High-density lipoprotein-cholesterol (HDL-C) levels inversely correlate with atherosclerotic cardiovascular disease (CVD).1 HDL plays a key role in reverse cholesterol transport by promoting cholesterol efflux from peripheral cells, including cholesterol-laden macrophages, and delivering acquired cholesterol to liver for excretion, a process that is believed to be atheroprotective.2 However, whether low HDL-C is merely a bystander or is causal in CVD remains controversial.3 Genetic factors that influence HDL-C levels are not consistently associated with altered CVD risk, and failure of the cholesterol ester transfer protein inhibitor torcetrapib and, more recently, niacin to reduce cardiovascular events, despite their HDL-raising effects, has raised doubts about the therapeutic potential of raising HDL.4,5 We recently demonstrated that measurement of HDL efflux capacity is a stronger predictor of CVD than plasma HDL-C levels,6 strengthening the argument that measures of HDL functionality may be more useful than HDL-C levels as predictors of risk and better targets of novel therapies. In any case, regulation of HDL metabolism and function and their relationship to atherosclerosis remains incompletely understood.
The Essential Role of the ATP-Binding Cassette Transporter 1 (ABCA1) in HDL Metabolism
The essential role of ABCA1 in HDL metabolism was first identified when genetic mutations of ABCA1 were found to underlie Tangier disease,7 which is characterized by markedly reduced plasma levels of HDL-C and its major protein apolipoprotein A-I (apoA-I) because of profound hypercatabolism. ABCA1 promotes cellular cholesterol efflux to lipid-free apoA-I, generating nascent HDL particles.8 The accumulation of cholesterol in macrophages and cells of the reticuloendothelial system in Tangier disease is due to the lack of ABCA1 in the macrophage. However, macrophage-cholesterol stores are minor, and, therefore, macrophages trivially contribute to circulating HDL-C.9 In elegant studies using conditional ABCA1 knockout mice, it was shown that the loss of hepatic ABCA1 resulted in ≈70% to 80% reduction in plasma HDL-C,10 and the loss of intestinal ABCA1 resulted in ≈15% to 20% reduction in plasma HDL-C.11 Thus, the concept was established that the 2 tissues that synthesize and secrete apoA-I—the liver and intestine—require ABCA1 at the site to immediately lipidate newly secreted apoA-I and protect it from rapid catabolism. Given the additive contribution of hepatic and intestinal ABCA1 to the HDL-C pool, a quantitatively important role for other organs in modulating HDL-C seemed unlikely. However, conditional deletion of ABCA1 in the brain12 surprisingly reduced HDL-C. In the current issue of Circulation, Chung et al13 now report that specific deletion of adipose ABCA1 significantly reduces HDL-C. Thus, the model of peripheral lipidation of HDL must again be revised.
Adipocytes Contribute to HDL-C Levels Through ABCA1-Mediated Cholesterol Efflux
Much evidence has pointed toward a potential contribution of adipose in mediating HDL lipidation. Adipose tissue is the major store for cholesterol within the body, and therefore represents a large pool of substrate to support HDL biogenesis. Adipose tissue expresses high levels of key cholesterol transporters ABCA1 and scavenger receptor class B type I (SR-BI; but not ABCG1),14 providing a gateway for cholesterol to efflux onto HDL. Expression of both ABCA1 and SR-BI increases during adipogenesis and adipocytes support HDL lipidation in vitro.14 Furthermore, adipocytes promote HDL lipidation in vivo, and the absence of either adipocyte ABCA1 or SR-BI impairs this process.14 ABCA1–/– adipocytes exhibited impaired efflux to apoA-I in vitro, but unaltered efflux to HDL, whereas SR-BI–/– adipocytes effluxed normally to apoA-I but failed to efflux to HDL. ABCG1–/– adipocytes exhibited normal efflux to both HDL and apo-A1, and protein levels were negligible in wild-type adipocytes.14 These findings highlighted the importance of ABCA1 and SR-BI pathways at facilitating adipocyte cholesterol efflux to HDL; however, the study was inherently limited in its capacity to quantify the contribution of adipose to the plasma HDL-C pool.
The timely research article by Chung et al has evaluated whether adipose ABCA1 regulates circulating HDL-C in vivo. To address this hypothesis, mice with specific deletion of adipose ABCA1 (ABCA1–A/–A) were generated, and significant reductions in plasma HDL-C (–15%) and apoA-I (–13%) levels were observed. Little difference in plasma decay of 125I-HDL tracer was evident between genotypes, indicating that the reduction of HDL-C was attributable to impaired HDL biogenesis and not to increased clearance. Furthermore, adipose explants were capable of mediating HDL biogenesis in vitro, and this process was impaired with ABCA1 deficiency. Interestingly, depot-specific differences in adipose ABCA1 expression was observed, with higher expression in metabolically active epididymal and mesenteric depots compared with the less metabolically active subcutaneous depot, suggesting that adipose depots differentially contribute to HDL biogenesis.
