Abstract
Background:
Psoriasis is a common dermatologic disorder, with fluctuating response to treatment. Considering the proven immunomodulatory effects of oral simvastatin in psoriasis, this trial study was enrolled to determine whether the topical form has also antipsoriatic effects. Vitamin D analogs known to be effective and are considered the first line of therapy in mild to moderate cases. In this study, the efficacy of topical calcipotriol 0.005% ointment (as a standard method of treatment for psoriasis) versus combination of calcipotriol plus topical simvastatin is compared in the treatment of psoriasis.
Materials and Methods:
A total of 80 subjects with symmetric psoriasis who had body surface involvement up to 20% were divided randomly into 2 groups. Group A were treated with calcipotriol 0.005% ointment twice daily and Group B with calcipotriol 0.005% ointment twice daily and simvastatin 3% ointment twice daily for 12 weeks. The results were evaluated by a Blind Dermatologist using psoriasis area severity index (PASI) score at baseline, 4th, 8th and 12th week of treatment. In a similar way, a subjective assessment performed by patients based on photo-evaluation at the end of the study.
Results:
Despite a continuous reduction in PASI score in both groups, according to both physician (P = 0.603) and patient (P = 0.243) assessment topical simvastatin was not statistically more effective than conventional treatment of psoriasis at the end of the study.
Conclusion:
This study indicates that topical simvastatin is not associated with significant impacts in the treatment of psoriasis as compared to oral form. This study indicates that psoriasis is a systemic disorder with variable skin manifestations.
Keywords: Calcipotriol, psoriasis, simvastatin
INTRODUCTION
The chronic, long-lasting character of psoriasis, along with its physical, psychological and social repercussions, constitutes difficulties in controlling the disease.[1,2,3,4,5] Psoriasis is an immunologically mediated skin disease affecting the skin, hairs, nails and joints. The world-wide estimated prevalence of this common skin disease is around 2%.[6]
Regarding the effectiveness and safety of topical calcipotriol, this drug has been become the first line therapy for mild to moderate cases of psoriasis since 1990.[6] However, the efficacy of calcipotriol remains modest.[7]
The advent of biological medications in the area of psoriasis treatment, have led to the development of investigations on the drugs that play role in modulating of cytokines.[8,9] With clarification of the immunomodulatory aspect of statins besides antihyperlipidemic effects,[10,11,12,13,14,15] several studies on the potential aspect of oral simvastatin in the treatment of psoriasis have been carried out.[16,17,18]
Due to the fact that psoriasis is a dermatologic disease, there is a common predilection to conduct surveys on topical therapies that are effective, safe and inexpensive. In spite of the confirmed effectiveness of oral simvastatin in the treatment of psoriasis, data in favor of the anti-inflammatory and wound healing potentials of the topical application of simvastatin has been confirmed.[19,20] Taken together, by incorporation of simvastatin in ointment formulation, a topical form of the drug is formed.[20]
The goal of the current study was thus to determine if the combination of topical simvastatin with calcipotriene is more effective than calcipotriene alone in patients with psoriasis. Both have no significant adverse effects. Furthermore, the sample size was large to reach the conclusion.
MATERIALS AND METHODS
This randomized, controlled trial was conducted on patients with psoriasis referring to the outpatient Department of Dermatology of Azahra Teaching Hospital in Isfahan (Iran), during March 2012 to December 2012. Patients included to in this study were subjects of 20-60 years old, with mild to moderate psoriasis. Patients should have had less than 20% of the involved body surface, with plaque size greater than 2 cm × 2 cm, but lesser than 15 cm × 15 cm. The study was approved by the Ethical Committee of Isfahan University of Medical Sciences. Research project number was 9028.
Patients with a current diagnosis of unstable or pustular psoriasis, only involvement of scalp, nail, flexural or palmoplantar areas, pregnancy and breast feeding, impaired kidney function and allergy to the vitamin D3 analogs were excluded from the study.
Apart from the predictable association of psoriasis with obesity and metabolic syndrome, a detailed history was taken from each patient regarding involved or deteriorating factors (Bacterial infections, human immunodeficiency virus infection, pregnancy, hypocalcemia and causative drug consumption including terbinafine, non-steroidal anti-inflammatory drugs, anti-malarias, angiotensin-converting enzyme inhibitors, lithium, interferon [IFN] and beta blockers).
