63(2):465–472. doi: 10.1073/pnas.63.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M., Fried J., Nudel U., Rifkind R. A., Marks P. A. Transient inhibition of initiation of S-phase associated with dimethyl sulfoxide induction of murine erythroleukemia cells to erythroid differentiation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):248–252. doi: 10.1073/pnas.74.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M., Nudel U., Fibach E., Rifkind R. A., Marks P. A. Changes in DNA associated with induction of erythroid differentiation by dimethyl sulfoxide in murine erythroleukemia cells. Cancer Res. 1978 Mar;38(3):835–840. [PubMed] [Google Scholar]
- Tung J. S., Knight C. A. Effect of charge on the determination of molecular weight of proteins by gel electrophoresis in SDS. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1117–1121. doi: 10.1016/0006-291x(71)90020-9. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Sessa G., Bevans V. Effect of DMSO on the stabilization of lysosomes by cortisone and chloroquine in vitro. Ann N Y Acad Sci. 1967 Mar 15;141(1):326–332. doi: 10.1111/j.1749-6632.1967.tb34897.x. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Reitz M. S., Paran M., Gallo R. C. Mechanism of stimulation of murine type-C RNA tumor virus production by glucocorticoids: post-transcriptional effects. J Virol. 1974 Oct;14(4):802–812. doi: 10.1128/jvi.14.4.802-812.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]




