Abstract
Purpose/Objective
Lymphedema following breast cancer treatment can be an irreversible condition with a negative impact on quality of life. The goal of this study was to identify radiotherapy-related risk factors for lymphedema.
Methods and Materials
From 2005–2012, we prospectively performed arm volume measurements on 1,476 breast cancer patients at our institution using a Perometer. Treating each breast individually, 1099/1501 (73%) received radiotherapy. Arm measurements were performed pre- and post-operatively. Lymphedema was defined as ≥10% arm volume increase occurring >3 months post-operative. Univariate and multivariate Cox proportional hazard models were used to evaluate risk factors for lymphedema.
Results
At a median follow-up of 25.4 months (range 3.4–82.6), the 2-year cumulative incidence of lymphedema was 6.8%. Cumulative incidence by radiotherapy type was: 3.0% (no radiotherapy), 3.1% (breast or chest wall alone), 21.9% (supraclavicular (SC)), and 21.1% (SC and posterior axillary boost (PAB)). On multivariate analysis, the hazard ratio for RLNR (SC±PAB) was 1.7 (p = 0.025) compared to breast/chest wall radiation alone. There was no difference in lymphedema risk between SC and SC+PAB (p=0.96). Other independent risk factors included early post-operative swelling (p <0.0001), higher BMI (p<0.0001), greater number of lymph nodes dissected (p =0.018), and axillary lymph node dissection (p=0.0001).
Conclusions
In a large cohort of breast cancer patients prospectively screened for lymphedema, RLNR significantly increased risk of lymphedema compared to breast/chest wall radiation alone. When considering use of RLNR, clinicians should weigh the potential benefit of RLNR for control of disease with the increased risk of lymphedema.
Keywords: Lymphedema, Quality of Life, Radiation Therapy, Regional Lymph Node Radiation
INTRODUCTION
Women treated for breast cancer have increasingly successful outcomes; therefore, late toxicities of treatment are assuming greater importance. Lymphedema causes significant physical and psychosocial side effects and is of concern for women undergoing breast cancer therapy (1–4). Data regarding risk of lymphedema with the inclusion of adjuvant radiotherapy are limited and often based on retrospective cohorts, varying definitions of lymphedema, and heterogeneous patient populations.
Of particular interest is the risk of lymphedema with extended radiotherapy fields. Regional lymph node radiation (RLNR) is routinely used in patients with ≥4 positive lymph nodes (LN) found at the time of lumpectomy or mastectomy with axillary lymph node dissection (ALND) (5). These clinical practices are based on trials that demonstrated decreased rates of loco-regional failure, increased disease free survival (DFS), and increased overall survival (OS) for high-risk or LN positive patients with the receipt of post-mastectomy radiotherapy (6, 7). As women undergoing RLNR most often also undergo sentinel lymph node biopsy (SLNB) or ALND, which have a reported incidence of lymphedema up to 11% and 30% respectively (8–10), quantifying the additional risk with inclusion of RLNR has significant clinical implications.
In this study, we utilized a large cohort of breast cancer patients prospectively screened for lymphedema and analyzed arm volume changes to quantify the risk of lymphedema with inclusion of adjuvant radiotherapy. Types of radiotherapy analyzed included receipt of no radiation, partial breast irradiation (PBI), whole breast irradiation (WBI) or chest wall radiation alone, or WBI/chest wall radiation with RLNR. Among patients who received RLNR, we sought to determine whether the addition of a posterior axillary boost (PAB) to supraclavicular irradiation (SC) increased the risk of lymphedema compared to SC alone.
METHODS AND MATERIALS
Patients
We included 1476 women who underwent surgery for unilateral or bilateral breast cancer from 2005–2012. Patients were recruited at the time of initial consultation in a multi-disciplinary clinic and all underwent surgery at our institution. All patients, regardless of T or N stage, were included excepting patients with pre-existing lymphedema. Treating each breast individually, we had 1501 unique cases. Each patient had >3 months of post-surgical follow-up.
Lymphedema definition and measurement
Per standard of care at our institution, all newly diagnosed breast cancer patients undergo screening for lymphedema using serial Perometer arm volume measurements. A perometer is an optoelectronic system that uses infrared beams to measure the limb and calculates the volume based on these measurements. Arm volume is measured pre-operatively and at regular intervals corresponding with routine oncology follow-up visits during and after completion of treatment. This protocol was approved by the Massachusetts General Hospital Institutional Review Board and has been previously published (11).
