Abstract
Context:
Coronary artery disease (CAD) is among the leading causes of mortality and morbidity worldwide. Traditional risk markers explain only a proportion of total cardiovascular risk. Thus, development and improvement of early diagnostic strategies and targeted initiation of preventive measures would be of great benefit.
Objective:
We aimed to identify molecular lipids that are associated with fatal outcome of CAD patients. Furthermore, the effect of different lipid-lowering drugs on novel risk lipids was evaluated.
Methods:
Serum samples of 445 CAD subjects participating in a long-term follow-up of the Ludwigshafen Risk and Cardiovascular Health (LURIC) study were analyzed. In addition, samples obtained from a separate randomized parallel three-group study of subjects treated with simvastatin (n = 24), ezetimibe (n = 24), or their combination (n = 24) were studied. Furthermore, samples from the LURIC participants with a loss-of-function mutation (R46L) in the PCSK9 gene (n = 19) were analyzed and compared with major allele carriers (n = 868).
Results:
Distinct ceramide species were significantly associated with the fatal outcome of CAD patients. Simvastatin lowered plasma ceramides broadly by about 25%, but no changes in ceramides were observed in the ezetimibe group. PCSK9 deficiency was significantly associated (−13%) with lowered low-density lipoprotein cholesterol accompanied by a significant 20% reduction in CAD outcome risk-related ceramides.
Conclusions:
These data suggest that distinct ceramides associate significantly with CAD outcome independently of traditional risk factors and that the mechanism of lipid lowering is important.
Worldwide, coronary artery disease (CAD) is among the leading causes of morbidity and mortality (1). Early preventive measures depend on accurate identification of individuals who have an increased risk. Serum total cholesterol and low-density lipoprotein cholesterol (LDL-C) significantly associate with atherosclerosis and its clinical manifestations such as acute coronary events and have long been used as the basis of risk stratification. However, these traditional risk measures fail to recognize a substantial proportion of patients at high risk for coronary events. For example, in a study in patients hospitalized with CAD, almost half of the patients had admission LDL-C less than 100 mg/dL, whereas less than a quarter had LDL-C greater than 130 mg/dL (2). Thus, there is a need for more precise understanding of the roles of different lipid species in atherosclerosis beyond LDL-C and high-density lipoprotein cholesterol (HDL-C). This would improve risk assessment as well as prevention and treatment of CAD. In fact, lipidomes of eukaryotic cells include thousands of lipid species that serve as cellular building blocks, store energy, and function as bioactive molecules (3, 4), and therefore, it is reasonable to assume that some of these numerous molecular lipid species are closely involved in the development of atherosclerosis, as has been suggested recently (5–7).
We performed a study on prospective clinical samples to specifically evaluate the value of molecular lipids in prediction of death from cardiovascular reasons in CAD patients with an established disease. In addition, we tested the effect of different lipid-lowering treatments on the identified novel risk lipids to evaluate whether it matters by which mechanism plasma lipids are lowered.
Materials and Methods
Subjects
The Ludwigshafen Risk and Cardiovascular Health (LURIC) study is an ongoing prospective study of currently more than 3000 individuals of German ancestry in whom the cardiovascular and metabolic phenotypes CAD, myocardial infarction (MI), dyslipidemia, hypertension, metabolic syndrome, and diabetes mellitus have been defined or ruled out using standardized methodologies (8). All the patients were in secondary prevention, ie, had known CAD.
Patients with German ancestry were recruited at the cardiac center in Ludwigshafen from 1997 to 2002. After obtaining a written informed consent, baseline examination was done consisting of a standardized individual and family history questionnaire and extensive sampling of fasted venous blood in the early morning. The coronary artery status was evaluated by angiography. The study was approved by the Ethics Review Committee at the “Landesärztekammer Rheinland-Pfalz.” Written informed consent was obtained from each of the participants.
For this study we first selected male CAD patients (n = 258) from the LURIC cohort who died due to cardiovascular disease (CVD) reasons within the first 3 years of follow-up. The control group consisted of male CAD patients who did not die during the follow-up. Frequency matching was used for matching case and control groups for age, body mass index (BMI), statin use, and smoking. The total number of controls (n = 187) remained smaller than the total number of cases. This was due to the exclusion of numerous stable diabetic patients who had had events indicative of plaque vulnerability such as MI and/or stroke prior to the study entry. Coronary angiography was used to verify CAD (stenosis > 20% in one or more coronary arteries) in both study groups. The characteristics of the study subjects are given in Table 1. Cases were classified based on information on mortality from local health registries. Cardiovascular deaths were defined as sudden cardiac death, fatal MI, death due to congestive heart failure, death immediately after intervention to treat CAD, fatal stroke, and other causes of deaths due to cardiac disease. Two experienced clinicians blinded of the study data independently went through death certificates to classify deaths to CVD and non-CVD causes (9).
