Abstract
Bacopa monnieri (L.) is an important medicinal plant mainly known as a memory enhancing herb. It is important to see the effect of metal pollution on its active constituents. In this context, efforts have been made to observe the effect of Cd on the triterpenoid saponins bacoside A and bacopaside I in this plant. The influence of the metal on growth parameters like protein, chlorophyll content, and biomass has also been observed. It is interesting to note that the bacoside A and bacopaside I gradually increased by the Cd treatment up to 10 μM and then decreased at higher concentrations, that is, 50 and 100 μM, but the concentration of these components was more in all the treated plants as compared to control. On the contrary, protein, chlorophyll content, and biomass decreased with the increase in metal concentration and exposure duration due to metal toxicity.
1. Introduction
Bacopa monnieri (L) Pennell (Fam. Scrophulariaceae) is growing in the marshy places, which may be natural, or manmade with high possibility of heavy metal contamination as manmade marshy places are generally created in industrial areas where industrial effluents were discharged with high metal content. In such places one can see the luxuriant growth of B. monnieri. In this context, it becomes necessary to check the effect of heavy metals on general growth and active constituents of the plant, because demand of this plant is quite high in market and plant collectors collect it without considering polluted or nonpolluted site. Cadmium is one of the heavy metals that is of great concern in the environment because of its toxicity to all the plants, animals, and human beings. Cd enters in the environment through industrial waste from processes such as electroplating, manufacturing of plastics, mining, paint pigments, alloy preparation, and batteries that contain cadmium [1, 2]. Cadmium is also used for luminescent dials, in photography and rubber curing, and as fungicides [1]. The most likely origin of the excess Cd is from heavy applications of cheap, contaminated phosphate fertilizers [3, 4]. It is considered to be highly mutagenic and designated as human carcinogen by International Agency for Research on Cancer [5, 6]. It is retained for many years in the human body and may induce chronic toxicity [7, 8]. Elevated levels of Cd in humans can cause kidney damage, and low levels of Cd in the diet are linked renal to dysfunction. Other diseases associated with Cd exposure are pulmonary emphysema and the notorious Itai-Itai (“ouch-ouch”) disease [9]. Therefore, effective and economical techniques are needed to remediate Cd-contaminated soils.
B. monnieri is a small creeping herb, commonly growing in marshy places up to an altitude of 1500 m. It is an important Ayurvedic drug and traditionally it is reported to be used in skin diseases, fever, inflammation, anaemia, urinary disorder, and psychiatric disorders [10]. It is also considered to be cardiotonic, a potent nervine tonic, and useful for asthma, hoarseness, insanity, and epilepsy [11]. Ethnobotanically the leaves are used in speech disorders [12]; in premature ejaculation [13]; flatulence [14]; abdominal pain [15]; cough and cold [16–18], and leaf juice is used in rheumatism [19, 20] revitalizer of intellectual faculty [21]. The major therapeutically important chemical constituents of this plant are triterpenoid saponins bacosides. The pharmacological [22, 23] and clinical studies [24, 25] on the extract and bacoside A and B have been published. The extracts have economic importance too as it is widely available in the international nutraceutical markets.
Although some reports are available on the accumulation and toxicity of heavy metals in B. monnieri [26–32], but no report is available on the effect of Cd on the active principles of B. monnieri. Keeping this in mind an attempt was made to check the effect of Cd on growth and active constituents of B. monnieri, that is, bacoside A and bacopaside I.
2. Material and Methods
Plants of Bacopa monnieri were collected from unpolluted sites and grown in hydroponic cultures for several months in the field laboratory. The healthy vegetative clones of B. monnieri were further acclimatized in 3% Hoaglands' nutrient medium [33] under standard physiological conditions providing 16 hrs light period (114 μmol M−2 s−1) and 8 hrs dark photoperiod, 26 ± 2°C for 6 weeks.
