Abstract
An imminent food crisis reinforces the need for novel strategies to increase crop yields worldwide. Effective control of pest insects should be part of such strategies, preferentially with reduced negative impact on the environment and optimal protection and utilization of existing biodiversity. Enhancing the presence and efficacy of native biological control agents could be one such strategy. Plant strengthener is a generic term for several commercially available compounds or mixtures of compounds that can be applied to cultivated plants in order to ‘boost their vigour, resilience and performance’. Studies into the consequences of boosting plant resistance against pests and diseases on plant volatiles have found a surprising and dramatic increase in the plants' attractiveness to parasitic wasps. Here, we summarize the results from these studies and present new results from assays that illustrate the great potential of two commercially available resistance elicitors. We argue that plant strengtheners may currently be the best option to enhance the attractiveness of cultivated plants to biological control agents. Other options, such as the genetic manipulation of the release of specific volatiles may offer future solutions, but in most systems, we still miss fundamental knowledge on which key attractants should be targeted for this approach.
Keywords: crop protection, biological control, plant strengtheners, elicitors, plant volatiles, parasitoids
1. Introduction
As repeatedly stressed by the Food and Agricultural Organization of the United Nations, the biggest contemporary challenge for humanity is to safeguard food security for current and future generations. A growing demand and a steady increase of the world population requires that food production per area of cultivated land will have to increase drastically, preferentially with minimal impact on natural ecosystems [1–4]. One way to achieve higher yields will be the reduction of crop losses caused by pathogens and pests, which still amount to an estimated 25–40% of total production. To this end, new and preferably sustainable and environment-friendly strategies for crop protection have to be developed. Effective biological control of arthropod pests is generally accepted as such a sustainable and ecologically sound approach to reduce crop damages. Increasing the effectiveness of biological control agents, such as predators and parasitoids, not only involves increasing their numbers in agro-ecosystems, but also enhancing their foraging success.
The natural enemies of herbivores commonly make use of plant-produced volatiles in order to locate their victims. More specifically, upon herbivore attack, plants emit specific blends of volatiles that attract natural enemies of the herbivores, a phenomenon that was first shown for predatory mites [5] and parasitoids of caterpillars [6]. The importance of herbivore-induced plant volatiles as foraging cues has since been demonstrated in many systems, not only as attractants for natural enemies [7–9], but also, in certain cases, as repellents for herbivores [10,11]. These effects have prompted the notion that inducible volatile signals can be enhanced in crop plants in order to improve the biological control of insect pests [9,12–16]. Indeed, genetic manipulation has been used successfully to alter the emissions of volatile compounds, and thereby enhance the attraction of natural enemies [17–21].
The approach has also been tested in the field, yielding some very promising results (see below). However, certain inducible plant volatiles that attract beneficial insects may also attract arthropod pests [22–24]. This is not necessary a problem, because, if appropriately exploited in pest control strategies, this latter phenomenon can be used to develop trap crops, for instance, in push–pull approaches [25,26], as described below. It is also desirable that the manipulated emissions are inducible by the pest, so that their natural enemies are offered a reliable signal and are attracted only when they are likely to encounter prey or hosts on the crop [27,28].
Transgenic approaches show great promise [4], but are subjected to continuous criticism. Scepticism and fear are being maintained by controversial, often flawed, but highly publicized studies that reinforce the notion that transgenic plants may cause environmental and health problems [29–31]. It is therefore unlikely that a transgenic approach to enhance the attractiveness of crop plants to beneficial insects will be adopted in the near future, especially not in Europe. It may, however, be possible to manipulate and enhance the existing indirect defence capabilities of plants by switching on relevant genes via the application of chemical elicitors [32]. Several of such elicitors have been shown to greatly enhance resistance in plants, in particular against pathogens [33–36]. With this in mind, we and others have studied the possibility to alter volatile emissions with the use of chemical elicitors, in particular, plant strengtheners that are already being used to improve crop performance. In this paper, we review the research in this area and present a series of laboratory experiments that show long-time persistence of enhanced parasitoid attraction after treating maize plants with two such plant strengtheners.
(a). What volatiles should we target?
In most cases, it is still far from clear which specific volatiles are most essential for attraction, in particular where it concerns parasitoids [37,38]. Yet, several compounds are of obvious importance in some specific systems. One of those is methyl salicylate, which, for instance, has been found to be particularly important for the attraction of predatory mites [39,40]. This compound is produced via the salicylic acid (SA) pathway, in contrast to the octadecanoid pathway (involving jasmonic acid) that is responsible for the production of volatiles that are typically induced by caterpillar feeding [41]. Indeed, methyl salicylate helps predatory mites to distinguish between mite-damaged and caterpillar-damaged plants. Yet, some studies suggest that the response of the predatory mites is not genetically fixed, but is learned by association [42], as is the case for many parasitoids [43,44]. Field studies by James and co-workers [45–48] confirmed a role of methyl salicylate in mediating natural enemy recruitment. They placed methyl salicylate-releasing dispensers in vineyards and hopyards and found an increased presence of arthropod predators and certain parasitoids. However, as the authors recognize, this positive effect may not (only) be an effect of direct attraction, but could also have been owing to the effect of the plant hormone on the direct and indirect plant defence responses. A field study with different maize inbred lines suggested that parasitism by the parasitoid Cotesia marginiventris may be positively influenced by the emission of methyl salicylate [49]. On the other hand, attraction of the parasitoid Diadegma semiclausum is negatively affected by methyl salicylate [11], again indicating that methyl salicylate is not of universal importance to attract all natural enemies.
The most common compounds that are specifically induced in plants by herbivore feeding are terpenoids [50]. This has led to the assumption that they are key to the attraction of natural enemies. Indeed, some of the earliest volatiles implicated in the attraction of natural enemies are terpenoids, such as (E)-ocimene, which is prominently released by mite-infested plants [5]. Because of their dominancy in attractive odour blends of caterpillar-infested plants [41], terpenoids were also thought to be of key importance for the attraction of generalist parasitoids, but evidence is accumulating that this may not necessarily be the case [51]. Indeed, genetic transformation of Arabidopsis plants with typical maize terpene synthase genes revealed that dominant sesquiterpenes might mainly be of importance after parasitoids learn to associate them with caterpillar presence [18,52]. The innate responses of parasitoids seem to depend on minor compounds that remain to be identified [38].
One particularly promising terpenoid is (E)-β-farnesene. This sesquiterpene serves as an alarm pheromone for many aphid species, which release it upon attack by predators, causing neighbouring aphids to disperse [53]. (E)-β-Farnesene also has a repellent effect on aphids in search of a host plant and can attract and arrest aphid natural enemies [19,54]. Beale et al. [19] successfully transformed Arabidopsis thaliana plants with a gene from peppermint to constitutively release (E)-β-farnesene, making the plants repellent to aphids and attractive to an aphid parasitoid. Field trials with similarly transformed wheat plants are underway.