Table 1.
Patient Characteristics
| Cases (n = 258) | Controls (n = 187) | P Value | |
|---|---|---|---|
| Men, % | 100 | 100 | |
| Age, y | 67.4 | 64.8 | n.s. |
| BMI, kg/m2 | 27.5 | 27.5 | n.s. |
| LDL-C, mg/dL | 112.0 | 117.9 | n.s. |
| HDL-C, mg/dL | 35.6 | 38.5 | .001 |
| TC, mg/dL | 185.2 | 197.0 | .002 |
| TG, mg/dL | 170.2 | 181.9 | n.s. |
| ApoA1, mg/dL | 120.2 | 129.9 | <.001 |
| ApoB, mg/dL | 103.6 | 107.7 | n.s. |
| CRP, mg/dL | 1.6 | 0.5 | <.001 |
| Statin users, % | 52.3 | 50.1 | n.s. |
| Other lipid-lowering drugs | 1.9 | 2.7 | n.s. |
| Smokers, % | 30.2 | 30.5 | n.s. |
| DM2, % | 34.9 | 16.6 | <.001 |
| CAD, % | 100 | 100 | |
| Framingham scorea | 20 | 20 | n.s. |
| SCOREb | 7.1 | 6.7 | n.s. |
Plasma samples of healthy males from a single-center, randomized, parallel, three-group study performed at the University of Cologne (10) were analyzed to evaluate the potential of ezetimibe (10 mg; n = 24), simvastatin (40 mg; n = 24), or their combination (n = 24) to reduce plasma concentrations of molecular lipids species that had been identified to be related to cardiovascular death in the LURIC cohort. This study was accepted by the Ethical Committee of the University of Cologne. The dose of ezetimibe and simvastatin was administered in the evening. Blood was drawn before and after the treatment period that lasted for 14 days.
Furthermore, we used the LURIC study genome-wide association studies database and identified subjects who were carrying the previously described loss-of-function mutation R46L (rs11591147) (11) of the PCSK9 gene to reveal the possible lipidomic effect of PCSK9 deficiency in men. Altogether 19 heterozygous male mutation carriers and 868 male homozygous major allele carriers were identified from our database with both genetic information and serum lipidomic data available.
Lipid extraction and mass spectrometry
An aliquot of plasma or serum was subjected to lipid extraction. Known amounts of internal standards were added to the samples before extraction and the final lipid extracts were dried under nitrogen. The extracts were reconstituted as described (12). Sphingolipids were analyzed as described elsewhere (13) on a 4000 QTRAP mass spectrometer (Applied Biosystems/MDS Analytical Technologies) equipped with an ultrahigh pressure liquid chromatography system, CTC PAL autosampler (Leap Technologies), and Rheos Allegro ultrahigh pressure liquid chromatography (Flux Instruments) using multiple reaction monitoring. Shotgun lipidomics was performed by multiple precursor ion and neutral loss scanning as described elsewhere (14) on a QTRAP 5500 mass spectrometer (Applied Biosystems/MDS Analytical Technologies) equipped with a robotic nanoflow ion source NanoMate HD (Advion). Mass spectrometry data files were processed using MultiQuant 1.1.0.26 or Lipid Profiler (15) (Applied Biosystems/MDS Analytical Technologies). Identified lipids were quantified by normalizing against their respective internal standard and volume for plasma or serum. Quality control (QC) samples were used to monitor the overall quality of the lipid extraction and mass spectrometry analyses (16). The QC samples were mainly used to remove technical outliers and lipid species that were detected below the lipid class-based lower limit of quantification. In total, 14 QC samples evenly distributed along analytical runs of the study were analyzed. The average coefficient of variation of all the lipids detected in the study samples was 20%.
Statistical analyses
Lipid class concentrations were calculated by summing up the concentrations of corresponding molecular lipids. For analysis of CAD mortality and PCSK9 mutation data, an unpaired Student t test was performed on log-transformed concentrations and lipid to lipid ratios because variables were approximately log normal. Equality of variance was tested, and pooled t test was used in case of equal variance and Satterthwaite t test was used in case of unequal variance. CAD mortality case and controls groups were frequency matched; therefore, an unpaired t test was used. The t test results are presented as volcano plots and heat maps (Figures 1–3). In volcano plots, the magnitude of relative difference between groups (horizontal axis) is plotted against the statistical significance (vertical axis). Odds ratios were calculated using logistic regression model with and without adjustment for age, BMI, fasting glucose, HDL-C, LDL-C, C-reactive protein, and triglycerides. A paired t test was conducted for comparing baseline and after-treatment lipid concentrations in subjects receiving ezetemibe, simvastatin, or their combination. Baseline and treatment values were approximately normally distributed. Normality of the lipid concentration distributions were tested with a Shapiro-Wilk test. A value of P < .05 was considered significant. The false discovery rate q values were calculated to correct the multiple hypotheses testing results. All the analyses were performed using SAS 9.2 (SAS Institute).