Different concentrations of Cd, that is, 5 μM, 10 μM, 50 μM, and 100 μM, were prepared in 3% Hoagland's solution using AR grade cadmium chloride (CdCl2). Only 3% Hoagland's solution without addition of Cd served as control. Young plants of B. monnieri with 14–16 internodes and 2-3 basal rooted nodes were incorporated in each concentration under the abovementioned standard physiological conditions. The whole experiment was repeated at least three times. Aeration was provided to all the plants. The plants were harvested after 48, 96, and 168 hrs for estimation of metal uptake, protein and chlorophyll content, and biomass (fresh weight). Bacoside A and bacopaside I contents were measured in control, 5 μM 10 μM, 50 μM, and 100 μM Cd concentrations after one week.
Biomass was calculated on fresh weight basis using electronic balance. The fresh plant was used for the estimation of chlorophyll content. 100 mg fresh weight of the plant was extracted in 80% chilled acetone and estimated following the method of Arnon [34]. Protein content in the plants of B. monnieri was estimated by the method of Lowry et al. [35] using bovine serum albumin (BSA) as a standard. For metal uptake the harvested plant material was washed with deionized water, weighed and dried at 70°C for 48 hrs, and digested with HNO3 : HCLO4 (10 : 1 v/v mixture). Cd was estimated using Perkin Elmer 2380 Atomic Absorption Spectrophotometer (detection limit of Cd: 0.0005 ppm).
For quantitative thin layer chromatography (TLC) for bacoside A and bacopaside I, ten grams of powdered plant material of control and Cd treated plant was extracted with 50 mL methanol on water bath consecutively three times. Extracts were filtered and concentrated at low temperature and reduced pressure. Bacoside A and bacopaside I standard solutions of 1 mg/mL concentration were prepared by dissolving the standards in methanol. 10 μL of standards and plant extracts was applied on HPTLC precoated silica gel plates (E-MERCK F254) with the help of CAMAG Linomat V Applicator. The plates were developed to a distance of 9.0 cm in the solvent system Chloroform : Methanol : Water (7 : 3 : 0.5) in previously saturated twin trough chamber (CAMAG). The plates were scanned at the wave length 500 nm using CAMAG TLC Scanner 3 with software winCATS. Photographs of TLC plates were taken by the CAMAG Reprostar-3.
2.1. Statistical Analysis
The experiment was set up as randomized block design. To confirm the variability of data and validity of results all the data were subjected to analysis of variance (ANOVA) followed by Newman Keuls' test for individual comparison. Comparison between control and treatment was done by LSD test [36].
3. Results and Discussion
Higher Cd treatments, that is, 50 and 100 μM, produce visible morphological symptoms like decay of basal portion of shoot and leaves due to Cd toxicity after one week. The intensity of Cd toxicity enhanced with increase in the exposure duration. Browning and stunting of roots were also observed in 50 μM Cd and above after one week. The significant decrease in the biomass of plant is also observed at higher concentrations (P < 0.05) (Table 1). Decrease in biomass also confirms that Cd affects the growth of plants at higher concentrations. This is in accordance with the studies of Haag-Kerwer et al., [37] in which Cd accumulation results in the decrease in growth rate and reduction in transpiration of mustard plants. A significant (P < 0.01) decrease in the chlorophyll content with the increase in the metal concentration (Table 2) was observed in the present study. This might be due to interaction of Cd with –SH group of various enzymes involved in the chlorophyll biosynthesis [38]. The decrease in the protein content of the plant with increase in the metal concentration was also highly significant (P < 0.01) (Table 3). A significant decrease in the protein content may be due to Cd-induced oxidation of proteins mediated by H2O2 and due to increased proteolytic activity which has been proposed as an index of oxidative stress [39].
Table 1.
Effect of cadmium on biomass (g fw) in B. monnieri at different concentrations and exposure periods.
| Cd concentrations (μM) | Exposure periods (h) | |||
|---|---|---|---|---|
| 0 | 48 | 96 | 168 | |
| 0 μM | 1.81 ± 0.14 | 1.87 ± 0.17 | 2.07 ± 0.25 | 2.14 ± 0.24 |
| 5 μM | 2.03 ± 0.11 | 2.06 ± 0.12 | 2.08 ± 0.03 | 2.17 ± 0.03 |
| 10 μM | 1.75 ± 0.52 | 1.75 ± 0.52 | 1.79 ± 0.52 | 1.86 ± 0.57 |
| 50 μM | 1.53 ± 0.42 | 1.52 ± 0.42 | 1.20 ± 0.23A | 1.13 ± 0.16a |
| 100 μM | 1.31 ± 0.19 | 1.31 ± 0.19 | 0.98 ± 0.07B | 0.94 ± 0.06b |
All values are means of triplicate ± SD.
