Abstract
One of the oldest models of schizophrenia is based on the effects of serotonergic hallucinogens such as mescaline, psilocybin, and (+)-lysergic acid diethylamide (LSD), which act through the serotonin 5-HT2A receptor. These compounds produce a “model psychosis” in normal individuals that resembles at least some of the positive symptoms of schizophrenia. Based on these similarities, and because evidence has emerged that the serotonergic system plays a role in the pathogenesis of schizophrenia in some patients, animal models relevant to schizophrenia have been developed based on hallucinogen effects. Here we review the behavioral effects of hallucinogens in four of those models, the receptor and neurochemical mechanisms for the effects, and their translational relevance. Despite the difficulty of modeling hallucinogen effects in nonverbal species, animal models of schizophrenia based on hallucinogens have yielded important insights into the linkage between 5-HT and schizophrenia and have helped to identify receptor targets and interactions that could be exploited in the development of new therapeutic agents.
Keywords: LSD, habituation, prepulse inhibition, interval timing, head twitch
INTRODUCTION
Substantial evidence indicates that the serotonergic system is involved in the pathophysiology of schizophrenia, but determining the exact role that serotonin (5-HT) plays in the disorder has proven elusive. One of the oldest models of schizophrenia is based on the observation that serotonergic hallucinogens can provoke a “model psychosis” in normal humans (Geyer and Vollenweider, 2008). The German psychiatrist Kurt Beringer was the first to comment on the similarities between the effects of mescaline and the symptoms of schizophrenia (Beringer, 1923, 1927). Although it was unknown at the time, it is now recognized that mescaline, (+)-lysergic acid diethylamide (LSD) (Figure 1), and other serotonergic hallucinogens exert their characteristic effects by activating the 5-HT2A receptor (reviewed by: Nichols, 2004; Halberstadt and Geyer, 2011). Soon after the discovery of LSD by Albert Hofmann (Stoll and Hofmann, 1943), it was administered to volunteers by the psychiatrist Walter Stoll. Stoll confirmed that LSD produced mescaline-like effects but was much more potent, and found that the effects of LSD resemble the symptoms of schizophrenia (Stoll, 1947). Likewise, as had been proposed several decades earlier with mescaline (Knauer and Maloney, 1913), Stoll recommended that psychiatrists self-experiment with LSD in order to gain insight into the mental states and experiences of their patients.
Figure 1.
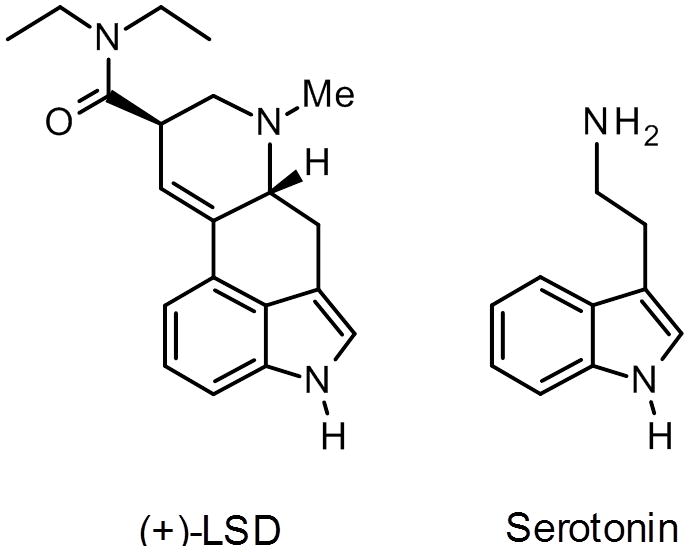
Chemical structures of (+)-lysergic acid diethylamide (LSD, left panel) and serotonin (right panel).
Many other groups subsequently characterized the effects of LSD, mescaline, and psilocybin and concluded that these hallucinogens produced mental states resembling the earliest phases of schizophrenia (Rinkel et al., 1952, 1955; Osmond and Smythies, 1952; Keeler, 1965; Bowers and Freedman, 1966). Other clinicians, however, noted that differences exist between the effects of hallucinogens and the symptoms of schizophrenia, leading them to question the validity of the model psychosis (Mayer-Gross, 1951). One of the most prominent critics was Hollister, who argued that auditory but not visual hallucinations are most prominent in schizophrenia, whereas the opposite is true of hallucinogens (Hollister, 1962). Nevertheless, there are often visual disturbances during the acute phase of schizophrenia, including hallucinations and synesthesias (McCabe et al., 1972; Freedman and Chapman, 1973). A second criticism made by Hollister is that hallucinogens rarely produce the social and emotional withdrawal but these symptoms are often found in schizophrenia patients. Subsequent investigations have shown that hallucinogens sometimes produce withdrawal and catatonia-like states, especially when administered at higher doses (Gouzoulis-Mayfrank et al., 1998a).
Since NMDA antagonists such as phencyclidine (PCP) and ketamine mimic most aspects of schizophrenia (Javitt and Zukin, 1991; Halberstadt, 1995; Javitt, 2007), it has been proposed that these dissociative anesthetics may be more appropriate models of schizophrenia. Nevertheless, it has been argued that NMDA antagonists and serotonergic hallucinogens may model different subtypes of schizophrenia, with NMDA antagonists producing effects most similar to the disorganized or undifferentiated subtype of schizophrenia and hallucinogens modeling the paranoid subtype (Abi-Saab et al 1998). In order to directly compare these two models, Gouzoulis-Mayfrank conducted a double-blind crossover study with S-ketamine and the hallucinogen N,N-dimethyltryptamine (DMT) in normal volunteers (Gouzoulis-Mayfrank et al., 2005). This comparison showed that the effects of DMT primarily resembled the positive symptoms of schizophrenia, whereas S-ketamine produced effects that more closely resembled the negative symptoms of schizophrenia (Gouzoulis-Mayfrank et al., 2005), indicating that these drugs model different aspects of schizophrenia. Gouzoulis-Mayfrank and colleagues (Gouzoulis-Mayfrank et al., 1998a) have also used the Altered States of Consciousness (APZ) rating scale to assess whether psychotic patients experience psychedelic experiences similar to those induced by hallucinogens. The APZ was developed by Dittrich to assess altered states of consciousness independent of their etiology (Dittrich, 1998), and is sensitive to the subjective effects of serotonergic hallucinogens including psilocybin, mescaline, and DMT (Hermle et al., 1992; Vollenweider et al., 1997; Gouzoulis-Mayfrank et al., 1999, 2005; Grob et al., 2011). Patients with acute schizophrenia, schizophreniform disorder, or schizoaffective disorder had significantly higher APZ scores than normal controls. Additionally, APZ scores were found to be significantly correlated with scores on the Brief Psychiatric Rating Scale, which measures psychotic symptoms. These findings demonstrate that psychotic patients experience hallucinogen-like alterations of perception and consciousness.
