Abstract
Background and Purpose: Obtaining renal access is one of the most important and complex steps in learning percutaneous nephrolithotomy (PCNL). Ideally, this skill should be practiced outside the operating room. There is a need for anatomically accurate and cheap models for simulated training. The objective was to develop a cost-effective, anatomically accurate, nonbiologic training model for simulated PCNL access under fluoroscopic guidance.
Methods: Collecting systems from routine computed tomography urograms were extracted and reformatted using specialized software. These images were printed in a water-soluble plastic on a three-dimensional (3D) printer to create biomodels. These models were embedded in silicone and then the models were dissolved in water to leave a hollow collecting system within a silicone model. These PCNL models were filled with contrast medium and sealed. A layer of dense foam acted as a spacer to replicate the tissues between skin and kidney.
Results: 3D printed models of human collecting systems are a useful adjunct in planning PCNL access. The PCNL access training model is relatively low cost and reproduces the anatomy of the renal collecting system faithfully. A range of models reflecting the variety and complexity of human collecting systems can be reproduced. The fluoroscopic triangulation process needed to target the calix of choice can be practiced successfully in this model.
Conclusions: This silicone PCNL training model accurately replicates the anatomic architecture and orientation of the human renal collecting system. It provides a safe, clean, and effective model for training in accurate fluoroscopy-guided PCNL access.
Introduction
Percutaneous nephrolithotomy (PCNL) was introducd in the early 1980s and has become established as the procedure of choice for removing larger stones or those that are difficult to access with ureteroscopy.1 The most technically challenging part of the procedure is obtaining percutaneous access to the kidney. The challenge is to convert two-dimensional images into a mental three-dimensional (3D) image to triangulate and puncture the calix of choice. This is performed by either radiologists or urologists, depending on the local expertise.2 It has been suggested that a trainee has to perform about 24 PCNL procedures to obtain a good proficiency, becomes competent after 60 cases, and excellence is obtained at >100 cases.3
Traditionally, the patient is in the prone position for PCNL, but more recently the modified supine position has been popularized that allows both ureteroscopic and percutaneous access simultaneously. This may have some surgical and anesthetic advantages.4,5 The access is traditionally guided by either fluoroscopy or ultrasonography. Recently, new localization techniques have been described that might simplify percutaneous access.6,7
Until these new technologies become mainstream, there is a need for a good training model for PCNL. Several training models have been described.3,8 These range from the virtual trainers, to the ex vivo animal organ models through to nonbiologic models. Each of these has its drawbacks. The virtual training models are expensive, the animal models do not reproduce the human renal anatomy accurately and require fresh animal organs, and the current plastic models are often expensive, single use, and do not accurately reproduce the collecting system.
The objective of this project was to produce a cost-effective, anatomically accurate nonbiologic model for training in PCNL access.
Methods
Extraction of collecting system from CT scans
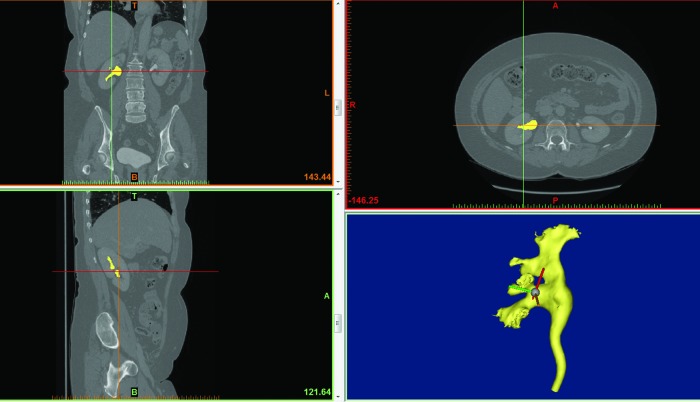
Routine anonymized CT urograms were taken from the Oxford University Hospitals Trust electronic system (Centricity PACS, GE Healthcare). Mimics software version 16.0 (Materialise, Belgium) was used to reformat the images and extract the collecting system anatomy (Fig. 1). These images were “cleaned-up” using the software to remove nonconnecting contrast material and to produce a single virtual collecting system structure. The file was exported as an STL (Standard Triangulation Language) file into open source software for 3D printing (Replicator G) (Fig. 2).
