Abstract
The light field on coral reefs varies in intensity and spectral composition, and is the key regulating factor for phototrophic reef organisms, for example scleractinian corals harbouring microalgal symbionts. However, the actual efficiency of light utilization in corals and the mechanisms affecting the radiative energy budget of corals are underexplored. We present the first balanced light energy budget for a symbiont-bearing coral based on a fine-scale study of the microenvironmental photobiology of the massive coral Montastrea curta. The majority (more than 96%) of the absorbed light energy was dissipated as heat, whereas the proportion of the absorbed light energy used in photosynthesis was approximately 4.0% under an irradiance of 640 µmol photons m−2 s−1. With increasing irradiance, the proportion of heat dissipation increased at the expense of photosynthesis. Despite such low energy efficiency, we found a high photosynthetic efficiency of the microalgal symbionts showing high gross photosynthesis rates and quantum efficiencies (QEs) of approximately 0.1 O2 photon−1 approaching theoretical limits under moderate irradiance levels. Corals thus appear as highly efficient light collectors with optical properties enabling light distribution over the corallite/tissue microstructural canopy that enables a high photosynthetic QE of their photosynthetic microalgae in hospite.
Keywords: light energy budget, microsensors, light utilization, thermal boundary layer, photosynthetic efficiency, bio-optics
1. Introduction
Coral reefs are among the most productive and diverse ecosystems on the Earth despite being situated mainly in oligotrophic tropical waters. The evolutionary success of this important ecosystem is largely attributed to the successful symbiosis between the coral host (a cnidarian) and its photosynthetic microalgal endosymbionts (dinoflagellates in the genus Symbiodinium). These so-called zooxanthellae excrete photosynthates, which can provide up to 95% of the energy demand of their cnidarian hosts [1,2]. Photosynthesis is the process where solar energy is converted into chemical energy and stored as biomass in phototrophic organisms. It is driven by photons absorbed by pigment–protein complexes resulting in a charge separation at the two reaction centres of the photosystems [3,4]. At low irradiance, the photosynthesis is limited by the rate of energy supply to the photosystems, whereas at higher irradiances enzymatic reactions limit the rate of energy transformation and thus lead to the increasing saturation of photosynthesis with irradiance [3]. Excess absorbed light energy that is not used for photosynthesis, especially at high photon fluxes, is dissipated as heat and fluorescence. Dissipation of excess light energy as heat is in part because of various photoregulatory mechanisms termed non-photochemical quenching (NPQ). NPQ is used by photosynthetic cells under high light to avoid photodamage, such as degradation of pigments and enzymes by reactive oxygen species produced in de-excitation of the triplet state of Chl (3Chl*) [3,5].
Photosynthetic organisms employ various mechanisms to avoid photodamage, where heat dissipation through NPQ is just one effective short-term way to get rid of excess energy. Long-term regulation, and thereby protection, can be achieved by regulating the amount of light-harvesting and carotenoid pigments [6,7]. Corals acclimatized to high irradiance often appear more transparent than those acclimatized to low light conditions owing to lower pigment concentrations per cell or the spatial organization of chloroplasts ensuring a lower absorption cross section [8]. Another long-term regulatory mechanism in corals is to upregulate the expression of protective coral host pigments, which absorb light in the blue to orange region of the photosynthetically active radiation (PAR) spectrum without inducing oxygenic photosynthesis [9–10]. Host pigment absorption and subsequent energy dissipation via reflection or fluorescence of photons may thus result in lower photosynthetic quantum efficiencies (QEs) but host pigments may also ensure photoprotection in high irradiance environments [11,12] and might give rise to scattering phenomena and wavelength transformations that could enhance photosynthesis [11,13]. However, some other members of the large family of GFP-like host pigments fulfil presumably different as yet unresolved functions in reef corals [14].
Absorbed solar radiation can also drive an increase in the surface temperature of corals relative to the ambient seawater [15–17] and such warming correlates linearly with incident irradiance [15]. The increase in surface temperature is counterbalanced by convective heat transfer to the surrounding water, leading to the establishment of a thermal boundary layer (TBL) [15]. The presence of a TBL limits the rate of convective heat dissipation from the coral surface, as the TBL acts as an insulating barrier, the thickness of which is decreasing with the inverse power of the flow velocity [18]. The TBL behaves analogous to the diffusive boundary layer, which at low flow velocity impedes mass transfer and thereby affects coral gas exchange and nutrient uptake [19–21] as well as the rate of photosynthesis and respiration [15,22,23].
Corals in shallow reef habitats are exposed to high downwelling irradiance of more than 2000 µmol photons m−2 s−1, especially during midday low-tide periods [16]. The variability of light in the reef environment is not only underlying diurnal dynamics modulated by tides but corals also experience pulses of high intensity light exposure owing to wave focusing producing short duration flashes of more than 9000 µmol photons m−2 s−1, with up to more than 350 light flashes per minute in shallow waters [24]. Excess absorbed light energy can lead to photoinhibition and damage the photosystems and coral light absorption can increase the temperature in the coral microenvironment, potentially aggravating negative responses to elevated seawater temperatures [15,18]. High irradiance in combination with elevated water and coral tissue temperatures can induce a cascade of stress responses in corals ultimately leading to the breakdown of the algal–cnidarian symbiosis (owing to the excretion of symbionts and/or pigment degradation of the algal symbiont), which is termed as coral bleaching [25–28]. Moreover, nutrient starvation reduces the photosynthetic efficiency of zooxanthellae and renders corals more susceptible to bleaching [29]. This demonstrates the importance of understanding the mechanisms regulating radiative energy dissipation and the fate of absorbed light energy within corals.
It has been shown that corals are highly efficient at collecting [30,31] and using solar radiation [32,33]. It is known that more than 90% of incident PAR (400–700 nm) can be absorbed by corals and calculations have suggested that their QEs are close to theoretical limits (i.e. 0.125 O2 photon−1 as eight photons are needed to separate the electrons required to produce one O2 molecule [3,32,34]). There is also mounting evidence that corals have unique optical properties that could relate such high efficiency to efficient light capture [21,30,35,36]. However, a closed radiative energy budget for corals and direct microscale measurements of the photosynthetic QE of zooxanthellae in hospite are lacking.
In this study, we apply an experimental approach developed for photosynthetic biofilms [37] to determine the first balanced light energy budget of a coral as a function of incident irradiance and flow velocity. This was obtained by combining fibre-optic and electrochemical microsensor measurements of light reflectance and absorption, rates of gross photosynthesis (GPP) and coral tissue surface warming. Such detailed measurements of the main energy dissipating mechanisms in coral tissue allowed us to estimate the proportion of the absorbed light energy used by photosynthesis and dissipated as heat, respectively. Furthermore, such measurements provided the first measurements of the local QE of zooxanthellae photosynthesis at depth within coral tissue.
2. Material and methods
2.1. Coral samples
Coral specimens were collected from shallow waters (less than 3 m depths) on the reef flat of the Heron Island lagoon, Great Barrier Reef, Australia (152°06′ E, 20°29′ S). The coral Montastrea curta was chosen as highly suitable for intratissue microsensor measurements owing to its thick tissue and minimal mucus secretion. Specimens were transported to the coral holding facility at the University of Technology, Sydney, where corals were acclimated and maintained under continuous flow at 25°C, salinity of 33 and moderate levels of downwelling irradiance (150–200 µmol photons m−2 s−1; 400–700 nm; 12 D : 12 L cycle).
2.2. Experimental set-up
Small fragments of M. curta were placed in a custom-made black acrylic flow-through chamber for at least 45 min prior to the microsensor measurements to ensure steady-state O2 and temperature conditions (as confirmed from repeated microprofile measurements). Corals were illuminated with a defined irradiance regime and were continuously flushed with aerated seawater (25°C and a salinity of 33) at an average flow velocity of either approximately 0.4 or 0.8 cm s−1 as maintained by a submerged water pump in a 20 l thermostated aquarium reservoir.