Although the use of hallucinogens as a model of psychosis was somewhat controversial during the 1950s, there was much less controversy regarding the possibility that 5-HT itself plays a role in the illness. Serotonin was first isolated from serum in 1948 by Rapport (Rapport et al., 1948), and the next year it was identified as 5-hydroxytryptamine (Rapport, 1949). The similarity of the chemical structures of 5-HT and LSD (Fig. 1), the fact that 5-HT is present in the brains of dogs, rabbits, and rats (Twarog and Page, 1953), and the finding that LSD blocked the contractile effect of LSD on smooth muscle (Gaddum, 1953), led Woolley and Shaw (1954) to propose that 5-HT plays a role in mental processing and possibly in the pathogenesis of schizophrenia (Woolley and Shaw, 1954). The link between 5-HT and schizophrenia was supported by the subsequent discovery that reserpine, an indole alkaloid isolated from Rauwolfia serpentina that has antipsychotic properties (Braun, 1960; Gore et al., 1957), causes massive depletion of 5-HT (Pletcher et al., 1955). One of the strongest arguments for the involvement of 5-HT in schizophrenia was the discovery of atypical antipsychotics such as clozapine, risperidone, and olanzapine, which act in part by blocking 5-HT2A receptors with some selectivity over the dopamine (DA) D2 receptor (Meltzer et al., 1989, Meltzer, 1991, 1999; Seeman, 2002). Atypical antipsychotics are associated with a lower risk of extrapyramidal side-effects compared with typical antipsychotics, which may be attributable at least partially to 5-HT2A antagonism (Meltzer, 1999; Roth and Meltzer, 2000; Abi-Dargham and Krystal, 2000). Animal studies have indicated that selective 5-HT2A antagonists have antipsychotic-like effects (Varty et al., 1999; Geyer et al., 2001). A subsequent clinical trial confirmed that the selective 5-HT2A antagonist M100,907 (volinanserin, formerly MDL 100,907) was more effective than placebo at treating schizophrenia, but did not show significantly greater efficacy than the typical antipsychotic haloperidol in neuroleptic-responsive patients (de Paulis, 2001). Development of the 5-HT2A/2C antagonist eplivanserin (SR-46349) as a treatment for schizophrenia was also discontinued after it was found to be less effective than haloperidol in neuroleptic-responsive patients (Meltzer et al., 2004). Although the antipsychotic efficacy of 5-HT2A antagonist monotherapy is apparently rather modest, it is possible that certain subpopulations of psychotic patients may respond more favorably. For example, 5-HT2A receptors may play a specific role in psychosis associated with Parkinson’s disease (Ballanger et al., 2010; Huot et al., 2010; Mcfarland et al., 2011), and the 5-HT2A inverse agonist pimavanserin (ACP-103) reduces delusions and hallucinations in Parkinsonian patients (Meltzer et al., 2011).
Because of the apparent similarities between the effects of hallucinogens and some of the symptoms of schizophrenia, several animal models relevant to schizophrenia have been developed based on hallucinogen effects (Geyer and Moghaddam, 2002; Geyer and Vollenweider, 2008; Halberstadt and Geyer, 2013b). These models have facilitated investigation of the role that 5-HT plays in schizophrenia, helped to characterize important interactions between 5-HT and other transmitter systems, and identified novel pharmacotherapeutics that act through receptors for 5-HT and other transmitters. Here, we review four of the animal behavioral models.
Startle Habituation
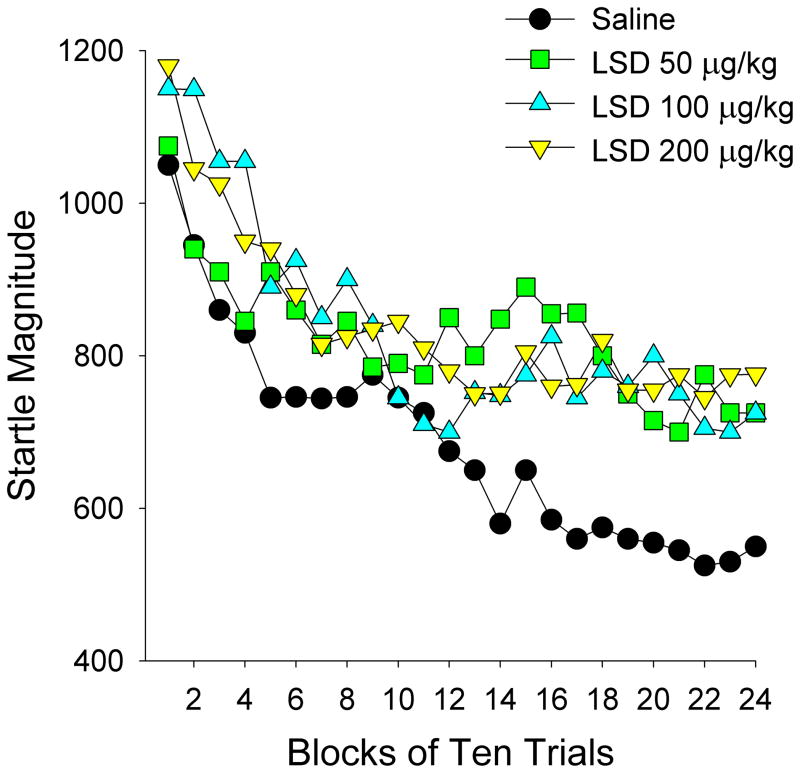
The startle response is a transient motor response exhibited by humans and other animal species in response to loud acoustic stimuli (acoustic startle) or unexpected tactile stimuli (tactile startle). Repeated exposure to a startling stimulus often leads to a marked response decrement, a process known as habituation (Szabo and Kolta,1967; Groves and Thompson, 1970; Davis and Heninger, 1972; Rankin et al., 2009). Schizophrenia patients often display an impaired ability to filter out extraneous or irrelevant stimuli, potentially contributing to the distractibility, sensory flooding, and cognitive fragmentation found in many of these patients (McGhie and Chapman, 1961). There is extensive evidence that patients with schizophrenia display startle reflex habituation deficits that may contribute to the sensory overload. Comparison of the eyeblink component of the acoustic startle reflex in schizophrenia and control subjects revealed that startle habituation is significantly impaired in schizophrenia patients (Geyer and Braff, 1982). Subsequent studies confirmed that habituation of the startle response evoked by acoustic stimuli or electrocutaneous stimulation is deficient in schizophrenia patients relative to normal controls (Bolino et al., 1992, 1994; Parwani et al., 2000; Taiminen et al., 2000; Ludewig et al., 2003; Meincke et al., 2004). Because habituation is a cross-species phenomenon that can be assessed in humans and in laboratory animals using similar procedures, startle habituation in animals has been used to model the information processing deficits that occur in schizophrenia. Tactile and acoustic startle response magnitudes in rats are increased by a variety of serotonergic hallucinogens, including members of the indoleamine (LSD, DMT, and psilocin) and phenylalkylamine (mescaline, 2,5-dimethoxy-4-methylamphetamine (DOM), and 2,5-dimethoxy-4-ethylamphetamine (DOET)) chemical classes (Davis and Sheard, 1974; Geyer et al., 1978). Importantly, acute administration of LSD to rats reduced habituation of tactile startle provoked by air-puffs (Figure 2) (Geyer et al., 1978; Geyer and Braff, 1987), an effect that is lost when LSD is administered chronically (Braff and Geyer, 1980). Mescaline also attenuates habituation of acoustic startle in rats (Davis, 1987), and this effect is blocked by the 5-HT2A/2C antagonists ritanserin, ketanserin, LY 53857, and cinanserin. Psilocybin, however, did not have significant effects on startle reactivity or habituation when tested in human subjects (Gouzoulis-Mayfrank et al., 1998c; Vollenweider et al., 2007; Quednow et al., 2012).
Figure 2.
The effects of LSD on the startle response in rats are shown for 24 blocks of 10 trials each. Each point represents the mean startle amplitude. Male Sprague-Dawley rats (200–250 g) were treated (1 ml/kg i.p.) with vehicle (isotonic saline) or LSD tartrate. Ten min later, the animals were placed in a stabilimeter chamber for a 5-min acclimation period, and then exposed to 240 air-puff stimuli (20-ms, 50 psi) with a 15 s inter-trial interval. This study was originally reported in: Geyer and Braff, 1987.
Prepulse Inhibition
The presentation of a weak prestimulus at a brief interval (30–500 ms) prior to a startle-inducing stimulus will attenuate the resulting startle response. This phenomenon, known as prepulse inhibition (PPI), has been used as an operational measure of sensorimotor gating and may reflect mechanisms that exist to regulate sensory input by filtering out extraneous or distracting stimuli (Swerdlow and Geyer, 1998). PPI is a cross-species phenomenon that is extremely robust, unlearned, and ubiquitous (Geyer et al., 2001; Swerdlow et al., 2001). Consistent with the view that schizophrenia is a gating or filtering disorder (Carlsson, 1995), PPI has been found to be deficient in schizophrenia patients (Braff et al., 1978; Braff and Geyer, 1990; Bolino et al., 1994; Parwani et al., 2000; Ludewig et al., 2003; Quednow et al., 2006).