Could Adipocyte Dysfunction Reduce Cholesterol Efflux and Help Explain Low HDL-C Levels in Obesity and Metabolic Syndrome?
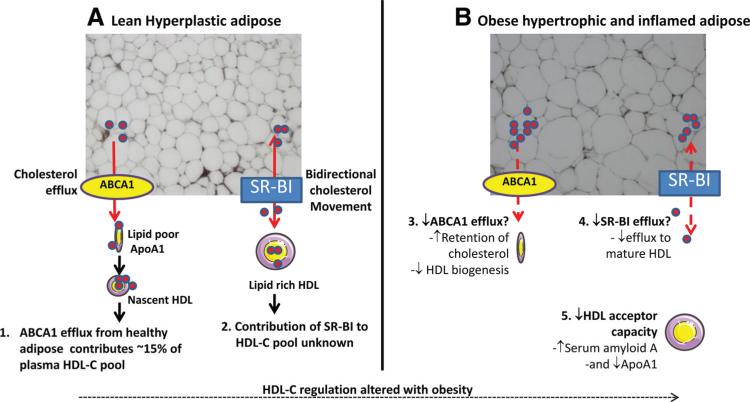
HDL-C levels are often reduced in the obese setting. If the findings by Chung el al in mice translate in humans, and adipose tissue contributes to HDL biogenesis, why is HDL-C not elevated with obesity because of enhanced substrate availability? A similar paradigm is seen for adipose-derived adiponectin, wherein increased adipose mass is associated with reduced adiponectin levels. Indeed, adiponectin, which is one of primary indicators of adipose health, strongly positively correlates with HDL-C.15 Adipose health may therefore be a better indicator of HDL-C, rather than the obese phenotype per se, which may explain why certain metabolically healthy obese cohorts maintain plasma HDL-C. Chronic inflammation is another hallmark of unhealthy adipose,16 and is probably a major mechanism responsible for lowering HDL-C during obesity (Figure).
Figure.
Adipose tissue as a critical regulator of HDL lipidation and potential mechanisms of obesity associated dyslipidemia. A1, Chung et al have demonstrated an essential role for adipose ABCA1 in HDL biogenesis, contributing to approximately 15% of the plasma HDL-C pool. A2, The contribution of adipose SR-BI to HDL lipidation is unknown, but may be negligible given its potential to support bidirectional HDL-C flux. Obesity is associated with lower HDL-C, which may be attributable to a combination of mechanisms. B3, Impaired ABCA1-mediated efflux may account for increased retention of cholesterol within adipose and reduced HDL biogenesis. B4, With obesity, cholesterol levels increase within adipose, which may drive the cholesterol efflux function of SR-BI in a concentration gradient-dependent manner. If SR-BI function is reduced because of adipose inflammation, this may result in impaired efflux to mature HDL particles. B5, Chronic inflammation associated with obesity also alters HDL acceptor function. The combination of these effects likely contributes to HDL-C lowering with obesity. ABCA1 indicates ATP-binding cassette transporter 1; HDL, high-density lipoprotein; HDL-C, high-density lipoprotein-cholesterol; SR-BI, scavenger receptor class B type I.
The primary function of adipose is to store fatty acids in the form of triglycerides. Excessive energy load, in combination with inflammation-impaired de novo adipogenesis, results in the hypertrophy of existing adipocytes that eventually fail to store fatty acids, resulting in their leakage into circulation and infiltration into other organs. Within the liver, fatty acids can be repackaged into triglycerides (TG) and resecreted on very low density lipoprotein particles. One mechanism for HDL lowering with obesity is currently attributed to enhanced very low density lipoprotein production with cholesterol ester transfer protein-mediated exchange of TG for cholesteryl ester on HDL particles. Cholesterol ester transfer protein activity is elevated with obesity,17 making this mechanism quite plausible.
Given the chronic inflammatory phenotype associated with obesity, it is possible that inflammation may alter HDL efflux function. Indeed, we demonstrated the reduced capacity of inflamed HDL particles to promote cholesterol efflux from macrophages after endotoxin-evoked inflammation in both mice and humans coincident with enrichment of HDL with serum amyloid A and displacement of apoA-I.18 Thus, inflammatory-driven changes in HDL composition may represent another mechanism of HDL-C lowering with obesity.