Illumination was provided by a fibre-optic tungsten halogen lamp (KL-2500, Schott GmbH, Germany) equipped with an internal heat filter and a collimating lens positioned vertically above the flow-through chamber (figure 1). The light intensity of the lamp could be regulated without spectral distortion by a built-in filter wheel with pinholes of various sizes. The downwelling quantum irradiance in the PAR range (400–700 nm) (Ed in µmol photons m−2 s−1) was measured with a calibrated quantum irradiance meter (ULM-500, Walz GmbH, Germany) equipped with a planar cosine collector (LI-192S, LiCor, USA). The experimental irradiances (160, 320, 640, 1280 and 2400 µmol photons m−2 s−1) were achieved by adjusting the aperture on the fibre-optic halogen lamp without any spectral distortion. The downwelling spectral irradiance at the above-mentioned quantum irradiance levels was also measured in radiometric energy units (in W m−2 nm−1) with a calibrated spectroradiometer (Jaz A0523, Ocean Optics, Dunedin, FL, USA). The coral fragment was positioned at the centre of the light beam. The complete set-up was covered with a black cloth to avoid stray light.
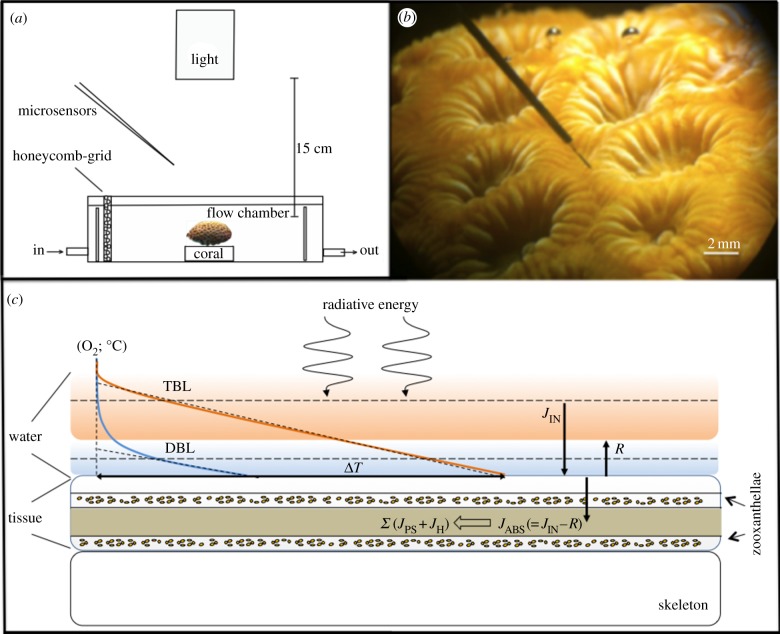
Figure 1.
Experimental setup. (a) Schematic of the experimental setup visualizing the relative position of light source, microsensors and coral fragment. (b) A scalar irradiance microsensor inserted into the coenosarc tissue of a M. curta coral. (c) Conceptual diagram showing the fate of light energy (abbreviations explained in text).
2.3. Microsensor measurements
Spectral scalar irradiance, E0(λ), was measured with a fibre-optic scalar irradiance microprobe (integrating sphere diameter approx. 100 µm [38,39]) connected to a fibre-optic spectrometer (USB 2000+, Ocean Optics, USA). We used a black non-reflective light well to measure the incident downwelling spectral irradiance, Ed(λ) table 1, at the same distance from the light source as done with the measurements on the coral surface; in a collimated light beam the downwelling scalar irradiance and the downwelling irradiance are identical [40]. The spectral reflectance was measured with a fibre-optic field radiance microprobe [41,42].
Table 1.
Terms, definitions and units. PAR denotes photosynthetically active radiation (400–700 nm).
| Ed(λ) | downwelling spectral scalar irradiance | (µmol photons m−2 s−1 nm−1) |
| E0(λ) | spectral scalar irradiance at depth, z | (µmol photons m−2 s−1 nm−1) |
| EE(λ) | absolute downwelling irradiance | (W m−2 nm−1) |
| E0(PAR) | photon scalar irradiance (PAR) | (µmol photons m−2 s−1) |
| JABS | absorbed light energy, vector irradiance (PAR) | (J m−2 s−1) |
| EABS(z) | local density of absorbed light | (µmol photons m−3 s−1) |
| R(PAR) | PAR irradiance reflectance | (W m−2 nm−1) |
| K0(PAR) | diffuse attenuation coefficient (PAR) | (mm−1) |
| A(PAR) | absorption coefficient (PAR) | (mm−1) |
| K0(λ) | spectral attenuation coefficient | (mm−1) |
| JGPP | areal rates of GPP | (nmol O2 cm−2 s−1) |
| JPS | areal rates of GPP, in energy units | (J m−2 s−1) |
| Pmax | photochemical energy conservation | (J m−2 s−1) |
| PS(z) | volumetric rate of GPP at depth z | (nmol O2 cm−3 s−1) |
| PSmax | local photosynthesis maximum | (nmol O2 cm−3 s−1) |
| JH | energy dissipation as heat | (J m−2 s−1) |
| η(z) | local photosynthetic quantum efficiency | (O2 photon−1) |
Oxygen concentrations were measured with Clark-type microelectrodes (tip diameter approx. 25 µm, OX-25, Unisense A/S, Aarhus, Denmark) with a fast response time (less than 0.5 s) and a low stirring sensitivity (less than 1–2%) [43,44]. The microsensor was connected to a pA-meter (Unisense A/S) and was linearly calibrated, at experimental temperature and salinity, from measurements in aerated (free-flowing part of the flow chamber) and anoxic seawater (flushed with N2).
Temperature measurements were performed with a thermocouple microsensor (tip diameter approx. 50 µm; T50, Unisense A/S) connected to a thermocouple meter (Unisense A/S). The temperature microsensors were linearly calibrated against a high precision thermometer (Testo 110, Testo AG, Germany; accuracy ±0.2°C) in seawater at different temperatures. Both temperature and O2 microsensors were connected to an A/D converter (DCR-16, Pyroscience GmbH, Germany) interfaced with a PC running data acquisition software (ProFix, Pyroscience GmbH).
Microsensor measurements of spectral scalar irradiance, spectral radiance, O2 and temperature were done with the sensors approaching the coral surface at a 45° angle relative to the vertically incident light beam to avoid self-shading. The microsensors were mounted on a PC-interfaced motorized micromanipulator (MU-1, PyroScience, GmbH) controlled by dedicated data acquisition and positioning software (ProFix, Pyros-Science GmbH); the software automatically corrected for the sensor inclination and all depths are given in vertical distances.
The microsensor was positioned at the coral tissue surface (defined as 0 µm depth) by means of the micromanipulator and observed using a stereo-microscope (7×–90×, AmScope, Irvine, CA, USA). Microsensor measurements of temperature, O2 and scalar irradiance on and within coral coenosarc tissue were performed as described previously [15,35] (see figure 1). All profiles were measured in vertical steps of 100 µm. Within the tissue, microprofiles were performed from the tissue surface until the skeleton was reached, which could be observed by a slight bending of the microsensors and enhanced noise in the O2 signal.