Lymphedema was defined as a ≥10% arm volume increase occurring >3 months post-operative based on consensus in the literature (12, 13). For patients who underwent unilateral breast surgery, arm volume change was quantified using the relative volume change (RVC) equation (11). Briefly, RVC = [(A(2)U(1)/U(2)A(1)) – 1] where A(1), A(2) are pre-operative (1) and post-operative (2) arm volumes on the surgical side and U(1), U(2) are arm volumes on the contralateral side at corresponding time points. This equation accounts for pre-operative asymmetry between arms and uses contralateral arm volume as a control to account for factors unrelated to lymphedema, such as weight gain. The RVC equation is not applicable in patients who undergo bilateral breast surgery due to a lack of a contralateral control arm. Therefore the weight-adjusted volume change (WAC) equation was utilized for patients who underwent bilateral breast surgery (14). Briefly, WAC = [(A(2)W(1)/W(2)A(1)) – 1] where A(1), A(2) are pre-operative (1) and post-operative (2) arm volumes on the surgical side, and W(1), W(2) are the patient’s weight at the corresponding time points. The equivalency of ≥10% according to the RVC and WAC equations as a criterion for lymphedema has been previously demonstrated (14).
Arm volume change was calculated at each follow-up visit. Measurements obtained ≤3 months after surgery were not utilized to determine lymphedema incidence. A ≤10% arm volume increase occurring during this period was considered early postoperative swelling, and included as a separate variable in the analysis.
Radiation Treatment
Of the 1099 breasts that received radiation, 796 (72%) were at the Massachusetts General Hospital (MGH), 92 (8%) at MGH-affiliated institutions, and 211 (19%) at outside institutions. Each breast was categorized as: no radiation; PBI, WBI or chest wall radiation alone (B/CW); or B/CW with RLNR. RLNR was classified as SC only or SC with a PAB. After 2008, supraclavicular level I, II, and III axillary LN’s at our institution were contoured the majority of the time according to the RTOG atlas (15). The lateral border of the SC field was defined by the treating physician. The SC and level III LN’s received a dose of 50–50.4 Gy in 25–28 fractions (1.8–2.0 Gy per fraction) through an anterior oblique field angled 10–15 degrees to avoid the spinal cord. The dose was calculated at a depth of mostly 3 cm or a depth to include the majority of the contoured supraclavicular area, which varied from 2–5 cm. The PAB was completed using a posterior approach and the dose was calculated at midplane. Photon energies of 6 or 10 MV photons were typically utilized. Until 2009, patients who received PBI were enrolled in a dose-escalation clinical trial (16), and received a total dose of 32, 36, or 40 Gy using 4 Gy per fraction BID with a 3-D conformal technique using a combination of photons and electrons (17). Thereafter patients were treated off-trial using 36 Gy in 9 fractions BID. Radiation specifics are given in Table 1.
Table 1.
Radiation dose and fractionation by treatment field.
| Median Dose in Gy (Range) | Fractions (Range) | |
|---|---|---|
| Partial breast irradiation (PBI) | 36.0 (32.0–40.0) | 9 (8–10) |
| Whole breast/chest wall tangents | 50.0 (41.9–61.2) | 25 (16–28) |
| Whole breast/chest wall boost | 10.0 (2.5 – 20.0) | 5 (3–10) |
| Supraclavicular (SC) | 50.0 (42.0–50.4) | 25 (22–28) |
| Posterior axillary boost (PAB) | 46.2 (35.0 – 48.6) | 28 (22–28) |
Statistical Analysis
Univariate and multivariate analyses were used to identify independent predictors of lymphedema by using Cox proportional hazards methodology. Factors evaluated in these analyses included patient-related factors (age at diagnosis, body mass index (BMI), and presence of post-operative swelling, defined as a ≥10% arm volume increase occurring fewer than or equal to three months from surgery), tumor-related factors (tumor size and number of pathologically involved lymph nodes), and treatment-related factors (type of breast surgery, management of the axilla, number of lymph nodes removed, use of systemic therapy, timing of systemic therapy, hormonal therapy, and radiation field(s)). Factors included were based on review of prior literature regarding lymphedema-associated risk factors. Age at diagnosis, BMI, tumor size, the number of lymph nodes dissected and the number of pathologically involved nodes were analyzed as continuous variables such that the hazard ratios computed for these variables reflect the change in lymphedema risk associated with a one-unit increase in the variable. Time-dependent covariates were included for use of systemic therapies and radiation fields such that cases were included in the unexposed group prior to initiation of a given treatment and in the exposed group thereafter. All the aforementioned factors were included in the initial univariate model. Only variables that were significant in the univariate analysis were included in the multivariate analysis. Tests of interaction were performed between the radiation groups and number of nodes removed, number of positive nodes, and breast surgery, and there were no significant interactions. The Kaplan-Meier method was used to estimate and plot cumulative incidence of lymphedema by radiation field. Patients were censored at the time of last follow-up measurement.