With regard to type I error (alpha) = 0.05, study power = 80% and expected difference of 30% in response rate, sample size was calculated as 40 subjects in each group. The study was approved by the Ethical Committee of Isfahan University of Medical Sciences. Research project number was 9028.
After initial screening visit, this double-blind clinical trial was initiated with 80 eligible subjects (aged 20-58 years). Patients were divided into two different treatment groups, using a table of random numbers. Finally, 71 patients with plaques of psoriasis sized greater than 2 cm × 2 cm, lesser than 15 cm × 15 cm on their bodies completed treatment modality. 4 subjects excluded from the study due to aggravation of lesions and 5 subjects were not willing to complete the study.
In each group, 40 patients for 12 weeks of treatment were enrolled in the study. All patients of 2 groups have been received calcipotriol 0.005% ointment (Psoriament 50 MCG/G 30G Oint; Aburaihan Co, Iran) twice daily in the morning and before sleeping. In addition, patients in Group B received have been received simvastatin 3% ointment twice daily.[20] Patients were advised to report the occurrence of any unwilling adverse effects (redness, burning, itching and erosion) during the 12 weeks of the study.
Evaluation of improvement and side-effects were conducted at baseline and 4th, 8th and 12th week of treatment. In each visit, digital photographs were taken by a facial photo fixture using a Canon Power shot G12 stand-off camera.
The improvement scoring system based on changes in erythema, induration and scaling was conducted through psoriasis area and severity index (PASI).[21] PASI as an accepted benchmark for clinical research study of psoriasis is considered for evaluation of results of any treatment modality in psoriasis. However, it is not commonly used in the daily management of patients in the clinical setting.
In order to evaluate patient satisfaction surveys, assessment of the treatment areas using comparative photographs were conducted by patient based satisfaction and 12 weeks after starting of treatment (Patient Global Assessment). The improvement was scored from 0 to 10 by a visual analog scale (0 as no improvement and 10 as the best possible improvement).[22]
A consort diagram was provided [Figure 1] and the statistical analysis was carried out by SPSS for Windows software (SPSS Inc., Chicago, IL, USA, version 18.0) by using Chi-square, independent t-test, ANCOVA and repeated measures ANOVA analyses. The significance level was set at P value of less than 0.05.
Figure 1.
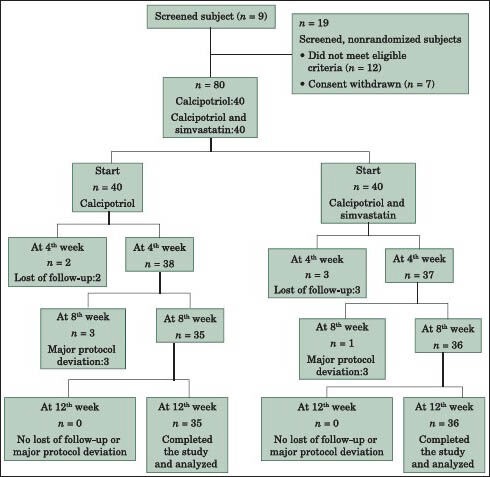
The consort diagram
RESULTS
Eighty subjects (100%) with a body surface area involvement of <20% were recruited in the study. At the end of study, 9 subjects failed to complete the study. 4 subjects (3 subjects in Group A and 1 subject in Group B) excluded from the study due to persistence or aggravation of lesions and 5 subjects (2 subjects in Group A and 3 subjects in Group B) gave up the study due to scheduling difficulties.
The mean age of patients was 31.55 ± 9.5 years in Group A and 32.5 ± 10.7 years in Group B. The difference in mean ages of the two groups was not statistically significant (P = 0.85). Group A included 19 (47.5%) female and 21 (52.5%) male patients. Group B included 22 (55%) female and18 (45%) male patients. There was no significant difference between sex ratios in the two groups (P = 0.5).
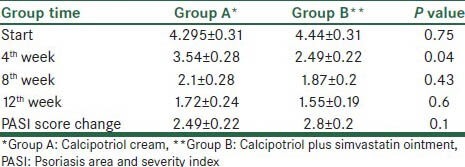
The mean PASI score before treatment was not statistically different between the 2 groups (P = 0.746). A steadily reduction in the mean PASI score in both groups was detected. By means of repeated measure ANOVA test, the reduction in PASI values from base to end of study in both Group A (P = 0.001) and Group B (P = 0.001) confirmed. At the end of 12th week of treatment, compared to the baseline a mean percentage of PASI reduction of 63% and 70% was noted in Groups A and B respectively. By independent t-test, at the end of 4th week, patients in Group B more likely experienced improvement (P = 0.004), but no statistically difference at the end of 8th (P = 0.43) and 12th (P = 0.603) week was noted [Table 1].