FIG. 1.
Images from a computed tomography (CT) urogram in three planes. The collecting system from the right kidney is highlighted and extracted and shown in the bottom right image.
FIG. 2.
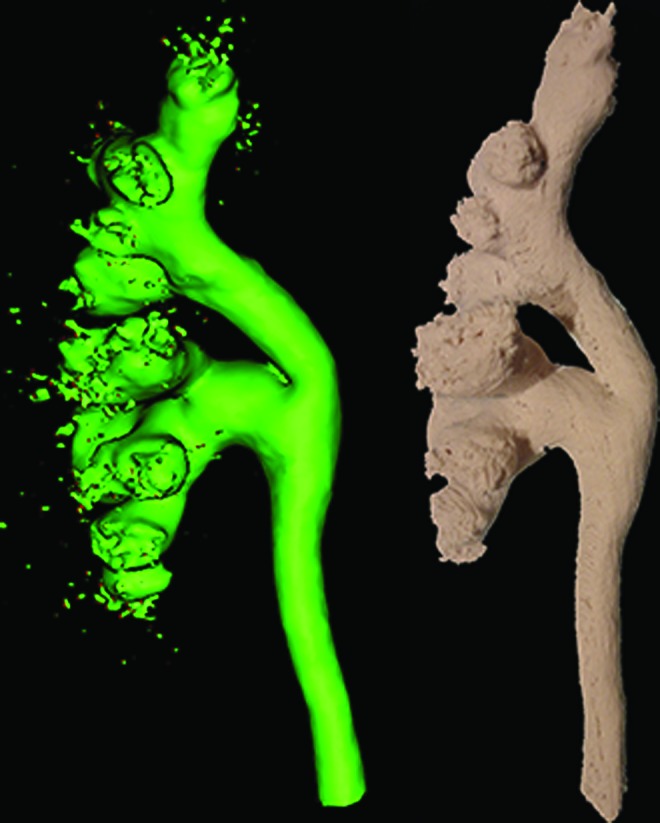
The STL file of a collecting system generated (left) is used to print a three-dimensional model (right) in a water soluble plastic (anterior views of both). A scale model of high fidelity is produced.
3D printing
STL files were printed using a 3D printer (Replicator, Makerbot, USA) in a water soluble polyvinyl alcohol plastic available as a 1.75 mm filament (Fig. 2). A variety of collecting systems were obtained from CT urograms (Fig. 3).
FIG. 3.

The variety of pelvicalyceal architecture is apparent from a selection of 3D models printed.
PCNL model
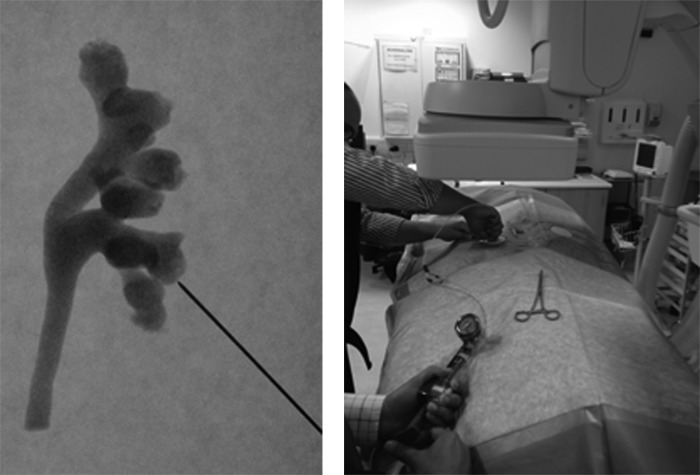
The 3D model of the collecting system was embedded in the correct anatomic orientation in silicone (Shore 27, Scarva, Ireland) in a plastic box. The two components of the silicone rubber system were mixed according to the manufacturer's recommendations. The silicone was left to solidify overnight. The silicone model was then removed from the plastic box and transferred to a bucket containing water. Two access ports were cut in the silicone, and the 3D plastic model was then dissolved from within the silicon model with water. The water was irrigated through the model with an aquarium pump (200 L/hour) to speed up the process. The model was then filled with contrast medium (Omnipaque, GE Healthcare) and sealed with waterproof tape The model was then covered in a layer of dense foam (Easyfoam, UK) to replicate the tissues between skin and kidney. This can be made of different thicknesses to simulate the triangulation process in patients of different body habitus. To simulate a more accurate operative experience, the model was covered in a surgical drape (Fig. 4).