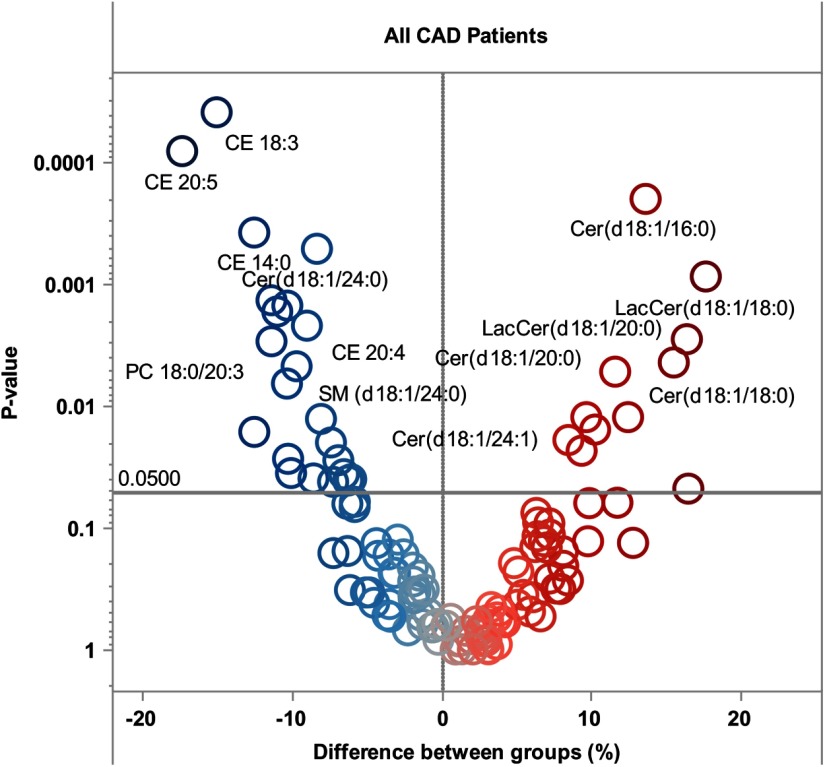
Figure 1.
Volcano heat map indicating molecular lipid species difference (percentage) between stable and high-risk CAD patients. Right panel (red color) indicates lipids that associate with CVD outcome risk in CAD patients. Left panel (blue color) indicates lipids that associate with protection. Lipid concentrations in controls are taken as a reference; thus, positive values correspond to higher values in cases vs controls.
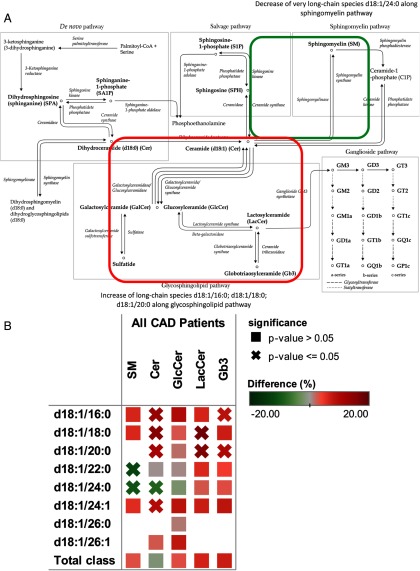
Figure 2.
Differential behavior of sphingolipid species in sphingolipid pathway. A, Sphingolipid pathway map. B, Heat map of sphingolipid differences.
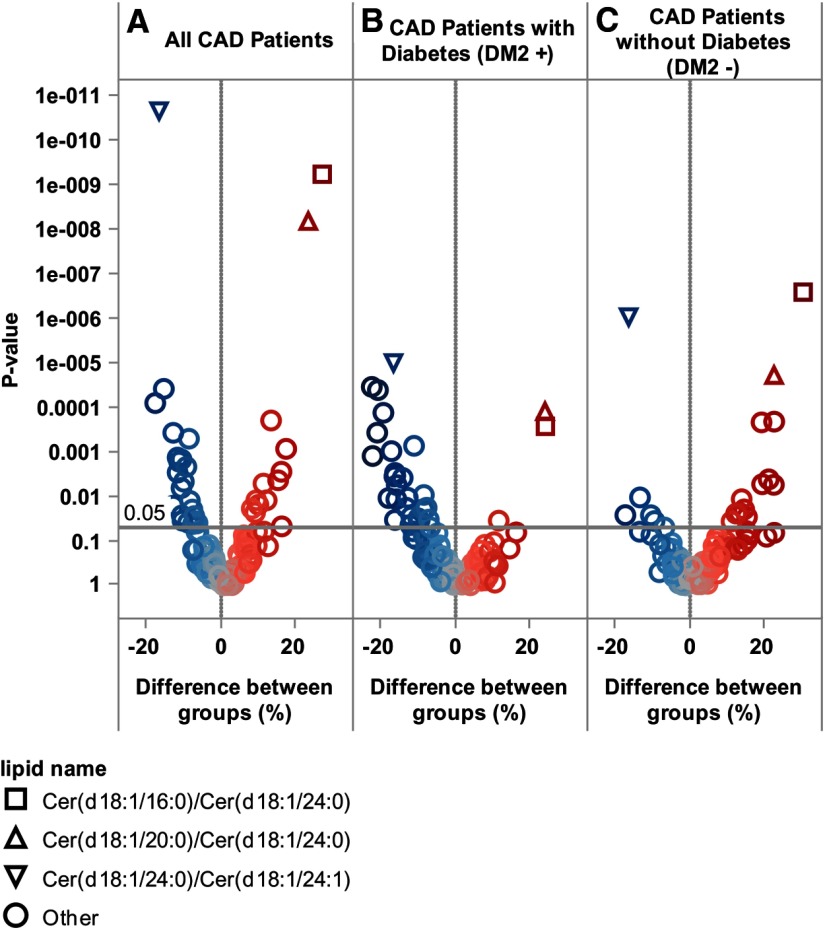
Figure 3.