Table 1.
Psoriasis parameters of comparison of calcipotriol and calcipotriol plus simvastatin
With regard to the improvement score from 0 to 10 by the visual analog scale, more patients in Group B at the end of the study reported scales greater than 5 rather than patients in Group A (32 subjects versus 25 subjects). In addition, nobody in Group B reported improvement less than 3 according to improvement score. However, by means of independent t-test, patient's satisfaction survey was compatible with dermatologist evaluation. In other words, statistical analysis revealed no significant difference between 2 groups (P = 0.24).
Although, there was no serious adverse reactions, some reports of burning in both groups was noted. Among 6 subjects complained of mild irritation in Group A, 4 subjects reported involvement in the facial region. By comparison, 5 subjects reported mild burning in Group B with 4 of them were related to the facial region. However, overall tolerability was good and nobody gave up the study in both groups due to side-effects.
DISCUSSION
Psoriasis, a common papulosquamous disorder responds to therapy with fluctuating results.[6] Although, topical vitamin D analogs including calcipotriol and topical corticosteroids are the cornerstone of treatment of mild to moderate psoriasis,[6] the overall outcome still remains modest.[8] Indeed, a request for finding a cheap, safe and common treatment is without cease.
For decades psoriasis has been considered an epidermal disease. With the advent of vitamin D analogs in the early 1990s, a revolution occurred in the treatment of psoriasis with drugs those have limited toxicity rather than corticosteroids. However, the former drugs including calcipotriol mainly regulate proliferation and differentiation of the keratinocytes.[6,23] Following the success in the cognition of the role of immune factors in the pathogenesis of psoriasis, psoriasis is now considered a polygenic disease with both impairment of immune function and keratinocyte biology.[24]
In the immunopathogenesis model, psoriasis is considered a Th1-mediated disease.[22,23] Moreover, NK T cells, neutrophils and nitric oxide are more expressed in psoriatic plaques.[6] IFN-γ, which is released by both T cells and NK cells, by activation of the signal transducers and activators of transcription factor family, induce the more accumulation of T cells and nitric oxide expression.[6]
Other than inhibiting of 3-hydroxy-3-methyl glutaryl coenzyme A, other interesting mechanisms by which statins are therapeutic are anti-inflammatory properties. By means of flow cytometry, it was shown that simvastatin are direct inhibitors of IFN-γ.[26] Furthermore, statins down-regulates the expression of adhesion molecules intercellular adhesion molecule-1 (ICAM-1), lymphocyte function associated antigen-1 (LFA-1) and monocyte chemotactic protein-1, on leucocytes and endothelial cells.[27] By binding to LFA-1, the LFA/ICAM interaction inhibited.[15] Moreover, considering psoriasis as a Th1 disease with overproduction of IL-17 and IFN-γ, statins shift Th1/Th2 balance.[25]
In the present study to our knowledge, we provide the first clinical evidence that measures quantitatively the effectiveness of topical simvastatin in the treatment of psoriasis. Since, the effectiveness of topical calcipotriol as the first line of therapy in mild to moderate psoriasis has been confirmed, both groups received calcipotriol. By adding topical simvastatin to the subjects of Group B, the efficacy of combination therapy (including calcipotriol and topical simvastatin) in comparison of mono-therapy (including calcipotriol) has become measurable.
Calcipotriol included among the most noticeable approaches for mild to moderate plaque psoriasis. The reduced PASI value for topical calcipotriol in literature was near 60%.[6]
Juxtaposition of the results of the Group A (reflecting only the effect of calcipotriol) and the findings of the literature, figures an incredible compatibility.
Previous studies focused on the effectiveness of combination of calcipotriol and corticosteroid in the treatment of psoriasis. Fenton and Plosker showed that up to 4 weeks of therapy with calcipotriol/betamethasone dipropionate provides an effective and well tolerated treatment. This paper emphasized on the long term safety of this combination.[28] Recently, in another study by Feldman et al. foam formulation of calcipotriol was safe and effective for the treatment of mild to moderate plaque-type psoriasis for up to 8 weeks.[29]
In our study, despite the more rapid reduction in PASI score in patients of Group B at the end of 4th week of treatment, the rate of improvement at the end of the study was not significant.