Overall, very few examples exist of specific compounds that can be enhanced to attract a broad range of beneficial arthropods. As long as this is the case, it might therefore be more advantageous to pursue a general increase of herbivore-induced volatiles. Several field studies demonstrate that plant attractiveness to natural enemies of herbivores can indeed be increased and that this leads to higher herbivore mortality.
(b). Field studies
There is still limited, but convincing field evidence for a role of plant volatiles in increasing predation and parasitism in the field. In each case, hormonal pathways leading to volatile emissions were manipulated. In a first such study, Thaler [55] applied the plant defence hormone jasmonic acid to tomato plants and found that this increased parasitism of sentinel caterpillars placed near the treated plants twofold. In a natural system involving the wild tobacco plant Nicotiana attenuata, Halitschke et al. [22] silenced two key enzymes of the octadecanoid defence pathway, NaLOX3 and NaHPL, in order to attenuate plant defences, including the release of volatile organic compounds. They tested these silenced plants alongside their non-transformed counterparts and found that green leaf volatiles as well as terpenoids attract natural enemies of herbivores but also the herbivores themselves. In recent follow-up work, the same research group [56] could demonstrate that when tobacco plants are impaired in the production of volatiles that are attractive to predatory bugs they are subjected to increased herbivory and produce fewer flowers, which is correlated with a loss in seed production.
It has been frequently proposed that enhancing the release of volatiles in crop plants may help to improve biological control [9,12–16]. A study on maize has provided a demonstration that a transgenic manipulation of volatile releases can indeed help to enhance crop protection. Degenhardt et al. [20] transformed a maize line with a gene from oregano to restore the emission of (E)-caryophyllene. This sesquiterpene is naturally emitted by the roots of many maize varieties and their wild ancestor, teosinte, when the roots are damaged, thereby attracting entomophagous nematodes, which in turn reduce the abundance of root feeding herbivores [20]. In field trials, the transformed maize plants, which constitutively produce (E)-caryophyllene, were found to be more effective at attracting entomopathogenic nematodes, thereby significantly reducing root damage inflicted by the western corn rootworm Diabrotica virgifera, a major belowground pest of maize [20].
The possibility to manipulate the attractiveness of plants to economically important insects was also evident from a study on rice volatiles by Xiao et al. [21]. They found that genetically altering the emissions of the monoterpene alcohol (S)-linalool and the sesquiterpene (E)-caryophyllene had a significant effect on the attraction of a major Asian pest of rice, the rice brown planthopper Nilaparvata lugens, as well as some of its natural enemies, in particular, an egg parasitoid and spiders. Depending on the compound that was altered, the composition of the insect community changed either in favour or the detriment of the pest insect, implying a great application potential to control the rice brown planthopper and possibly other pests. The fact that (E)-caryophyllene was not only attractive to natural enemies, but also to the pest itself complicates the issue. The same was found for (E)-caryophyllene emitted by maize roots damaged by western corn rootworm: belowground, this volatile not only attracts entomopathogenic nematodes [20,27], but also rootworm larvae, which use the signal to aggregate on maize root systems, but only at intermediate concentrations [23,24]. This phenomenon could be useful in the development of a belowground push–pull approach, which obviously would work at much shorter ranges than aboveground systems, where pests and their natural enemies move considerably longer distances. Yet, aboveground (E)-caryophyllene also appears to attract adult herbivores, including Diabrotica looking for oviposition sites, and could be used to divert them from non-producing plants [28,57].
The push–pull idea has received a tremendous boost after its very successful adoption in subsistence maize farming in Africa [25,26]. In this specific example, maize plots were bordered by Napier grass, which was found to be highly attractive to stemborers. Most important to the success of this approach is the fact that this attractive grass is, in fact, toxic to the pest and unsuitable as a host. Initially, within the plot, maize was intercropped with the repellent molasses grass, which further reduced maize infestation by the stemborers [25]. Molasses grass was eventually replaced by Desmodium spp., legumes that are not only repellent to the pests, but also contribute to soil fertility and stability as well as effectively suppressing the plant parasitic Striga weed [58,59].
The rice example shows that an effective push–pull strategy can also be obtained with a transgenic approach, at least in the case of the rice brown planthopper [21]. However, for leaf-feeding pests, the transgenic approach appears to be more complicated, as leaf-volatile blends induced by chewers are more complex, making it difficult to pinpoint the key compounds that are involved in the attraction of predators and parasitoids [50,60].
(c). Plant strengtheners: the case of maize
Rostás & Turlings [61] studied how parasitoid attraction to maize plants is effected by treating the plants with benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH), a chemical mimic of SA that has been used successfully to induce resistance to a wide range of diseases on field crops [62,63]. It was assumed that due to negative cross talk [64] the activation of the SA pathway would suppress jasmonic acid activation, and therewith herbivore-induced volatile emissions. Surprisingly, BTH-treated maize plants, after they have been infested by caterpillars, were highly attractive to females of the braconid parasitoid Microplitis rufiventris, much more so than untreated plants [61]. As expected, BTH treatment did not increase the emission of volatiles, but, in fact, it significantly reduced the emission rates of two volatile compounds (indole and (E)-caryophyllene) in maize seedlings. Apparently, this was not the case for key compounds responsible for parasitoid attraction and possibly the reduced release of several less relevant compounds may have increased the attractiveness of such key attractants. These findings support the conclusion from work by D'Alessandro et al. [38] that the dominating compounds in the herbivore-induced volatile emissions from maize plants are not essential for parasitoid attraction and may, in fact, reduce attractiveness by masking more important parasitoid attractants.
The initial study by Rostás & Turlings [61] was followed up by Sobhy et al. [65], who tested BTH, as well as another chemical plant elicitor, laminarin, which induces the accumulation of phytoalexins and expression of a set of pathogenesis-related proteins [66] through the activation of the SA pathway [67]. The aim was to find out whether treatment with these plant enhancers has a general positive effect on the attractiveness of plants to parasitoids. As before, it was found that, compared with control plants, both BTH- and laminarin-treated plants released lower amounts of volatiles, in particular, indole and some sesquiterpenes, and again this increased the attractiveness of maize plants to parasitic wasps. This effect of treatment was found for all three of the tested parasitoid species [65]. Overall, the results imply that maize plants that produce less of the dominating volatiles show increased attractiveness to a wide range of parasitoids. Most promising, was the fact that BTH treatment not only increased wasp attraction, but also increases the direct defence against larvae of Spodoptera littoralis after 2 days feeding.
The promising results from laboratory studies prompted a field study in which treatment with BTH in maize plants as well as treatment with methyl jasmonate (MeJA) was tried out in a subtropical region of Mexico with high insect pressures [68]. As in the laboratory, application of BTH slightly reduced volatile emission in maize, whereas MeJA increased the emission compared with control treatments. However, these changes in volatile emissions had no consistent effects on infestation by Spodoptera frugiperda larvae, the main insect pest found on the maize seedlings in this region, and the treatments only marginally effected parasitism rates. These somewhat disappointing results may be explained by the severe biotic and abiotic conditions during the time of the experiment, which may have masked the effects of the treatments [68]. To test whether this is indeed the case, further field experiments, using early (seed) treatments, before pest infestations, will be needed.