RESULTS
Of the 1476 patients, 1262 (86%) underwent unilateral breast surgery and 214 (14%) underwent bilateral breast surgery for unilateral (n=189) or bilateral breast cancer (n=25). 1099/1501 (73%) individual breast surgeries received post-operative radiotherapy. Clinical and pathologic characteristics are given in Table 2. Median postoperative follow-up was 25.4 months (range: 3.0 – 82.6 months), with a median of 4 post-operative measurements per patient (range: 1–25).
Table 2.
Clinical and pathologic characteristics of the study cohort.
| Median (Range) | n = 1501 (100%) | |
|---|---|---|
| Clinical Characteristics | ||
| Age at diagnosis, years | 54 (23–89) | - |
| BMI at diagnosis, kg/m2 | 26.1 (16.5–59.0) | - |
| Pathologic Characteristics | ||
| Invasive tumor size*, cm | 1.5 (0.01–10.5) | - |
| Breast Surgery | ||
| Lumpectomy | - | 927 (62%) |
| Mastectomy | - | 574 (38%) |
| Axillary Surgery | ||
| None | - | 178 (12%) |
| SLNB | - | 928 (62%) |
| ALND | - | 395 (26%) |
| #LN’s removed, SLNB | 2 (1–13) | - |
| #LN’s removed, ALND | 16 (3–43) | - |
| # Positive LN’s, ALND | 2 (0–39) | - |
| Radiation Therapy | ||
| None | - | 402 (27%) |
| Partial breast (PBI) | - | 92 (6%) |
| Whole breast/ chest wall only (B/CW) | - | 698 (47%) |
| B/CW + SC | - | 194 (13%) |
| B/CW + SC +PAB | - | 115 (8%) |
| Neoadjuvant Chemotherapy | ||
| Yes | - | 151 (10%) |
| No | - | 1350 (90%) |
| Adjuvant Chemotherapy | ||
| Yes | - | 571 (38%) |
| No | - | 930 (62%) |
| Hormonal Therapy | ||
| Yes | - | 1110 (74%) |
| No | - | 391 (26%) |
Abbreviations: BMI = Body Mass Index, ALND = Axillary lymph node dissection, SLNB = Sentinel lymph node biopsy, LN = lymph node, SC= Supraclavicular, PAB = Posterior Axillary Boost
Excludes tumor size for patients who underwent neoadjuvant chemotherapy
Lymphedema Incidence
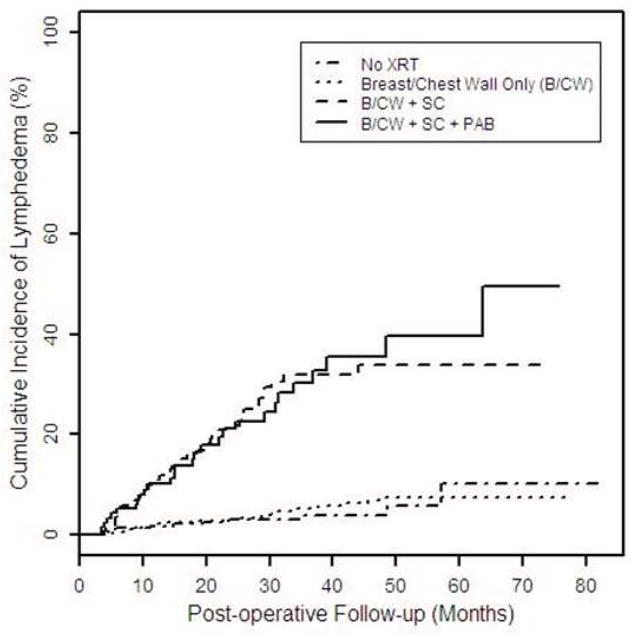
The overall cumulative incidence of lymphedema increased from 6.8% (95% CI: 5.5–8.4 %) at 24 months to 13.7% (95% CI: 11.1–16.9%) at 60 months post-operative (Figure 1). The median time to the development of lymphedema was 24.1 months postoperative (range: 3.0–82.6). The 2-year cumulative incidence of lymphedema was higher with RLNR as SC (21.9%, 95% CI: 16.0–29.5%) or SC +PAB (21.1%, 95% CI: 13.8–31.5%) compared with no radiation (3.0%, 95% CI: 1.6–5.6%) or PBI/WBI/chest wall radiation alone (3.1%, 95% CI: 2.0–4.7%).