Although the 2 indirect mechanisms outlined above likely contribute to the relationship between obesity and low HDL-C, it remains possible that obesity directly influences the ability of adipocytes to efflux cholesterol to HDL. The direct effects of inflammation on the cholesterol efflux capacity of adipocytes in vitro have been inconclusive and highly dependent on cytokine concentration and cell differentiation status.14 High-cholesterol feeding of rabbits, however, resulted in reduced adipocyte efflux to HDL coincident with reduced SR-BI expression.19 These findings suggest that inflammation and high-fat feeding modulate the efflux capacity of adipose tissue; however, studies in obese mice and humans are required to corroborate this theory.
Could Reduced Cholesterol Efflux From Adipocytes Impair Adipose Function?
The ability of adipose tissue to expand is an important determinant of metabolic health. peroxisome proliferator-activated receptor γ agonists stimulate the formation of new adipocytes from progenitor cells (hyperplasia), and thus enhance the storage capacity of adipose and promote insulin sensitivity. We recently demonstrated that peroxisome proliferator-activated receptor γ agonist treatment increased the partitioning of HDL-cholesterol away from liver/feces into adipose, coincident with enhanced adipocyte SR-BI expression.20 Peroxisome proliferator-activated receptor γ agonists likely increase adipose TG and cholesterol levels by promoting their storage within newly generated adipocytes. Retention of cholesterol within adipose was also evident in ABCA–A/–A mice. Presumably, lack of ABCA1 entrapped cholesterol within hypertrophic adipocytes, as opposed to peroxisome proliferator-activated receptor γ agonists, which promote SR-BI–mediated cholesterol influx into differentiating adipocytes. Although the net result on systemic cholesterol is similar, the consequences on adipose health and insulin sensitivity probably differ substantially. It will be important to establish the consequences of enhanced lipid partitioning within ABCA–A/–A adipose on metabolic health (adipocyte size, TG storage, systemic insulin resistance, systemic inflammation, adipokine profiling, and systemic low-density lipoprotein cholesterol, very low density lipoprotein, TG, and free fatty acid levels). Furthermore, the contribution of adipose SR-BI, a bidirectional cholesterol transporter, to HDL lipidation and adipose functionality remains to be investigated. Our findings suggest that adipocyte SR-BI might sustain lipidation of larger HDL particles within circulation,14 although SR-BI also mediates uptake of cholesterol from HDL into adipocytes.20
Adipose ABCA1: A Therapeutic Target to Raise HDL-C?
The findings by Chung et al suggest that raising adipose ABCA1 would enhance nascent HDL particle formation and raise HDL-C levels. Whether promoting adipose cholesterol efflux transporter pathways such as ABCA1 actually raises HDL-C levels and, if so, whether this has positive effects on reducing atherosclerosis, remain to be elucidated. Arguably, in individuals who are obese, who generally have excessive cholesterol ester transfer protein activity, promotion of ABCA1-mediated cholesterol efflux from adipocytes to HDL may result in increased transfer of adipocyte-derived cholesterol from HDL to apoB-containing lipoproteins, negating any positive benefits of elevated HDL on reverse cholesterol transport.
ABCA1 has long been recognized as a potential therapeutic target for the treatment of atherosclerotic CVD. However, targeting ABCA1 for therapeutic purposes is complex. Although upregulation of ABCA1 in arterial macrophages would probably be beneficial with regard to atherosclerosis, the impact of hepatic ABCA1 upregulation is less clear. Although it would probably promote HDL biogenesis and increase HDL-C levels, it might also redirect cholesterol destined for bile back into the plasma compartment, where it could end up on atherogenic apoB lipoproteins. The emergence of adipose ABCA1 as a regulator of plasma HDL-C levels suggests a new therapeutic target that is not, as yet, mature. More comprehensive analysis of the functional consequences of ABCA1 deficiency on adipose biology, and atherosclerosis, as well, would be required to corroborate adipose ABCA1 as a potential target.