Adenote the significance (P < 0.05) at 50 μM cadmium as compared to control after 96 hours.
Bdenotes the significance (P < 0.01) at 100 μM cadmium as compared to control after 96 hours.
adenotes the significance (P < 0.05) at 50 μM cadmium as compared to control after 168 hours.
bdenotes the significance (P < 0.05) at 100 μM cadmium as compared to control after 168 hours.
Table 2.
Effect of cadmium on the chlorophyll content (mg g−1 fw) in B. monnieri at different concentrations and exposure periods.
| Cd concentration (μM) | Exposure periods (h) | ||
|---|---|---|---|
| 48 | 96 | 168 | |
| 0 | 1.23 ± 0.02 | 1.36 ± 0.04 | 1.37 ± 0.03 |
| 5 μM | 1.25 ± 0.03A | 1.32 ± 0.02B | 1.36 ± 0.01C |
| 10 μM | 1.24 ± 0.04A | 1.22 ± 0.02B | 1.10 ± 0.02C |
| 50 μM | 1.21 ± 0.01A | 0.99 ± 0.01B | 0.74 ± 0.02C |
| 100 μM | 1.00 ± 0.02A | .72 ± 0.01B | 0.51 ± 0.04C |
All values are means of three replicates ± SD; LSD P < 0.01.
Adenotes significance (P < 0.01) at different Cd concentrations as compared to control after 48 hours.
Bdenotes significance (P < 0.01) at different Cd concentrations as compared to control after 96 hours.
Cdenotes significance (P < 0.01) at different Cd concentrations after 168 hours.
Table 3.
Effect of Cd on the protein content (mg g−1 fw) of B. monnieri at different concentrations and exposure periods.
| Cd concentration (μM) | Exposure periods (h) | ||
|---|---|---|---|
| 48 | 96 | 168 | |
| Control 0 | 12.33 ± 0.04 | 12.33 ± 0.01 | 12.50 ± 0.08 |
| 5 μM | 11.99 ± 0.01A | 11.09 ± 0.01A | 10.21 ± 0.01A |
| 10 μM | 11.80 ± 0.09A | 11.54 ± 0.20A | 9.25 ± 0.01A |
| 50 μM | A10.07 ± 0.03A | 8.09 ± 0.01A | 7.06 ± 0.03A |
| 100 μM | 9.66 ± 0.07A | 7.51 ± 0.01A | 5.10 ± 0.01A |
All the values are means of three replicates, LSD P < 0.01; Adenotes significance (P < 0.01) at different Cd concentrations and exposure periods as compared to control.
The uptake of Cd by the plant was doze and duration dependent and also highly significant (P < 0.01). At the lowest ambient concentration of 5 μM the uptake was as high as 169.91 μg/g dry wt, within 48 hrs. However maximum uptake was 1779.70 μg/g, after 168 h observed in 100 μM concentrations (Table 4). Although Cd is a highly toxic metal for the growth and development of plant but it is highly accumulated by aquatic macrophytes, which has also been previously reported by various workers [40–44]. Accumulation of cadmium by B. monnieri has also been reported earlier by Sinha and Chandra [26]; Sinha [29]; Ali et al. [30]; and Singh et al. [32]. It can be said with these results that B. monnieri can work as potential accumulator of Cd and can be used in the phytoremediation. Phytoremediation is the use of green plants to detoxify a degraded or polluted environment [45]. The main advantages of phytoremediation are that the procedure is carried out in situ and it is inexpensive compared to other technologies for remediation [45]. Another important advantage of phytoremediation is that soils retain their fertility after metal removal.
Table 4.