Volcano heat map indicating molecular lipids and ceramide to ceramide ratio differences between all studied stable CAD patients and high-risk CAD patients (A), differences between stable CAD patients and vulnerable CAD patients with diabetes (B), and patients without diabetes (C). Lipid concentrations in controls are taken as a reference; thus, positive values correspond to higher values in cases vs controls.
Results
Molecular lipid species are potential indicators of CAD outcome risk
The classical risk markers were unable to distinguish CAD patients who died during the follow-up from stable CAD patients who had structural coronary disease at baseline. Modestly higher total cholesterol concentrations (6%, P = .002) were recorded in the case groups and HDL-C and apoA1 were slightly lower in cases compared with controls (−7.5%, P = .001, and −7.5%, P = .001, respectively) (Table 1). Furthermore, traditional risk scores were not different between the groups. Both case and control groups had median Framingham 10-year risk of coronary heart disease (17) of 20%. The estimation of the 10-year risk of fatal cardiovascular disease in Europe (Systematic Coronary Risk Evaluation, SCORE) (18) was 7.1% in cases and 6.7% in controls, and the slight difference was not statistically significant.
More pronounced differences between groups were observed through lipidomic analyses (Figure 1 and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Lactosylceramides LacCer (18:1/18:0) and LacCer (18:1/20:0) were among the risk-associated lipids together with Cer (18:1/16:0) and Cer (18:1/18:0). Several molecular lipid species such as CE20:5 (−17.4%, P = .0001) and CE18:3 (−15.1%, P = .000037) displayed reduced concentrations in cases as compared with the control group. Abundance of several ceramide species containing specific fatty acids was higher along the axis of the glycosphingolipid pathway (Figure 2) in which ceramide is converted to galactosylceramide and glucosylceramide, glucosylceramide is converted to lactosylceramide, and lactosylceramide is metabolized to globotriaosylceramide (Gb3). No significant differences were observed in corresponding sphingomyelin species. For example, d18:1/22:0-containing species were higher in ceramide, lactosylceramide, and globotriaosylceramide classes. A similar pattern is demonstrated by other long-chain containing ceramides (d18:1/16:0 and d18:1/18:0). However, Cer(d18:1/24:0) lipid species concentration was lower in cases reflected in lower concentration of sphingomyelin containing the same fatty acid SM(d18:1/24:0) (Figure 2B).
To account for lipid concentration differences due to metabolome influencing factors, we also studied patients with and without diabetes mellitus type 2. Interestingly, when these patients were studied separately, very distinct lipidomic profiles were recorded for the two groups. In nondiabetic CAD patients, the risk of fatal outcome was associated with elevated sphingolipids, whereas in diabetic CAD patients, the risk was mainly associated with reduced cholesteryl ester species (Figure 3). Because we noted that factors like diabetes, smoking, and lipid-lowering treatment among others, could influence the expression of the risk-associated lipids, we decided to calculate ceramide and cerebroside ratios to check whether these would be less dependent on different patient characteristics while still indicative of risk. It turned out that the ratios of Cer(18:1/16:0)/Cer(18:1/24:0) and Cer(18:1/22:0)/Cer(18:1/24:0) were significantly related to increased risk of CVD death in all subjects and subgroups. Cer(18:1/24:0)/Cer(18:1/24:1) was indicative of a reduced risk of CVD death, regardless of diabetes status (Figure 3). Importantly, odds ratios for CVD death of these ratios remained significant after adjustment for traditional CVD death risk factors, such as LDL-C (Table 2).
Table 2.
