Abstract
Context:
Hashimoto's thyroiditis is less prevalent in tobacco smokers. Anatabine, an alkaloid found in Solanaceae plants including tobacco, has been reported to ameliorate a mouse model of Hashimoto's thyroiditis.
Objective:
The effects of anatabine in patients with Hashimoto's thyroiditis were studied.
Design, Setting, Patients, and Intervention:
This was a double-blind, randomized, placebo-controlled multisite study. A total of 146 patients (70 treated with anatabine and 76 with placebo) completed the study. Approximately 50% of patients in each group were taking levothyroxine. Anatabine lozenges (9–24 mg/d) or placebo, each containing vitamins A and D3, were administered orally 3 times a day for 3 months.
Main Outcome Measures:
Serum thyroperoxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) levels were assessed. Safety was assessed through adverse events, clinical laboratory evaluations, and vital sign measurements.
Results:
Anatabine-treated patients had a significant reduction in absolute serum TgAb levels from baseline by study end relative to those receiving placebo (P = .027); however, there were no significant changes or differences in treatment group means for TPOAb or TgAb levels. Mean ± SD TgAb values decreased by 46.2 ± 101.1 and 3.9 ± 83.9 World Health Organization units for the anatabine and placebo groups, respectively. Significantly more patients had a >20% drop in TgAb levels in the anatabine than placebo group (P = .023). Overall, the anatabine supplement was safe and well tolerated, although significantly (P < .05) more patients in the anatabine group reported adverse events.
Conclusions:
These results demonstrate an immunological effect of anatabine on TgAb levels. Further studies are warranted to determine the longer-term effects and possible actions of anatabine on the course of Hashimoto's thyroiditis.
Treatment for chronic lymphocytic (Hashimoto's) thyroiditis consists of l-thyroxine replacement when hypothyroidism develops (1). Tobacco smoking has numerous effects on thyroid volume, function, and disease and a protective effect on development of Hashimoto's thyroiditis and thyroid antibodies (2). Nicotine has anti-inflammatory effects (3) but cannot be recommended because it is addictive (4) and toxic (5, 6). Anatabine, another Solanaceae alkaloid with a similar chemical structure, may have immunomodulatory properties. In a mouse model of thyroiditis, anatabine reduced the incidence and severity of thyroiditis and lowered the levels of thyroglobulin antibodies (TgAbs) (7). We designed a clinical trial to assess the effects of anatabine dietary supplementation in patients with Hashimoto's thyroiditis.
Materials and Methods
Study sites, patients, and objectives
This was a multicenter, double-blind, placebo-controlled, randomized clinical trial enrolling patients with Hashimoto's thyroiditis. Institutional review board approval was obtained, and all study patients provided signed informed consent. Patients were recruited from 9 endocrinology clinics in the United States between March 2012 and August 2012.
The primary objective was to collect information on the effects of anatabine supplementation in patients with Hashimoto's thyroiditis. Patients taking l-thyroxine were included, but only if their dose was ≤1.0 μg/kg/d to exclude individuals with thyroid destruction incapable of responding to any intervention. The main inclusion and exclusion criteria are provided in Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Study design and randomization
Patients underwent 5 study site visits over 4 months. At visit 1 (screening), demographics, vital signs, medical and medication history, and blood and urine samples were collected, and ultrasonography was scheduled. At visit 2 (randomization), patients were randomly assigned to either the anatabine or placebo group. Thereafter, patients returned monthly for visits 3, 4, and 5 to complete the study procedures.
Anatabine and placebo lozenge
Anatabine was provided by Rock Creek Pharmaceuticals and formulated into a flavored mannitol granulation lozenge that also contained fractional replacement doses of vitamins A (834 IU) and D3 (66 IU), in both active and placebo units to reduce the chance that vitamin deficiencies might obscure an anatabine effect on autoimmunity. Anatabine lozenges were administered orally 3 times daily to a target total dose of 0.17 to 0.25 mg/kg/d. To reduce nicotinic type effects (eg, dizziness and nausea), patients started with 9 mg/d and advanced to the target dose during week 2. Patients who took less than 70% of assigned treatment (pill count) were excluded from the efficacy analysis.