Accumulation of Cd (µg g−1 dw) in B. monnieri at different concentrations and exposure periods.
| Cd concentrations (μM) | Exposure periods (h) | ||
|---|---|---|---|
| 48 | 96 | 168 | |
| 0.0 | ND | ND | ND |
| 5 μM | 169.91 ± 3.05 | 280.35 ± 25.34 | 330.07 ± 31.58 |
| 10 μM | 290.32 ± 19.08 | 667.62 ± 47.13 | 718.56 ± 2.74 |
| 50 μM | 584.47 ± 51.55 | 1140.16 ± 33.92 | 1729.21 ± 50.23 |
| 100 μM | 971.84 ± 20.16 | 1303.07 ± 84.41 | 1779.91 ± 49.07 |
All values are means of triplicate ± SD; LSD P < 0.01.
The TLC fingerprint profile showed the increase in the content of the bioactive compounds, that is, bacoside A and bacopaside I in all Cd treated plants (Figure 1). The retention factor values and the colour of components present in B. monnieri extracts are shown in Table 5.
Figure 1.
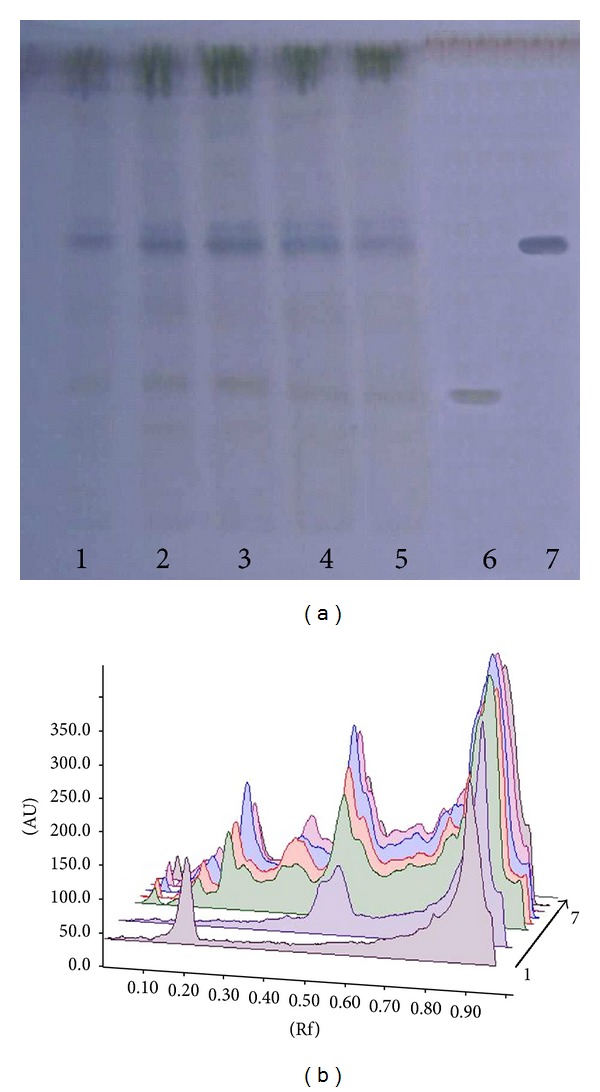
TLC of methanolic extract of control and Cd treated plants of Bacopa monnieri. (a) Fingerprint profile, 1: control, 2: 5 μM Cd, 3: 10 μM Cd, 4: 50 μM Cd, 5: 100 μM, 6: bacoside A, and 7: bacopaside I. (b) Densitometric scan at 500 nm.
Table 5.
R f values and the colour of components present in B. monnieri extracts.
| Colour | R f |
|---|---|
| Yellowish brown | 0.20 |
| Brown (I) | 0.26 bacopaside I |
| Yellow | 0.39 |
| Purple blue (a) | 0.57 bacoside A |
| Blue (a) | 0.60 A |
| Light blue | 0.67 |
| Light blue | 0.71 |
| Light blue | 0.78 |
| Greenish | 0.82 |
Bold fonts refer to R fs of Bacoside and Bacopaside.
It is a well-known fact that secondary metabolites are formed under various stresses as a defense mechanism [46]. In the present study it is interesting to note that the aforesaid compounds were gradually increased by the Cd treatment up to 10 μM and then decreased at higher concentrations, that is, 50 μM and 100 μM (Figure 2). This indicates that the synthesis of secondary metabolites enhances initially up to a certain limit due to abiotic stress and then decreases due to Cd toxicity in higher concentrations. There are several examples available where plants synthesize and accumulate secondary metabolites upon treatment with heavy metals [47–50].