FIG. 4.
(Left) The water-soluble model is dissolved away from a silicone surround and then filled with contrast to allow visualization under fluoroscopy. This is the posterior view of the collecting system printed in Figure 2. The needle can be seen approaching the calix of interest. This replicates the prone position of PCNL puncture. (Right) The model being used by a trainee on the Oxford Stone Course to establish a percutaneous nephrolithotromy tract.
Results
This silicone model allows training in PCNL access under fluoroscopic guidance with the equipment used in routine clinical practice. The consumable costs of the model are low (around £60/€70/$100), but there are capital costs of the Mimics software (£6000/€7100/$9600) and 3D printer (£2000/€2400/$3200). The print time for the 3D model is approximately 1 to 2 hours. Multiple models can be printed at once, and the printer works unsupervised. The silicone takes a few minutes to mix as a liquid and then solidifies overnight. The model takes 1 to 2 days to dissolve with the aquarium pump (£12/€14/$19) to irrigate the model. Each model can be used for multiple punctures (around 20) without leakage of contrast. Once a tract is dilated, with a balloon dilator or serial dilators, leakage of contrast occurs, however. There are no ethical issues.
The surgeon can puncture a calix in the model under fluoroscopic guidance and, importantly, the anatomic orientation and scale of the collecting system is preserved. The model received a high evaluation score when used on a national training course.
Discussion
Successful PCNL surgery relies on accurate planning and competency in accurately puncturing the kidney to gain access. This project has developed models that may improve PCNL surgery.
The ability to rapidly convert two-dimensional CT images into a 3D biomodel either on-screen or using a 3D printer allows better understanding of the anatomy of a collecting system and an appreciation of the orientation of the calices that is harder to obtain from either ultrasonographic or fluoroscopic images alone. Human collecting systems are very varied in appearance (Fig. 3), and it has been suggested that a 3D model may help the surgeon in planning accurate access.9 Identification of posterior and anterior calices is readily appreciated in a printed model, and the orientation of the calices to the renal pelvis can be visualized when planning access. 3D modeling has been used in other areas of surgery (particularly orthopedics and maxillofacial surgery) in planning and assessing outcomes of procedures.10,11 This study has described one way in which this technology might be applied to endourology. Recent advances in software and 3D printing have enabled rapid and cheap production of these useful models.
The diversity of renal pelvic architecture is readily apparent from the models printed (Fig. 3). Models can be produced to allow surgeons to practice their technique in systems of different grades of complexity. Thus, a training program could be developed.
Several models for PCNL training have been developed over the years.3,12,13 These models fall into three main categories: Virtual reality trainers, artificial models, and those using animal organs. The virtual reality trainers (eg, Perc Mentor) are expensive (∼£63,000/∼€75,000/∼$100,000), the artificial models currently are single use and expensive (eg, Limbs and things - £674/€800/$1080–2013 prices), and the animal models fail to accurately reproduce the human anatomy, are single use and messy.
The main advantage of the model described here is that it is the first model that reproduces the human anatomy accurately. Also, it is clean and relatively low in cost. The anatomic accuracy seen on fluoroscopy (Fig. 4) makes it particularly useful for practicing triangulation techniques. Creation of bespoke 3D anatomic models and PCNL training models from individuals' CT scans may be of particular use when planning particularly complex or high-risk procedures. Moreover, anatomically accurate models such as those described here may be a cheaper and more representative alternative when developing new technologies to facilitate caliceal targeting.7 It is hoped that this anatomically accurate model can be adapted and developed for training in flexible ureteroscopy.