(d). Identifying the ideal elicitor: a genetic screening approach
In the cases discussed so far, known elicitors and plant enhancers were used and were found to have a positive effect on natural enemy attraction, but the mechanisms and further implications of these effects remain largely unknown. Xin et al. [69] introduced an approach that targets genes which are specifically involved in the production of volatiles of known importance, and screened for chemicals that activate these genes. They used this approach on rice plants and developed a high-throughput chemical genetics screening system based on a herbivore-induced (S)-linalool synthase promoter fused to a β-glucuronidase reporter construct to test candidate synthetic compounds for their potential to induce rice defences. (S)-Linalool was chosen, because it is one of the key volatiles that is emitted by rice plants in response to attack by brown planthopper N. lugens [70–74] and is highly attractive to its main egg parasitoid Anagrus nilaparvatae [75]. Of the tested compounds, the widely used herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) was found to be particularly active. It induced a strong defensive reaction and a significant increase in volatile production. Induced plants were more resistant to a caterpillar pest, but became highly attractive to N. lugens as well as to its parasitoid A. nilaparvatae. In a subsequent field experiment, 2,4-D application showed great potential to draw away N. lugens from non-treated plants and turned the treated plants into deadly traps by also attracting large numbers of parasitoids [69]. As mentioned above, this volatile-mediated trap cropping or push–pull approach has already shown great potential in subsistence maize farming in Africa [25,59], and could be used at larger scales with the use of appropriate elicitors.
(e). Persistence and early (seed) treatment
The highly promising results from our laboratory studies with maize [61] were not as evident in the field study that was conducted in Mexico [68]. Our main explanation for this is that the plants were treated too late and that most of the plants had already suffered insect attack under high insect pressures. This will need to be further tested with additional field trials. We expect that by treating the plants earlier, even at the seed stage, might yield better results. This would require, however, that the treatment effect is long-lasting. To test this, we conducted the following series of experiments.
2. Material and methods
(a). Plants and insects
Zea mays var. Delprim plants were grown in commercial potting soil (Ricoter, Aussaaterde, Aarberg, Switzerland) in plastic pots (11 cm height, 4 cm diameter) at 25 ± 2°C, 60 ± 5% r.h., 16: 8 h L : D, and 926 µmol m−2 s−1 in a climate chamber (CLF plant climatics, Percival). Maize plants used for the experiments were 10–12 days old and had three fully developed leaves. For each experiment, we selected plants of approximately the same size. Eggs of S. littoralis Boisd. (Lepidoptera: Noctuidae) were supplied by Syngenta (Stein, Switzerland). Newly hatched larvae were reared in transparent plastic boxes on a wheat germ-based artificial diet until they had reached the second instar, at which point they were used for experiments. The larval endoparasitoids, M. rufiventris Kok., Cotesia marginiventris (Cresson; Braconidae: Hymenoptera) and Campoletis sonorensis (Cameron; Ichneumonidae: Hymenoptera) were reared as described in Sobhy et al. [65]. Thirty minutes before the bioassays, cages with 2–4 day old parasitoid adults were transferred to the laboratory for acclimatization.
(b). Application of plant strengtheners
BTH (BION) was obtained from Syngenta, Switzerland, as a water-dispersible granular formulation containing 50% active ingredient. Laminarin (IODUS 40) was obtained from Stähler, Switzerland, as a soluble liquid formulation containing 3.5% active ingredient. To evaluate the use of both plant strengtheners in an agricultural context, we tested two modes of application: first, we applied the plant strengtheners at different time points (6 days and 2 days) before the bioassays. In order to test plants at the same age, they were sprayed 6 and 10 days old after germination, respectively. The chemicals were applied at concentrations of 0.15 g l−1 (BTH) and 20 ml l−1 (Laminarin) [65]. These concentrations correspond to the recommended doses by the manufacturers for application in agriculture. Control plants were not sprayed at all. In a second experiment, maize seeds were soaked in chemical solutions for 12 h until complete absorption of the liquid [76]. Laminarin treatment was performed using a solution of 20 ml, using a concentration of 0.02% (v/v) in distilled water. BTH treatment was performed using a solution of 0.15 g ml−1 dissolved in distilled water [77]. A control treatment was performed using seeds soaked in distilled water. Treated and control seeds were sown separately and used for experiments after 10–12 days.
(c). Olfactometer bioassays
A series of experiments using a six-arm olfactometer was conducted to evaluate the effect of the plant enhancers on the attractiveness of maize plants to parasitic wasps. For all experiments, 3–5 days old mated naive female wasps were used. For the choice bioassays, six female wasps were removed from their cage with an aspirator and released into the central choice chamber of the olfactometer. The wasps moved up to the top of the chamber attracted by the diffuse light coming from above. Depending on the attractiveness of the different odour sources, they then walked into one of the six arms connected to the central chamber. The central choice chamber was connected via a Tygon tube to a water-filled glass U-tube that served as a pressure gauge to balance incoming and outgoing air, minimizing pressure differences with the outside. Each group of wasps was given 30 min to make a choice. Wasps that did not enter an arm after this time were removed from the central part of the olfactometer and considered as individuals that made ‘no choice’. Five groups of six wasps were tested on each experimental day. Each olfactometer experiment was repeated six times on different experimental days, each time with a new set of treated plants as odour sources and with new wasps. The position of the odour source was changed clockwise after each day of testing to avoid position effects. All bioassays were performed between 9.00 and 17.00.
Using the above procedure, three treatment combinations were tested individually for the three different parasitoid species. First, plants that had been sprayed with plant strengtheners for 2 or 6 days were infested with 10 second-instar S. littoralis larvae on the evening before the experiment. After infestation, plants were kept under laboratory conditions (25 ± 2°C, 16 L : 8 D h). The following odour sources were then offered simultaneously to the parasitoids: (i) a maize plant damaged by S. littoralis caterpillars and treated with a chemical 2 days before, (ii) a maize plant damaged by S. littoralis and treated with a chemical 6 days before, (iii) an untreated maize plant damaged by caterpillars (as control), and (iv) three empty control vessels. BTH and laminarin were assayed in separate experiments. A second set of experiments were conducted in the same way, apart from the fact that plants were induced artificially by scratching the abaxial side of fully developed leaves (20 mm2) with a scalpel blade without damaging the midrib and applying 10 µl of S. littoralis larval regurgitant using a micropipette. Regurgitant had previously been collected with a micropipette from fourth instar larvae that had been feeding on maize leaves for at least 24 h and was stored at −80°C until use [78]. The above-mentioned treatment was applied the evening before and a second time on the morning of each experimental day, about 3 h before the start of bioassays. The third experimental set-up included seed-treated plants that were induced by adding 10 second-instar larvae of S. littoralis to each plant as above. The odour sources were: (i) attacked maize plants from BTH-treated seeds, (ii) attacked maize plants from laminarin-treated seeds, and (iii) attacked maize plants from seeds soaked in distilled water (control treatment), as well as three empty arms as blank controls.