Animals treated with hallucinogens show reductions in PPI, indicating that hallucinogens reduce the gating or filtering of sensory stimuli. LSD, 2,5-dimethoxy-4-iodoamphetamine (DOI), 2,5-dimethoxy-4-bromoamphetamine (DOB), and mescaline disrupt PPI in rats (Rigdon and Weatherspoon, 1992; Sipes and Geyer, 1994; Johansson et al., 1995; Varty and Higgins, 1995; Ouagazzal et al., 2001; Palenicek et al., 2008; Halberstadt and Geyer, 2010). The selective 5-HT2A antagonists M100,907 and MDL 11,939 block the effects of DOI and LSD on PPI (Sipes and Geyer, 1995; Padich et al., 1996; Ouagazzal et al., 2001; Halberstadt and Geyer, 2010), whereas 5-HT1A or 5-HT2C antagonists are ineffective at preventing their effects. The reduction of PPI induced by DOI is also blocked by the atypical antipsychotics aripiprazole, risperidone, and clozapine, but not by the D2 antagonist haloperidol or the D2/3 antagonist raclopride (Varty and Higgins, 1995; Kohnomi et al., 2008). The hallucinogen 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) also disrupts PPI in rats, but this effect is dependent on 5-HT1A receptor activation since it is prevented by the selective 5-HT1A antagonist WAY-100635 and not by M100,907 (Krebs-Thomson et al., 2006). The involvement of 5-HT1A receptors in mediating the effects of 5-MeO-DMT on PPI is consistent with numerous findings that the behavioral effects of 5-MeO-DMT are primarily attributable to 5-HT1A activation (Winter et al., 2000; Halberstadt et al., 2011; van den Buuse et al., 2011).
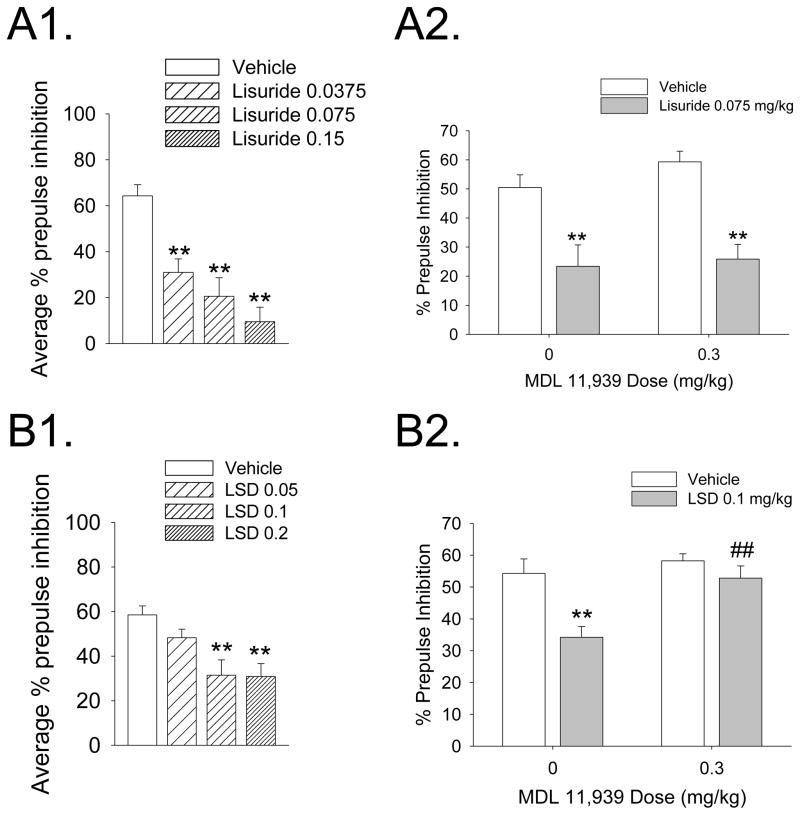
Lisuride is an LSD congener that acts as a 5-HT2A agonist but does not have hallucinogenic effects in humans. González-Maeso et al. (2007) have proposed that lisuride does not act as a hallucinogen because of agonist-directed trafficking of 5-HT2A responses; i.e., certain 5-HT2A agonists are hallucinogenic because they activate specific signaling pathways that are not recruited by lisuride. Interestingly, although both LSD and lisuride disrupt PPI in rats, they do so by different receptor mechanisms; the PPI disruption induced by lisuride was not blocked by MDL 11,939 or the selective 5-HT1A antagonist WAY-100635 but was prevented by pretreatment with the selective DA D2/3 receptor antagonist raclopride (Figure 3; Halberstadt and Geyer, 2010).
Figure 3.
Effects of lisuride (A) and LSD (B) on prepulse inhibition in rats. (A1) Effect of lisuride (0.0375, 0.075, and 0.15 mg/kg, s.c.) on average prepulse inhibition. (A2) Effects of the selective 5-HT2A antagonist MDL 11,939 on the disruption of PPI induced by lisuride. (B1) Effect of LSD (0.05, 0.1, and 0.2 mg/kg, s.c.) on average prepulse inhibition. (A2) Effects of the selective 5-HT2A antagonist MDL 11,939 on the disruption of PPI induced by LSD. Values represent mean ± SEM for each group. Drug doses are in milligram per kilogram. *p < 0.05, **p < 0.01, significantly different from vehicle control; ##p < 0.01, significantly different from LSD-treated animals. Male Sprague-Dawley rats (250–275 g) were placed in a stabilimeter chamber 30 min after treatment with MDL 11,939, 10 min after treatment with lisuride hydrogen maleate, or 5 min after treatment with LSD tartrate. After a 5-min acclimation period to 65-dB broadband background noise, %prepulse inhibition was assessed using a combination of startle trials (a 40-ms 120-dB pulse of broadband white noise) and prepulse trials (a 20-ms acoustic prepulse at either 68, 71, or 77 dB, an 80-ms delay, and then a 40-ms 120-dB startle pulse) presented in a pseudo-randomized order. Data from: Halberstadt and Geyer, 2010.
Studies in humans have demonstrated that hallucinogens can alter PPI, although the effect is highly dependent on the specific testing parameters used. One study with psilocybin found that the hallucinogen increased PPI when a 100 ms interstimulus interval (ISI) was used (Gouzoulis-Mayfrank et al., 1998c). Another study confirmed that psilocybin increased PPI at long ISIs (120–2000 ms), but also found that psilocybin reduced PPI when shorter ISIs of 30 ms were used (Vollenweider et al., 2007). Importantly, the ability of psilocybin to reduce PPI at a 30 ms ISI is completely blocked by ketanserin (Quednow et al., 2012), confirming the involvement of 5-HT2A/2C receptors in mediating this effect. Given the similarity of hallucinogen effects on PPI in humans and rats, hallucinogen effects on PPI have been used as a model of the positive symptoms of schizophrenia. Importantly, it was recently reported that specific 5-HT2A polymorphisms modulate PPI levels in normal volunteers and in patients with schizophrenia (Quednow et al., 2008, 2009). These findings raise the possibility that changes in 5-HT2A signaling could contribute to the PPI disruption observed in schizophrenia.