Figure 2.
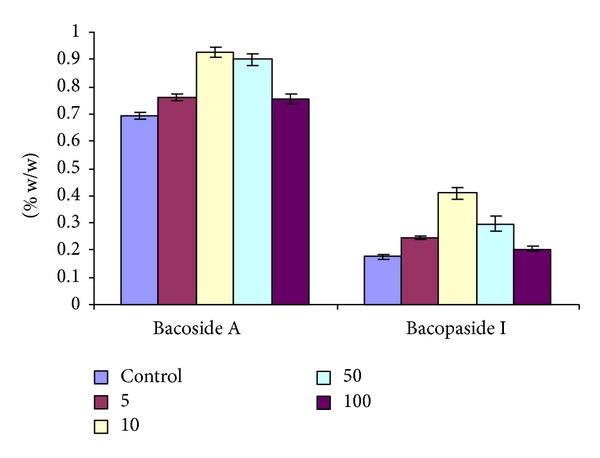
Effect of cadmium on the bacoside A and bacopaside I content in B. monnieri.
Thus, it is clear from this study that B. monnieri may become a very important plant for phytoremediation and remove Cd from the polluted site, and it is a good indication that its active constituents increase in this condition. Pharmaceutical companies may use these plants for extracting its active compounds, even if grown on polluted sites.
Acknowledgments
P. Gupta acknowledges Department of Science and Technology, New Delhi, for financial support under Women Scientist Scheme. The authors are thankful to the Director of National Botanical Research Institute, Lucknow, for all his encouragement and necessary facilities. P. Gupta acknowledges the support and keen interest shown by the Head the Department of Botany Lucknow University and acknowledges him for providing the basic facilities.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Adriano DC. Trace Elements in Terrestrial Environments. Biogeochemistry, Bioavailability, and Risks of Metals. New York, NY, USA: Springer; 2001. [Google Scholar]
- 2.Cordero B, Lodeiro P, Herrero R, Sastre de Vicente ME. Biosorption of cadmium by fucus spiralis. Environmental Chemistry. 2004;1(3):180–187. [Google Scholar]
- 3.Booth B. The added danger of counterfeit cigarettes. Environmental Science & Technology. 2005;39(2, article A34) doi: 10.1021/es053168z. [DOI] [PubMed] [Google Scholar]
- 4.Stephens WE, Calder A, Newton J. Source and health implications of high toxic metal concentrations in illicit tobacco products. Environmental Science and Technology. 2005;39(2):479–488. doi: 10.1021/es049038s. [DOI] [PubMed] [Google Scholar]
- 5.IARC. Beryllium cadmium Mercury exposure in glass manufacturing industry. Monographs on evaluation of carcinogenic risk to humans, vol. 58, pp. 119–239, IARC, Lyon, France, 1993. [PMC free article] [PubMed]
- 6.Filipič M, Hei TK. Mutagenicity of cadmium in mammalian cells: implication of oxidative DNA damage. Mutation Research. 2004;546(1-2):81–91. doi: 10.1016/j.mrfmmm.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Jackson AP, Alloway BJ. The transfer of cadmium from agricultural soils to the human food chain. In: Adriano DC, editor. Biogeochemistry of Trace Metals. Boca Raton, Fla, USA: Lewis Publishers; 1992. [Google Scholar]
- 8.FAO/WHO. Position Paper on Cadmium. Hague, The Netherlands: SPB Acadamic; 1995. Joint committee on food additives and contaminants. [Google Scholar]
- 9.Yeung AT, Hsu C-N. Electrokinetic remediation of cadmium-contaminated clay. Journal of Environmental Engineering. 2005;131(2):298–304. [Google Scholar]
- 10.API. Ayurvedic Pharmacopoeia of Part I. Vol. 2. India: Ministry of Health and family welfare Government of India; 1999. [Google Scholar]
- 11.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. 6th edition. New Delhi, India: CSIR; 2002. [Google Scholar]
- 12.Upadhya AS, Vartak VD, Kamboj V, Kar MS. Ethno-medico botanical studies in Western Maharashtra India. Ethnobotany. 1994;6:25–31. [Google Scholar]
- 13.Mohan K, Singh AK. Ethno-medico-botany of Tharus. Advances in Plant Science. 1996;9:1–6. [Google Scholar]
- 14.Chetty KM, Chetty ML, Sudhakar A, Ramesh C. Ethno-medico botany of some aquatic angiospermae in Chittoor district of Andhra Pradesh, India. Fitoterapia. 1998;69(1):7–12. [Google Scholar]
