Abstract
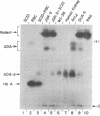
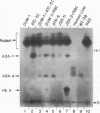
In human tissues, adenosine deaminase (ADA) (adenosine aminohydrolase; EC 3.5.4.4) activity can be separated by gel electrophoresis into several isozymes. A structural gene (ADA) on chromosome 20 codes for the “erythrocyte” isozyme, ADA-1, which is also expressed in some nonerythroid tissues. Nonerythroid cells also differentially express five ADA “tissue isozymes” of a greater molecular weight than ADA-1. Each ADA tissue isozyme has a characteristic electrophoretic mobility and tissue distribution. It has been suggested that these ADA tissue isozymes are composed of ADA-1 and other components. We report that the expression of one of these tissue isozymes, ADA-d, is dependent upon ADA on chromosome 20 and another gene on chromosome 6 which functions in the assembly of the ADA tissue isozymes. In human-mouse hybrids segregating human chromosomes, chromosome 6+,20+ hybrids express both ADA-1 and ADA-d; chromosome 6-,20+ hybrids express only ADA-1; while 6+,20- hybrids have no human ADA activity. ADA-d formation also occurs in vitro by self-assembly when an extract of human erythrocytes or chromosome 6-,20+ hybrids is mixed with a homogenate of chromosome 6+,20- hybrids. The gene on chromosome 6, designated ADCP, codes for an adenosine deaminase complexing protein. The product of ADCP presumably combines with ADA-1 to form the ADA tissue isozymes. The data are consistent with the hypothesis that the distribution of enzymatic activity between ADA-1 and the tissue isozymes depends on the expression of the gene for ADA complexing protein, while the differences in the electrophoretic mobilities of the ADA isozymes, except ADA-1, are generated, as suggested by others, by the degree of glycosylation of the complexing protein.
Keywords: somatic cell hybrids, gene mapping, immune deficiency disease
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELLA E., MARKERT C. L. Dissociation of lactate dehydrogenase into subunits with guanidine hydrochloride. Biochem Biophys Res Commun. 1961 Nov 20;6:171–176. doi: 10.1016/0006-291x(61)90123-1. [DOI] [PubMed] [Google Scholar]
- Champion M. J., Shows T. B. Electrophoretic abnormalities of lysosomal enzymes in mucolipidosis fibroblast lines. Am J Hum Genet. 1977 Mar;29(2):149–163. [PMC free article] [PubMed] [Google Scholar]
- Champion M. J., Shows T. B. Mannosidosis: assignment of the lysosomal alpha-mannosidase B gene to chromosome 19 in man. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2968–2972. doi: 10.1073/pnas.74.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. M., Lin C. C., Sybert V., Orecchio E. J. Two human X-autosome translocations identified by autoradiography and fluorescence. Am J Hum Genet. 1972 Sep;24(5):583–597. [PMC free article] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Adenosine deaminase isozymes in human tissues. Ann Hum Genet. 1971 Oct;35(2):207–219. doi: 10.1111/j.1469-1809.1956.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. Conversion of human erythrocyte-adenosine deaminase activity to different tissue-specific isozymes. Evidence for a common catalytic unit. J Clin Invest. 1975 Mar;55(3):661–667. doi: 10.1172/JCI107974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Levytaka V., Pollara B., Meuwissen H. J. Evidence for control of several different tissue-specific isozymes of adenosine deaminase by a single genetic locus. Nat New Biol. 1973 Dec 19;246(155):200–202. doi: 10.1038/newbio246200a0. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Harris H. A third phosphoglucomutase locus in man. Ann Hum Genet. 1968 May;31(4):359–367. doi: 10.1111/j.1469-1809.1968.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., Brown J. A., Eddy R. L., Haley L. L., Byers M. G., Goggin A. P., Shows T. B. Human beta-glucuronidase: assignment of the structural gene to chromosome 7 using somatic cell hybrids. Biochem Genet. 