Unadjusted and Adjusted Odds Ratios for CVD Death of Ceramide Ratios
| Lipid | Odds Ratio | OR per SD | P Value | OR Second Quartile | OR Third Quartile | OR Fourth Quartile | P Value for Trend |
|---|---|---|---|---|---|---|---|
| Cer(d18:1/16:0)/Cer(d18:1/24:0) | OR | 1.90 (1.53–2.35) | <.0001 | 1.36 (0.80–2.30) | 2.45 (1.43–4.21) | 4.52 (2.54–8.06) | <.0001 |
| Adjusted OR | 1.62 (1.28–2.06) | <.0001 | 1.49 (0.85–2.61) | 2.42 (1.36–4.33) | 2.99 (1.59–5.60) | <.0001 | |
| Cer(d18:1/20:0)/Cer(d18:1/24:0) | OR | 1.82 (1.47–2.26) | <.0001 | 1.63 (0.96–2.78) | 3.65 (2.09–6.37) | 3.82 (2.18–6.67) | <.0001 |
| Adjusted OR | 1.50 (1.18–1.90) | .0009 | 1.41 (0.81–2.47) | 2.91 (1.61–5.28) | 2.21 (1.18–4.15) | <.0001 | |
| Cer(d18:1/24:0)/Cer(d18:1/24:1) | OR | 0.49 (0.39–0.61) | <.0001 | 0.62 (0.34–1.14) | 0.24 (0.13–0.42) | 0.20 (0.11–0.35) | <.0001 |
| Adjusted OR | 0.65 (0.50–0.83) | .0003 | 0.89 (0.46–1.71) | 0.43 (0.22–0.81) | 0.40 (0.20–0.77) | <.0001 |
Abbreviation: OR, odds ratio. Models adjusted for age, BMI, fasting glucose, HDL-C, LDL-C, C-reactive protein, triglycerides, and systolic and diastolic blood pressure.
Predictive potential for CAD mortality was tested by receiver-operating characteristic analysis for the ceramide ratios and compared with LDL-C. The area under curve and 95% confidence limits were 0.67 (range 0.62–0.72) for Cer(d18:1/16:0)/Cer(d18:1/24:0), 0.65 (range 0.60–0.71) for Cer(d18:1/20:0)/Cer(d18:1/24:0), and 0.68 (range 0.63–0.73) for Cer(d18:1/24:0)/Cer(d18:1/24:1), whereas for LDL-C, these values were 0.55 (range 0.50–0.61).
Loss-of-function mutation of PCSK9 gene efficiently lowered risk-related lipids
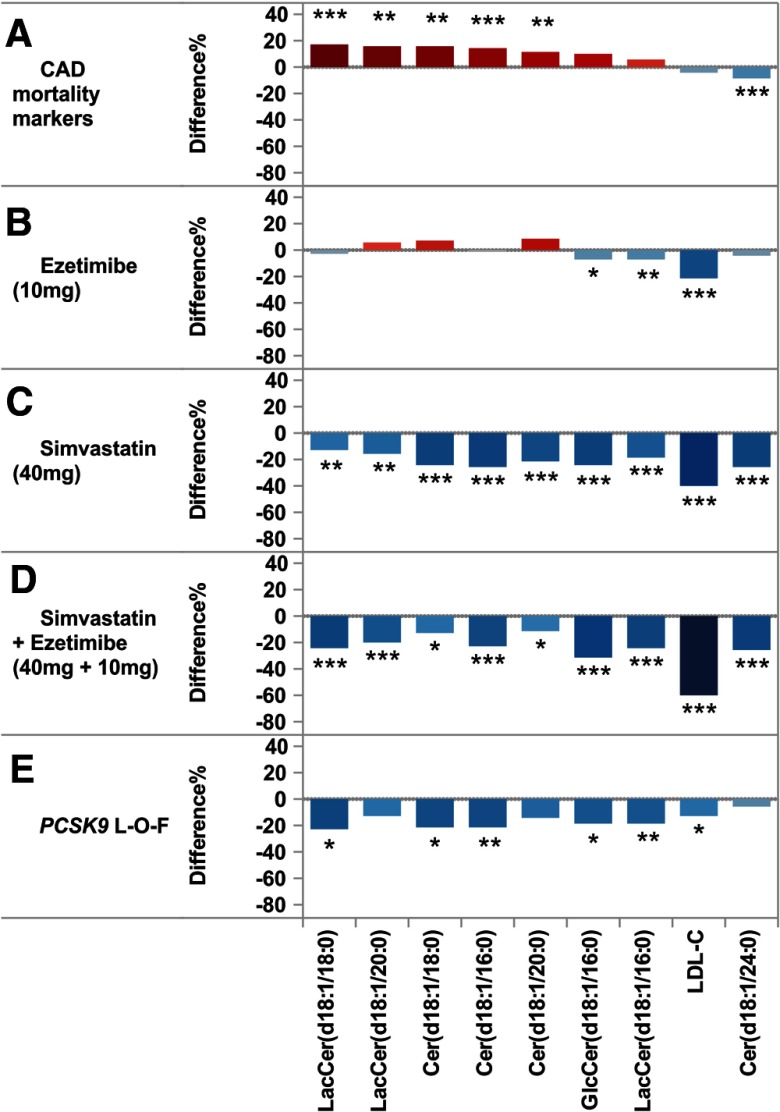
Finally, the effect of lipid-lowering treatments on ceramides and cerebrosides associated with increased risk of CVD death in statin-naïve subjects as well as on LDL-C was evaluated (Figure 4). As a comparison basis, we used the most significant lipids that separate the high-risk CAD patients from stable CAD patients (Figure 4A).