Head Twitch Response
Hallucinogens induce stereotypical motor responses in many mammalian species, including ear scratching (mice), limb flicks (cats), or head bobs (rabbits). In rats and mice, administration of a variety of hallucinogens produces a paroxysmal rotational head movement known as the head twitch response (HTR)(Corne and Pickering, 1967; Yamomoto and Ueki, 1975; Bedard and Pycock, 1977; Canal and Morgan, 2012; Halberstadt and Geyer, 2013a). Although the HTR is typically assessed by direct observation and hence experiments can be time-consuming, it was recently reported that a head-mounted magnet and a magnetometer coil can be used to detect the behavior with extremely high sensitivity and specificity (Halberstadt and Geyer, 2013a). The hallucinogen-induced HTR is blocked by selective 5-HT2A antagonists (Schreiber et al., 1995; Fox et al., 2009) and is absent in 5-HT2A knockout mice (González-Maeso et al., 2007; Keiser et al., 2009; Halberstadt et al., 2011), suggesting that this behavior is a consequence of 5-HT2A activation. 5-HT2A receptors in the prefrontal cortex (PFC) may be responsible for mediating the HTR induced by hallucinogens, as evidenced by the fact that infusion of DOI directly into this region induces the behavior in rats (Willins and Meltzer, 1997), and loss of the HTR in 5-HT2A knockout mice can be rescued by selective restoration of the receptor in cortical regions (González-Maeso et al., 2007). In recent years, the HTR has been widely adopted as a rodent behavioral proxy for hallucinogen effects in humans. In fact, there is evidence that the HTR is one of the few behaviors that can reliably and distinguish hallucinogenic and non-hallucinogenic 5-HT2A agonists (González-Maeso et al., 2007). Nevertheless, there is little evidence to support using the HTR as an animal model of hallucinations or of mental states that are directly relevant to schizophrenia. For example, many non-hallucinogenic compounds that increase 5-HT release and indirectly activate the 5-HT2A receptor, including d-fenfluramine (Darmani, 1997) and even some benzodiazepines (Tadano et al., 2001), can induce the HTR. Furthermore, although many antipsychotics can block the hallucinogen-induced HTR due to their 5-HT2A antagonist activity, selective 5-HT2A antagonists such as M100,907 have only limited efficacy as antipsychotics when administered to schizophrenia patients.
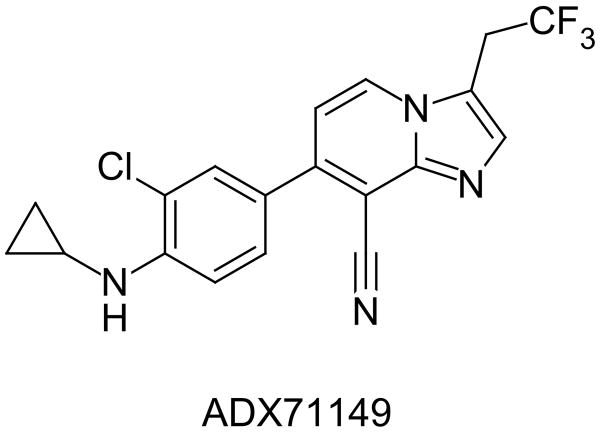
There is, however, substantial evidence that the HTR has utility as a behavioral tool to study the neural basis for hallucinogen effects, which may have direct relevance to understanding the positive symptoms of schizophrenia. For example, the HTR induced by DOI in rats and mice is suppressed by the selective metabotropic glutamate (mGlu)2/3 receptor agonists LY354740 and LY379268 (Fig. 4) and enhanced by the selective mGlu2/3 antagonist LY341495 (Gewirtz and Marek, 2000; Klodzinska et al., 2002). Likewise, the mGlu2 positive allosteric modulator (PAM) biphenyl-indanone A inhibits the HTR induced by (−)-DOB (Benneyworth et al., 2007). Chronic treatment with the mGlu2/3 antagonist LY341495 has been shown to down-regulate cortical 5-HT2A sites and attenuate the HTR induced by LSD in mice (Moreno et al., 2013). Deletion of the mGlu2 gene in mice has been shown to produce a reduction of the HTR to LSD and DOI and a profound loss of high-affinity 5-HT2A binding sites in frontal cortex (Moreno et al., 2011a). Indeed, there is extensive electrophysiological, neurochemical, and behavioral evidence that mGlu2/3 receptors regulates the response to 5-HT2A activation (Marek et al., 2000; Gewirtz et al., 2002; Klodzinska et al., 2002; Winter et al., 2004; Benneyworth et sl., 2007; Molinaro et al., 2009; Wischhof et al., 2011; Wischhof and Koch, 2012). These findings are significant because there is some evidence that mGlu2/3 agonists may possess antipsychotic efficacy. Although pomaglumetad methionil (LY2140023; Fig. 4), a methionine amide prodrug for the selective orthosteric mGlu2/3 agonist LY404039, reduced schizophrenia symptoms in an initial phase II trial (Patil et al., 2007), follow-up studies were either inconclusive (Kinon et al., 2011) or failed to show evidence for efficacy (Lilly, 2012). Although Lilly has discontinued further clinical trials, it appears that the clinical response to pomaglumetad methionil may depend on the presence of specific single nucleotide polymorphisms (SNPs) in the 5-HT2A receptor (Liu et al., 2012). Importantly, according to a recent press release, a phase II trial conducted by Janssen Pharmaceuticals demonstrated that the selective mGlu2 PAM ADX71149 (Figure 5) has efficacy in medicated schizophrenia patients with residual negative symptoms (Addex, 2012), although peer-reviewed data have yet to appear in the literature. One potential explanation for the interactions between mGlu2 and 5-HT2A is that these receptors may be co-localized in cortical neurons, where they can form functional complexes (Gonzalez-Maeso et al., 2008; Moreno et al., 2012). There is evidence that the behavioral effects of some antipsychotic drugs in mice may be directly mediated by these mGlu2/5-HT2A complexes (Fribourg et al., 2011). The receptor heterodimers may play a specific role in mediating the HTR because the loss of the behavioral response in mGlu2 knockout mice can be rescued by viral-mediated over-expression of mGlu2 in frontal cortex, whereas expression of a mutated form of mGlu2 that is incapable of forming complexes with 5-HT2A did not rescue the behavior (Moreno et al., 2012). Nonetheless, it is possible that functional or circuit interactions may actually be involved in mediating the interactions between 5-HT2A and mGlu2 receptors, and further work is required to conclusively demonstrate that mGlu2 and 5-HT2A heterodimers are responsible for mediating the crosstalk between these systems (Delille et al., 2012, 2013).
Figure 4.
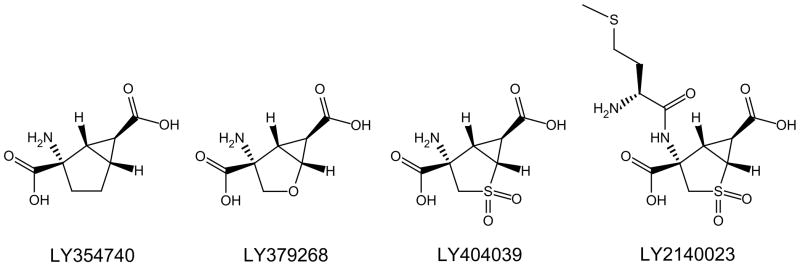
Chemical structures of orthosteric mGlu2/3 receptor agonists.
Figure 5.
Chemical structure of the selective mGlu2 receptor positive allosteric modulator ADX71149.