Wolkenstein et al. has noticed the diminished risk of psoriasis associated with statin intake.[30] In another study Naseri et al., evaluated the efficacy of oral simvastatin on improvement of psoriasis.[28] This paper has shown significantly more reduction of PASI score in the group received oral simvastatin compared to group who did not (P = 0.001).[18]
In comparison to aforementioned studies on the beneficial impact of oral simvastatin in psoriasis, the number of patients with remarkable response in our study was not significant. This difference in response could reflect the fact that psoriasis is a systemic disorder with dermatologic manifestation. In overview of immunorelated mechanisms that play the role in the pathogenesis of psoriasis, investigators noticed that even associated findings such as obesity mirrored underlying inflammatory base.[31] In other words; topical treatment in such condition is not as effective as systemic form.
Considering the studies that confirmed the effectiveness of topical statins in the treatment of dermatologic disorders are limited to local conditions such as contact dermatitis and wound healing.[19,20]
However, many patients still consider psoriasis only a dermatologic disorder. With the advent of nanoparticle preparation of topical drugs in solid lipid nanoparticles, a topical form of the drug with more bioavailability is formed.[32] Despite the fact that the bioavailability, even more than oral form is predictable by the nanoparticle loaded preparations according to past pharmaceutical surveys,[32] no clinical trial study in the treatment of psoriasis has not recruited to date. With regard to insignificant results of the common topical form of simvastatin in the treatment of psoriasis, in future studies the effectiveness of topical form of statins, should be followed in the nanoparticle loaded preparations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–7. doi: 10.1016/s0190-9622(99)70112-x. [DOI] [PubMed] [Google Scholar]
- 2.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: Results of a 1998 national psoriasis foundation patient-membership survey. Arch Dermatol. 2001;137:280–4. [PubMed] [Google Scholar]
- 3.Choi J, Koo JY. Quality of life issues in psoriasis. J Am Acad Dermatol. 2003;49(Suppl 2):57–61. doi: 10.1016/s0190-9622(03)01136-8. [DOI] [PubMed] [Google Scholar]
- 4.de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: A systematic literature review. J Investig Dermatol Symp Proc. 2004;9:140–7. doi: 10.1046/j.1087-0024.2003.09110.x. [DOI] [PubMed] [Google Scholar]
- 5.Mease PJ, Menter MA. Quality-of-life issues in psoriasis and psoriatic arthritis: Outcome measures and therapies from a dermatological perspective. J Am Acad Dermatol. 2006;54:685–704. doi: 10.1016/j.jaad.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 6.van de Kerkhof P, Nestle F. Psoriasis. In: Bolognia J, Jorizzo J, Schaffer J, editors. Dermatolog. 3rd edition. Elsevier Saunders; 2012. pp. 135–56. [Google Scholar]
- 7.Barnes L, Altmeyer P, Fôrstrôm L, Stenström MH. Long-term treatment of psoriasis with calcipotriol scalp solution and cream. Eur J Dermatol. 2000;10:199–204. [PubMed] [Google Scholar]
- 8.Gottlieb AB. Psoriasis: Emerging therapeutic strategies. Nat Rev Drug Discov. 2005;4:19–34. doi: 10.1038/nrd1607. [DOI] [PubMed] [Google Scholar]
- 9.Sterry W, Barker J, Boehncke WH, Bos JD, Chimenti S, Christophers E, et al. Biological therapies in the systemic management of psoriasis: International consensus conference. Br J Dermatol. 2004;151(Suppl 69):3–17. doi: 10.1111/j.1365-2133.2004.06070.x. [DOI] [PubMed] [Google Scholar]
- 10.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 11.Dobreanu M, Dobreanu D, Fodor A, Bacarea A. Integrin expression on monocytes and lymphocytes in unstable angina short term effects of atorvastatin. Rom J Intern Med. 2007;45:193–9. [PubMed] [Google Scholar]
- 12.Yamashita M, Otsuka F, Mukai T, Otani H, Inagaki K, Miyoshi T, et al. Simvastatin antagonizes tumor necrosis factor-alpha inhibition of bone morphogenetic proteins-2-induced osteoblast differentiation by regulating Smad signaling and Ras/Rho-mitogen-activated protein kinase pathway. J Endocrinol. 2008;196:601–13. doi: 10.1677/JOE-07-0532. [DOI] [PubMed] [Google Scholar]