(d). Odour trapping and analysis
Volatiles emitted by the various odour sources were trapped for 3 h during the bioassays on Super Q adsorbent filters (25 mg, 80–100 mesh; Alltech Associates, Deerfield, IL, USA) [79]. Before use, traps were washed with 3 ml dichloromethane. In all experiments, a filter was attached to the horizontal port at the top of each odour source vessel. Purified air entered the bottles at a rate of 1.1 l min−1, and air carrying the volatiles was pulled through each trap at a rate of 0.7 l min−1 (Analytical Research System, Gainesville, FL, USA). Traps were extracted with 150 µl dichloromethane (Super solvent; Merck, Dietikon, Switzerland), and 200 ng of n-octane and n-nonyl acetate (Sigma, Buchs, Switzerland) in 10 µl dichloromethane were added to each sample as internal standards. Samples were either analysed immediately or stored at −80°C in small vials (Supelco, Amber Vial, 7 ml with solid cap w/PTFE Liner). Odour samples were analysed using a gas chromatograph (Agilent 7890A) coupled to a mass spectrometer (Agilent 5975C VL MSD). After injection of 2 µl of sample, the temperature was maintained at 40°C for 3.5 min, and then increased to 100°C at 8°C per minute and subsequently to 200°C at 5°C per minute followed by a post-run of 5 min at 250°C. Helium at constant flow (0.9 ml min−1) was used as carrier gas. The volatiles were identified by comparing their mass spectra with those of the NIST05 library and by comparing their retention times with those of previous analyses [38,65]. The total emission for each compound was estimated as the sum of the amounts for all compounds released during the collection period (3 h), assuming equal ionization efficiency in the source of the mass spectrometer for the different compounds.
(e). Statistical analyses
The functional relationship between parasitoid responses and the different volatile sources offered in the six-arm olfactometer was examined with a generalized linear model as described earlier [79]. The model was fitted by maximum quasi-likelihood estimation in the software package R (R: a language and environment for statistical computing, v. 2.9.0, Zurich, Switzerland, 2009, http://www.R-project.org), and its adequacy was assessed through likelihood ratio statistics and examination of residuals. One-way ANOVAs and Tukey's post hoc comparisons were performed to compare means of volatile emission results when the data were normally distributed and the variances were homogeneous. In case that assumptions for normally distributed data with homogeneous variances could not be fulfilled, we used the non-parametric Kruskal–Wallis or Mann–Whitney rank sum tests and then compared treatment effects using Dunn's test. These analyses were performed with SigmaPlot v. 12 (SPSS Inc., Chicago, IL, USA).
3. Results
(a). Foliar and seed application of plant strengtheners sustainably enhances parasitoid attraction
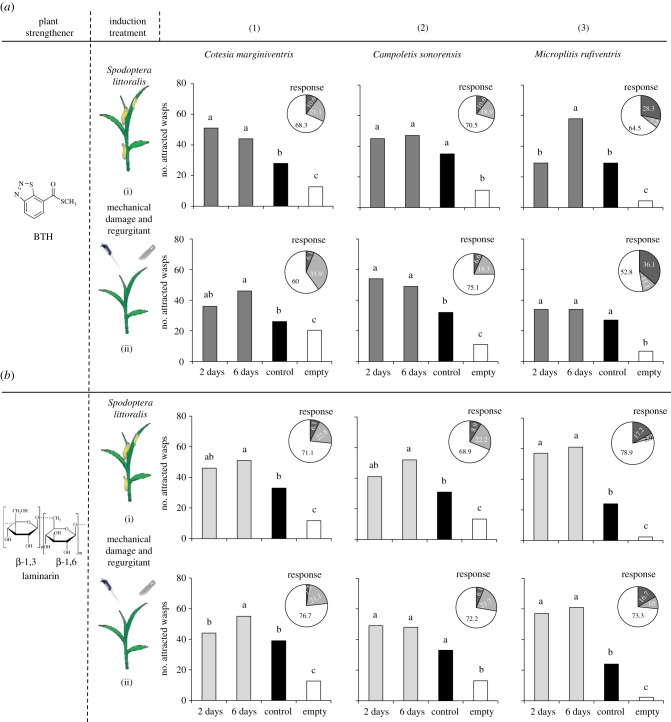
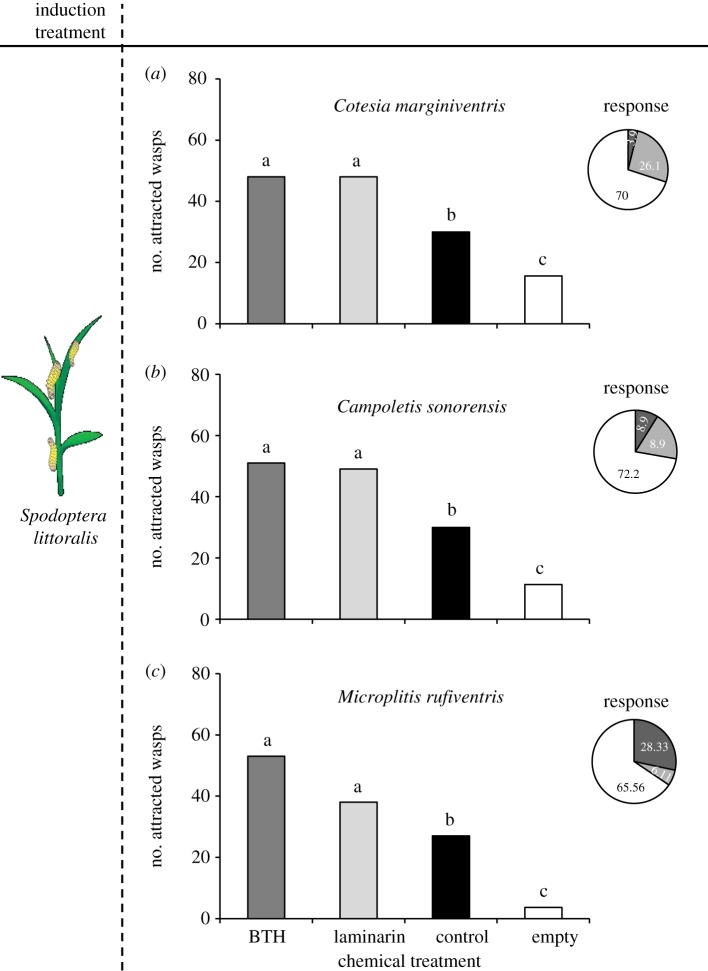
Foliar application of BTH at two different intervals increased the attractiveness of S. littoralis infested plants to three different parasitoid species. In general, the effect was stronger when plants had been treated 6 days before elicitation with real or simulated herbivory (figure 1). Microplitis rufiventris reacted more strongly to plants induced with S. littoralis, whereas C. sonorensis was more sensitive to mechanically damaged BTH-treated plants. Similar to BTH, laminarin increased the attractiveness of herbivore-induced plants 6 days after application to all three parasitoids, with the exception of C. sonorensis, which was not significantly attracted to mechanically induced laminarin-treated plants (figure 1b(ii)). Similarly, treating maize seeds with either BTH or laminarin increased the attractiveness of herbivore-infested plants for all three tested parasitoid species (figure 2). Previous experiments show that neither laminarin nor BTH itself are attractive to the parasitoids and that they do not significantly alter the overall larval feeding, although a small, but significant difference was found after 2 days of feeding [61,65]. These results are highly encouraging in the light of an easy to apply treatment of maize with plant strengtheners for long-lasting enhanced attractiveness to parasitoids.