Although there is substantial evidence that some forms of schizophrenia have genetic etiologies, environmental events, especially during pregnancy, also play a role. Two rodent models—maternal variable stress and prenatal immune challenge—have been developed to study whether adverse prenatal events can produce schizophrenia-like effects. Interestingly, it was recently shown that the HTR is altered in both models. Maternal variable stress and prenatal immune activation with polyinosinic:polycytidylic acid significantly increased the HTR evoked by DOI in adult mice, and reduced the antipsychotic-like behavioral effects of the mGlu2/3 agonist LY379268 (Moreno et al., 2011b; Holloway et al., 2013). These behavioral alterations were accompanied by up-regulation of the 5-HT2A receptor and down-regulation of the mGlu2 receptor (Moreno et al., 2011b). A similar pattern of changes in 5-HT2A and mGlu2 binding and mRNA expression has been found in the prefrontal cortex of unmedicated schizophrenia patients post-mortem (Gonzales-Maeso et al., 2008; Muguruza et al., 2012). Gonzalez-Maeso and colleagues have also reported that crosstalk between mGlu2 and 5-HT2A receptors is altered in schizophrenia patients (Moreno et al., 2012). Taken together, these findings indicate that alterations of 5-HT2A receptor signaling may contribute to the pathophysiology of schizophrenia. However, the finding that the 5-HT2A receptor is upregulated in schizophrenia needs to be replicated because numerous post-mortem studies have found either no change or reductions of 5-HT2A binding site densities and mRNA expression in the cortex of schizophrenia patients (reviewed by: Quednow et al., 2010). Likewise, other groups have reported that cortical mGlu2-like immunoreactivity and mRNA expression levels are not downregulated in schizophrenia subjects post-mortem (Crook et al., 2002; Gupta et al., 2005; Ghose et al., 2008, 2009). Although many of the earlier studies were confounded by antipsychotic treatment, which could potentially reduce 5-HT2A expression, PET studies with [18F]altanserin, [18F]septoperone, or [11C]N-methylspiperone in antipsychotic-naive subjects found either no change (Trichard et al., 1998; Lewis et al., 1999; Okubo et al., 2000; Erritozoe et al., 2008) or reductions (Ngan et al., 2000; Rasmussen et al., 2010) of radiotracer binding to cortical 5-HT2A receptors.
Interval Timing
The perception of time is essential for survival and is required for the precise organization of sequences of activity as well as the anticipation of behavioral outcomes and future events. Time perception occurs over multiple timescales, ranging from milliseconds to days (Buhusi and Meck 2005), and encompasses a diverse variety of functions such as sensory and motor timing, and circadian activity. Interval timing falls within this larger framework of temporal processing and refers to the discrimination of durations, typically in the seconds to minutes range. Deficits of timing have been reported in patients with a variety of neuropsychiatric disorders. Given the crucial importance of temporal processing to the regulation of behavior and interaction with the world, timing impairment would have significant consequences for these patient populations.
It has been proposed that impaired temporal processing is a core deficit of schizophrenia (Carroll et al 2008; Bonnot et al 2011; Ward et al 2012). Schizophrenia patients consistently overestimate and under-produce temporal durations in behavioral studies (Densen 1977; Wahl and Sieg 1980; Tysk 1983; Rammsayer 1990; Carroll et al 2009; Waters and Jablensky 2009), and interval timing is less accurate and more variable in schizophrenia patients than in normal controls (Tysk 1984; Davalos et al 2003; Carroll et al 2008, 2009; Lee et al., 2009; Davalos et al 2011). The fact that the timing deficits occur over multiple time scales (<100 ms to several minutes) and have been demonstrated using tasks with varying degrees of difficulty indicates that the timing impairment is not a consequence of more generalized mnemonic or attentional deficits (Carroll et al 2009; Davalos et al 2011). Furthermore, timing impairments occur independently of working memory deficits (Elvevag et al 2003). There is also evidence that schizophrenia patients show less activation of brain regions thought to be involved in timing when performing an auditory time estimation task (Volz et al., 2001; Davalos et al., 2011). Finally, schizophrenia patients exhibit impaired processing of the temporal relationship between sensory stimuli (Braus, 2002; Tenckhoff et al., 2002; Todd 2006; Schmidt et al 2011) and impaired ability to predict when events will occur (Turgeon et al 2012). Together, these findings demonstrate that there is a fundamental deficit of timing and temporal perception in schizophrenia.
There are several potential functional consequences of impaired temporal perception in schizophrenia. Timing deficits could impair perceptual and cognitive processing and alter the temporal coordination of behavior, contributing to the behavioral disorganization, contextually inappropriate behavior, and planning deficits observed in schizophrenia. Additionally, accurate temporal perception is required to infer causality (e.g., Maeda et al 2012) and the sensory consequences of actions (Waters & Jablensky 2009). Disturbed interval timing could potentially alter the perceived sequence of mental thoughts and sensory events, resulting in erroneous causal attributions (Haggard et al 2003; Waters & Jablensky 2009) and delusional thinking. Laboratory studies have shown that even minor changes in inter-sensory temporal relationships can produce perceived violations of temporal contiguity in normal subjects (Cunningham et al., 2001), and it is possible that changes in timing in schizophrenia patients could potentially give rise to feelings that thoughts or actions are being controlled by outside forces.
There is evidence that the serotonergic system modulates temporal perception and interval timing (Ho et al 2002; Sysoeva et al 2010). One line of evidence has emerged from the differential-reinforcement-of-low-rate 72-s (DRL 72-s) paradigm (in which rats must wait 72 s between responses to obtain reinforcement), which is used as a screen for antidepressant drugs. A variety of serotonergic ligands, including M100,907 and the 5-HT releasing drug fenfluramine, alter the performance of rats under the DRL 72-s schedule (Richards et al., 1993; Marek et al., 2005), which may reflect a change in the accuracy of temporal discrimination. Additionally, serotonergic hallucinogens markedly alter the subjective experience of time (Heimann, 1994). Under the influence of mescaline or LSD, human subjects reported that these drugs could speed up or slow down the passage of time, or even produce a feeling of timelessness (Serko, 1913; Beringer, 1927; DeShon et al., 1952; Hoch et al., 1952; Kenna and Sedman, 1964). Boardman and colleagues found that administration of low p.o. doses of LSD to volunteers increased the variability of 1 min duration judgments but did not consistently produce underestimations or overestimations (Boardman et al., 1957). By contrast, subjects given 1 or 2 μg/kg LSD p.o. reliably under-produced longer durations (15–240 min)(Aronson et al., 1959). More recent studies have shown that psilocybin disrupts interval timing in human volunteers (Wittmann et al 2007; Wackerman et al 2008).
Hallucinogens also disrupt interval timing in rodent models. Interval timing is often assessed in rodents using immediate and retrospective timing schedules. An example of an immediate timing schedule is the free-operant psychophysical task, where intermittent reinforcement is provided for responding on two levers, and the animal must respond on lever A during the first half of each trial and on lever B during the second half of the trial (Stubbs, 1980). The discrete-trials task is an example of a retrospective timing schedule; in this task, a lamp is illuminated for a variable duration, and then two levers are presented. Responding on lever A is reinforced if the stimulus duration is shorter than a specific value; responding on lever B is reinforced if the stimulus duration is longer than the value (Body et al 2002a). For both tasks, timing is measured by T50 (the time when %B responding is equal to 50%), a measure of timing accuracy, and by the Weber fraction, a measure of timing precision. Since similar tasks are used to assess interval timing in humans (e.g., Penney et al 2008; Sysoeva et al 2010), the results of these timing tasks are directly translatable across species. In rats, DOI alters performance in the free-operant timing task (Body et al 2003, 2006a,b; Cheung et al., 2007) and the discrete-trials task (Asgari et al 2006; Hampson et al 2010). In the discrete-trials task, DOI increases the Weber fraction (indicating increased variability of timing), but does not consistently displace T50. DOI does not alter performance on a similar non-temporal task (light-intensity discrimination), demonstrating that DOI is specifically altering timing and not the mnemonic or attentional processes required to perform the task (Hampson et al 2010). In the free-operant procedure, DOI reduced T50, suggesting an increase in the speed of the internal clock. The effects of DOI on interval timing are blocked by ketanserin (Body et al., 2003; Asgari et al., 2006) and M100,907 (Body et al 2006a,b; Asgari et al 2006). It is not clear why DOI has qualitatively different effects on performance in the discrete-trials and free-operant procedures, but it is not unusual to find that pharmacological agents do not uniformly alter timing maintained under different reinforcement schedules (Body et al., 2013). Despite these differences, it is clear that DOI alters timing in rats in a 5-HT2A receptor-dependent manner. Fenfluramine also disrupts interval timing in rats and this effect is blocked by ketanserin (Body et al 2004), indicating that endogenous 5-HT alters timing by activating 5-HT2A/2C receptors. 5-HT2A receptor polymorphisms are linked to altered timing in humans (Sysoeva et al 2010), further demonstrating that the 5-HT2A system plays an important role in regulating temporal perception.
