Abstract
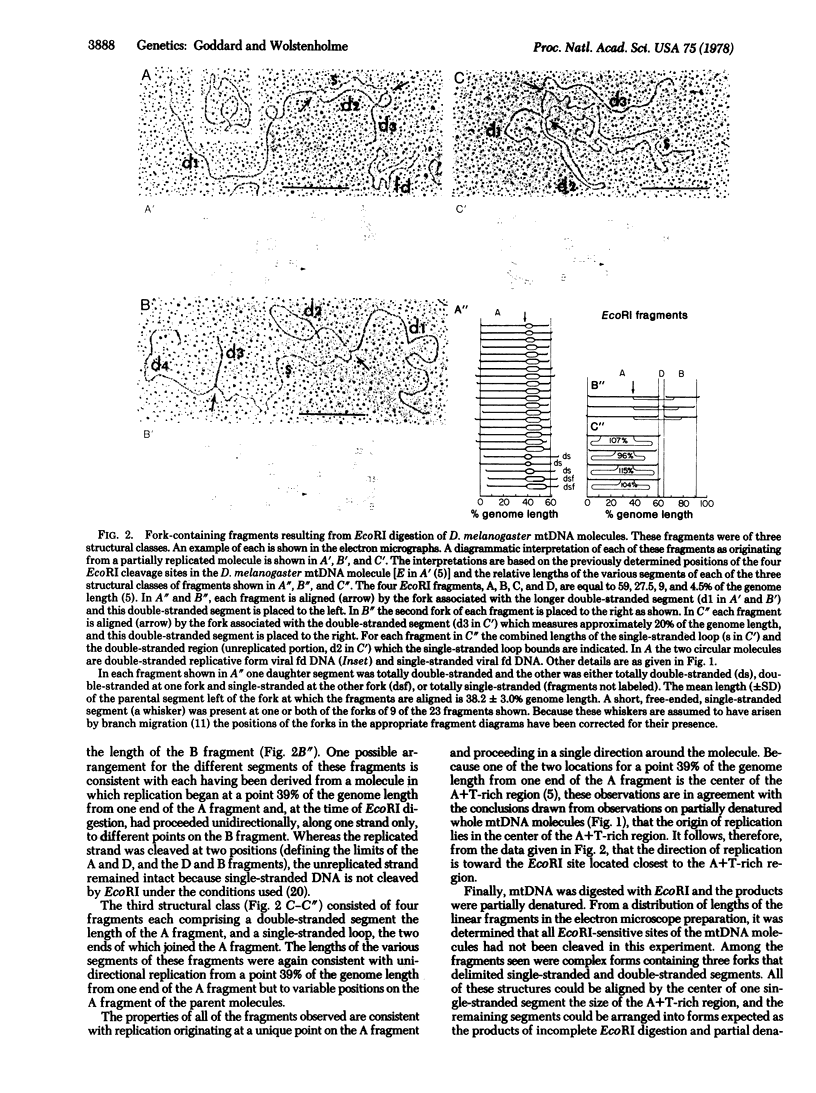
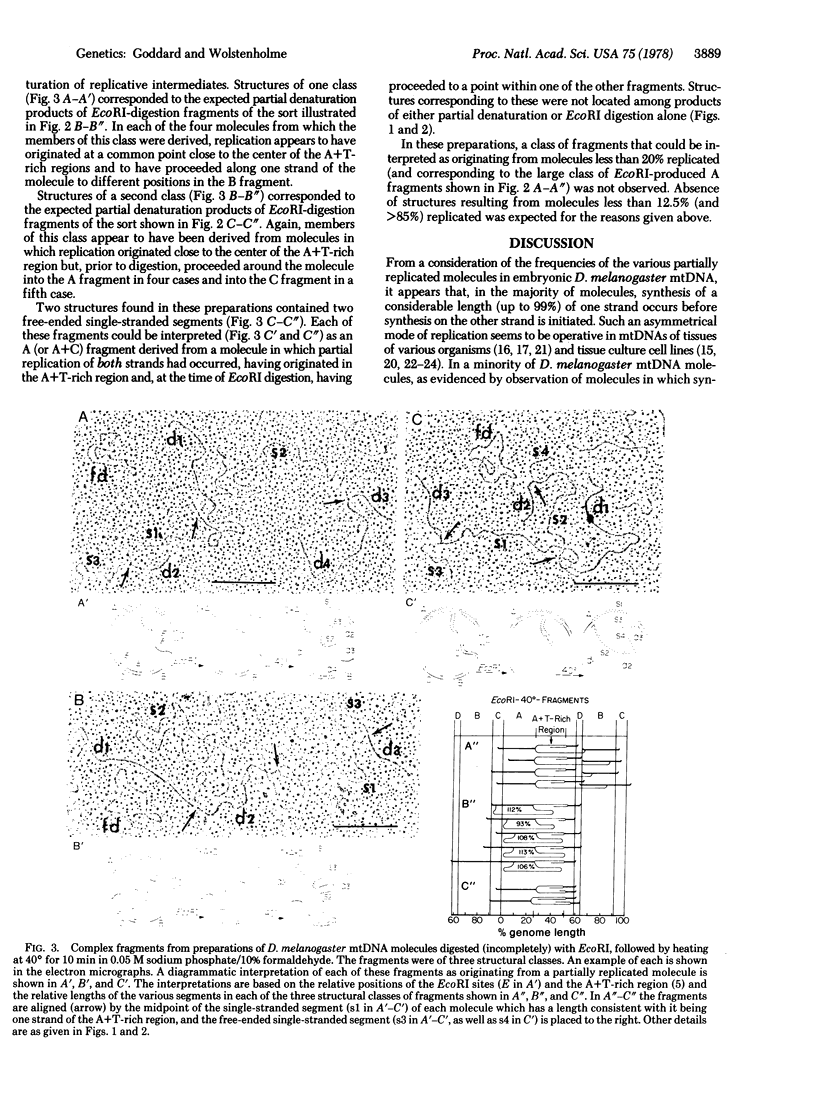
From a consideration of the various structural forms of partially replicated mitochondrial DNA (mtDNA) molecules from Drosophila melanogaster embryos observed in the electron microscope, it appears that the majority of molecules are replicated by a highly asymmetrical mode in which synthesis on one strand is up to 99% complete before synthesis on the second strand is initiated. Replication of the minority of molecules involves a more nearly symmetrical synthesis of the two complementary strands. The D. melanogaster mtDNA molecules have physical features with respect to which the origin and direction of replication could be mapped. These features are (i) a single region accounting for approximately 25% of the circular contour length and rich in adenine + thymine, and (ii) four EcoRI sites, all of which lie outside of this region. Molecules of this mtDNA were subjected to partial denaturation, EcoRI digestion, or partial denaturation after EcoRI digestion and the products were examined in the electron microscope. Complex forms interpretable as originating from replicative intermediates were observed. The size and structure of the components of these complex forms were wholly consistent with the interpretation that, in all of these mtDNA molecules, replication originates at, or close to, the center of the adenine + thymine-rich region and proceeds unidirectionally around the molecule toward the EcoRI site lying closest to the adenine + thymine-rich region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: asynchronous replication of strands, segregation of circular daughter molecules, aspects of topology and turnover of an initiation sequence. J Mol Biol. 1974 Jul 15;86(4):801–824. doi: 10.1016/0022-2836(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Vinograd J. Restriction endonuclease cleavage maps of animal mitochondrial DNAs. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4617–4621. doi: 10.1073/pnas.71.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultmann H., Laird C. D. Mitochondrial DNA from Drosophila melanogaster. Biochim Biophys Acta. 1973 Mar 19;299(2):196–209. doi: 10.1016/0005-2787(73)90342-0. [DOI] [PubMed] [Google Scholar]