Figure 1.
(a,b) Responses of naive female parasitoid wasps tested in a six-arm olfactometer. Values shown represent the number of parasitoids attracted to a particular odour. Wasps were allowed to choose between odours of plants sprayed with strengtheners 2 or 6 days before infesting the plants with caterpillars (i) or simulating herbivory by mechanically damaging the plants and treating them with caterpillar regurgitant (ii); control, untreated maize plants; empty, empty control vessel (mean value of three vessels). Pie charts indicate percentages of female wasps (dark grey, females choose the empty bottles; light grey, non-responding females; white, responding females). Different letters indicate significant differences between treatments (p < 0.05).
Figure 2.
(a–c) Responses of naive females of three species of parasitic wasps tested in a six-arm olfactometer. Values shown are numbers of parasitoids attracted to a particular odour. Wasps were allowed to choose between odours of BTH: maize plants that germinated from BTH-treated seeds, laminarin: maize plants that germinated from laminarin-treated seeds, control: maize plants that germinated from distilled water-treated seeds, and empty: empty control vessel (mean value of three vessels). Pie charts indicate percentages of female wasps (dark grey, non-responding females; light grey, females choose the empty bottles; black, responding females). Different letters indicate significant differences between treatments (p < 0.05).
(b). Plant strengtheners alter volatile emission
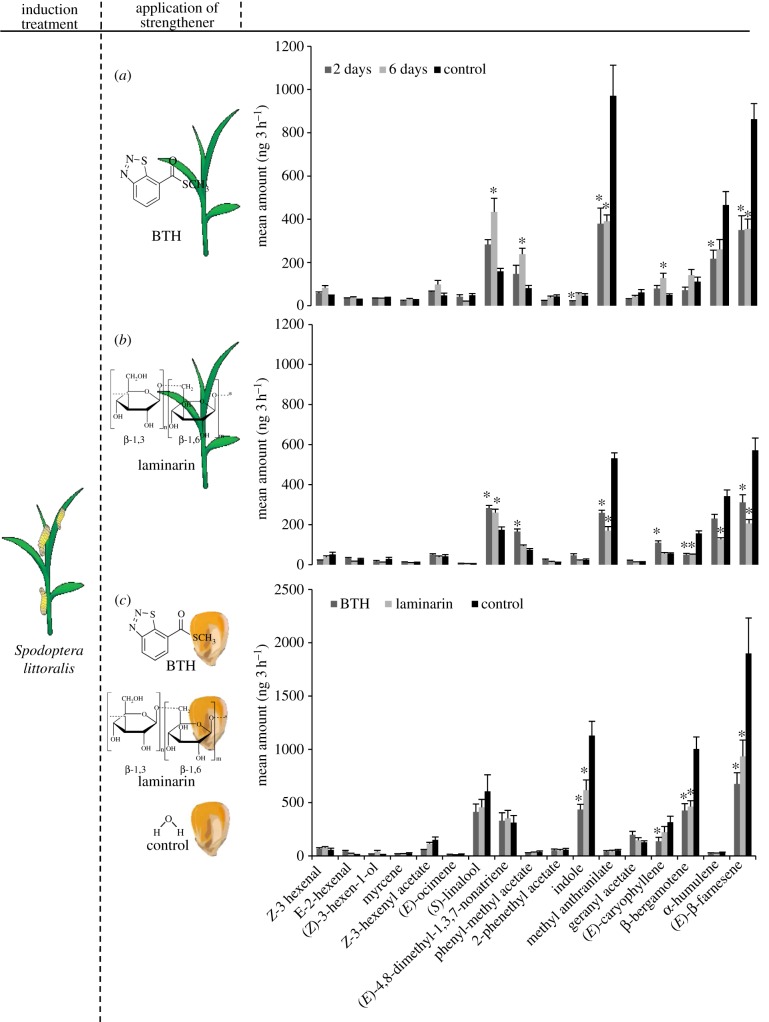
Previous experiments revealed minor changes in constitutive volatile emission of BTH- or laminarin-treated, undamaged maize plants, but surprisingly some herbivore-induced volatiles were found to be suppressed after treatment [61,65]. This study confirms this suppression, both in the case of foliar and seed application of the plant strengtheners (figure 3). The most dramatic responses were found for the aromatic compound indole and the sesquiterpene (E)-β-farnesene, both of which were emitted in lower quantities in plant strengthener-treated plants, irrespective of the timing and mode of application. The emission of (E)-caryophyllene and β-bergamotene was also reduced, albeit not in all cases. The emission of the terpenes linalool and (E)-4,8-dimethyl-1,3,7-nonatriene and geranyl acetate, on the other hand, was enhanced by foliar application of plant strengtheners, pointing at a possible role of these volatiles in parasitoid attraction.
Figure 3.
Mean emission (±s.e.; ng) of Spodoptera littoralis induced odours emitted by 11 day old maize seedlings at different time points after plant strengthener application (a,b) and after seed treatment (c). Asterisks indicate differences between controls and chemically treated plants (p < 0.05; n = 18).
4. Discussion
The notion that plant volatile emissions can be enhanced or otherwise manipulated to help combat insect pests has been around for some time, also in the context of biological control [80,81]. The targeted manipulation of specific compounds has already shown promising results in laboratory and greenhouse assays [17–21]. A few field studies show direct evidence that engineering volatile emissions can strongly affect the attraction of biological control agents. For instance, the restoration of a root-produced signal that attracts entomopathogenic nematodes was found to significantly improve nematode-mediated protection of maize roots against an exceedingly important coleopteran pest [20]. Also, first field tests with genetically altered volatile emissions in rice show promise for the control for one of the most important rice pests, the rice brown plant-hopper [21]. In general, however, it remains largely unclear what compounds should be enhanced, especially to improve the attraction of parasitic wasps [38]. A transgenic approach remains controversial and requires major R&D investments that are rarely cost effective. Therefore, the coincidental discovery [61] that treatment with plant strengtheners can, besides enhancing the direct resistance of plants against pests and diseases, dramatically increase the attractiveness to a spectrum of parasitoid species shows great promise for application. Here, we show that the effect is long-lasting, persisting over weeks, and would therefore be particularly promising to enhance parasitoid-mediated protection of plants at an early stage when the plants are most vulnerable to yield-reducing caterpillar damage. Particularly promising is the fact that the effect can also be achieved by soaking the seeds in solutions with the elicitors. The effect remains unexplained, as for now we cannot pinpoint the chemical changes that are responsible for the enhanced parasitoid attraction. Without information on the illusive compounds that are of key importance for the attraction of these generalist parasitoids, treatment with the studied elicitors of resistance seems to be the best option to improve plant resistance and performance, and simultaneously improve the biological control of important lepidopteran pests.