2.4. Irradiance calculations
The spectral scalar irradiance, E0(λ), was measured in vertical depth steps throughout the coral tissue and calculated as the fraction of the incident downwelling irradiance, E0(λ)/Ed(λ). By multiplying the normalized scalar irradiance spectra with the measured spectra of absolute downwelling irradiance at the coral tissue surface (in W m−2 nm−1) as measured by a calibrated spectrometer (Jaz, Ocean optics, USA), we obtained the absolute energy levels of scalar irradiance at the different measuring depths. We converted the absolute scalar irradiance spectra to photon scalar irradiance spectra (in µmol photons m−2 s−1 nm−1) by using Planck's equation:
where Eλ is the energy of a photon, λ the wavelength, h Planck's constant (6.626 × 10−34 W s2) and c the speed of light in vacuum (in m s−1). The light attenuation in the tissue was calculated by integrating the spectral photon irradiance over PAR (420–700 nm) yielding a measure of PAR photon scalar irradiance (in µmol photons m−2 s−1), i.e. light energy available for oxygenic photosynthesis at a given tissue depth. Light less than 420 nm was strongly absorbed in the upper tissue and light measurements in this wavelength range exhibited increasing amounts of stray light from within the spectrometer and were therefore not included.
The PAR irradiance reflectance of the coral tissue surface was calculated as
where Eu(λ) is the upwelling irradiance at the coral tissue surface, here estimated as the backscattered spectral radiance measured at the coral tissue surface [42], and Ed(λ) is the downwelling irradiance estimated as the backscattered spectral radiance measured over a white reflectance standard (Spectralon; Labsphere, North Sutton, NH, USA). R(PAR) measurements rely on the assumption that the backscattered light from the tissue surface was entirely diffused [30,35,40].
The absorbed light energy (JABS; in J m−2 s−1) within the coral tissue and thus available for photosynthesis was calculated by subtracting the downwelling and upwelling irradiance at the tissue surface, as calculated by
where Ed(λ) and R(λ) are the downwelling spectral irradiance and irradiance reflectance, respectively. The parameter JABS is equivalent to the so-called vector irradiance, which is a measure of the net downwelling energy flux [40].
2.5. Temperature and O2 calculations
GPP was measured with O2 microsensors using the light–dark shift method, which allows photosynthesis estimates independent of light respiration [45]. Areal rates of GPP (JGPP; in nmol O2 cm−2 s−1) were calculated by depth integration of the volumetric rates (in nmol O2 cm−3 s−1) measured in different depths over the euphotic zone, i.e. throughout the photosynthetic coral tissue.
The total amount of energy used by photosynthesis in the coral tissue (JPS; in J m−2 s−1) was calculated by multiplying the areal GPP rate with the Gibbs free energy (482.9 kJ (mol O2)−1), i.e. the energy released through O2 and adenosine triphosphate formation [37,46]:
Light energy that was not used in photosynthesis resulted in a local increase of the coral tissue surface temperature leading to the establishment of a TBL [15]. The heat dissipation (JH,up; in J m−2 s−1), i.e. the heat flux from the coral tissue into the water column, was estimated from the temperature flux across the TBL and was calculated by Fourier's law of conduction:
where k is the thermal conductivity in seawater (0.6 W m−1 K−1 [47]) and 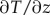 is the measured linear temperature gradient in the TBL.
is the measured linear temperature gradient in the TBL.
As microsensor measurements of heat conduction into coral skeleton are very challenging because of high risk of sensor breakage, the downward flux of heat into the coral skeleton was calculated as JH,down = JABS – (JH,up + JPS). The total amount of energy dissipated as heat in the coral tissue (JH; in J m−2 s−1) was then calculated as JH = JH,up + JH,down.
2.6. Light energy budget and photosynthetic efficiency calculations
To estimate the overall radiative energy utilization efficiency of the system, the balanced light energy budget was determined by the following equations:
where JIN is the total incoming light energy flux; JH is the amount of the incoming light energy dissipated as heat; JPS is the amount of the incoming light energy used by photosynthesis; R is the amount of incident light energy backscattered from the coral tissue surface and thus lost from the system; and JABS is the amount of the incoming light energy absorbed by the system. The final balanced light energy budget characterized the tissue as a homogeneous layer, ignoring any vertical microheterogeneity in symbiont distribution and was defined as
assuming a 1 : 1 stoichiometry of CO2 fixation and O2 production, i.e. the energy stored in the light-dependent reaction is completely used for CO2 fixation in the dark reaction, and that autofluorescence from the tissue is negligible [37].
ɛPS and ɛH represent the efficiencies of photosynthetic energy conservation and heat dissipation at a given absorbed light energy (JABS), respectively, and were calculated as [37]
The photon scalar irradiance of PAR within the coral tissue E0(PAR) was fitted to an exponential decay function to estimate the diffuse attenuation coefficient of E0(PAR), K0(PAR) in units of mm−1
The absorption coefficient of PAR within the coral tissue was estimated from the coral tissue irradiance reflectance (R) and the scalar irradiance attenuation coefficient, K0(PAR) as [37]
assuming that photons at each depth have equal probability to propagate in all directions, i.e. a totally diffuse light field within the tissue.
The spectral attenuation coefficient (K0(λ)) of scalar irradiance with depth ( ) was calculated as
) was calculated as
where E0(λ)1 and E0(λ)2 are the spectral scalar irradiance measured at depths 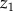 and
and 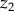 , respectively [40]. We used these data to identify depths within the coral tissue with the strongest light attenuation and spectral signatures of photopigments.
, respectively [40]. We used these data to identify depths within the coral tissue with the strongest light attenuation and spectral signatures of photopigments.
The local density of absorbed light EABS(z) (in µmol photons m−3 s−1) at particular depths in the coral tissue was calculated as
assuming a totally diffuse light field inside the tissue, i.e. photons at the given depths had equal probabilities of propagation in all directions [37].
Finally, the local photosynthetic QE, η(z) (O2 photon−1), was calculated by dividing the locally measured volumetric GPP rates with EABS(z)
3. Results
3.1. Spectral light microenvironment
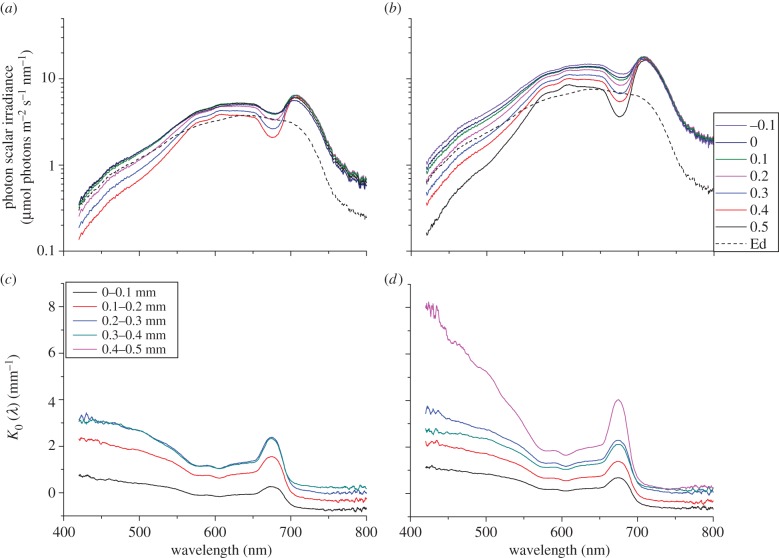
In the uppermost coral tissue layers (approx. 0–0.3 mm) there was a local enhancement in scalar irradiance relative to the incident irradiance with maximum values at the coral surface reaching 135 and 191% of the incident downwelling irradiance at 640 and 1280 µmol photons m−2 s−1, respectively (figure 2a,b). Scalar irradiance attenuation spectra showed that the highest light attenuation occurred at the tissue–skeleton interface, especially around the absorption maxima of the predominant photopigments Chl a (440 and 675 nm), Chl c (635 nm) and the dinoflagellate carotenoid peridinin (490 nm) (figure 2c,d).
Figure 2.