Study outcomes and assays
The main experimental outcomes were serum TgAb and thyroperoxidase antibody (TPOAb) levels. Other measures included serum TSH, free T4, free T3, and inflammatory biomarker (high-sensitivity C-reactive protein, IL-1β, IL-6, and IL-18) levels and ultrasonographic thyroid volume, echogenicity, and vascularity. The North Coast Clinical Laboratory (Sandusky, Ohio) performed the measurements of thyroid function and high-sensitivity C-reactive protein. Assays for TgAbs and TPOAbs and the 3 interleukins were performed at Johns Hopkins Immunological Disorder Laboratory (Baltimore, Maryland). Thyroid ultrasonography was performed at the 9 sites, and scans were sent to a central radiologist who read them blinded (Supplemental Table 2).
Statistical analysis
The data set included thyroid-related variables (TgAbs, TPOAbs, TSH, free T4, free T3, volume, vascularity, and echogenicity), demographic variables (sex, age, race, and ethnicity), body mass index, inflammatory markers (high-sensitivity C-reactive protein, IL-1β, IL-6, and IL-18), and safety outcomes. Urinary iodine was not measured.
We compared nonadjusted continuous variables between the 2 treatment groups using a paired t test when variables were normally distributed, a Wilcoxon rank sum test when variables were not normally distributed, and a χ2 test for categorical variables.
All statistical analyses were performed using Stata 12 (StataCorp) or JMP 7 (SAS Institute).
Results
Demographics and baseline characteristics
Among the 230 patients screened, 165 were randomly assigned in the study, and 146 completed the efficacy evaluation (70 receiving anatabine and 76 placebo) (Supplemental Figure 1). Patients were predominantly female, Caucasian, and non-Hispanic (Supplemental Table 2). Seventy-seven patients reported taking levothyroxine (36 taking anatabine and 41 taking placebo). There were no significant differences between groups with respect to demographic and baseline characteristics.
Thyroid autoantibodies
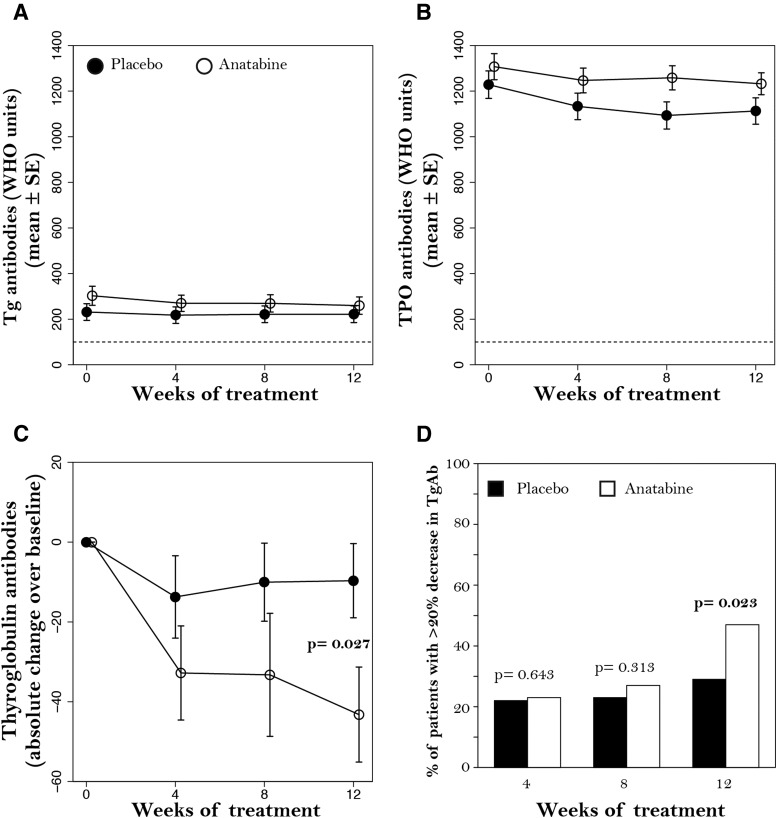
The absolute change in mean TgAb levels between baseline and weeks 4, 8, and 12 revealed a significantly greater reduction in TgAbs in patients taking anatabine relative to those taking placebo by week 12 (P = .027) (Figure 1C). TgAb values decreased by 46.2 ± 101.1 World Health Organization (WHO) units for the anatabine group and only 3.9 ± 83.9 WHO units for the placebo group by week 12. The proportion of patients with a >20% decrease in TgAb levels was greater in the anatabine group (47%) than in the placebo group (28%) at week 12 (P = .023) (Figure 1D). Because of the significant heteroscedasticity in TgAb values, data were categorized based on the reduction from baseline: ≥25, ≥50, ≥75, and ≥100 WHO unit reductions. By week 12, the percentages of patients in the anatabine group with reductions in TgAb levels of ≥25, ≥50, ≥75, and ≥100 WHO units were significantly greater than those in the placebo group for each category (all P < .05) (Supplemental Figure 2). No significant differences were found for TPOAb levels.