Figure 1.
Cumulative incidence of lymphedema stratified by type of radiotherapy.
The 2-year cumulative incidence of lymphedema by radiation field and axillary surgery is listed in Table 3. For SLNB, the 2-year cumulative incidence was 6.1% (95% CI: 1.5–23.4%) with RLNR (n=46) and 2.8% (95% CI: 1.6–4.8%) with PBI/WBI/chest wall alone (n=569). For ALND, the incidence was 24.3% (95% CI: 18.9–30.9%) with RLNR (n=262) and 7.3% (95% CI: 3.1–16.8%) with WBI/chest wall alone (n=73).
Table 3.
Cumulative incidence of lymphedema at 24 months post-operative stratified by type of radiotherapy and axillary surgery.
| Cumulative Incidence of Lymphedema (%, 95% Confidence Interval) (Number in subgroup) | |||
|---|---|---|---|
| Radiation Field(s) | Entire cohort (n=1501) | SLNB (n=928) | ALND (n=395) |
| No radiation | 3.0 (1.6–5.6) (n=402) | 0.00 (0.00–0.00) (n=313) | 18.27 (9.7–32.9) (n=60) |
| B/CW only | 3.1 (2.0–4.7) (n=790) | 2.82 (1.7–4.8) (n=569) | 7.33 (3.1–16.8) (n=73) |
| B/CW + SC | 21.9 (16.0–29.5) (n=194) | 7.14 (1.0–40.9) (n=25) | 23.93 (17.5–32.2) (n=169) |
| B/CW + SC + PAB | 21.1 (13.8–31.5) (n=115) | 4.76 (0.7–29.3) (n=21) | 24.89 (16.2–37.1) (n=94) |
Abbreviations: SLNB = Sentinel Lymph Node Biopsy, ALND = Axillary Lymph Node Dissection, B/CW= PBI or Whole breast/chest wall, SC= Supraclavicular, PAB= Posterior Axillary Boost
Univariate and Multivariate Analysis
Univariate results are included in Table 4. In a multivariate model using no radiation as the reference group, PBI (p = 0.67) and WBI/chest wall radiation alone (p=0.92) did not significantly increase lymphedema risk. Given these results, a multivariate model was constructed with PBI and WBI/chest wall radiation groups combined as the reference group based on perceived clinical relevance. Additionally, as the type of breast surgery was not significant in the multivariate model (p = 0.99) and there was not a significant interaction between type of breast surgery and radiation, all patients, regardless of surgical type, were included in the final multivariate model. The results of the multivariate analysis are seen in Table 5. The addition of RLNR (SC or SC + PAB) when compared to breast/chest wall radiation alone significantly increased lymphedema risk with a hazard ratio (HR) of 1.70 (95% CI: 1.07 – 2.70, p = 0.025).
Table 4.
Univariate results for association of factors with risk of lymphedema.
| Variable | Hazard Ratio | 95% Confidence Interval | p value |
|---|---|---|---|
| Clinical Characteristics | |||
| Age at diagnosis* | 1.01 | 0.99–1.02 | 0.38 |
| BMI* | 1.06 | 1.04–1.09 | <0.0001 |
| Pathologic Characteristics | |||
| Invasive Tumor Size* | 1.32 | 1.22–1.44 | <0.0001 |
| Breast Surgery | |||
| Mastectomy vs. Lumpectomy | 1.87 | 1.31–2.67 | 0.0006 |
| Axillary Surgery | |||
| SLNB vs. no axillary surgery | 0.74 | 0.31–1.81 | 0.51 |
| ALND vs. SLNB | 9.17 | 5.88–14.29 | <0.0001 |
| # LNs removed* | 1.10 | 1.08–1.11 | <0.0001 |
| # Positive LNs* | 1.09 | 1.05–1.13 | <0.0001 |
| Radiation Therapy** | |||
| Partial breast only | 0.38 | 0.09–1.56 | 0.18 |
| Whole breast/chest wall only | 0.85 | 0.51–1.42 | 0.53 |
| Whole breast/chest wall +SC | 5.05 | 3.11–8.19 | <0.0001 |
| Whole breast/chest wall +SC +PAB | 4.98 | 2.92–8.47 | <0.0001 |
| Systemic Therapy | |||
| Neoadjuvant Chemotherapy | 2.61 | 1.68–4.07 | <0.0001 |
| Adjuvant Chemotherapy | 2.35 | 1.62–3.39 | <0.0001 |
| Hormonal Therapy | 1.68 | 1.13–2.51 | 0.01 |
| Early Post-operative Swelling | |||
| ≥10%, ≤3 months post-operative | 12.6 | 6.06–26.1 | <0.0001 |
Abbreviations: BMI= Body Mass Index, SLNB = Sentinel lymph node biopsy, ALND = Axillary lymph node dissection, LN = Lymph node, SC = supraclavicular, PAB = posterior axillary boost
Analyzed as continuous variables; HR reflects the change in lymphedema risk associated with a one-unit increase
Reference group: no radiotherapy
Table 5.