- 13.Asarch A, Barak O, Loo DS, Gottlieb AB. Th17 cells: A new therapeutic target in inflammatory dermatoses. J Dermatolog Treat. 2008;19:318–26. doi: 10.1080/09546630802206660. [DOI] [PubMed] [Google Scholar]
- 14.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 15.Namazi MR. Statins: Novel additions to the dermatologic arsenal? Exp Dermatol. 2004;13:337–9. doi: 10.1111/j.0906-6705.2004.00208.x. [DOI] [PubMed] [Google Scholar]
- 16.Späh F. Inflammation in atherosclerosis and psoriasis: Common pathogenic mechanisms and the potential for an integrated treatment approach. Br J Dermatol. 2008;159(Suppl 2):10–7. doi: 10.1111/j.1365-2133.2008.08780.x. [DOI] [PubMed] [Google Scholar]
- 17.Shirinsky IV, Shirinsky VS. Efficacy of simvastatin in plaque psoriasis: A pilot study. J Am Acad Dermatol. 2007;57:529–31. doi: 10.1016/j.jaad.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 18.Naseri M, Hadipour A, Sepaskhah M, Namazi MR. The remarkable beneficial effect of adding oral simvastatin to topical betamethasone for treatment of psoriasis: A double-blind, randomized, placebo-controlled study. Niger J Med. 2010;19:58–61. doi: 10.4314/njm.v19i1.54216. [DOI] [PubMed] [Google Scholar]
- 19.Otuki MF, Pietrovski EF, Cabrini DA. Topical simvastatin: Preclinical evidence for a treatment of skin inflammatory conditions. J Dermatol Sci. 2006;44:45–7. doi: 10.1016/j.jdermsci.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Adami M, Prudente Ada S, Mendes DA, Horinouchi CD, Cabrini DA, Otuki MF. Simvastatin ointment, a new treatment for skin inflammatory conditions. J Dermatol Sci. 2012;66:127–35. doi: 10.1016/j.jdermsci.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Fredriksson T, Pettersson U. Severe psoriasis – Oral therapy with a new retinoid. Dermatologica. 1978;157:238–44. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 22.Flytström I, Stenberg B, Svensson Å, Bergbrant IM. Patients’ visual analogue scale: A useful method for assessing psoriasis severity. Acta Derm Venereol. 2012;92:347–8. doi: 10.2340/00015555-1237. [DOI] [PubMed] [Google Scholar]
- 23.Kragballe K. New York: Marcel Dekker; 2000. Vitamin D in Dermatology. [Google Scholar]
- 24.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger JG, Bowcock A. Psoriasis pathophysiology: Current concepts of pathogenesis. Ann Rheum Dis. 2005;64(Suppl 2):ii30–6. doi: 10.1136/ard.2004.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwak B, Mulhaupt F, Veillard N, Pelli G, Mach F. The HMG-CoA reductase inhibitor simvastatin inhibits IFN-gamma induced MHC class II expression in human vascular endothelial cells. Swiss Med Wkly. 2001;131:41–6. doi: 10.4414/smw.2001.06144. [DOI] [PubMed] [Google Scholar]
- 27.Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–30. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 28.Fenton C, Plosker GL. Calcipotriol/betamethasone dipropionate: A review of its use in the treatment of psoriasis vulgaris. Am J Clin Dermatol. 2004;5:463–78. doi: 10.2165/00128071-200405060-00012. [DOI] [PubMed] [Google Scholar]
- 29.Feldman SR, Matheson R, Bruce S, Grande K, Markowitz O, Kempers S, et al. Efficacy and safety of calcipotriene 0.005% foam for the treatment of plaque-type psoriasis: Results of two multicenter, randomized, double-blind, vehicle-controlled, phase III clinical trials. Am J Clin Dermatol. 2012;13:261–71. doi: 10.2165/11630710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Wolkenstein P, Revuz J, Roujeau JC, Bonnelye G, Grob JJ, Bastuji-Garin S, et al. Psoriasis in France and associated risk factors: Results of a case-control study based on a large community survey. Dermatology. 2009;218:103–9. doi: 10.1159/000182258. [DOI] [PubMed] [Google Scholar]
- 31.Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-alpha blockade on metabolic syndrome components in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61–73. doi: 10.1111/j.1529-8019.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 32.Gambhire M, Bhalekar M, Shrivastava B. Bioavailability assessment of simvastatin loaded solid lipid nanoparticles after oral administration. J Pharm Sci. 2011;6:251–8. [Google Scholar]