Figure 1.
TgAbs and TPOAbs in patients treated with anatabine (○) or placebo (●). A and B, means ± SEM over time for TgAbs and TPOAbs. C, Absolute change in TgAbs between subsequent visits and baseline. D, Percentage of patients with a >20% decrease in TgAbs.
Subgroup analysis of patients taking levothyroxine (n = 36) compared with those not taking levothyroxine (n = 34) revealed a substantial decrease in TgAb levels from baseline at week 12 in the anatabine group receiving levothyroxine therapy (P = .008). Additional analysis conducted with 31 patients in the anatabine group taking levothyroxine doses of ≤1.0 μg/kg/d (range, 0.29–1.0 μg/kg) revealed a greater effect of anatabine on TgAb levels. This analysis showed that decreases in TgAb levels from baseline at weeks 4, 8, and 12 were significant (P < .05 for all). Further, there was a significant difference in mean TgAb levels for patients taking anatabine and levothyroxine ≤1.0 μg/kg/d vs those not taking levothyroxine (−80.6 vs −23.1 WHO units, respectively; P = .02). No relationship between the anatabine dose and TgAb lowering was observed.
Serological thyroid function tests, inflammatory biomarkers, and thyroid ultrasonography
There were no significant changes or treatment group differences in serum thyroid function tests and inflammatory biomarkers or ultrasonography measures (Supplemental Table 3).
Adverse events (AEs) and safety
More patients in the anatabine group (81%) reported AEs relative to those in the placebo group (44%, P < .05) (Table 1). The most common AEs in patients taking anatabine were dizziness (36%), nausea (8%), and headaches (7%) during dose titration and paresthesia (7%).
Table 1.
Overall Summary of AEs for All Patients Who Took at Least 1 Dose of Their Assigned Study Treatment (n = 165)
| Parametera | Anatabine (n = 84), n (%) | Placebo (n = 81), n (%) |
|---|---|---|
| AE | 68 (80.9) | 36 (44.4) |
| Treatment-related AEb | 52 (61.9) | 13 (16.0) |
| Most commonly reported treatment-related AE | ||
| Dizziness | 30 (35.7) | 2 (2.5) |
| Nausea | 7 (8.3) | 2 (2.5) |
| Headache | 6 (7.1) | 2 (2.5) |
| Paresthesia (tingling) | 6 (7.1) | 0 |
| Insomnia | 3 (3.6) | 0 |
| Moderate or severe treatment-related AE | 23 (27.4) | 11 (13.6) |
| Serious AEc | 1 (1.2) | 0 |
| AE that led to withdrawal from study | 7 (8.3) | 1 (1.2) |
| Dose reduction due to AEd,e | 29 (34.5) | 2 (2.5) |
| Death outcome | 0 | 0 |
For each parameter, patients who had at least 1 AE were included in the analysis; ie, patients could have experienced multiple events within a particular parameter but were only counted once.
Determined by the investigator as either possibly or probably related to the study treatment.
One patient in the anatabine group reported a serious AE (chest pain) that was considered unrelated to study treatment, did not result in withdrawal from the study, and did not recur after resumption of active study product.
Anatabine dose reductions occurred with similar frequency regardless of dose group assignment and occurred most often for patients before their week 4 site visit (ie, the first in-person visit after initiation of dosing).
Five of the patients in the anatabine group who had dose reductions subsequently withdrew from the study. The efficacy analysis group for anatabine (n = 70) consisted of 24 (34%) patients who had a dose reduction and 46 (66%) patients who completed the study at their maximum assigned dose.