Multivariate results for factors associated with risk of lymphedema.
| Variable | Hazard ratio | 95% Confidence interval | p value |
|---|---|---|---|
| No radiation* | 0.99 | 0.59–1.67 | 0.9825 |
| RLNR* | 1.70 | 1.07–2.70 | 0.0253 |
| B/CW + SC | 1.68 | 1.00 – 2.83 | 0.0492 |
| B/CW + SC + PAB | 1.72 | 1.00 – 2.96 | 0.0484 |
| ALND | 3.52 | 1.84–6.73 | 0.0001 |
| #LN’s removed** | 1.04 | 1.01–1.07 | 0.0184 |
| BMI** | 1.06 | 1.03–1.09 | <.0001 |
| ≥10% swelling, ≤3 mo post-op | 10.3 | 4.52–23.3 | <.0001 |
Abbreviations: RLNR = Regional lymph node radiation, ALND = Axillary lymph node dissection, LN = Lymph node, BMI = Body Mass Index
Radiation reference group: Partial breast (PBI) and whole breast/chest wall only
Analyzed as continuous variables; HR reflects the change in lymphedema risk associated with a one-unit increase
Other factors significant for increased lymphedema risk on multivariate analysis included early post-operative swelling (HR=10.3, p<0.0001), higher pre-operative BMI (HR=1.06, p<0.0001), number of axillary LN’s dissected (HR=1.04, p=0.02), and undergoing ALND (HR=3.5, p=0.0001).
DISCUSSION
The current study utilizes a cohort of 1476 breast cancer patients prospectively screened for lymphedema at a single institution. Patients in our study were treated for breast cancer between 2005 and 2012, and thereby represent a population treated with modern radiotherapy and surgical techniques over a relatively short time period. Risk factors for lymphedema including adjuvant radiotherapy have been previously evaluated; however, most studies utilized retrospectively collected data and/or variable and largely subjective methods to measure and define lymphedema (12, 18–20).
The cumulative incidence of lymphedema in our series was 6.8% at 24 months and 13.7% at 60 months post-operative. This is lower than has been reported in previous studies, likely due to our inclusion of a large cohort of women who did not undergo axillary surgery (n=178). When stratified by radiotherapy field, the 2-year cumulative incidence was 3.0%, 3.1%, 21.9%, and 21.1% for no radiation, breast/chest wall radiation alone, B/CW + SC, and B/CW + SC + PAB, respectively. To evaluate the risk of lymphedema with inclusion of RLNR, we utilized a reference group comprised of patients who received PBI or WBI/chest wall radiation alone since RLNR is unlikely to be used without also irradiating the breast or chest wall. In our series, the inclusion of a SC field, with or without the inclusion of a PAB, significantly increased the risk of lymphedema compared to breast/chest wall radiation alone. The addition of a PAB to a SC field did not increase the risk of lymphedema when compared to a SC field alone.
When further stratified by type of axillary surgery, for patients who underwent ALND, the 2-year cumulative incidence was higher with RLNR (as SC or SC+PAB) compared to breast/chest wall radiation alone, 24.3% versus 7.3%, respectively. A similar trend was observed for patients who underwent SLNB (2.8% without RLNR versus 6.1% with RLNR); however, confidence intervals were overlapping due to a small number of patients who underwent SLNB with RLNR (n=46).