Summary and Conclusions
Nearly a century has passed since it was first recognized that hallucinogens produce a schizophrenia-like state that can be used to model psychosis. Since that time, there have been substantial advances in neuropharmacology and biological psychiatry, but laboratory models based on the effects of hallucinogenic drugs still play an important role in modern work to characterize the etiology of the illness and identify novel pharmacotherapeutics. Despite the continuing use of hallucinogens as models of psychotic disorders, it could be argued that the most important legacy of the work with hallucinogens during the first half of the twentieth century is the recognition that 5-HT acts a transmitter substance in the brain and that it might play a role in the group of schizophrenias. Although the degree to which serotonergic alterations contribute to the development and symptoms of schizophrenia remains unclear, it is now known the effects of hallucinogens in humans are mediated primarily by the serotonin 5-HT2A receptor. Importantly, in the four behavioral models discussed above—startle habituation, prepulse inhibition of startle, head twitch response, and interval timing—the 5-HT2A receptor has been identified as playing a fundamental role in mediating hallucinogen effects. In addition to the role that the 5-HT2A receptor plays in mediating hallucinogen effects, this receptor is an important target of atypical antipsychotic drugs, and there is at least some evidence that interactions with this site may contribute to their therapeutic profile. Moreover, it is now recognized that interactions between 5-HT2A and mGlu receptors may play a role in the development of schizophrenia and in the putative antipsychotic efficacy of mGlu2/3 agonists. The fact that an animal behavioral model based on hallucinogen effects played a major role in the discovery and characterization of these novel interactions demonstrates the continuing importance of this type of model and indicates that it will likely be even more important in the future.
Acknowledgments
This work was supported by National Institute on Drug Abuse Award DA002925, the Brain & Behavior Research Foundation, and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
STATEMENT OF INTEREST
Dr. Halberstadt has received research grant support from NIDA and the Brain & Behavior Research Foundation. Dr. Geyer has received consulting compensation from Abbott, Acadia, Addex, Cerca, Lundbeck, Merck, Neurocrine, Omeros, Takeda, and Teva, and holds an equity interest in San Diego Instruments. Dr. Geyer also has research grant support from Intracellular Therapeutics, Johnson & Johnson, NIDA, NIMH, and the U.S. Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
References
- Abi-Dargham A, Krystal J. Serotonin receptors as targets of antipsychotic medication. In: Lidow MS, editor. Neurotransmitter Receptors in Actions of Antipsychotic Medications. CRC Press LLC; Boca Raton, Florida: 2000. pp. 79–107. [Google Scholar]
- Abi-Saab WM, D’Souza DC, Moghaddam B, Krystal JH. The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry. 1998;31 (Suppl 2):104–109. doi: 10.1055/s-2007-979354. [DOI] [PubMed] [Google Scholar]
- Addex. Addex Reports Top-line Data from a Successful Phase 2a Clinical Study with ADX71149 in Schizophrenia Patients. 2012 http://www.addextherapeutics.com/investors/press-releases/news-details/article/addex-reports-top-line-data-from-a-successful-phase-2a-clinical-study-with-adx71149-in-schizophrenia/ (Retrieved May 27, 2013)
- Aronson H, Silverstein AB, Klee GD. Influence of lysergic acid diethylamide (LSD-25) on subjective time. AMA Arch Gen Psychiatry. 1959;1:469–472. doi: 10.1001/archpsyc.1959.03590050037003. [DOI] [PubMed] [Google Scholar]
- Asgari K, Body S, Bak VK, Zhang ZQ, Rickard JF, Glennon JC, Fone KC, Bradshaw CM, Szabadi E. Effects of 5-HT2A receptor stimulation on the discrimination of durations by rats. Behav Pharmacol. 2006;17:51–59. doi: 10.1097/01.fbp.0000189810.69425.89. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Strafella AP, van Eimeren T, Zurowski M, Rusjan PM, Houle S, Fox SH. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67:416–421. doi: 10.1001/archneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- Bedard P, Pycock CJ. “Wet-dog” shake behavior in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology. 1977;16:663–670. doi: 10.1016/0028-3908(77)90117-4. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Beringer K. Experimentelle Psychosen durch Mescalin. Z Ges Neurol Psychiat. 1923;84:426–433. [Google Scholar]
- Beringer K. Seine Geschiehte und Erseheinungsweise. Berlin: Springer; 1927. Der Meskalinrausch. [Google Scholar]
- Boardman WK, Goldstone S, Lhamon WT. Effects of lysergic acid diethylamide (LSD) on the time sense of normals; a preliminary report. AMA Arch Neurol Psychiatry. 1957;78:321–324. doi: 10.1001/archneurpsyc.1957.02330390103013. [DOI] [PubMed] [Google Scholar]
- Body S, Asgari K, Cheung TH, Bezzina G, Fone KF, Glennon JC, Bradshaw CM, Szabadi E. Evidence that the effect of 5-HT2 receptor stimulation on temporal differentiation is not mediated by receptors in the dorsal striatum. Behav Processes. 2006b;71:258–267. doi: 10.1016/j.beproc.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Body S, Cheung TH, Bezzina G, Asgari K, Fone KC, Glennon JC, Bradshaw CM, Szabadi E. Effects of d-amphetamine and DOI (2,5-dimethoxy-4-iodoamphetamine) on timing behavior: interaction between D1 and 5-HT2A receptors. Psychopharmacology. 2006a;189:331–343. doi: 10.1007/s00213-006-0575-0. [DOI] [PubMed] [Google Scholar]
- Body S, Cheung TH, Valencia-Torres L, Olarte-Sánchez CM, Fone KC, Bradshaw CM, Szabadi E. Pharmacological studies of performance on the free-operant psychophysical procedure. Behav Processes. 2013 doi: 10.1016/j.beproc.2013.02.004. in press pii: S0376-6357(13)00025-9. [DOI] [PubMed] [Google Scholar]
- Body S, Chiang TJ, Mobini S, Ho MY, Bradshaw CM, Szabadi E. Effect of 8-OH-DPAT on temporal discrimination following central 5-hydroxytryptamine depletion. Pharmacol Biochem Behav. 2002a;71:787–793. doi: 10.1016/s0091-3057(01)00674-8. [DOI] [PubMed] [Google Scholar]
- Body S, Kheramin S, Ho MY, Miranda F, Bradshaw CM, Szabadi E. Effects of a 5-HT2 receptor agonist, DOI (2,5-dimethoxy-4-iodoamphetamine), and antagonist, ketanserin, on the performance of rats on a free-operant timing schedule. Behav Pharmacol. 2003;14:599–607. doi: 10.1097/00008877-200312000-00004. [DOI] [PubMed] [Google Scholar]
- Body S, Kheramin S, Ho MY, Miranda Herrera F, Bradshaw CM, Szabadi E. Effects of fenfluramine on free-operant timing behaviour: evidence for involvement of 5-HT2A receptors. Psychopharmacology. 2004;176:154–165. doi: 10.1007/s00213-004-1871-1. [DOI] [PubMed] [Google Scholar]
- Bolino F, Di Michele V, Di Cicco L, Manna V, Daneluzzo E, Casacchia M. Sensorimotor gating and habituation evoked by electro-cutaneous stimulation in schizophrenia. Biol Psychiatry. 1994;36:670–679. doi: 10.1016/0006-3223(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Bolino F, Manna V, Di Cicco L, Di Michele V, Daneluzzo E, Rossi A, Casacchia M. Startle reflex habituation in functional psychoses: a controlled study. Neurosci Lett. 1992;145:126–128. doi: 10.1016/0304-3940(92)90002-o. [DOI] [PubMed] [Google Scholar]
- Bonnot O, de Montalembert M, Kermarrec S, Botbol M, Walter M, Coulton N. Are impairments in time perception in schizophrenia a neglected phenomenon? J Physiol (Paris) 2011;105:164–169. doi: 10.1016/j.jphysparis.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Bowers MB, Jr, Freedman DX. “Psychedelic” experiences in acute psychoses. Arch Gen Psychiatry. 1966;15:240–248. doi: 10.1001/archpsyc.1966.01730150016003. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Acute and chronic LSD effects on rat startle: data supporting an LSD--rat model of schizophrenia. Biol Psychiatry. 1980;15:909–916. [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braun M. Reserpine as a therapeutic agent in schizophrenia. Am J Psychiatry. 1960;116:744. doi: 10.1176/ajp.116.8.744. [DOI] [PubMed] [Google Scholar]