Figure 4.
The effect of different LDL-C-lowering methods on CAD outcome-related lipidomic markers. A, Lipid profile in high-risk vs stable CAD patients. Results shown for top ranked CAD risk lipids, LDL-C, and ceramide (18:1/24:0). Effect of ezetimibe (10 mg) (B), simvastatin (40 mg) (C), simvastatin + ezetimibe (40 mg+10 mg), (D) and PCSK9 loss-of-function mutation (R46L) (E) on CAD mortality risk lipids.
For evaluation of ezetimibe and simvastatin effects on these putative risk lipids we used plasma samples from a randomized clinical trial comparing simvastatin 40 mg, ezetimibe 10 mg, and their combination (Supplemental Table 2). As expected, simvastatin 40 mg lowered LDL-C significantly by 40%, and this reduction was accompanied by a significant reduction (∼25%) also in all ceramides and cerebrosides recorded in this study (Figure 4C). Ezetimibe treatment (10 mg) in turn lowered LDL-C by 21% but affected only very modestly the plasma levels of CVD death-related lipid species (Figure 4B). In fact, Cer18:1/18:0 and Cer18:1/20:0 concentrations were slightly elevated in ezetimibe-treated subjects by 7.6% and 8.8%, respectively, whereas Cer18:1/16:0 levels remained unchanged during this treatment. There was no statistically significant difference between treatment effects of simvastatin alone or in combination with ezetimibe on molecular lipids. However, combined treatment resulted in significantly lower LDL-C levels than simvastatin or ezetimibe alone (Figure 4D).
Drugs that inhibit PCSK9 are being developed as an alternative to statin lipid-lowering therapy, and we studied the effects of PCSK9 inhibition on the risk lipids (Supplemental Table 3). The effect was investigated by using subjects with the PCSK9 allelic variants from the LURIC patient population (Figure 4E). The PCSK9 loss-of-function mutation (R46L) resulted in a significant reduction of ceramides indicative of CAD outcome risk comparable with that observed in simvastatin-treated subjects. Interestingly, the mutation carriers had only modestly lower LDL-C levels (12.9%) compared with major allele carriers while being still efficient in risk lipid reduction. Furthermore, PCSK9 deficiency lowered the reduced CAD outcome risk-associated Cer(18:1/24:0) nonsignificantly by 6.4%, whereas simvastatin and simvastatin-ezetimibe combination both resulted in significant (>25%) reductions.
Discussion
The present study demonstrates that specific molecular lipid species are significantly associated with mortality in CAD. Importantly, the predictive potential of distinct ceramides was superior to the currently used standard LDL-C measurement, underscoring the value of molecular lipidomic analyses. Moreover, we show that these molecular lipid species are highly influenced by the choice of lipid-lowering treatment and that simple numerical LDL-C lowering per se is not necessarily translated to modulation of all risk-associated lipid species.
In earlier experimental studies, ceramides and other sphingolipids have been associated with the development of atherosclerosis, and several enzymes in the ceramide synthetic pathway have been tested as potential drug targets in animal models (19–21). The present study suggests that different molecular ceramides associate with CAD outcome risk, and thus, they may play a significant role also in plaque vulnerability in addition to development of atherosclerotic disease. Notably, for some ceramide species containing specific fatty acids, higher concentrations were observed along the axis of glycosphingolipid pathway (Figure 2) with no significant differences observed in corresponding sphingomyelin species, although lower concentrations of other ceramide species with longer-chain fatty acids were reflected in lower concentrations of the corresponding sphingomyelin. These findings together with the fact that total sphingolipid levels remained unchanged indicate that alteration in sphingolipid balance is species dependent.
A role of the glycosphingolipid pathway in atherosclerosis was previously suspected based on the following observations: lactosylceramide and glucosylceramide accumulate in the atherosclerotic plaque (22), and both lactosylceramide and glucosylceramide suppress production of macrophage apoE and lead to an accumulation of cholesterol in macrophage foam cells (23). Importantly, inhibition of the glycosphingolipid pathway was previously shown to decrease atherosclerosis in mice (24). To the best of our knowledge, our data for the first time link specific molecular lipid species from the glucosphingolipid pathway measured in human serum to CAD mortality. On the other hand, a reverse association of long-chain ceramide and sphingomyelin d18:1/24:0 with CAD mortality demonstrates the complexity of this disease, which is not seen by analyzing the lipid classes alone. Further studies are needed to evaluate the potential connections between the ceramide species and thrombogenesis, fibrinolysis, and plaque instability that may explain the observed linkage between death from CVD reasons and molecular ceramides.