These findings are consistent with several prior studies. In a retrospective study of 727 patients treated from 1982–1995, Coen et al used a post-operative arm circumference measurement (without pre-operative assessment) to evaluate predictors of lymphedema in BCT patients (19). The authors found that addition of RLNR significantly increased the risk of lymphedema from 1.8% to 8.9% with inclusion of RLNR (SC with or without PAB). Another study by Hayes et al evaluated a cohort of 2,579 breast cancer patients treated between 1970–2005, classifying lymphedema based on physician examination, without pre-operative assessment (18). Consistent with our findings, the authors concluded that inclusion of RLNR significantly increased the risk of lymphedema (p = 0.0001), with no significant difference between SC and SC+PAB (p=0.1).
In contrast, Shah et al utilized 1,497 patients treated from 1980–2006 with BCT, and did not find that RLNR was a significant risk factor for lymphedema (20). Increased rates with SC (9.9% versus 7.0%, p=0.29) and PAB (14.7% versus 7.3%, p=0.40) were found compared with breast radiation alone. The study did not utilize a pre-operative assessment and defined lymphedema based on physical examination with or without circumference measurements. Graham et al evaluated the impact of SC radiotherapy volumes on lymphedema in a cross-sectional study of 91 breast cancer patients who had post-operative (but no pre-operative) arm circumference measurements (21). They found that SC volumes limited laterally by the coracoid process did not increase lymphedema risk expected from ALND, whereas expanding the field or including a PAB did.
Our cohort uniquely included 402 patients who did not receive radiation, primarily mastectomy patients with limited nodal involvement and older women undergoing lumpectomy. Utilizing this cohort as a reference group, PBI and WBI/chest wall radiation alone did not significantly increase lymphedema risk. Non-receipt of radiotherapy is rarely a comparison group in other studies; however, our findings are consistent with those from Liljegren et al which demonstrated that postoperative breast radiotherapy alone did not contribute to lymphedema (22). It has been suggested that use of PBI may reduce rates of lymphedema secondary to lower doses to the axilla (23). Our series demonstrated low rates for patients who underwent BCT with PBI or WBI, whether following SLNB or no axillary surgery. The PBI cohort in our study was comprised of patients who underwent lumpectomy for low-risk, node-negative disease; therefore further research is warranted to determine whether PBI reduces lymphedema risk compared with WBI for patients with higher-risk or node-positive disease as in the NSABP B-39/RTOG 0413 trial [Clinicaltrials.gov ID #: NCT00103181].
Other independent risk factors for lymphedema in our series included higher pre-operative BMI, ALND, and early post-operative swelling. A previous study by Mahamaneerat et al evaluated post-operative arm swelling as a risk factor for lymphedema, and found that a ≥5% increase in arm volume ≤1 month after surgery was associated with a 1.4-fold increased risk of lymphedema (24). Interestingly, in this study, early post-operative swelling conferred the highest risk of lymphedema for all variables analyzed, with a hazard ratio of 10.3. Higher BMI and ALND have also been consistently reported as risk factors in previous studies (8, 12, 25, 26). We found that even after controlling for type of axillary surgery in a multivariate model, lymphedema risk remained significantly increased with a greater number of LN’s removed, which has also been demonstrated in prior studies (18, 25). Although these data are unlikely to change the SLNB or ALND procedure, it may allow for identification of women for whom closer surveillance is appropriate.
Findings from several recent studies may impact practices regarding treatment of the axilla. The ACOSOG Z0011 trial demonstrated no difference in the rates of regional recurrence, DFS, or OS in 891 women with limited nodal involvement on SLNB randomized to completion ALND or no further axillary treatment (27, 28). Although it was recommended that patients not receive a third field, they may have had deep or partially deep tangents which can cover a significant part of the axilla (29). Rates on lymphedema in this trial have not yet been reported. Preliminary findings presented by Rutgers et al on the AMAROS Phase III trial comparing axillary radiation to ALND after a positive SLNB demonstrated no differences in OS or DFS (30). Lymphedema rates were higher for ALND (28%) without RLNR versus SLNB with RLNR (14%) at 5 years (p<0.0001). Whelan et al reported on the MA.20 intergroup trial, which randomized high-risk women (defined by positive LN or negative LN with high-risk features), who underwent BCT to receive WBI with or without RLNR (31). Inclusion of RLNR improved locoregional DFS, distant DFS, and trended towards a statistically significant improvement in OS. The rate of lymphedema was 4.1% and 7.3% for WBI and WBI + RLNR, respectively (p=0.004). We similarly observed an increased lymphedema incidence for patients who underwent ALND and RLNR (24.3%) compared to ALND and B/CW radiation alone (7.3%) and/or SLNB and RLNR (6.1%), although these rates include mastectomy patients unlike the MA.20 trial and the number of patients in each subgroup are small. A better understanding of the morbidities secondary to combinations of surgical and radiotherapy treatments of the axilla is increasingly important due to the likely change in practice patterns resulting from the publication of these trials, including more frequent use of RLNR.