Photon scalar irradiance and spectral attenuation in coral tissue. Photon scalar irradiance spectra measured at two different downwelling photon irradiances of (a) 640 and (b) 1280 µmol photons m−2 s−1. The dashed line is the incident downwelling spectral irradiance. Spectral attenuation coefficient of scalar irradiance K0(λ) (mm−1) measured at two different downwelling photon irradiances of (c) 640 and (d) 1280 µmol photons m−2 s−1. Legends show the measurement depth below the tissue surface (0 mm = coral tissue surface). (Spectra represent mean values; n = 3.)
Below 0.1 mm depth, E0(PAR) was attenuated exponentially with depth in the coral tissue (figure 3) with attenuation coefficients of 0.79 and 1.18 mm−1 at an incident irradiance of 640 and 1280 µmol photons m−2 s−1, respectively. This corresponds to a decrease from 135 to 90% and 191 to 91% of the incident irradiance over a tissue thickness of 0.4 and 0.5 mm, respectively. The absorption coefficient A(PAR) in the coral tissue was calculated to be 0.628 and 0.949 mm−1 under a downwelling irradiance of 640 and 1280 µmol photons m−2 s−1, respectively.
Figure 3.
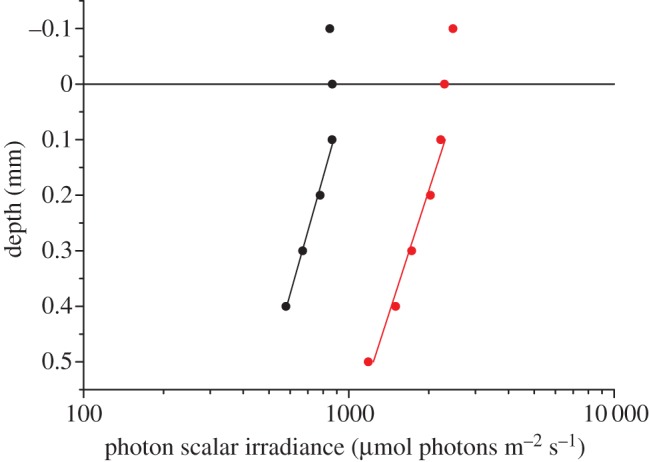
Vertical distribution of photon scalar irradiance (400–700 nm), E0(PAR), in coral tissue under an incident downwelling irradiance of 640 µmol photons m−2 s−1 (black symbols) and 1280 µmol photons m−2 s−1 (red symbols) (n = 3, R2 of exponential fits (solid lines) were 0.99 and 0.98, respectively). y = 0 indicates the position of the tissue surface.
The coral tissue irradiance reflectance, R(PAR), was approximately 12 and 11% at 640 and 1280 µmol photons m−2 s−1, respectively. Reflectance levels were constant over an irradiance range 160–2400 µmol photons m−2 s−1 and no significant correlation between reflection and incident irradiance was found (p > 0.05, electronic supplementary material, figure S1).
3.2. Temperature microenvironment
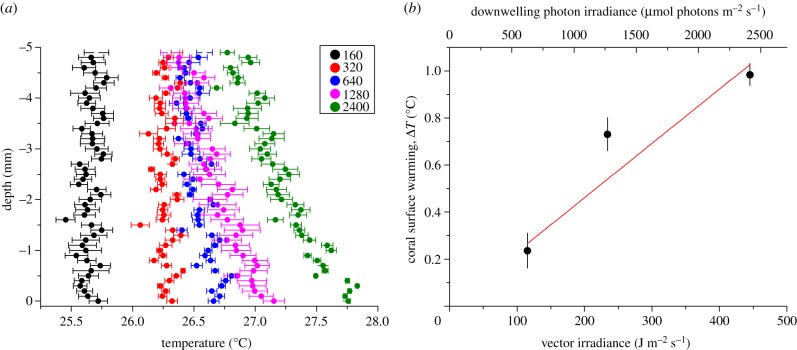
A slight surface heating of the coral tissue relative to the ambient seawater was observed, which increased with irradiance. A TBL could only be identified at irradiance levels more than 320 µmol photons m−2 s−1 (figure 4a), reaching a thickness of approximately 3 mm at a flow velocity of 0.4 cm s−1. There was a positive linear correlation between the coral surface warming, ΔT, and the incident irradiance with a heating slope of 0.0023°C (J m−2 s−1)−1, and increasing temperature gradients of 0.24–0.98°C between coral tissue and the ambient water under increasing irradiance (R2 = 0.98; figure 4b).
Figure 4.
(a) Vertical microprofiles of temperature (°C) through the TBL under five different downwelling photon irradiances (160, 320, 640, 1280 and 2400 µmol photons m−2 s−1) and a flow velocity of 0.4 cm s−1. Symbols and error bars indicate mean ± s.d.; n = 3. (b) Measured temperature gradients (°C) between the ambient seawater and the coral tissue surface measured at three vector irradiances (116, 234 and 445 J m−2 s−1). R2 of linear fit = 0.98. Error bars are ± s.d. (n = 3).
3.3. O2 microenvironment and photosynthesis
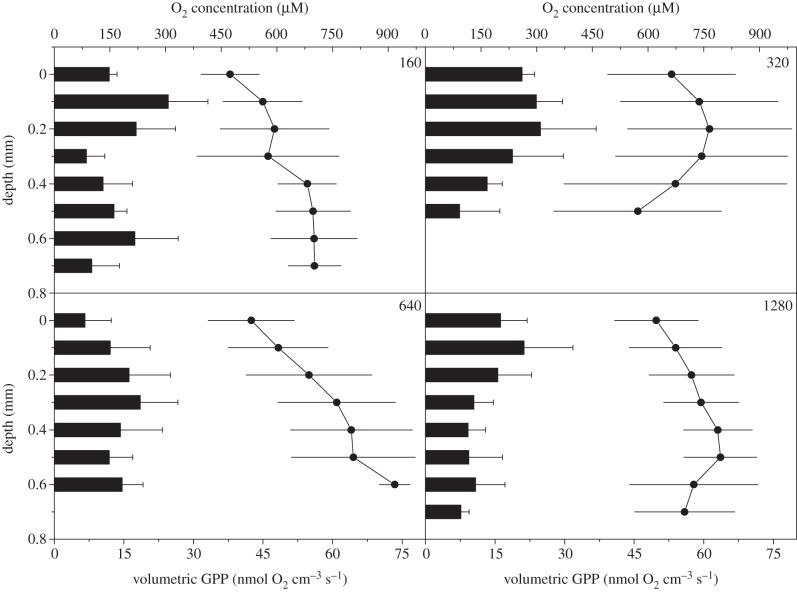
Local volumetric rates of GPP ranged between 7 and 25 nmol O2 cm−3 s−1 and O2 production was detected at all vertical measurement positions within the 0.5–0.7 mm thick coenosarc tissue (figure 5). Generally, GPP peaked approximately 0.1–0.3 mm below the tissue surface; however the vertical distribution of photosynthesis differed under the different experimental irradiance regimes. The O2 microenvironment within the tissue ranged between 500 and 900 µM (240–430% air saturation) and showed in most cases an increasing trend towards the tissue–skeleton interface (figure 5).
Figure 5.
Vertical microprofiles of volumetric gross photosynthesis rates (in nmol O2 cm−3 s−1) (black bars; bottom x-axis) and the corresponding O2 concentrations (in µM) (line and symbols; top x-axis) measured under four different downwelling photon irradiances (160, 320, 640 and 1280 µmol photons m−2 s−1). Error bars indicate ± s.d. (n = 3).