- 15.Pareek A. Preliminary ethnobotanical notes on the plants of aquatic habitats of Rajasthan. Journal of Phytolological Research. 1994;7:73–76. [Google Scholar]
- 16.Malhotra SK, Moorthy S. Some useful and medicinal plants of Chandrapur district (Maharashtra state) Bulletin of Botanical Survey India. 1973;15:13–21. [Google Scholar]
- 17.Singh PB, Aswal BS. Medicinal plants of Himachal Pradesh used in Indain pharmaceuticals (Bacopa monniera Wettst.) Part I. Journal of Research in Ayurveda and Siddha. 1992;1:133–148. [Google Scholar]
- 18.Singh PB. Medicinal plants of Ayurvedic importance from Mandi district of Himachal Pradesh. Bulletin of Medicinal and Ethnobotanical Research. 1993;13:172–208. [Google Scholar]
- 19.Bedi SJ. Ethnobotany of the Ratan Mahal Hills, Gujarat, India. Economic Botany. 1978;32(3):278–284. [Google Scholar]
- 20.Shah GL, Menon AR, Gopal GV. An account of the Ethnobotany of Saurashtra in Gujarat state (India) Journal of Economic and Taxonomy Botany. 1981;2:173–182. [Google Scholar]
- 21.Sharma R, Chaturvedi C, Tewari PV. Efficiency of Bacopa monnieri in revitalizing intellectual function in children. Journal Research Edn Ind Medica. 1987;6:1–10. [Google Scholar]
- 22.Singh HK, Rastogi RP, Srimal RC, Dhawan BN. Effect of bacosides A and B on avoidance responses in rats. Phytotherapy Research. 1988;2(2):70–75. [Google Scholar]
- 23.Singh HK, Dhawan BN. Neuropsychopharmacological effects of the ayurvedic nootropic Bacopa monniera Linn. (Brahmi) Indian Journal of Pharmacology. 1997;29(5):S359–S365. [Google Scholar]
- 24.Nathan PJ, Clarke J, Lloyd J, Hutchison CW, Downey L, Stough C. The acute effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy normal subjects. Human Psychopharmacology. 2001;16(4):345–351. doi: 10.1002/hup.306. [DOI] [PubMed] [Google Scholar]
- 25.Stough C, Lloyd J, Clarke J, et al. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology. 2001;156(4):481–484. doi: 10.1007/s002130100815. [DOI] [PubMed] [Google Scholar]
- 26.Sinha S, Chandra P. Removal of Cu and Cd from water by Bacopa monnieri L. Water, Air, and Soil Pollution. 1990;51(3-4):271–276. [Google Scholar]
- 27.Gupta M, Sinha S, Chandra P. Uptake and toxicity of metals in Scirpus lacustris L. and Bacopa monnieri L. Journal of Environmental Science and Health A. 1994;29(10):2185–2202. [Google Scholar]
- 28.Sinha S, Gupta M, Chandra P. Bioaccumulation and biochemical effects on mercury in the plant Bacopa monnieri L . Environ Toxicol Water Qual. 1996;11:105–112. [Google Scholar]
- 29.Sinha S. Accumulation of Cu, Cd, Cr, Mn and Pb from artificially contaminated soil by Bacopa monnieri . Environmental Monitoring and Assessment. 1999;57(3):253–264. [Google Scholar]
- 30.Ali G, Srivastava PS, Iqbal M. Responses of Bacopa monniera cultures to cadmium toxicity. Bulletin of Environmental Contamination and Toxicology. 2001;66(3):342–349. doi: 10.1007/s001280011. [DOI] [PubMed] [Google Scholar]
- 31.Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MNV. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiology and Biochemistry. 2006;44(1):25–37. doi: 10.1016/j.plaphy.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Eapen S, D’Souza SF. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere. 2006;62(2):233–246. doi: 10.1016/j.chemosphere.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Hoagland DR, Arnon DI. The Water-Culture Method For Growing Plants Without Soil. Vol. 347. California Agricultural Experiment Station Publications; 1950. [Google Scholar]