Our study has potential limitations. There is potential bias associated with non-random assignment of radiation fields. Additionally, based on practice patterns, the number of patients who underwent SLNB and received RLNR was small, making subgroup analysis challenging and warranting further investigation. Lastly, the size of the supraclavicular field, and in particular, the lateral border, was not examined in the current study. This may have an impact on lymphedema risk and will be the focus of further research.
Our study has a number of strengths. Patients underwent pre-operative and regular post-operative measurements utilizing a Perometer. The validity and accuracy of the Perometer in quantifying arm volume has been rigorously demonstrated (32). The importance of obtaining pre-operative assessments to account for asymmetry between arms, and incorporating change in contralateral arm size to account for factors unrelated to lymphedema has also been demonstrated (11, 14, 33, 34). The RVC and WAC equations used in this study account for both of these factors. Previous often-cited studies evaluating radiotherapy-related risk factors for lymphedema have utilized patients who underwent breast cancer treatment over several decades, and lack prospectively-collected and accurate assessments with incorporation of a pre-operative assessment (18–20). In contrast, the current study utilizes patients treated over a 7-year period and reflects modern surgical and radiotherapy techniques, including an increasing utilization of SLNB as opposed to ALND, and inclusion of patients who received PBI and those with positive SLNB who received RLNR instead of ALND.
In conclusion, the addition of RLNR, either with SC or SC + PAB, significantly increased the risk of lymphedema when compared to breast or chest wall radiation alone. Other independent risk factors included higher pre-operative BMI, undergoing ALND, number of LN dissected, and early post-operative swelling. When considering inclusion of RLNR, clinicians should weigh the potential benefit of RLNR for control of disease with the increased risk of lymphedema, particularly in women with other potential risk factors for lymphedema. Women who do receive RLNR should be prospectively monitored for lymphedema to ensure early detection and possible intervention. Future studies should further evaluate the risk of lymphedema following SLNB with RLNR compared with completion ALND in the setting of BCT or mastectomy.
Summary.
This prospective study evaluated the risk of lymphedema in women diagnosed and treated for breast cancer. It demonstrated that regional lymph node irradiation, either with inclusion of a supraclavicular field or posterior axillary boost, increased the risk of lymphedema. These conclusions were based on a large cohort of patients treated with modern surgical and radiotherapy techniques and Perometer arm volume measurements. Closely following women at risk of lymphedema will allow for earlier detection and intervention.
Acknowledgments
This project was supported by Award R01CA139118 (AGT), and Award P50CA089393 (AGT) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institutes of Health. In addition, we would like to acknowledge Nadine R. Taghian, whose continuous dedication helped expedite completion of this project. The authors are greatly appreciative of her efforts.
Footnotes
Meeting Presentation: These data were presented at ASTRO’s 55th Annual Meeting in Atlanta, Georgia, on September 24, 2013 during a Scientific Session.