3.4. Energy budget
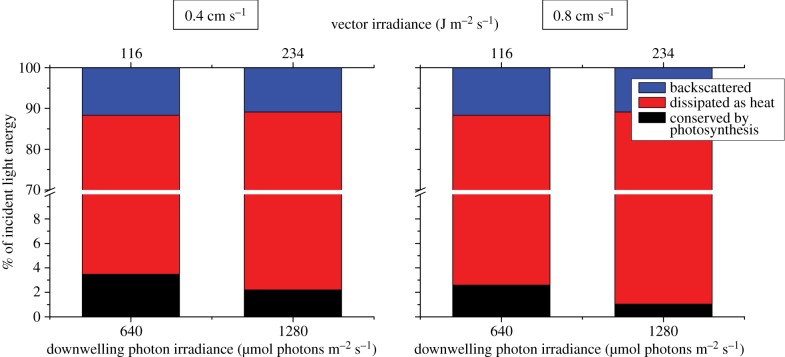
Based on detailed measurements of light, photosynthesis and temperature, we calculated a balanced light energy budget in % of the incident light energy for incident downwelling photon irradiances of 640 and 1280 µmol photons m−2 s−1 (equivalent to a vector irradiance of 116 and 234 J m−2 s−1, respectively) (figure 6). About 3.5 and 2.2% of the incident irradiance was conserved by photosynthesis, while 84.9 and 86.9% was dissipated as heat under an incident photon irradiance of 640 and 1280 µmol photons m−2 s−1, respectively (flow velocity of approx. 0.4 cm s−1). The remaining 11.6 and 10.9% of the incident light energy was backscattered by the tissue surface and thus not absorbed.
Figure 6.
Light energy budget in % of the incident irradiance calculated for two downwelling photon irradiances (640 and 1280 µmol photons m−2 s−1) and at two different flow velocities (0.4 and 0.8 cm s−1). Blue bars are the fraction of energy backscattered by the coral surface, red bars are the fraction of energy dissipated as heat and black bars are fraction of energy conserved by photosynthesis. Note the break in the y-axis.
At increased flow velocity (approx. 0.8 cm s−1), the proportion of the incident light energy that was photochemically conserved decreased to 2.6 and 1.0% under an incident photon irradiance of 640 and 1280 µmol photons m−2 s−1, respectively. Generally, the energy budget was dominated by heat dissipation and the proportion of energy conserved by photosynthesis decreased with increasing incident irradiance favouring dissipation of heat (figure 6 and table 2). The maximum efficiency of photochemical energy conservation, 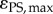 , and the minimum efficiency of heat dissipation,
, and the minimum efficiency of heat dissipation, 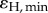 , were 0.04 and 0.96, respectively (table 2).
, were 0.04 and 0.96, respectively (table 2).
Table 2.
Balanced light energy budget (in energy units), overall photosynthetic energy use and heat dissipation (in % of absorbed light energy) and efficiencies of photochemical energy conservation and heat dissipation (ɛPS and ɛH) at two different incident irradiances and flow velocities.
|
JPS (J m−2 s−1) |
JH (J m−2 s−1) |
ɛPS |
ɛH |
ɛPS
+
ɛH |
|||||
|---|---|---|---|---|---|---|---|---|---|
| JABS (J m−2 s−1) | 0.4 cm s−1 | 0.8 cm s−1 | 0.4 cm s−1 | 0.8 cm s−1 | 0.4 cm s−1 | 0.8 cm s−1 | 0.4 cm s−1 | 0.8 cm s−1 | both flow velocities |
| 116 | 4.55 (3.9%) | 3.39 (2.9%) | 111.0 (96.1%) | 112.2 (97.1%) | 0.04 | 0.03 | 0.96 | 0.97 | 1.0 |
| 234 | 5.77 (2.5%) | 2.71 (1.2%) | 228.1 (97.5%) | 231.2 (98.8%) | 0.02 | 0.01 | 0.98 | 0.99 | 1.0 |
Based on detailed light and photosynthesis measurements, we estimated the QE of photosynthesis in particular depths in the coral tissue (figure 7). The local QEs varied over depth, with an increasing trend towards the tissue–skeleton interface and showing decreasing QE values at increasing incident irradiances. The maximum photosynthetic QE was 0.102 O2 photon−1 (320 µmol photons m−2 s−1), approaching the theoretical maximum of 0.125 O2 photon−1.
Figure 7.
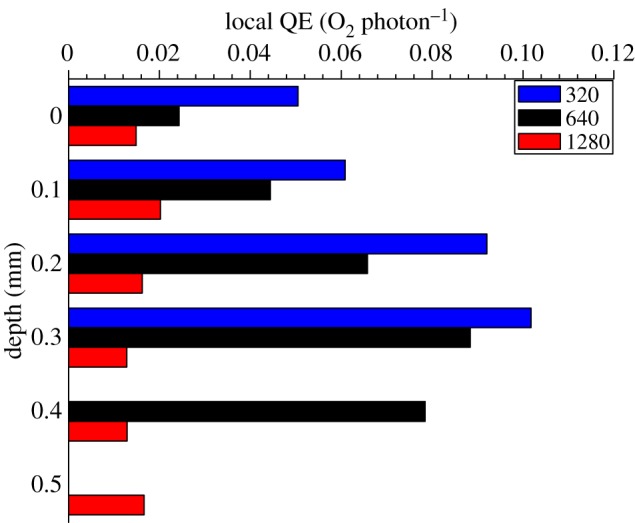
Vertical microprofiles of the local photosynthetic quantum efficiency (QE) (η) in the studied coral (in units of mol O2 produced per mol photon absorbed) under three different incident photon irradiances noted as colour bar legends (in µmol photons m−2 s−1). y = 0 indicates the position of the tissue surface.
4. Discussion
This fine-scale study of the energy budget and photosynthetic efficiency of the symbiont-bearing scleractinian coral M. curta presents the first detailed account of the fate of incident and absorbed light within coral tissues. Despite the finding that a relatively high proportion of the incident irradiance (approx. 11%) was backscattered at the tissue surface and thus not absorbed (figure 6; electronic supplementary material, figure S1) we found that corals are highly efficient at using solar radiation (figure 7).
Within the coral tissue, the absorbed light differed from the incident irradiance with respect to both spectral composition and intensity, where scalar irradiance was enhanced (135 and 191% for incident irradiances of 640 µmol photons m−2 s−1 and 1280 µmol photons m−2 s−1, respectively) at and just below the coral tissue surface (figure 2a,b). Such enhancement suggests intense scattering and redistribution of photons in the upper layers of the tissue [35]. Tissue scattering increases the local density and residence time of photons because of increased photon pathlength per vertical distance traversed [40]. These findings are similar to recent studies by Wangpraseurt et al. [35,36] observing scalar irradiance levels reaching up to 200% of the incident downwelling irradiance in the upper coral tissue layers (0–100 µm). We observed the highest attenuation of scalar irradiance in the lowest tissue depth interval, i.e. at the tissue–skeleton interface (figure 2c,d), where strong backscatter would further enhance light absorption efficiency by symbionts in the coral tissue [30].
We found a pronounced positive correlation between increasing incident irradiance and dissipation of heat, leading to the establishment of approximately 3 mm thick TBL at incident irradiances more than 320 µmol photons m−2 s−1 (figure 4a). We also measured a linear increase in tissue surface temperature, resulting in convective heat dissipation over a TBL, with an average slope of 2.3 × 10−3°C (J m−2 s−1)−1 and a maximum temperature difference of +0.98°C (figure 4b). Hemispherical corals studied by Jimenez et al. [15,18] showed a similar maximum surface warming, ΔT, between the surface tissue and the ambient water of +0.9°C, with a TBL thickness of approximately 3 mm and an average slope of approximately 1.7 × 10−3°C (J m−2 s−1)−1 at equivalent flow velocities and absorbed irradiances.
The rate of photosynthesis within the illuminated coenosarc coral tissue showed a relatively uniform distribution of photosynthesis throughout the tissue and O2 concentrations ranged from 500 to 900 µM (240–430% air saturation; figure 5). This correlated with the attenuation of PAR observed within the coenosarc tissue (figures 2 and 3), where approximately 90% of the incident irradiance remained at the tissue–skeleton interface.