- 34.Arnon DI. Copper enzyme in isolated chloroplast: polyphenol oxidase in Beta vulgaris. Plant Physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of biological chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 36.Zar JH. Biostatistical Analysis. Prentice Hall Publishers; 1974. [Google Scholar]
- 37.Haag-Kerwer A, Schäfer HJ, Heiss S, Walter C, Rausch T. Cadmium exposure in Brassica juncea causes a decline in transpiration rate and leaf expansion without effect on photosynthesis. Journal of Experimental Botany. 1999;50(341):1827–1835. [Google Scholar]
- 38.Griffiths WT. Characterization of the terminal stages of chlorophyll(ide) synthesis in etioplast membrane preparations. Biochemical Journal. 1975;152(3):623–635. doi: 10.1042/bj1520623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, Del Río LA, Sandalio LM. Cadmium-induced subcellular accumulation of O2.- and H2O2 in pea leaves. Plant, Cell and Environment. 2004;27(9):1122–1134. [Google Scholar]
- 40.Mayes RA, McIntosh AW, Anderson VL. Uptake of cadmium and lead by a rooted aquatic macrophyte (Elodea canadensis) Ecology. 1977;58(5):1176–1180. [Google Scholar]
- 41.Nakada M, Fukaya K, Takeshita S, Wada Y. The accumulation of heavy metals in the submerged plant (Elodea nuttallii) Bulletin of Environmental Contamination and Toxicology. 1979;22(1-2):21–27. doi: 10.1007/BF02026901. [DOI] [PubMed] [Google Scholar]
- 42.Kay H, Haller WT, Garrard LA. Effects of heavy metals on water hyacinths (Eichhornia crassipes (mart.) solms) Aquatic Toxicology. 1984;5(2):117–128. [Google Scholar]
- 43.Nir R, Gasith A, Perry AS. Cadmium uptake and toxicity to water hyacinth: effect of repeated exposures under controlled conditions. Bulletin of Environmental Contamination and Toxicology. 1990;44(1):149–157. doi: 10.1007/BF01702375. [DOI] [PubMed] [Google Scholar]
- 44.Garg P, Tripathi RD, Rai UN, Sinha S, Chandra P. Cadmium accumulation and toxicity in submerged plant Hydrilla verticillata (L.F.) Royle. Environmental Monitoring and Assessment. 1997;47(2):167–173. [Google Scholar]
- 45.Brooks RR. General introduction. In: Brooks RR, editor. Plants That Hyperaccumulate Heavy Metals. Wallingford, UK: CAB International; 1998. pp. 1–14. [Google Scholar]
- 46.Trease GE, Evans GE. Text Book of Pharmacognosy. 2nd edition. London, UK: Tindall and Cox; 1989. [Google Scholar]
- 47.Mithöfer A, Schulze B, Boland W. Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Letters. 2004;566(1–3):1–5. doi: 10.1016/j.febslet.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Rai V, Vajpayee P, Singh SN, Mehrotra S. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Science. 2004;167(5):1159–1169. [Google Scholar]
- 49.Rai V, Khatoon S, Bisht SS, Mehrotra S. Effect of cadmium on growth, ultramorphology of leaf and secondary metabolites of Phyllanthus amarus Schum. and Thonn. Chemosphere. 2005;61(11):1644–1650. doi: 10.1016/j.chemosphere.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 50.Sinha S, Saxena R. Effect of iron on lipid peroxidation, and enzymatic and non-enzymatic antioxidants and bacoside: a content in medicinal plant Bacopa monnieri L. Chemosphere. 2006;62(8):1340–1350. doi: 10.1016/j.chemosphere.2005.07.030. [DOI] [PubMed] [Google Scholar]