- Braus DF. Temporal perception and organisation, neuronal synchronization and schizophrenia. Fortschr Neurol Psychiatr. 2002;70:591–600. doi: 10.1055/s-2002-35172. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012;4:556–576. doi: 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A. Neurocircuitries and neurotransmitter interactions in schizophrenia. Int Clin Psychopharmacol. 1995;10 (Suppl 3):21–28. [PubMed] [Google Scholar]
- Caroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain Cogn. 2009a;70:181–190. doi: 10.1016/j.bandc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Caroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009b;71:345–353. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Bezzina G, Body S, Fone KC, Bradshaw CM, Szabadi E. Tolerance to the effect of 2,5-dimethoxy-4-iodoamphetamine (DOI) on free-operant timing behaviour: interaction between behavioural and pharmacological mechanisms. Psychopharmacology. 2007;192:521–535. doi: 10.1007/s00213-007-0743-x. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–1508. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- Crook JM, Akil M, Law BC, Hyde TM, Kleinman JE. Comparative analysis of group II metabotropic glutamate receptor immunoreactivity in Brodmann’s area 46 of the dorsolateral prefrontal cortex from patients with schizophrenia and normal subjects. Mol Psychiatry. 2002;7:157–164. doi: 10.1038/sj.mp.4000966. [DOI] [PubMed] [Google Scholar]
- Cunningham DW, Billock VA, Tsou BH. Sensorimotor adaptation to violations of temporal contiguity. Psychol Sci. 2001;12:532–535. doi: 10.1111/1467-9280.d01-17. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Deficits in D-fenfluramine-sensitive pool of brain 5-HT following withdrawal from chronic cocaine exposure. Life Sci. 1997;61:2575–2582. doi: 10.1016/s0024-3205(97)01012-6. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Kisley MA, Ross RG. Effects of interval duration on temporal processing in schizophrenia. Brain Cogn. 2003;52:295–301. doi: 10.1016/s0278-2626(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Rojas DC, Tregellas JR. Temporal processing in schizophrenia: effects of task-difficulty on behavioral discrimination and neuronal responses. Schizophrenia Res. 2011;127:123–130. doi: 10.1016/j.schres.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Mescaline: excitatory effects on acoustic startle are blocked by serotonin2 antagonists. Psyhopharmacology. 1987;93:286–291. doi: 10.1007/BF00187244. [DOI] [PubMed] [Google Scholar]
- Davis M, Heninger GR. Comparison of response plasticity between the eyeblink and vertex potential in humans. Electroencephalography Clin Neurophysiol. 1972;33:283–293. doi: 10.1016/0013-4694(72)90155-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Sheard MH. Effects of lysergic acid diethylamide (LSD) on habituation and sensitization of the startle response in the rat. Pharmacol Biochem Behav. 1974;2:675–683. doi: 10.1016/0091-3057(74)90037-9. [DOI] [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology. 2012;62:2184–2191. doi: 10.1016/j.neuropharm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Delille HK, Mezler M, Marek GJ. The two faces of the pharmacological interaction of mGlu2 and 5-HT2A - Relevance of receptor heterocomplexes and interaction through functional brain pathways. Neuropharmacology. 2013;70:296–305. doi: 10.1016/j.neuropharm.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Densen ME. Time perception and schizophrenia. Percept Motor Skills. 1977;44:436–438. doi: 10.2466/pms.1977.44.2.436. [DOI] [PubMed] [Google Scholar]
- de Paulis T. M-100907 (Aventis) Curr Opin Investig Drug. 2001;2:123–132. [PubMed] [Google Scholar]
- Dietrich A, Allen JD. Functional dissociation of the prefrontal cortex and the hippocampus in timing behavior. Behav Neurosci. 1998;112:1043–1047. doi: 10.1037//0735-7044.112.5.1043. [DOI] [PubMed] [Google Scholar]
- Dittrich A. The standardized psychometric assment of altered states of consciounesss (ASCs) in humans. Pharmacopsychiatry. 1998;31 (Suppl 2):80–84. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- Duncan EJ, Szilagyi S, Efferen TR, Schwartz MP, Parwani A, Chakravorty S, Madonick SH, Kunzova A, Harmon JW, Angrist B, Gonzenbach S, Rotrosen JP. Effect of treatment status on prepulse inhibition of acoustic startle in schizophrenia. Psychopharmacology. 2003;167:63–71. doi: 10.1007/s00213-002-1372-z. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, McCormack T, Gilbert A, Brown GD, Weinberger DR, Goldberg TE. Duration judgements in patients with schizophrenia. Psychol Med. 2003;33:1249–1261. doi: 10.1017/s0033291703008122. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Rasmussen H, Kristiansen KT, Frokjaer VG, Haugbol S, Pinborg L, Baaré W, Svarer C, Madsen J, Lublin H, Knudsen GM, Glenthoj BY. Cortical and subcortical 5-HT2A receptor binding in neuroleptic-naive first-episode schizophrenic patients. Neuropsychopharmacology. 2008;33:2435–2441. doi: 10.1038/sj.npp.1301656. [DOI] [PubMed] [Google Scholar]
- Freedman B, Chapman LJ. Early subjective experience in schizophrenic episodes. J Abnorm Psychol. 1973;82:46–54. doi: 10.1037/h0034952. [DOI] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, MacKerell AD, Jr, Brezina V, Sealfon SC, Filizola M, González-Maeso J, Logothetis DE. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH. Antagonism between lysergic acid diethylamide and 5-hydroxytryptamine. J Physiol. 1953;121:15P. [PubMed] [Google Scholar]
- Gewirtz JC, Chen AC, Terwilliger R, Duman RC, Marek GJ. Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacol Biochem Behav. 2002;73:317–326. doi: 10.1016/s0091-3057(02)00844-4. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Braff DL. Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology. 1982;19:1–6. doi: 10.1111/j.1469-8986.1982.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophrenia Bulletin. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Moghaddam B. Animal models relevant to schizophrenia disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins; Philadelphia: 2002. pp. 689–701. [Google Scholar]
- Geyer MA, Petersen LR, Rose GJ, Horwitt DD, Light RK, Adams LM, Zook JA, Hawkins RL, Mandell AJ. The effects of lysergic acid diethylamide and mescaline-derived hallucinogens on sensory-integrative function: tactile startle. J Pharmacol Exp Ther. 1978;207:837–847. [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Ghose S, Crook JM, Bartus CL, Sherman TG, Herman MM, Hyde TM, Kleinman JE, Akil M. Metabotropic glutamate receptor 2 and 3 gene expression in the human prefrontal cortex and mesencephalon in schizophrenia. Int J Neurosci. 2008;118:1609–1627. doi: 10.1080/00207450802330702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose S, Gleason KA, Potts BW, Lewis-Amezcua K, Tamminga CA. Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: a mechanism for antipsychotic drug action? Am J Psychiatry. 2009;166:812–820. doi: 10.1176/appi.ajp.2009.08091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gore P, Egan GP, Walton D. The place of reserpine in the treatment o the chronic patient. Am J Psychiatry. 1957;114:333–337. doi: 10.1176/ajp.114.4.333. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Habermeyer E, Hermle L, Steinmeyer AM, Kunert HJ, Sass H. Hallucinogenic drug induced states resemble acute endogenous psychoses: Results of an empirical study. Eur Psychiatry. 1998a;13:399–406. doi: 10.1016/S0924-9338(99)80686-5. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar KA. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Thelen B, Lindenblatt H, Kovar KA, Sass H, Geyer MA. Effects of the hallucinogen psilocybin on habituation and prepulse inhibition of the startle reflex in humans. Behav Pharmacol. 1998c;9:561–566. doi: 10.1097/00008877-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Hermle L, Thelen B, Sass H. History, rationale and potential of human experimental hallucinogenic drug research in psychiatry. Pharmacopsychiatry. 1998b;31 (Suppl 2):63–68. doi: 10.1055/s-2007-979348. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, Hermle L, Spitzer M, Sass H. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology. 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68:71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychological Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse. 2005;57:123–131. doi: 10.1002/syn.20164. [DOI] [PubMed] [Google Scholar]