Another main finding of this study is that two different lipid-lowering drugs display distinct effects on the CVD risk-associated plasma ceramides, beyond their LDL-C-lowering effects. Simvastatin 40 mg resulted in a broad lowering of all recorded ceramide and cerebroside species; however, the magnitude of reduction was clearly lower than that for LDL-C. On the other hand, we demonstrated in this study that a very modest LDL-C effect due to PCSK9 deficiency resulted in a larger proportional reduction in CAD outcome-related plasma ceramides. This suggests that there is an interaction between the means of LDL receptor (LDLR) up-regulation and the levels of circulating ceramides.
Both statins and PCSK9 inhibition increase LDLR-mediated hepatic lipid uptake, and it could thus be expected that they result in similar lipid-lowering profiles. However, statins are acting indirectly via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase and inhibition of this rate-limiting enzyme of the mevalonate pathway has multiple effects on a number of genes related to lipid metabolism in hepatocytes. This is due to subsequent activation of the sterol regulatory element binding protein (SREBP)-1- and -2-mediated gene transcription (25, 26). SREBP-2 activates LDLR synthesis, but SREBP-1 activation is related to fatty acid synthesis. PCSK9 inhibition, in turn, may act more precisely on LDLR in hepatocytes and thus may lead to more targeted lipid lowering without more systemic effects on lipid metabolism. Therefore, PCSK9 inhibition seems a promising lipid-lowering method of choice from the plasma lipidomic point of view. In particular, this is the case if this inhibition leads to similar plasma lipidomic composition as the well-characterized loss-of-function mutation.
Ezetimibe seemed to act only weakly on risk-related ceramide species. However, these data are based on a 2-week intervention, and thus, the long-term effect must await confirmation in longer studies. On the other hand, Taylor et al demonstrated recently that in the Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol-6 trial, ezetimibe treatment resulted in a paradoxical progression of carotid intima-media thickness in association with both greater LDL-C reduction and cumulative drug exposure (27). The authors concluded that their findings may suggest the presence of off-target actions of ezetimibe. Based on our current results, one could hypothesize that the paradoxical effect on carotid intima-media thickness is due to the minimal effects on ceramides despite the substantial LDL-C-lowering effects.
This study suggests that LDL-C and traditional risk factors provide only limited predictive information on fatal CVD complications in patients with established CAD. However, molecular lipid assays can be used to improve the identification of CAD patients at high risk, and such assay results may also serve as better efficacy indicators for lipid-modifying drugs. It is important to note that although traditional risk scores are developed for the general population, for the purpose of this study, we selected only diseased patients and investigated association of lipids with stable or unstable disease. As a result, both patient groups were at high risk from the start of the follow-up as indicated by Framingham and European risk score values.
A limitation of our study is that only male subjects from only one cohort were evaluated. We selected men for reducing variability in the data because atherosclerotic development is gender specific (28), and traditionally risk scores are evaluated separately for men and women (17, 18). Thus, our results should be validated in an independent large cohort and extended to women. In the present work, the statin effect was evaluated in healthy male subjects treated for 2 weeks. Longer exposure time, greater subject numbers, and possibly study in patients with CAD will be needed to further understand the dynamics of modulation of the risk lipids.
Acknowledgments
Author contributions included the following: R.L., K.E., K.T., R.H., I.G.-B., H.K.B., M.E.K., and W.M. wrote the manuscript; R.L. and W.M. designed the research; K.E., D.K., T.S., I.G.-B., H.K.B., and M.E.K. performed the research; and R.L., K.T., M.S., and M.E.K. analyzed the data.
This work was supported by European Union (integrated project Atheroremo, Grant 201668) and by Tekes, Finnish Funding Agency for Technology, and Innovation.
Disclosure Summary: R.L., K.E., K.T., M.S., D.K., T.S., and R.H are employees of Zora Biosciences Oy. I.G.-B., H.K.B., M.E.K., and W.M. have nothing to declare.