Acknowledgements
We thank Thomas Degen and Matthias Held for their invaluable advice and assistance with the writing of this paper.
Funding statement
Our work on understanding the importance of plant volatiles in plant–insect interactions and our efforts to exploit such volatiles for crop protection is supported by the European Science Foundation (EuroCore project Eurovol) and by the National Centre of Competence in Research (NCCR) Plant Survival, a research programme of the Swiss National Science Foundation. The work of M.E. is supported by a Marie Curie Intra-European Fellowship (grant no. 273107).
References
- 1.Foley JA, et al. 2011. Solutions for a cultivated planet. Nature 478, 337–342. ( 10.1038/Nature10452) [DOI] [PubMed] [Google Scholar]
- 2.Godfray HCJ, et al. 2010. Food security: the challenge of feeding 9 billion people. Science 327, 812–818. ( 10.1126/Science.1185383) [DOI] [PubMed] [Google Scholar]
- 3.Gregory PJ, George TS. 2011. Feeding nine billion: the challenge to sustainable crop production. J. Exp. Bot. 62, 5233–5239. ( 10.1093/Jxb/Err232) [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 2009. Reaping the benefits: towards sustainable intensification of global agriculture. London, UK: The Royal Society. [Google Scholar]
- 5.Dicke M, Sabelis MW. 1988. How plants obtain predatory mites as bodyguards. Neth. J. Zool. 38, 148–165. ( 10.1163/156854288X00111) [DOI] [Google Scholar]
- 6.Turlings TCJ, Tumlinson JH, Lewis WJ. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250, 1251–1253. ( 10.1126/Science.250.4985.1251) [DOI] [PubMed] [Google Scholar]
- 7.Turlings TCJ, Wäckers FL. 2004. Recruitment of predators and parasitoids by herbivore-damaged plants. In Advances in insect chemical ecology (eds Cardé RT, Millar J.), pp. 21–75. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Arimura G, Matsui K, Takabayashi J. 2009. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol. 50, 911–923. ( 10.1093/Pcp/Pcp030) [DOI] [PubMed] [Google Scholar]
- 9.Dicke M, Baldwin IT. 2010. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci. 15, 167–175. ( 10.1016/J.Tplants.2009.12.002) [DOI] [PubMed] [Google Scholar]
- 10.De Moraes CM, Mescher MC, Tumlinson JH. 2001. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410, 577–580. ( 10.1038/35069058) [DOI] [PubMed] [Google Scholar]
- 11.Snoeren TAL, Mumm R, Poelman EH, Yang Y, Pichersky E, Dicke M. 2010. The herbivore-induced plant volatile methyl salicylate negatively affects attraction of the parasitoid Diadegma semiclausum. J. Chem. Ecol. 36, 479–489. ( 10.1007/S10886-010-9787-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degenhardt J, Gershenzon J, Baldwin IT, Kessler A. 2003. Attracting friends to feast on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 14, 169–176. ( 10.1016/S0958-1669(03)00025-9) [DOI] [PubMed] [Google Scholar]
- 13.Poppy GM, Sutherland JP. 2004. Can biological control benefit from genetically-modified crops? Tritrophic interactions on insect-resistant transgenic plants. Physiol. Entomol. 29, 257–268. ( 10.1111/J.0307-6962.2004.00382.X) [DOI] [Google Scholar]
- 14.Aharoni A, Jongsma MA, Bouwmeester HJ. 2005. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 10, 594–602. ( 10.1016/J.Tplants.2005.10.005) [DOI] [PubMed] [Google Scholar]
- 15.Turlings TCJ, Ton J. 2006. Exploiting scents of distress: the prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests. Curr. Opin. Plant Biol. 9, 421–427. ( 10.1016/J.Pbi.2006.05.010) [DOI] [PubMed] [Google Scholar]
- 16.Pickett JA, et al. 2006. Plant volatiles yielding new ways to exploit plant defence. In Chemical ecology: from gene to ecosystem (eds Dicke M, Takken W.), pp. 161–173. Berlin, Germany: Springer. [Google Scholar]
- 17.Kappers IF, Aharoni A, van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ. 2005. Genetic engineering of terpenoid metabolism attracts, bodyguards to Arabidopsis. Science 309, 2070–2072. ( 10.1126/Science.1116232) [DOI] [PubMed] [Google Scholar]
- 18.Schnee C, Köllner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J. 2006. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl Acad. Sci. USA 103, 1129–1134. ( 10.1073/pnas.0508027103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beale MH, et al. 2006. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc. Natl Acad. Sci. USA 103, 10 509–10 513. ( 10.1073/Pnas.0603998103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degenhardt J, Hiltpold I, Köllner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TCJ. 2009. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl Acad. Sci. USA 106, 13 213–13 218. ( 10.1073/Pnas.0906365106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao Y, et al. 2012. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 15, 1130–1139. ( 10.1111/J.1461-0248.2012.01835.X) [DOI] [PubMed] [Google Scholar]
- 22.Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT. 2008. Shared signals: 'alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol. Lett. 11, 24–34. ( 10.1111/J.1461-0248.2007.01123.X) [DOI] [PubMed] [Google Scholar]
- 23.Robert CAM, Erb M, Duployer M, Zwahlen C, Doyen GR, Turlings TCJ. 2012. Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytol. 194, 1061–1069. ( 10.1111/J.1469-8137.2012.04127.X) [DOI] [PubMed] [Google Scholar]
- 24.Robert CAM, Erb M, Hibbard BE, Wade French B, Zwahlen C, Turlings TCJ. 2012. A specialist root herbivore reduces plant resistance and uses an induced plant volatile to aggregate in a density-dependent manner. Funct. Ecol. 26, 1429–1440. ( 10.1111/j.1365-2435.2012.02030.x) [DOI] [Google Scholar]
- 25.Khan ZR, et al. 1997. Intercropping increases parasitism of pests. Nature 388, 631–632. ( 10.1038/41681) [DOI] [Google Scholar]
- 26.Cook SM, Khan ZR, Pickett JA. 2007. The use of push–pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375–400. ( 10.1146/Annurev.Ento.52.110405.091407) [DOI] [PubMed] [Google Scholar]
- 27.Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ. 2005. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434, 732–737. ( 10.1038/Nature03451) [DOI] [PubMed] [Google Scholar]