A limitation of the model described here is that the current model composition is not very suitable for ultrasonographic imaging. A new embedding medium for the 3D models that facilitates ultrasonography examination is under development. Like other nonbiologic models, the consistency of the foam and silicone does not replicate human tissues exactly but does replicate the difference in density felt between extrarenal tissue and the kidney when performing punctures. Also, although the model can tolerate multiple punctures (∼20), once a tract is dilated, contrast leaks out of the model and it cannot be reused. This model does require the trainee to work with radiation. The trainee, however, must learn to work safely with radiation when performing PCNL, and this forms part of the training. The model does not provide objective electronic feedback and scoring like the computerized surgical simulators but does allow the trainee to practice all of the steps of the procedure, which can be evaluated by a mentor.
Further improvements to the model could include the addition of “ribs” to the foam layer and simulation of respiratory motion. A graded training program could be developed and validated involving collecting systems of varying complexity, punctures below and above ribs, with and without simulated respiratory motion. Once access can be achieved reliably, trainees could practice removing embedded stones from the model. Future iterations of the model require a formal training evaluation to confirm the educational value of this approach.
Conclusion
This project has led to the production of 3D biomodels that provide better interpretation of the CT images in planning PCNL and the development of a low cost, anatomically accurate model for training in PCNL access.
Abbreviations Used
- CT
computed tomography
- PCNL
percutaneous nephrolithotomy
- STL
Standard Triangulation Language
- 3D
three-dimensional
Acknowledgments
The research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford.
Disclosure Statement
No competing financial interests exist.
References
- 1.Turk C, Knoll T, Petrik A, et al. . European Association of Urology. Guidelines on Urolithiasis 2013. Available from: http://www.uroweb.org/gls/pdf/21_Urolithiasis_LR.pdf Accessed: October31, 2013
- 2.de la Rosette J, Assimos D, Desai M, et al. . The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study: Indications, complications, and outcomes in 5803 patients. J Endourol 2011;25:11–17 [DOI] [PubMed] [Google Scholar]
- 3.de la Rosette JJ, Laguna MP, Rassweiler JJ, Conort P. Training in percutaneous nephrolithotomy—a critical review. Eur Urol 2008;54:994–1001 [DOI] [PubMed] [Google Scholar]
- 4.Valdivia JG, Scarpa RM, Duvdevani M, et al. . Supine versus prone position during percutaneous nephrolithotomy: A report from the clinical research office of the endourological society percutaneous nephrolithotomy global study. J Endourol 2011;25:1619–1625 [DOI] [PubMed] [Google Scholar]
- 5.Atkinson CJ, Turney BW, Noble JG, et al. . Supine vs prone percutaneous nephrolithotomy: An anaesthetist's view. BJU Int 2011;108:306–308 [DOI] [PubMed] [Google Scholar]
- 6.Müller M, Rassweiler MC, Klein J, et al. . Mobile augmented reality for computer-assisted percutaneous nephrolithotomy. Int J Comput Assist Radiol Surg 2013;8:663–675 [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues PL, Vilaça JL, Oliveira C, et al. . Collecting system percutaneous access using real-time tracking sensors: First pig model in vivo experience. J Urol 2013;190:1932–1937 [DOI] [PubMed] [Google Scholar]
- 8.Schout BM, Hendrikx AJ, Scherpbier AJ, Bemelmans BL. Update on training models in endourology: A qualitative systematic review of the literature between January 1980 and April 2008. Eur Urol 2008;54:1247–1261 [DOI] [PubMed] [Google Scholar]
- 9.Radecka E, Brehmer M, Holmgren K, et al. . Pelvicaliceal biomodeling as an aid to achieving optimal access in percutaneous nephrolithotripsy. J Endourol 2006;20:92–101 [DOI] [PubMed] [Google Scholar]
- 10.Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med 2013;41:894–902 [DOI] [PubMed] [Google Scholar]
- 11.Frank DO, Zanation AM, Dhandha VH, et al. . Quantification of airflow into the maxillary sinuses before and after functional endoscopic sinus surgery. Int Forum Allergy Rhinol 2013;3:834–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern J, Zeltser IS, Pearle MS. Percutaneous renal access simulators. J Endourol 2007;21:270–273 [DOI] [PubMed] [Google Scholar]
- 13.Mishra S, Jagtap J, Sabnis RB, Desai MR. Training in percutaneous nephrolithotomy. Curr Opin Urol 2013;23:147–151 [DOI] [PubMed] [Google Scholar]