The coral skeleton acts as a diffusion barrier for chemical species, leading to a relative build-up of solutes (such as O2) in the lower tissue layers (figure 5). This has recently been shown to increase the O2 concentration up to approximately 400% of air saturation at the tissue–skeleton interface [35]. Saturation of photosynthetic activity at high irradiance led to decreased photosynthetic efficiencies, i.e. a decreased proportion of the absorbed light energy was conserved by photosynthesis with increasing incident irradiances (figure 6 and table 2). At increased flow velocity (approx. 0.8 cm s−1), the proportion of the absorbed light energy conserved by photosynthesis decreased as compared to the lower flow velocity (approx. 0.4 cm s−1; figure 6). This was unexpected as a decrease in boundary layer thickness owing to increased flow rates normally leads to increased rates of photosynthesis and respiration as a response to alleviation of mass transfer resistance for O2 and dissolved inorganic carbon exchange [48,49]. However, we also saw a decreased euphotic zone (i.e. the photosynthetic tissue layer) from approximately 0.6 to 0.4 mm, possibly owing to coenosarc tissue contraction at higher flow. Recently, tissue contraction has been found to reduce the amount of lateral light transfer through coral tissue and could thus explain the observed reduction in local GPP rates [36].
The volumetric rates of photosynthesis at higher flow were of the same order as at the lower flow velocity, i.e. PSmax of 24.2 nmol O2 cm−3 s−1 (data not shown). At a flow velocity of approximately 0.8 cm s−1, the proportion of the absorbed light energy that was used in photosynthesis still represents a high energy efficiency (figure 6 and table 2) as compared to other photosynthetic systems such as biofilms and microbial mats, where less than 1.8% of the absorbed light energy was conserved by photosynthesis at equivalent vector irradiances [37].
In comparison with previous studies of the radiative energy budget of benthic photosynthetic systems, such as biofilms and microbial mats [37,50], we found much higher photosynthetic efficiencies in coral tissue. Locally measured volumetric rates of photosynthesis in the coral tissue were very high (up to 25 nmol O2 cm−3 s−1; figure 5) as compared to what has been reported in microbial mats and biofilms (Australian mat 6–13 nmol O2 cm−3 s−1, Abu Dhabi mat 2–6 nmol O2 cm−3 s−1 [37,50]) at equivalent absorbed irradiances. Even when considering the relatively restricted spatial extension of photosynthesis in corals (M. curta approx. 0.5 mm tissue thickness) as compared to an order of photic zone of approximately 1–2 mm in microbial mats and biofilms, the depth integrated areal rate of photosynthesis, i.e. the photosynthetic energy conservation of the coral system (Pmax = 5.8 J m−2 s−1; table 2), was about three times higher than what has been reported in biofilms and microbial mats at equivalent absorbed irradiances.
A high light usage efficiency in corals was also supported by our measurements of the local QE of photosynthesis showing much higher values than in biofilms or other compact photosynthetic systems of similar thickness and degree of compaction [50]. The local QE generally decreased with increasing incident downwelling irradiances but the maximum QE of 0.102 O2 per photon under a photon irradiance of 320 µmol photons m−2 s−1 (figure 7) approached the theoretical maximum of 0.125. Such high efficiency values are about an order of magnitude higher than previous estimates that were based on tissue extracts and thus ignored the role of coral tissue and skeleton optics in light absorption efficiency [51–54]. More similar but still lower QEs were reported when coral absorptance was based on skeleton reflectivity (approx. 0.07 mol O2 per absorbed mol photons [32]). Likewise, a study of intact colonies of the coral Montipora monasteriata in shaded environments also showed high QE values of 0.071–0.096 O2 per incident photon [55]. Such high efficiencies are comparable to macroalgal stands and terrestrial communities such as forests, which exhibit a canopy distribution of the photosynthetic active components facilitating a more uniform availability and use of light throughout the photic zone [56,57]. It is thus intriguing to speculate whether microscale canopy effects are at play in corals that facilitate the observed high photosynthetic efficiency.
Light regulation on a microscale occurs through several mechanisms in corals. Photons incident on the coral surface will be strongly scattered in the coral tissue. This enhances photon pathlength per vertical distance traversed and thus the average residence time of photons at a given point within the tissue increasing the probability of absorption for wavelengths overlapping with absorption maxima of symbiont photopigments or coral host pigments [35]. In combination with refractive index mismatches between coral tissue and water such photon trapping leads to near-surface maxima in scalar irradiance and enhanced spectral filtering [40]. Light can also be transferred laterally through coral tissue thereby leading to a more homogeneous distribution of incident irradiance over the coral [36]. Additionally, photons that have passed through tissue undergo multiple scattering in the coral skeleton, which acts as a Lambertian-like diffuser and thus facilitates further enhancement of light capture by zooxanthellae [30,58]. Corals thus have several distinct microscale mechanisms that can optimize light capture and usage.
Light regulating mechanisms also operate on larger scales. It is known that individual colonies show a plastic response to the ambient light climate, where for instance coral orientation, branch spacing and corallite architecture are regulated by the ambient light climate [59–63]. The concerted action of the mentioned micro- and macroscale light regulating mechanisms indeed indicates that symbiont-bearing corals have canopy-like optical properties not only at larger colony and reef scales but also at the scale of single polyps, where optical properties of tissue and skeleton act in with behavioural modulation of tissue contraction/expansion [36,64] to maintain high QEs and metabolic rates.
In conclusion, we present the first balanced radiative energy budget of a symbiont-bearing coral. The majority (more than 96%) of absorbed light energy was dissipated as heat and the proportion of the absorbed light energy that was photochemically conserved decreased with increasing incident irradiance favouring heat dissipation. Yet, coral symbionts are able to retain a very high photosynthetic activity and efficiency in hospite, and we propose that canopy-like effects involving the interplay between tissue and skeleton optical properties of the coral holobiont are important but largely unexplored factors affecting the successful algal–cnidarian symbiosis and its ability to adapt to different light regimes.
Acknowledgements
We thank the staff at Heron Island Research Station for excellent assistance during our field work and Dr V. Schrameyer and Dr D. Nielsen for thoughtful discussions. We acknowledge the help and assistance from the Aquatic Processes Group at the University of Technology, Sydney (UTS). The research was conducted under research permits for field work on the Great Barrier Reef, Australia (G11/34670.1 and G09/31733.1) and was funded by grants from Knud Højgaards Fond, Oticon Fonden, Thorsons Rejselegat, København Universitets Fælleslegat (Pastor Peter Albert Raashous legat) (K.E.B. and M.L.), the Danish Council for Independent Research | Natural Sciences (M.K.), the Australian Research Council (P.J.R. and M.K.) and the University of Technology, Sydney (K.E.B. and D.W.). K.E.B., M.L., D.W. and M.K. designed research and outlined experiments. K.E.B., M.L. and D.W. conducted experiments. K.E.B., M.L., D.W. and M.K. analysed data. P.J.R. and M.K. contributed new analytical tools. K.E.B., D.W. and M.K. wrote the paper with editorial inputs from M.L. and P.J.R.