Footnotes
- BMI
- body mass index
- CAD
- coronary artery disease
- CVD
- cardiovascular disease
- HDL-C
- high-density lipoprotein cholesterol
- LDL-C
- low-density lipoprotein cholesterol
- LDLR
- LDL receptor
- LURIC
- Ludwigshafen Risk and Cardiovascular Health
- MI
- myocardial infarction
- QC
- quality control
- SREBP
- sterol regulatory element binding protein.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136 905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157:111–117.e112 [DOI] [PubMed] [Google Scholar]
- 3. Harkewicz R, Dennis EA. Applications of mass spectrometry to lipids and membranes. Annu Rev Biochem. 2011;80:301–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Meer G. Cellular lipidomics. EMBO J. 2005;24:3159–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stubiger G, Aldover-Macasaet E, Bicker W, et al. Targeted profiling of atherogenic phospholipids in human plasma and lipoproteins of hyperlipidemic patients using MALDI-QIT-TOF-MS/MS. Atherosclerosis. 2012;224:177–186 [DOI] [PubMed] [Google Scholar]
- 6. Stegemann C, Drozdov I, Shalhoub J, et al. Comparative lipidomics profiling of human atherosclerotic plaques. Circulation Cardiovasc Genet. 2011;4:232–242 [DOI] [PubMed] [Google Scholar]
- 7. Meikle PJ, Wong G, Tsorotes D, et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscleros Thromb Vasc Biol. 2011;31:2723–2732 [DOI] [PubMed] [Google Scholar]
- 8. Winkelmann BR, Marz W, Boehm BO, et al. Rationale and design of the LURIC study—a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics. 2001;2:S1–S73 [DOI] [PubMed] [Google Scholar]
- 9. Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31:1237–1247 [DOI] [PubMed] [Google Scholar]
- 10. Berneis K, Rizzo M, Berthold HK, Spinas GA, Krone W, Gouni-Berthold I. Ezetimibe alone or in combination with simvastatin increases small dense low-density lipoproteins in healthy men: a randomized trial. Eur Heart J. 2010;31:1633–1639 [DOI] [PubMed] [Google Scholar]
- 11. Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272 [DOI] [PubMed] [Google Scholar]
- 12. Ekroos K, Chernushevich IV, Simons K, Shevchenko A. Quantitative profiling of phospholipids by multiple precursor ion scanning on a hybrid quadrupole time-of-flight mass spectrometer. Anal Chem. 2002;74:941–949 [DOI] [PubMed] [Google Scholar]
- 13. Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–224 [DOI] [PubMed] [Google Scholar]
- 14. Stahlman M, Ejsing CS, Tarasov K, Perman J, Boren J, Ekroos K. High-throughput shotgun lipidomics by quadrupole time-of-flight mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877:2664–2672 [DOI] [PubMed] [Google Scholar]
- 15. Ejsing CS, Duchoslav E, Sampaio J, et al. Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal Chem. 2006;78:6202–6214 [DOI] [PubMed] [Google Scholar]
- 16. Jung HR, Sylvanne T, Koistinen KM, Tarasov K, Kauhanen D, Ekroos K. High throughput quantitative molecular lipidomics. Biochim Biophys Acta. 2011;1811(11):925–934 [DOI] [PubMed] [Google Scholar]
- 17. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 18. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003 [DOI] [PubMed] [Google Scholar]
- 19. Billich A, Baumruker T. Sphingolipid metabolizing enzymes as novel therapeutic targets. Subcell Biochem. 2008;49:487–522 [DOI] [PubMed] [Google Scholar]
- 20. Bismuth J, Lin P, Yao Q, Chen C. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 2008;196:497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hojjati MR, Li Z, Zhou H, et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem. 2005;280:10284–10289 [DOI] [PubMed] [Google Scholar]
- 22. Chatterjee SB, Dey S, Shi WY, Thomas K, Hutchins GM. Accumulation of glycosphingolipids in human atherosclerotic plaque and unaffected aorta tissues. Glycobiology. 1997;7:57–65 [DOI] [PubMed] [Google Scholar]
- 23. Garner B, Mellor HR, Butters TD, Dwek RA, Platt FM. Modulation of THP-1 macrophage and cholesterol-loaded foam cell apolipoprotein E levels by glycosphingolipids. Biochem Biophys Res Commun. 2002;290:1361–1367 [DOI] [PubMed] [Google Scholar]
- 24. Bietrix F, Lombardo E, van Roomen CP, et al. Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 Leiden and low-density lipoprotein receptor −/− mice. Arteriosclerosis, Thrombos Vasc Biol. 2010;30:931–937 [DOI] [PubMed] [Google Scholar]
- 25. Ye J, DeBose-Boyd RA. Regulation of cholesterol and fatty acid synthesis. Cold Spring Harb Perspect Biol. 2011;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raghow R, Yellaturu C, Deng X, Park EA, Elam MB. SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol Metab. 2008;19:65–73 [DOI] [PubMed] [Google Scholar]
- 27. Taylor AJ, Villines TC, Stanek EJ. Paradoxical progression of atherosclerosis related to low-density lipoprotein reduction and exposure to ezetimibe. Eur Heart J. 2012;33:2939–2945 [DOI] [PubMed] [Google Scholar]
- 28. Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S62–S72 [DOI] [PubMed] [Google Scholar]