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed RL, Schmitz KH, Prizment AE, et al. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat. 2011;130:981–91. doi: 10.1007/s10549-011-1667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jäger G, Döller W, Roth R. Quality-of-life and body image impairments in patients with lymphedema. Lymphology. 2006;39:193–200. [PubMed] [Google Scholar]
- 3.Tobin MB, Lacey HJ, Meyer L, et al. The psychological morbidity of breast cancer-related arm swelling. Psychological morbidity of lymphoedema. Cancer. 1993;72:3248–52. doi: 10.1002/1097-0142(19931201)72:11<3248::aid-cncr2820721119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Khan F, Amatya B, Pallant JF, et al. Factors associated with long-term functional outcomes and psychological sequelae in women after breast cancer. Breast. 2012;21:314–20. doi: 10.1016/j.breast.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Ceilley E, Jagsi R, Goldberg S, et al. Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys. 2005;61:365–73. doi: 10.1016/j.ijrobp.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 6.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–55. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 7.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(2):116–26. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26:5213–9. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245:452–61. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ancukiewicz M, Russell TA, Otoole J, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:1436–43. doi: 10.1016/j.ijrobp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959–72. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 13.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–17. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- 14.Miller CL, Specht MC, Horick N, et al. A novel, validated method to quantify breast-cancer related lymphedema (BCRL) following bilateral breast surgery. Lymphology. (forthcoming) [PubMed] [Google Scholar]
- 15.Li XA, Tai A, Arthur DW, et al. Variability of target and normal structure delineation for breast cancer radiotherapy: an RTOG multi-institutional and multiobserver study. Int J Radiat Oncol Biol Phys. 2009;73(3):944–51. doi: 10.1016/j.ijrobp.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pashtan IM, Recht A, Ancukiewicz M, et al. External beam accelerated partial-breast irradiation using 32 gy in 8 twice-daily fractions: 5-year results of a prospective study. Int J Radiat Oncol Biol Phys. 2012;84:e271–7. doi: 10.1016/j.ijrobp.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taghian AG, Kozak KR, Katz A, et al. Accelerated partial breast irradiation using proton beams: Initial dosimetric experience. Int J Radiat Oncol Biol Phys. 2006;65:1404–10. doi: 10.1016/j.ijrobp.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Hayes SB, Freedman GM, Li T, et al. Does axillary boost increase lymphedema compared with supraclavicular radiation alone after breast conservation? Int J Radiat Oncol Biol Phys. 2008;72:1449–55. doi: 10.1016/j.ijrobp.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 19.Coen JJ, Taghian AG, Kachnic LA, et al. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. Int J Radiat Oncol Biol Phys. 2003;55:1209–15. doi: 10.1016/s0360-3016(02)04273-6. [DOI] [PubMed] [Google Scholar]
- 20.Shah C, Wilkinson JB, Baschnagel A, et al. Factors associated with the development of breast cancer-related lymphedema after whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2012;83:1095–100. doi: 10.1016/j.ijrobp.2011.09.058. [DOI] [PubMed] [Google Scholar]
- 21.Graham P, Jagavkar R, Browne L, et al. Supraclavicular radiotherapy must be limited laterally by the coracoid to avoid significant adjuvant breast nodal radiotherapy lymphoedema risk. Australas Radiol. 2006;50:578–82. doi: 10.1111/j.1440-1673.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 22.Liljegren G, Holmberg L. Arm morbidity after sector resection and axillary dissection with or without postoperative radiotherapy in breast cancer stage I. Results from a randomised trial. Uppsala-Orebro Breast Cancer Study Group. Eur J Cancer. 1997;33:193–9. doi: 10.1016/s0959-8049(96)00375-9. [DOI] [PubMed] [Google Scholar]
- 23.Shah C, Badiyan S, Khwaja S, et al. Breast Cancer Related Lymphedema: A Review of Recent Developments. Androl Gynecol Curr Res. 2013;1:2. [Google Scholar]
- 24.Mahamaneerat WK, Shyu C-R, Stewart BR, et al. Breast cancer treatment, BMI, post-op swelling/lymphoedema. J Lymphoedema. 2008;3:38–44. [PMC free article] [PubMed] [Google Scholar]
- 25.Disipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 26.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–63. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 27.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–32. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haffty BG, Hunt KK, Harris JR, et al. Positive sentinel nodes without axillary dissection: implications for the radiation oncologist. J Clin Oncol. 2011;29:4479–81. doi: 10.1200/JCO.2011.36.1667. [DOI] [PubMed] [Google Scholar]
- 30.Rutgers EJ, Donker M, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer patients: Final analysis of the EORTC AMAROS trial (10981/22023). J Clin Oncol; Proceedings of the ASCO Annual Meeting; 2013 May 31–June 4; Chicago, IL. 2013. (suppl; abstr LBA1001) [Google Scholar]
- 31.Whelan T, Olivotto I, Chapman J. NCIC-CTG MA.20: An intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol; Proceedings of the ASCO Annual Meeting; 2011 June 3–June 7; Chicago, IL. 2011. (suppl; abstr LBA 1003) [Google Scholar]
- 32.Tierney S, Aslam M, Rennie K, et al. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12:412–7. doi: 10.1016/s1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 33.Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–19. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 34.Ancukiewicz M, Miller CL, Skolny MN, et al. Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: the flaws in current studies and need for universal methodology. Breast Cancer Res Treat. 2012;135:145–52. doi: 10.1007/s10549-012-2111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]