Abstract
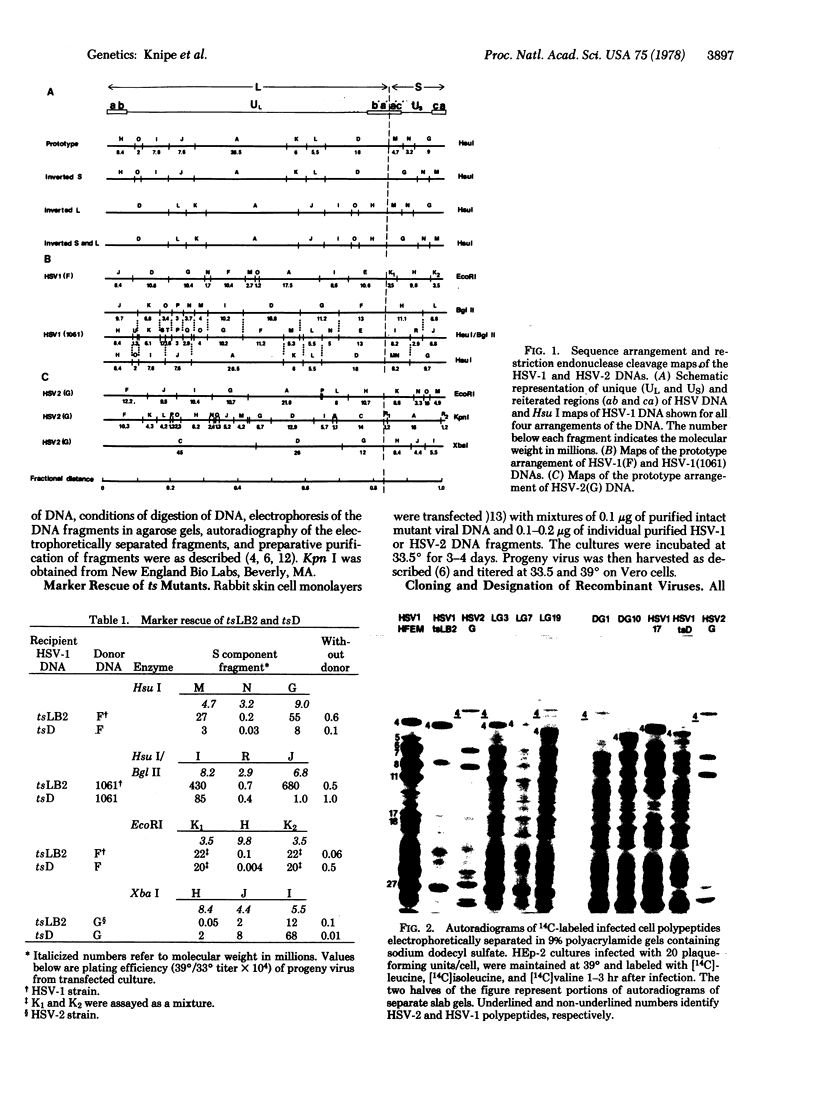
The DNAs of herpes simplex virus (HSV) 1 and 2 consist of two components, L and S, each composed of unique sequences bracketed by inverted repeats. In this study we have probed the structure of the reiterated regions of the S component in marker rescue experiments involving transfection of cells with mixtures of intact HSV-1 mutant viral DNA and individual DNA fragments generated by restriction endonuclease digestion of wild-type HSV-1 or HSV-2 DNAs. The results were as follows: (i) HSV is diploid for the wild-type sequences that rescue two temperature-sensitive (ts) mutants. DNA fragments from both reiterated regions of the S component of HSV-1(F) DNA can rescue tsLB2 and tsD mutants. (ii) Identity of the entire reiterated sequence at both ends of S is not obligatory because only one end of the S component of wild phenotype virus HSV-1(1061) rescues tsD even though both ends rescue tsLB2. (iii) Genes in both reiterated sequences can be expressed. We produced, by marker rescue experiments, recombinants with heterotypic ends of the S component, and these specified corresponding polypeptides characteristic of both HSV-1 and HSV-2. (iv) The reiterated sequences of the S component may contain a region of obligatory identity. Thus, several recombinant clones produced by rescue with HSV-2 DNA contained identical HSV-2 DNA insertions within both reiterated regions of the HSV-1 S component. Consistent with this conclusion, the termini of the S component in the heterodiploids described in iii were identical by restriction enzyme analysis. (v) The observation that HSV DNA can be expanded by at least 5 × 106 by means of insertion in the S component suggests that it can be a vehicle for exogenous DNA.
Keywords: reiterated sequences, marker rescue, recombination
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Randall R. E., Killington R. A., Watson D. H. Some properties of recombinants between type 1 and type 2 herpes simplex viruses. J Gen Virol. 1977 Sep;36(3):471–484. doi: 10.1099/0022-1317-36-3-471. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Buchman T. G., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus DNA. IX. Apparent exclusion of some parental DNA arrangements in the generation of intertypic (HSV-1 X HSV-2) recombinants. J Virol. 1977 Oct;24(1):231–248. doi: 10.1128/jvi.24.1.231-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Wadsworth S., Jacob R. J., Roizman B. Anatomy of herpes simplex virus DNA. II. Size, composition, and arrangement of inverted terminal repetitions. J Virol. 1975 Jun;15(6):1487–1497. doi: 10.1128/jvi.15.6.1487-1497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]