References
- 1.Muscatine L, McCloskey L, Marian R. 1981. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 26, 601–611. ( 10.4319/lo.1981.26.4.0601) [DOI] [Google Scholar]
- 2.Edmunds PJ, Spencer Davies P. 1986. An energy budget for Porites porites (Scleractinia). Mar. Biol. 92, 339–347. ( 10.1007/BF00392674) [DOI] [Google Scholar]
- 3.Falkowski P, Raven J. 1997. Aquatic photosynthesis. Oxford, UK: Blackwell Science. [Google Scholar]
- 4.Kirk J. 2007. Light and photosynthesis in aquatic ecosystems, 3rd ed Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Müller P, Li X, Niyogi K. 2001. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125, 1558–1566. ( 10.1104/pp.125.4.1558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nymark M, Valle K, Brembu T, Hancke K, Winge P, Andresen K, Johnsen G. 2009. An integrated analysis of molecular acclimation to high light in the marine diatom Phaeodactylum tricornutum. PLoS ONE 4, e7743 ( 10.1371/journal.pone.0007743.t001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartley GE, Scolnik PA. 1995. Plant carotenoids: pigments for photoprotection, visual attraction, and human health. Plant Cell 7, 1027–1038. ( 10.1105/tpc.7.7.1027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falkowski PG, Dubinsky Z. 1981. Light-shade adaptation of Stylophora pistillata, a hermatypic coral from the Gulf of Eilat. Nature 289, 172–174. ( 10.1038/289172a0) [DOI] [Google Scholar]
- 9.Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O. 2000. Fluorescent pigments in corals are photoprotective. Nature 408, 850–853. ( 10.1038/35048564) [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo C, Denzel A, Vogt A, Matz MV, Oswald F, Salih A, Nienhaus GU, Wiedenmann J. 2008. Blue light regulation of host pigment in reef-building corals. Mar. Ecol. Progr. Ser. 364, 97–106. ( 10.3354/meps07588) [DOI] [Google Scholar]
- 11.Dove SG, Lovell C, Fine M, Deckenback J, Hoegh-Guldberg O, Iglesias-Prieto R, Anthony KRN. 2008. Host pigments: potential facilitators of photosynthesis in coral symbioses. Plant Cell Environ. 31, 1523–1533. ( 10.1111/j.1365-3040.2008.01852.x) [DOI] [PubMed] [Google Scholar]
- 12.Smith E, D'Angelo C, Salih A, Wiedenmann J. 2013. Screening by coral green fluorescent protein (GFP)-like chromoproteins supports a role in photoprotection of zooxanthellae. Coral Reefs 32, 463–474. ( 10.1007/s00338-012-0994-9) [DOI] [Google Scholar]
- 13.Schlichter D, Fricke HW. 1990. Coral host improves photosynthesis of endosymbiotic algae. Naturwissenschaften 77, 447–450. ( 10.1007/BF01135950) [DOI] [Google Scholar]
- 14.Alieva NO, et al. 2008. Diversity and evolution of coral fluorescent proteins. PLoS ONE 3, e2680 ( 10.1371/journal.pone.0002680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimenez IM, Kühl M, Larkum AWD. 2008. Heat budget and thermal microenvironment of shallow-water corals: do massive corals get warmer than branching corals? Limnol. Oceanogr. 53, 1548–1561. ( 10.4319/lo.2008.53.4.1548) [DOI] [Google Scholar]
- 16.Jimenez IM, Larkum AWD, Ralph PJ, Kühl M. 2012. In situ thermal dynamics of shallow water corals is affected by tidal patterns and irradiance. Mar. Biol. 8, 1773–1782. ( 10.1007/s00227-012-1968-8) [DOI] [Google Scholar]
- 17.Jimenez IM, Larkum AWD, Ralph PJ, Kühl M. 2012. Thermal effects of tissue optics in symbiont-bearing reef building corals. Limnol. Oceanogr. 57, 1816–1825. ( 10.4319/lo.2012.57.6.1816) [DOI] [Google Scholar]
- 18.Jimenez IM, Kühl M, Larkum AWD, Ralph PJ. 2011. Effects of flow and colony morphology on the thermal boundary layer of corals. J. R. Soc. Interface 8, 1785–1795. ( 10.1098/rsif.2011.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkinson M, Bilger R. 1992. Effects of water velocity on phosphate uptake in coral reef-flat communities. Limnol. Oceanogr. 37, 273–279. ( 10.4319/lo.1992.37.2.0273) [DOI] [Google Scholar]
- 20.Kühl M, Cohen Y, Dalsgaard T, Jørgensen BB, Revsbech NP. 1995. The microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar. Ecol. Progr. Ser. 117, 159–172. ( 10.3354/meps117159) [DOI] [Google Scholar]
- 21.Wangpraseurt D, Weber M, Roy H, Polerecky L, de Beer D, Suharsono, Nugues MM. 2012. In situ oxygen dynamics in coral–algal interactions. PLoS ONE 7, e31192 ( 10.1371/journal.pone.0031192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edmunds PJ. 2005. Effect of elevated temperature on aerobic respiration of coral recruits. Mar. Biol. 146, 655–663. ( 10.1007/s00227-004-1485-5) [DOI] [Google Scholar]
- 23.Finelli C, Helmuth B, Pentcheff N, Wethey D. 2006. Water flow influences oxygen transport and photosynthetic efficiency in corals. Coral Reefs 25, 47–57. ( 10.1007/s00338-005-0055-8) [DOI] [Google Scholar]
- 24.Veal C, Carmi M, Dishon G, Sharon Y, Michael K, Tchernov D, Hoegh-Guldberg O, Fine M. 2010. Shallow-water wave lensing in coral reefs: a physical and biological case study. J. Exp. Biol. 213, 4303–4312. ( 10.1242/jeb.044941) [DOI] [PubMed] [Google Scholar]
- 25.Lesser M. 1996. Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol. Oceanogr. 41, 271–283. ( 10.4319/lo.1996.41.2.0271) [DOI] [Google Scholar]
- 26.Jones R, Hoegh-Guldberg O, Larkum AWD, Schreiber U. 1998. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 21, 1219–1230. ( 10.1046/j.1365-3040.1998.00345.x) [DOI] [Google Scholar]
- 27.Warner M, Fitt W, Schmidt G. 1999. Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc. Natl Acad. Sci. USA 96, 8007–8012. ( 10.1073/pnas.96.14.8007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weis VM. 2008. Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J. Exp. Biol. 211, 3059–3066. ( 10.1242/jeb.009597) [DOI] [PubMed] [Google Scholar]
- 29.Wiedenmann J, D'Angelo C, Smith EG, Hunt AN, Legiret FE, Postle AD, Achterberg EP. 2013. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 3, 160–164. ( 10.1038/nclimate1661) [DOI] [Google Scholar]
- 30.Enriquez S, Mendez ER, Iglesias-Prieto R. 2005. Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol. Oceanogr. 50, 1025–1032. ( 10.4319/lo.2005.50.4.1025) [DOI] [Google Scholar]
- 31.Stambler N, Dubinsky Z. 2005. Corals as light collectors: an integrating sphere approach. Coral Reefs 24, 1–9. ( 10.1007/s00338-004-0452-4) [DOI] [Google Scholar]
- 32.Rodriquez-Roman A, Hernandez-Pech X, Thome PE, Enriquez S, Iglesias-Prieto R. 2006. Photosynthesis and light utilization in the Caribbean coral Montastraea faveolata recovering from a bleaching event. Limnol. Oceanogr. 51, 2702–2710. ( 10.4319/lo.2006.51.6.2702) [DOI] [Google Scholar]
- 33.Pinchasov-Grinblat Y, Mauzerall D, Goffredo S, Falini G, Dubinsky Z. 2013. Photoacoustics: a novel application to the determination of photosynthetic efficiency in zooxanthellate hermatypes. Limnol. Oceanogr. Meth. 11, 374–381. ( 10.4319/lom.2013.11.374) [DOI] [Google Scholar]