Conclusions
The abundance of free cholesterol within adipose stores has made it a prime candidate organ to support HDL lipidation, but in vivo evidence to support these speculations has to date been preliminary.14 Chung et al have demonstrated an indisputable role for adipose ABCA1 in contributing to plasma HDL-C in vivo. Lack of adipose ABCA1 impaired cholesterol efflux to apoA-I, reduced circulating HDL-C, and caused a significant backlog of cholesterol within adipose stores. Whether inhibition of adipose-specific ABCA1 efflux is pro- or antiatherogenic, however, is debatable and requires more rigorous investigation. These findings demonstrate a direct capacity of adipose to modulate HDL-C and justify more intensive investigation into the intricate mechanisms by which adipose tissue health affects HDL metabolism and function and by which adipocyte cholesterol content affects adipocyte function. Such timely studies are warranted given the widespread exposure to the obesigenic environment and rising obesity rates worldwide, along with the strong association of obesity with both low levels of HDL-C and increased risk of atherosclerotic CVD. It also reinforces the notion that low HDL-C levels, like low adiponectin, may be a key barometer of adipose dysfunction, and thereby returns us to the question of whether low HDL-C is merely a bystander or is causal in the development of CVD.
Footnotes
Disclosures
Dr Rader has equity in Vascular Strategies. Drs McGillicuddy and Reilly report no disclosures.
References
- 1.Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1:16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 2.Rader DJ. Regulation of reverse cholesterol transport and clinical implications. Am J Cardiol. 2003;92:42J–49J. doi: 10.1016/s0002-9149(03)00615-5. [DOI] [PubMed] [Google Scholar]
- 3.Robinson JG. Low high-density lipoprotein cholesterol and chronic disease risk marker or causal? J Am Coll Cardiol. 2010;55:2855–2857. doi: 10.1016/j.jacc.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 4.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 5.NHLBI Communications Office NIH stops clinical trial on combination cholesterol treatment: lack of efficacy in reducing cardiovascular events prompts decision. [August 22, 2011];NIH News. 2011 May 26; http://www.nih.gov/news/health/may2011/nhlbi-26.htm.
- 6.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipo-protein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young SG, Fielding CJ. The ABCs of cholesterol efflux. Nat Genet. 1999;22:316–318. doi: 10.1038/11878. [DOI] [PubMed] [Google Scholar]
- 8.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 9.Haghpassand M, Bourassa PA, Francone OL, Aiello RJ. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels. J Clin Invest. 2001;108:1315–1320. doi: 10.1172/JCI12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singaraja RR, Van Eck M, Bissada N, Zimetti F, Collins HL, Hildebrand RB, Hayden A, Brunham LR, Kang MH, Fruchart JC, Van Berkel TJ, Parks JS, Staels B, Rothblat GH, Fievet C, Hayden MR. Both hepatic and extrahepatic ABCA1 have discrete and essential functions in the maintenance of plasma high-density lipoprotein cholesterol levels in vivo. Circulation. 2006;114:1301–1309. doi: 10.1161/CIRCULATIONAHA.106.621433. [DOI] [PubMed] [Google Scholar]
- 12.Karasinska JM, Rinninger F, Lutjohann D, Ruddle P, Franciosi S, Kruit JK, Singaraja RR, Hirsch-Reinshagen V, Fan J, Brunham LR, Bissada N, Ramakrishnan R, Wellington CL, Parks JS, Hayden MR. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J Neurosci. 2009;29:3579–3589. doi: 10.1523/JNEUROSCI.4741-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung S, Sawyer JK, Gebre AK, Maeda N, Parks JS. Adipose tissue ATP binding cassette transporter A1 contributes to high-density lipoprotein biogenesis in vivo. Circulation. 2011;124:1663–1672. doi: 10.1161/CIRCULATIONAHA.111.025445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, McGillicuddy FC, Hinkle CC, O'Neill S, Glick JM, Rothblat GH, Reilly MP. Adipocyte modulation of high-density lipoprotein cholesterol. Circulation. 2010;121:1347–1355. doi: 10.1161/CIRCULATIONAHA.109.897330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 16.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park KH, Shin DG, Kim JR, Hong JH, Cho KH. The functional and compositional properties of lipoproteins are altered in patients with metabolic syndrome with increased cholesteryl ester transfer protein activity. Int J Mol Med. 2010;25:129–136. [PubMed] [Google Scholar]
- 18.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao SP, Wu ZH, Hong SC, Ye HJ, Wu J. Effect of atorvastatin on SR-BI expression and HDL-induced cholesterol efflux in adipocytes of hyper-cholesterolemic rabbits. Clin Chim Acta. 2006;365:119–124. doi: 10.1016/j.cca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Toh SA, Millar JS, Billheimer J, Fuki I, Naik SU, Macphee C, Walker M, Rader DJ. PPARgamma activation redirects macrophage cholesterol from fecal excretion to adipose tissue uptake in mice via SR-BI. Biochem Pharmacol. 2011;81:934–941. doi: 10.1016/j.bcp.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]