- 28.Robert CAM, et al. 2013. Genetically engineered maize plants reveal distinct costs and benefits of constitutive volatile emissions in the field. Plant Biotechnol. J. 11, 628–639. ( 10.1111/pbi.12053) [DOI] [PubMed] [Google Scholar]
- 29.Losey JE, Rayor LS, Carter ME. 1999. Transgenic pollen harms monarch larvae. Nature 399, 214–214. ( 10.1038/20338) [DOI] [PubMed] [Google Scholar]
- 30.Ewen SWB, Pusztai A. 1999. Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet 354, 1353–1354. ( 10.1016/S0140-6736(98)05860-7) [DOI] [PubMed] [Google Scholar]
- 31.Séralini G-E, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois JS. 2012. Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food Chem. Toxicol. 50, 4221–4231. ( 10.1016/j.fct.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 32.Pickett JA, Poppy GM. 2001. Switching on plant genes by external chemical signals. Trends Plant Sci. 6, 137–139. ( 10.1016/S1360-1385(01)01899-4) [DOI] [PubMed] [Google Scholar]
- 33.Conrath U, Pieterse CMJ, Mauch-Mani B. 2002. Priming in plant-pathogen interactions. Trends Plant Sci. 7, 210–216. ( 10.1016/S1360-1385(02)02244-6) [DOI] [PubMed] [Google Scholar]
- 34.Walters D, Newton A, Lyon G. 2007. Induced resistance for plant defence: a sustainable approach to crop protection. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 35.Ton J, Jakab G, Toquin V, Flors V, Iavicoli A, Maeder MN, Metraux JP, Mauch-Mani B. 2005. Dissecting the β-aminobutyric acid-induced priming phenomenon in Arabidopsis. Plant Cell 17, 987–999. ( 10.1105/Tpc.104.029728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Rad U, Mueller MJ, Durner J. 2005. Evaluation of natural and synthetic stimulants of plant immunity by microarray technology. New Phytol. 165, 191–202. ( 10.1111/J.1469-8137.2004.01211.X) [DOI] [PubMed] [Google Scholar]
- 37.D'Alessandro M, Turlings TCJ. 2006. Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst 131, 24–32. ( 10.1039/B507589k) [DOI] [PubMed] [Google Scholar]
- 38.D'Alessandro M, Brunner V, von Merey G, Turlings TCJ. 2009. Strong attraction of the parasitoid Cotesia marginiventris towards minor volatile compounds of maize. J. Chem. Ecol. 35, 999–1008. ( 10.1007/S10886-009-9692-7) [DOI] [PubMed] [Google Scholar]
- 39.De Boer JG, Dicke M. 2004. The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J. Chem. Ecol. 30, 255–271. ( 10.1023/B:Joec.0000017976.60630.8c) [DOI] [PubMed] [Google Scholar]
- 40.De Boer JG, Posthumus MA, Dicke M. 2004. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J. Chem. Ecol. 30, 2215–2230. ( 10.1023/B:Joec.0000048784.79031.5e) [DOI] [PubMed] [Google Scholar]
- 41.Paré PW, Tumlinson JH. 1997. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 114, 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Wijk M, De Bruijn PJA, Sabelis MW. 2008. Predatory mite attraction to herbivore-induced plant odors is not a consequence of attraction to individual herbivore-induced plant volatiles. J. Chem. Ecol. 34, 791–803. ( 10.1007/s10886-008-9492-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turlings TCJ, Wäckers F, Vet LEM, Lewis WJ, Tumlinson JH. 1993. Learning of host-finding cues by hymenopterous parasitoids. In Insect learning: ecological and evolutionary perspectives (eds Papaj DR, Lewis A.), pp. 51–78. New York, NY: Chapman & Hall. [Google Scholar]
- 44.Vet LEM, Lewis WJ, Cardé RT. 1995. Parasitoid foraging and learning. In Chemical ecology of insects 2 (eds Cardé RT, Bell WJ.), pp. 65–101. New York, NY: Chapman & Hall. [Google Scholar]
- 45.James DG. 2005. Further field evaluation of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. J. Chem. Ecol. 31, 481–495. ( 10.1007/S10886-005-2020-Y) [DOI] [PubMed] [Google Scholar]
- 46.James DG. 2003. Field evaluation of herbivore-induced plant volatiles as attractants for beneficial insects: methyl salicylate and the green lacewing, Chrysopa nigricornis. J. Chem. Ecol. 29, 1601–1609. ( 10.1023/A:1024270713493) [DOI] [PubMed] [Google Scholar]
- 47.James DG, Price TS. 2004. Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J. Chem. Ecol. 30, 1613–1628. ( 10.1023/B:Joec.0000042072.18151.6f) [DOI] [PubMed] [Google Scholar]
- 48.James DG, Grasswitz TR. 2005. Synthetic herbivore-induced plant volatiles increase field captures of parasitic wasps. Biocontrol 50, 871–880. ( 10.1007/s10526-005-3313-3) [DOI] [Google Scholar]
- 49.Degen T, Bakalovic N, Bergvinson D, Turlings TCJ. 2012. Differential performance and parasitism of caterpillars on maize inbred lines with distinctly different herbivore-induced volatile emissions. PLoS ONE 7, e47589 ( 10.1371/journal.pone.0047589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dicke M. 2009. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 32, 654–665. ( 10.1111/J.1365-3040.2008.01913.X) [DOI] [PubMed] [Google Scholar]
- 51.D'Alessandro M, Turlings TCJ. 2005. In situ modification of herbivore-induced plant odors: a novel approach to study the attractiveness of volatile organic compounds to parasitic wasps. Chem. Senses 30, 739–753. ( 10.1093/Chemse/Bji066) [DOI] [PubMed] [Google Scholar]
- 52.Fontana A, Held M, Fantaye CA, Turlings TC, Degenhardt J, Gershenzon J. 2011. Attractiveness of constitutive and herbivore-induced sesquiterpene blends of maize to the parasitic wasp Cotesia marginiventris (Cresson). J. Chem. Ecol. 37, 582–591. ( 10.1007/S10886-011-9967-7) [DOI] [PubMed] [Google Scholar]
- 53.Pickett JA, Wadhams LJ, Woodcock CM, Hardie J. 1992. The chemical ecology of aphids. Annu. Rev. Entomol. 37, 67–90. ( 10.1146/annurev.ento.37.1.67) [DOI] [Google Scholar]
- 54.Al Abassi S, Birkett MA, Pettersson J, Pickett JA, Wadhams LJ, Woodcock CM. 2000. Response of the seven-spot ladybird to an aphid alarm pheromone and an alarm pheromone inhibitor is mediated by paired olfactory cells. J. Chem. Ecol. 26, 1765–1771. ( 10.1023/A:1005555300476) [DOI] [Google Scholar]
- 55.Thaler JS. 1999. Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399, 686–688. ( 10.1038/21420) [DOI] [Google Scholar]
- 56.Schuman MC, Barthel K, Baldwin IT. 2012. Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. eLife Sci. 1, e00007 ( 10.7554/eLife.00007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammack L. 2001. Single and blended maize volatiles as attractants for diabroticite corn rootworm beetles. J. Chem. Ecol. 27, 1373–1390. ( 10.1023/A:1010365225957) [DOI] [PubMed] [Google Scholar]