1977 Apr;15(3-4):367–382. doi: 10.1007/BF00484467. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., Brown J. A., Shows T. B. Assignment of hexosaminidase-B to chromosome 5, its segregation after diphtheria toxin selection, and the linkage of hexosaminidase-A, mannose phosphate isomerase, and pyruvate kinase (M2). Cytogenet Cell Genet. 1976;16(1-5):188–191. doi: 10.1159/000130587. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., Shows T. B. Lysosomal Acid phosphatase deficiency: liver specific variant in the mouse. Genetics. 1977 Oct;87(2):305–317. doi: 10.1093/genetics/87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara H., Ishikawa S., Shinkai K., Akedo H. Multiple forms of human adenosine deaminase. II. Isolation and properties of a conversion factor from human lung. Biochim Biophys Acta. 1973 Apr 12;302(2):429–442. doi: 10.1016/0005-2744(73)90172-1. [DOI] [PubMed] [Google Scholar]
- Paigen K., Swank R. T., Tomino S., Ganschow R. E. The molecular genetics of mammalian glucuronidase. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):379–392. doi: 10.1002/jcp.1040850406. [DOI] [PubMed] [Google Scholar]
- Parkman R., Gelfand E. W., Rosen F. S., Sanderson A., Hirschhorn R. Severe combined immunodeficiency and adenosine deaminase deficiency. N Engl J Med. 1975 Apr 3;292(14):714–719. doi: 10.1056/NEJM197504032921402. [DOI] [PubMed] [Google Scholar]
- Rattazzi M. C., Brown J. A., Davidson R. G., Shows T. B. Studies on complementation of beta hexosaminidase deficiency in human GM2 gangliosidosis. Am J Hum Genet. 1976 Mar;28(2):143–154. [PMC free article] [PubMed] [Google Scholar]
- Schrader W. P., Stacy A. R. Purification and subunit structure of adenosine deaminase from human kidney. J Biol Chem. 1977 Sep 25;252(18):6409–6415. [PubMed] [Google Scholar]
- Shows T. B., Brown J. A., Haley L. L., Byers M. G., Eddy R. L., Cooper E. S., Goggin A. P. Assignment of the beta-glucuronidase structural gene to the pter leads to q22 region of chromosome 7 in man. Cytogenet Cell Genet. 1978;21(1-2):99–104. doi: 10.1159/000130882. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Brown J. A. Human X-Linked genes regionally mapped utilizing X-autosome translocations and somatic cell hybrids. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2125–2129. doi: 10.1073/pnas.72.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Brown J. A. Mapping chromosomes 1 and 2 employing a 1/2 translocation in somatic cell hybrids. Birth Defects Orig Artic Ser. 1975;11(3):251–255. [PubMed] [Google Scholar]
- Shows T. B. Genetics of human-mouse somatic cell hybrids: linkage of human genes for lactate dehydrogenase-A and esterase-A 4 . Proc Natl Acad Sci U S A. 1972 Feb;69(2):348–352. doi: 10.1073/pnas.69.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E., Bobrow M., Goodfellow P. N., Bodmer W. F., Swallow D. M., Povey S., Noël B. Human gene mapping using an X/autosome translocation. Somatic Cell Genet. 1976 Mar;2(2):125–140. doi: 10.1007/BF01542626. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Evans L., Hopkinson D. A. Several of the adenosine deaminase isozymes are glycoproteins. Nature. 1977 Sep 15;269(5625):261–262. doi: 10.1038/269261a0. [DOI] [PubMed] [Google Scholar]
- Tischfield J. A., Creagan R. P., Nichols E. A., Ruddle F. H. Assignment of a gene for adenosine deaminase to human chromosome 20. Hum Hered. 1974;24(1):1–11. doi: 10.1159/000152631. [DOI] [PubMed] [Google Scholar]
- van Someren H., Westerveld A., Hagemeijer A., Mees J. R., Meera Khan P., Zaalberg O. B. Human antigen and enzyme markers in man-Chinese hamster somatic cell hybrids: evidence for synteny between the HL-A, PGM3, ME1, and IPO-B loci. Proc Natl Acad Sci U S A. 1974 Mar;71(3):962–965. doi: 10.1073/pnas.71.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]