- 34.Hochberg E, Atkinson M. 2008. Coral reef benthic productivity based on optical absorptance and light-use efficiency. Coral Reefs 27, 49–59. ( 10.1007/s00338-007-0289-8) [DOI] [Google Scholar]
- 35.Wangpraseurt D, Larkum A, Ralph P, Kühl M. 2012. Light gradients and optical microniches in coral tissues. Front. Microbiol. 3, 316 ( 10.3389/fmicb.2012.00316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wangpraseurt D, Larkum AWD, Franklin J, Szabo M, Ralph PJ, Kühl M. In press. Lateral light transfer ensures efficient resource distribution in symbiont-bearing corals. J. Exp. Biol. [DOI] [PubMed] [Google Scholar]
- 37.Al-Najjar M, de Beer D, Jørgensen BB, Kühl M, Polerecky L. 2010. Conversion and conservation of light energy in a photosynthetic microbial mat ecosystem. ISME J. 4, 440–449. ( 10.1038/ismej.2009.121) [DOI] [PubMed] [Google Scholar]
- 38.Lassen C, Ploug H, Jørgensen BB. 1992. A fibre optic scalar irradiance microsensor—application for spectral light measurements in sediments. FEMS Microbiol. Ecol. 86, 247–254. ( 10.1111/j.1574-6968.1992.tb04816.x) [DOI] [Google Scholar]
- 39.Lassen C, Ploug H, Jørgensen BB. 1992. Microalgal photosynthesis and spectral scalar irradiance in coastal marine sediments of Limfjorden, Denmark. Limnol. Oceanogr. 37, 760–772. ( 10.4319/lo.1992.37.4.0760) [DOI] [Google Scholar]
- 40.Kühl M, Jørgensen BB. 1994. The light field of microbenthic communities: radiance distribution and microscale optics of sandy coastal sediments. Limnol. Oceanogr. 39, 1368–1398. ( 10.4319/lo.1994.39.6.1368) [DOI] [Google Scholar]
- 41.Jørgensen BB, Marais D. 1988. Optical properties of benthic photosynthetic communities: fiber-optic studies of cyanobacterial mats. Limnol. Oceanogr. 33, 99–113. ( 10.4319/lo.1988.33.1.0099) [DOI] [PubMed] [Google Scholar]
- 42.Kühl M. 2005. Optical microsensors for analysis of microbial communities. Meth. Enzymol. 397, 166–199. () [DOI] [PubMed] [Google Scholar]
- 43.Revsbech NP. 1989. An oxygen microsensor with a guard cathode. Limnol. Oceanogr. 34, 474–478. ( 10.4319/lo.1989.34.2.0474) [DOI] [Google Scholar]
- 44.Revsbech NP. 1989. Diffusion characteristics of microbial communities determined by use of oxygen microsensors. J. Microbiol. Meth. 9, 111–122. ( 10.1016/0167-7012(89)90061-4) [DOI] [Google Scholar]
- 45.Revsbech NP, Jørgensen BB. 1983. Photosynthesis of benthic microflora measured with high spatial resolution by the oxygen microprofile method: capabilities and limitations of the method. Limnol. Oceanogr. 28, 749–756. ( 10.4319/lo.1983.28.4.0749) [DOI] [Google Scholar]
- 46.Thauer R, Jungermann K, Decker K. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41, 100–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young H, Freedman R, Ford L. 1996. University physics, with modern physics. Boston, MA: Addison Wesley. [Google Scholar]
- 48.Kühl M, Glud RN, Ploug H, Ramsing NB. 1996. Microenvironmental control of photosynthesis and photosynthesis-coupled respiration in an epilithic cyanobacterial biofilm. J. Phycol. 32, 799–812. ( 10.1111/j.0022-3646.1996.00799.x) [DOI] [Google Scholar]
- 49.Larkum AWD, Koch EM, Kühl M. 2003. Diffusive boundary layers and photosynthesis of the epilithic algal community of coral reefs. Mar. Biol. 142, 1073–1082. ( 10.1007/s00227-003-1022-y) [DOI] [Google Scholar]
- 50.Al-Najjar M, de Beer D, Kühl M, Polerecky L. 2012. Light utilization efficiency in photosynthetic microbial mats. Environ. Microbiol. 14, 982–992. ( 10.1111/j.1462-2920.2011.02676.x) [DOI] [PubMed] [Google Scholar]
- 51.Dubinsky Z, Falkowski P, Porter J, Muscatine L. 1984. Absorption and utilization of radiant energy by light- and shade-adapted colonies of the hermatypic coral Stylophora pistillata. Proc. R. Soc. Lond. B 222, 203–214. ( 10.1098/rspb.1984.0059) [DOI] [Google Scholar]
- 52.Wyman KD, Dubinsky Z, Porter JW, Falkowski PG. 1987. Light absorption and utilization among hermatypic corals: a study in Jamaica, West Indies. Mar. Biol. 96, 283–292. ( 10.1007/BF00427028) [DOI] [Google Scholar]
- 53.Dubinsky Z, Stambler N, Ben-Zion M, Mccloskey LR, Muscatine L, Falkowski P. 1990. The effect of external nutrient resources on the optical properties and photosynthetic efficiency of Stylophora pistillata. Proc. R. Soc. Lond. B 239, 231–246. ( 10.1098/rspb.1990.0015) [DOI] [Google Scholar]
- 54.Lesser MP, Mazel C, Phinney D, Yentsch CS. 2000. Light absorption and utilization by colonies of the congeneric hermatypic corals Montastraea faveolata and Montastraea cavernosa. Limnol. Oceanogr. 45, 76–86. ( 10.4319/lo.2000.45.1.0076) [DOI] [Google Scholar]
- 55.Anthony KRN, Hoegh-Guldberg O. 2003. Variation in coral photosynthesis, respiration and growth characteristics in contrasting light microhabitats: an analogue to plants in forest gaps and understoreys? Funct. Ecol. 17, 246–259. ( 10.1046/j.1365-2435.2003.00731.x) [DOI] [Google Scholar]
- 56.Sand-Jensen K, Krause-Jensen D. 1997. Broad-scale comparison of photosynthesis in terrestrial and aquatic plant communities. Oikos 80, 203–208. ( 10.2307/3546536) [DOI] [Google Scholar]
- 57.Krause-Jensen D, Sand-Jensen K. 1998. Light attenuation and photosynthesis of aquatic plant communities. Limnol. Oceanogr. 43, 396–407. ( 10.4319/lo.1998.43.3.0396) [DOI] [Google Scholar]
- 58.Marcelino LA, et al. 2013. Modulation of light-enhancement to symbiotic algae by light-scattering in corals and evolutionary trends in bleaching. PLoS ONE 8, e61492 ( 10.1371/journal.pone.0061492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Helmuth BST, Timmerman BEH, Sebens KP. 1997. Interplay of host morphology and symbiont microhabitat in coral aggregations. Mar. Biol. 130, 1–10. ( 10.1007/s002270050219) [DOI] [Google Scholar]
- 60.Anthony KRN, Hoogenboom MO, Connolly SR. 2005. Adaptive variation in coral geometry and the optimization of internal colony light climates. Funct. Ecol. 19, 17–26. ( 10.1111/j.0269-8463.2005.00925.x) [DOI] [Google Scholar]
- 61.Hoogenboom MO, Connolly SR, Anthony KRN. 2008. Interactions between morphological and physiological plasticity optimize energy acquisition in corals. Ecology 89, 1144–1154. ( 10.1890/07-1272.1) [DOI] [PubMed] [Google Scholar]
- 62.Ow Y, Todd P. 2010. Light-induced morphological plasticity in the scleractinian coral Goniastrea pectinata and its functional significance. Coral Reefs 29, 797–808. ( 10.1007/s00338-010-0631-4) [DOI] [Google Scholar]
- 63.Kaniewska P, Anthony KRN, Sampayo EM, Campbell PR, Hoegh-Guldberg O. In press. Implications of geometric plasticity for maximizing photosynthesis in branching corals. Mar. Biol. ( 10.1007/s00227-013-2336-z) [DOI] [Google Scholar]
- 64.Levy O, Dubinsky Z, Achituv Y. 2003. Photobehavior of stony corals: responses to light spectra and intensity. J. Exp. Biol. 206, 4041–4049. ( 10.1242/jeb.00622) [DOI] [PubMed] [Google Scholar]