- 58.Khan ZR, Pickett JA, Wadhams LJ, Hassanali A, Midega CAO. 2006. Combined control of Striga hermonthica and stemborers by maize-Desmodium spp. intercrops. Crop Prot. 25, 989–995. ( 10.1016/J.Cropro.2006.01.008) [DOI] [Google Scholar]
- 59.Khan ZR, Midega CAO, Bruce TJA, Hooper AM, Pickett JA. 2010. Exploiting phytochemicals for developing a ‘push–pull’ crop protection strategy for cereal farmers in Africa. J. Exp. Bot. 61, 4185–4196. ( 10.1093/Jxb/Erq229) [DOI] [PubMed] [Google Scholar]
- 60.D'Alessandro M, Held M, Triponez Y, Turlings TCJ. 2006. The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J. Chem. Ecol. 32, 2733–2748. ( 10.1007/S10886-006-9196-7) [DOI] [PubMed] [Google Scholar]
- 61.Rostas M, Turlings TCJ. 2008. Induction of systemic acquired resistance in Zea mays also enhances the plant's attractiveness to parasitoids. Biol. Control 46, 178–186. ( 10.1016/J.Biocontrol.2008.04.012) [DOI] [Google Scholar]
- 62.Friedrich L, et al. 1996. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J. 10, 61–70. ( 10.1046/J.1365-313x.1996.10010061.X) [DOI] [PubMed] [Google Scholar]
- 63.Tally A, Oostendorp M, Lawton K, Staub T, Bassi B. 1999. Commercial development of elicitors of induced resistance to pathogens. In Induced plant defenses against pathogens and herbivores: biochemistry, ecology, and agriculture (eds Agrawal AA, Tuzun S, Bent E.), pp. 357–369. St Paul, MN: APS Press. [Google Scholar]
- 64.Thaler JS, Humphrey PT, Whiteman NK. 2012. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 17, 260–270. ( 10.1016/J.Tplants.2012.02.010) [DOI] [PubMed] [Google Scholar]
- 65.Sobhy IS, Erb M, Sarhan AA, El-Husseini MM, Mandour NS, Turlings TCJ. 2012. Less is more: treatment with BTH and laminarin reduces herbivore-induced volatile emissions in maize but increases parasitoid attraction. J. Chem. Ecol. 38, 348–360. ( 10.1007/S10886-012-0098-6) [DOI] [PubMed] [Google Scholar]
- 66.Klarzynski O, Plesse B, Joubert JM, Yvin JC, Kopp M, Kloareg B, Fritig B. 2000. Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 124, 1027–1037. ( 10.1104/Pp.124.3.1027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ménard R, Alban S, de Ruffray P, Jamois F, Franz G, Fritig B, Yvin JC, Kauffmann S. 2004. β-1,3 glucan sulfate, but not β-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant Cell 16, 3020–3032. ( 10.1105/Tpc.104.024968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Merey G, Veyrat N, Mahuku G, Valdez RL, Turlings TCJ, D'Alessandro M. 2011. Dispensing synthetic green leaf volatiles in maize fields increases the release of sesquiterpenes by the plants, but has little effect on the attraction of pest and beneficial insects. Phytochemistry 72, 1838–1847. ( 10.1016/J.Phytochem.2011.04.022) [DOI] [PubMed] [Google Scholar]
- 69.Xin ZJ, Yu ZN, Erb M, Turlings TCJ, Wang BH, Qi JF, Liu SN, Lou YG. 2012. The broad-leaf herbicide 2,4-dichlorophenoxyacetic acid turns rice into a living trap for a major insect pest and a parasitic wasp. New Phytol. 194, 498–510. ( 10.1111/J.1469-8137.2012.04057.X) [DOI] [PubMed] [Google Scholar]
- 70.Lou YG, Du MH, Turlings TCJ, Cheng JA, Shan WF. 2005. Exogenous application of jasmonic acid induces volatile emissions in rice and enhances parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae. J. Chem. Ecol. 31, 1985–2002. ( 10.1007/S10886-005-6072-9) [DOI] [PubMed] [Google Scholar]
- 71.Lou YG, Ma B, Cheng JA. 2005. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J. Chem. Ecol. 31, 2357–2372. ( 10.1007/S10886-005-7106-Z) [DOI] [PubMed] [Google Scholar]
- 72.Lou YG, Hua XY, Turlings TCJ, Cheng JA, Chen XX, Ye GY. 2006. Differences in induced volatile emissions among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the field. J. Chem. Ecol. 32, 2375–2387. ( 10.1007/S10886-006-9151-7) [DOI] [PubMed] [Google Scholar]
- 73.Lu YJ, Wang X, Lou YG, Cheng JA. 2006. Role of ethylene signaling in the production of rice volatiles induced by the rice brown planthopper Nilaparvata lugens. Chin. Sci. Bull. 51, 2457–2465. ( 10.1007/S11434-006-2148-3) [DOI] [Google Scholar]
- 74.Zhou GX, Qi JF, Ren N, Cheng JA, Erb M, Mao BZ, Lou YG. 2009. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 60, 638–648. ( 10.1111/J.1365-313x.2009.03988.X) [DOI] [PubMed] [Google Scholar]
- 75.Lou Y, Cheng J, Du M. (eds). 1999. Role of rice volatiles in the host selection behavior of the parasitoid, Anagrus nilaparvatae Pang et Wang: isolation and identification of volatile rice synomone. First Annual Meeting of Science Society of China: Science and Technology Progress and Society and Economy Development Beyond 2000. Beijing, China: Science and Technology Press of China. [Google Scholar]
- 76.Buzi A, Chilosi G, De Sillo D, Magro P. 2004. Induction of resistance in melon to Didymella bryoniae and Sclerotinia sclerotiorum by seed treatments with acibenzolar-S-methyl and methyl jasmonate but not with salicylic acid. J. Phytopathol. 152, 34–42. ( 10.1046/J.1439-0434.2003.00798.X) [DOI] [Google Scholar]
- 77.Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, DeSamblanx GW, Buchala A, Metraux JP, Manners JM, Broekaert WF. 1996. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8, 2309–2323. ( 10.2307/3870470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turlings TCJ, Lengwiler UB, Bernasconi ML, Wechsler D. 1998. Timing of induced volatile emissions in maize seedlings. Planta 207, 146–152. ( 10.1007/S004250050466) [DOI] [Google Scholar]
- 79.Turlings TCJ, Davison AC, Tamo C. 2004. A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 29, 45–55. ( 10.1111/J.1365-3032.2004.0362.X) [DOI] [Google Scholar]
- 80.Vinson SB. 1976. Host selection by insect parasitoids. Annu. Rev. Entomol. 21, 109–133. ( 10.1146/Annurev.En.21.010176.000545) [DOI] [Google Scholar]
- 81.Nordlund DA, Lewis WJ, Altieri MA. 1988. Influences of plant-produced allelochemicals on the host/prey selection behaviour of entomophagous insects. In Novel aspects of insect–plant interactions (eds Barbosa P, Letourneau DK.), pp. 65–96. New York, NY: Wiley. [Google Scholar]