Seven (8%) patients taking anatabine and 1 (1%) patient taking placebo withdrew from the study because of AEs; these were all considered mild or moderate. One patient taking anatabine reported a serious AE (chest pain) that was evaluated and found to be of noncardiac origin, considered unrelated to study treatment, resolved without complications, and did not recur with resumption of anatabine.
There were no significant abnormalities in clinical laboratory values attributed to anatabine and no clinically significant effects of either treatment on vital sign measures.
Discussion
The results of this study show a selective decrease in TgAb but not TPOAb levels in patients with Hashimoto's thyroiditis after 12 weeks of anatabine supplementation. Anatabine is an alkaloid found in plants of the Solanaceae family, including tobacco, tomatoes, potatoes, peppers, and eggplants (8). Although the mechanism of action of dietary anatabine on thyroid autoimmunity remains to be elucidated, it may produce immunomodulatory effects through activation of α4β2 or α7 cholinergic receptors similar to nicotine and other structurally related agonists (9–11). In a mouse model of thyroiditis, anatabine decreases thyroidal IL-1β and IL-18 levels (7), and in other experimental disease models it suppresses the inflammatory transcription factors signal transducer and activator of transcription 3 and nuclear factor-κB (12–14).
Multiple epidemiological studies have reported a protective effect of smoking on autoimmune hypothyroidism and thyroid antibodies (Supplemental Table 4). Noteworthy are studies showing selective effects of smoking on TgAbs. Analysis of 4125 randomly selected Danes found a negative association between smoking and the presence of thyroid antibodies; the most pronounced association was found between smoking and TgAbs, irrespective of TPOAb status (15). Analysis of a prospective population-based cohort of 9362 pregnant mothers in Finland showed that mothers who smoked before pregnancy had a lower TgAb prevalence than nonsmokers (2.5% vs 4.7%, P < .001), whereas prevalences of TPOAb were similar (16).
Although TPOAbs and TgAbs are typically both measured for diagnostic purposes and often fluctuate in parallel with the disease course, the quantitative correlation between them is rather poor. Carlé et al (17) analyzed 145 patients with newly diagnosed autoimmune hypothyroidism and noted a correlation between TPOAbs and TgAbs (P < .001), but a low Pearson r2 value (0.11). Analysis of National Health and Nutrition Examination Survey data from 2007 to 2008 showed a similarly low r2 value of 0.21 in >6200 Americans (unpublished data).
A retrospective chart review study found that surgical removal or radioiodine ablation of the thyroid leads to gradual reductions in both TgAbs and TPOAbs over several years, with TgAb levels initially declining more rapidly than TPOAb levels (18). Based on those findings, it is possible that the differential response of TgAbs and TPOAbs that we observed after 12 weeks of treatment might have become less apparent after several additional months of anatabine supplementation, although that remains to be tested. It is also possible that anatabine specifically and directly affects thyroglobulin secretion resulting in a drop in TgAb production, as has been suggested regarding the effects of smoking on thyroid autoimmunity (15). Regardless of mechanism, anatabine in the diet reduced TgAbs much like smoking, without nicotine or exposure to toxic smoke constituents.
Anatabine supplementation was safe and well tolerated. Patients in the anatabine group reported more AEs relative to those in the placebo group during upward dose titration, mostly mild nicotinic effects that resolved with dose adjustment.
The primary limitation of this study is its short duration. Longer treatment is required to show a clinically meaningful effect on thyroid gland function and structure. In addition, vitamin D is known to affect immune function (19), and the addition of this vitamin to both the active and placebo formulations may have obscured a treatment effect because TPOAb levels decreased in both groups. Third, the dose of anatabine may have been too low, although the amount taken per day (0.17–0.25 mg/kg) was expected to be safe and effective based on case reports of individuals who experienced positive immunomodulatory effects within this dose range. Fourth, the inclusion of patients who were taking levothyroxine could have influenced the results, because TgAb levels were lower in individuals in the anatabine group who were receiving levothyroxine therapy. Further studies are needed to determine whether anatabine could be an adjunctive therapy to levothyroxine or whether its effects would be more or less robust without the addition of vitamins A and D3. Finally, because urinary iodide measurements were not performed, a potential effect of dietary iodine status on anatabine responsiveness could not be assessed.