- Buzzo K., Fouts D. L., Wolstenholme D. R. EcoRI cleavage site variants of mitochondrial DNA molecules from rats. Proc Natl Acad Sci U S A. 1978 Feb;75(2):909–913. doi: 10.1073/pnas.75.2.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Structural heterogeneity of mitochondrial DNA molecules within the genus Drosophila. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3623–3627. doi: 10.1073/pnas.73.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts D. L., Manning J. E., Wolstenholme D. R. Physicochemical properties of kinetoplast DNA from Crithidia acanthocephali. Crithidia luciliae, and Trypanosoma lewisi. J Cell Biol. 1975 Nov;67(2PT1):378–399. doi: 10.1083/jcb.67.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Mitochondrial DNA replication in Paramecium aurelia. Cross-linking of the initiation end. J Mol Biol. 1977 Jan 15;109(2):327–344. doi: 10.1016/s0022-2836(77)80037-5. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Unidirectionality of replication in mouse mitochondrial DNA. Nat New Biol. 1973 Jan 24;241(108):103–105. doi: 10.1038/newbio241103a0. [DOI] [PubMed] [Google Scholar]
- Klukas C. K., Dawid I. B. Characterization and mapping of mitochondrial ribosomal RNA and mitochondrial DNA in Drosophila melanogaster. Cell. 1976 Dec;9(4 Pt 1):615–625. doi: 10.1016/0092-8674(76)90044-1. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto L., Kasamatsu H., Pikó L., Vinograd J. Mitochondrial DNA replication in sea urchin oocytes. J Cell Biol. 1974 Oct;63(1):146–159. doi: 10.1083/jcb.63.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock W. J., Brutlag D., Goldring E., Appels R., Hinton C. W., Lindsley D. L. The organization of highly repeated DNA sequences in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:405–416. doi: 10.1101/sqb.1974.038.01.043. [DOI] [PubMed] [Google Scholar]
- Polan M. L., Friedman S., Gall J. G., Gehring W. Isolation and characterization of mitochondrial DNA from Drosophila melanogaster. J Cell Biol. 1973 Feb;56(2):580–589. doi: 10.1083/jcb.56.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A., Morrow J. F. Cleavage of replicating forms of mitochondrial DNA by EcoRI endonuclease. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4447–4451. doi: 10.1073/pnas.71.11.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Pulse-labeled components in the replication of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Jun 25;248(12):4512–4514. [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Replication of mitochondrial DNA in mouse L cells and their thymidine kinase - derivatives: displacement replication on a covalently-closed circular template. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3810–3814. doi: 10.1073/pnas.69.12.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Brutlag D., Clayton D. A. The mitochondrial DNA of Drosophila melanogaster exists in two distinct and stable superhelical forms. Cell. 1977 Oct;12(2):471–482. doi: 10.1016/0092-8674(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Fauron C. M. A partial map of the circular mitochondrial genome of Drosophila melanogaster. Location of EcoRI-sensitive sites and the adenine-thymine-rich region. J Cell Biol. 1976 Nov;71(2):434–448. doi: 10.1083/jcb.71.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Koike K., Cochran-Fouts P. Replication of mitochondrial DNA: replicative forms of molecules from rat tissues and evidence for discontinuous replication. Cold Spring Harb Symp Quant Biol. 1974;38:267–280. doi: 10.1101/sqb.1974.038.01.030. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Koike K., Cochran-Fouts P. Single strand-containing replicating molecules of circular mitochondrial DNA. J Cell Biol. 1973 Jan;56(1):230–245. doi: 10.1083/jcb.56.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R. Replicating DNA molecules from eggs of Drosophila melanogaster. Chromosoma. 1973 Jul 23;43(1):1–18. doi: 10.1007/BF01256731. [DOI] [PubMed] [Google Scholar]
- Wolstonholme D. R., Kirschner R. G., Gross N. J. Heart denaturation studies of rat liver mitrochondrial DNA. A denaturation map and changes in molecular configurations. J Cell Biol. 1972 May;53(2):393–406. doi: 10.1083/jcb.53.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A. Electron microscopic analysis of DNA replication in main band and satellite DNAs of Drosophila virilis. J Mol Biol. 1976 Dec;108(2):305–331. doi: 10.1016/s0022-2836(76)80123-4. [DOI] [PubMed] [Google Scholar]