This study shows that nutritional supplementation with anatabine significantly reduces circulating TgAb levels in patients with Hashimoto's thyroiditis. These results confirm an immunological effect of anatabine in humans as shown previously in a preclinical model of Hashimoto's thyroiditis (7). Collectively, the results of this trial suggest that further studies of anatabine in Hashimoto's thyroiditis and other autoimmune disorders, including studies that monitor changes noted above for longer time periods and elucidate its mechanism of action, are warranted.
Acknowledgments
We thank Jane Loescher for assistance with study procedures and data management, Kainen Gibson for statistical consulting and advice, and Dr. Maria Varga for assistance with protocol development and safety monitoring throughout the study.
This study was funded by Rock Creek Pharmaceuticals, Inc.
This study was registered with clinical trial registration number NCT01551498, clinicaltrials.gov.
The first draft of the manuscript was prepared by R.K.L. All the authors approved the study design, collected data reliably, and reviewed and were invited to provide revisions to the manuscript.
Disclosure Summary: A.E.C., R.K.L., and C.W. are employees of Rock Creek Pharmaceuticals. J.D.L., has received speaker fees on behalf of AbbVie, Inc. The other authors have nothing to disclose.
Footnotes
- AE
- adverse event
- TgAb
- thyroglobulin antibody
- TPOAb
- thyroperoxidase antibody
- WHO
- World Health Organization.
References
- 1. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;12:1200–1235 [DOI] [PubMed] [Google Scholar]
- 2. Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013;79:145–151 [DOI] [PubMed] [Google Scholar]
- 3. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karaconji IB. Facts about nicotine toxicity. Arh Hig Rada Toksikol. 2005;56:363–371 [PubMed] [Google Scholar]
- 6. Schep LJ, Slaughter RJ, Beasley DM. Nicotinic plant poisoning. Clin Toxicol (Phila). 2009;47:771–781 [DOI] [PubMed] [Google Scholar]
- 7. Caturegli P, De Remigis A, Ferlito M, et al. Anatabine ameliorates experimental autoimmune thyroiditis. Endocrinology. 2012;153:4580–4587 [DOI] [PubMed] [Google Scholar]
- 8. Andersson C, Wennström P, Gry J. Nicotine alkaloids in solanaceous food plants. TemaNord. 2003;531:1–37 [Google Scholar]
- 9. Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68:931–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hosur V, Loring RH. α4β2 nicotinic receptors partially mediate anti-inflammatory effects through Janus kinase 2-signal transducer and activator of transcription 3 but not calcium or cAMP signaling. Mol Pharmacol. 2011;79:167–174 [DOI] [PubMed] [Google Scholar]
- 11. Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paris D, Beaulieu-Abdelahad D, Bachmeier C, et al. Anatabine lowers Alzheimer's Aβ production in vitro and in vivo. Eur J Pharmacol. 2011;670:384–391 [DOI] [PubMed] [Google Scholar]
- 13. Paris D, Beaulieu-Abdelahad D, Mullan M, et al. Amelioration of experimental autoimmune encephalomyelitis by anatabine. PloS One. 2013;8:e55392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paris D, Beaulieu-Abdelahad D, Abdullah L, et al. Anti-inflammatory activity of anatabine via inhibition of STAT3 phosphorylation. Eur J Pharmacol. 2013;698:145–153 [DOI] [PubMed] [Google Scholar]
- 15. Pedersen IB, Laurberg P, Knudsen N, et al. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: a population study. Eur J Endocrinol. 2008;158:367–373 [DOI] [PubMed] [Google Scholar]
- 16. Männistö T, Hartikainen AL, Vääräsmäki M, et al. Smoking and early pregnancy thyroid hormone and anti-thyroid antibody levels in euthyroid mothers of the Northern Finland Birth Cohort 1986. Thyroid. 2012;22:944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlé A, Laurberg P, Knudsen N, et al. Thyroid peroxidase and thyroglobulin auto-antibodies in patients with newly diagnosed overt hypothyroidism. Autoimmunity. 2006;39:497–503 [DOI] [PubMed] [Google Scholar]
- 18. Chiovato L, Latrofa F, Braverman LE, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139:346–351 [DOI] [PubMed] [Google Scholar]
- 19. Castellani ML, Shaik-Dasthagirisaheb YB, Tripodi D, et al. Interrelationship between vitamins and cytokines in immunity. J Biol Regul Homeost Agents. 2010;24:385–390 [PubMed] [Google Scholar]