- Haggard P, Martin F, Taylor-Clarke M, Jeannerod M, Franck N. Awareness of action in schizophrenia. Neuroreport. 2003;14:1081–1085. doi: 10.1097/01.wnr.0000073684.00308.c0. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. The phencyclidine-glutamate model of schizophrenia. Clin Neuropharmacol. 1995;18:237–249. doi: 10.1097/00002826-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. LSD but not lisuride disrupts prepulse inhibition in rats by activating the 5-HT2A receptor. Psychopharmacology. 2010;208:179–189. doi: 10.1007/s00213-009-1718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors mediate the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology. 2013a;227:727–739. doi: 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Neuropharmacology of lysergic acid diethylamide (LSD) and other hallucinogens. In: Spanagel R, editor. Biological Research on Addiction. Vol. 2. Elsevier; London: 2013b. pp. 625–635. [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol. 2011;25:1548–1561. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson CL, Body S, Den Boon FS, Cheung THC, Bezzina G, Langley RW, Fone KCF, Bradshaw CM, Szabadi E. Comparison of the effects of 2,5-dimethoxy-4-iodoamphetamine and D-amphetamine on the ability of rats to discriminate the durations and intensities of light stimuli. Behv Pharmacol. 2010;21:11–20. doi: 10.1097/FBP.0b013e328334707a. [DOI] [PubMed] [Google Scholar]
- Heimann H. Experience of time and space in model psychoses. In: Pletscher A, Ladewig D, editors. 50 Years of LSD. Current Status and Perspectives on Hallucinogens. New York: Parthenon; 1994. pp. 59–66. [Google Scholar]
- Hermle L, Fünfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, Fehrenbach RA, Spitzer M. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1992;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Brain Res Cogn Brain Res. 2004;21:171–182. doi: 10.1016/j.cogbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E. 5-Hydroxytryptamine and interval timing behavior. Pharmacol Biochem Behav. 2002;71:773–785. doi: 10.1016/s0091-3057(01)00672-4. [DOI] [PubMed] [Google Scholar]
- Hoch P, Cattell JP, Pennes HH. Effects of mescaline and lysergic acid diethylamide (d. LSD-25) Am J Psychiatry. 1952;108:579–584. doi: 10.1176/ajp.108.8.579. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Drug-induced psychoses and schizophrenic reactions: a critical comparison. Ann N Y Acad Sci. 1962;96:80–92. doi: 10.1111/j.1749-6632.1962.tb50103.x. [DOI] [PubMed] [Google Scholar]
- Holloway T, Moreno JL, Umali A, Rayannavar V, Hodes GE, Russo SJ, González-Maeso J. Prenatal stress induces schizophrenia-like alterations of serotonin 2A and metabotropic glutamate 2 receptors in the adult offspring: role of maternal immune system. J Neurosci. 2013;33:1088–1098. doi: 10.1523/JNEUROSCI.2331-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Darr T, Hazrati LN, Visanji NP, Pires D, Brotchie JM, Fox SH. Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov Disord. 2010;25:1399–1408. doi: 10.1002/mds.23083. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav. 1995;52:649–654. doi: 10.1016/0091-3057(95)00160-x. [DOI] [PubMed] [Google Scholar]
- Jones CR, Rosenkranz K, Rothwell JC, Jahanshahi M. The right dorsolateral prefrontal cortex is essential in time reproduction: an investigation with repetitive transcranial magnetic stimulation. Exp Brain Res. 2004;158:366–372. doi: 10.1007/s00221-004-1912-3. [DOI] [PubMed] [Google Scholar]
- Keeler MH. Similarity of schizophrenia and the psilocybin syndrome as determined by objective methods. Int J Neuropsychiatry. 1965;1:630–634. [PubMed] [Google Scholar]
- Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KL, Edwards DD, Shoichet BK, Roth BL. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna JC, Sedman G. The subjective experience of time during lysergic acid diethylamide (LSD-25) intoxication. Psychopharmacologia. 1964;5:280–288. doi: 10.1007/BF02341260. [DOI] [PubMed] [Google Scholar]
- Kim J, Jung AH, Byun J, Jo S, Jung MW. Inactivation of medial prefrontal cortex impairs time interval discrimination in rats. Frontiers Behav Neurosci. 2009;3:1–9. doi: 10.3389/neuro.08.038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano K, Okamato H, Fukai T. Time representing cortical activities: two models inspired by prefrontal persistant activity. Biol Cybernetics. 2003;88:387–394. doi: 10.1007/s00422-002-0390-6. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav. 2002;73:327–332. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Kohnomi S, Suemaru K, Kawasaki H, Araki H. Effect of aripiprazole on 5-HT2 receptor-mediated wet-dog shake responses and disruption of prepulse inhibition. J Pharmacol Sci. 2008;106:645–650. doi: 10.1254/jphs.fp0071924. [DOI] [PubMed] [Google Scholar]
- Knauer A, Maloney WJMA. A prelimnary note on the psychic action of mescaline, with special reference to the mechanism of visual hallucinations. J Nerv Ment Dis. 1913;40:425–436. [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology. 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Lee KH, Bhaker RS, Mysore A, Parks RW, Birkett PBL, Woodruff PWR. Time perception and its neuropsychological correlates in patients with schizophrenia and in healthy volunteers. Psychiatry Res. 2009;166:174–183. doi: 10.1016/j.psychres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Lewis R, Kapur S, Jones C, DaSilva J, Brown GM, Wilson AA, Houle S, Zipursky RB. Serotonin 5-HT2 receptors in schizophrenia: a PET study using [18F]setoperone in neuroleptic-naive patients and normal subjects. Am J Psychiatry. 1999;156:72–78. doi: 10.1176/ajp.156.1.72. [DOI] [PubMed] [Google Scholar]
- Lilly. Lilly announces pomaglumetad methionil did not meet primary endpoint of clinical study. 2012 http://newsroom.lilly.com/releasedetail.cfm?releaseid=690836 (Retrieved May 27, 2013)
- Liu W, Downing AC, Munsie LM, Chen P, Reed MR, Ruble CL, Landschulz KT, Kinon BJ, Nisenbaum LK. Pharmacogenetic analysis of the mGlu2/3 agonist LY2140023 monohydrate in the treatment of schizophrenia. Pharmacogenomics J. 2012;12:246–254. doi: 10.1038/tpj.2010.90. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kato M, Muramatsu T, Iwashita S, Mimura M, Kashima H. Abberrant sense of agency in patients with schizophrenia: forward and backward over-attribution of temporal causality during intentional action. Psychiatry Res. 2012;198:1–6. doi: 10.1016/j.psychres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology. 2005;30:2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Mayer-Gross W. Experimental psychoses and other mental abnormalities produced by drugs. Br Med J. 1951;2:317–320. doi: 10.1136/bmj.2.4727.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MS, Fowler RC, Cadoret RJ, Winokur G. Symptom differences in schizophrenia with good and poor prognosis. Am J Psychiatry. 1972;128:1239–1243. doi: 10.1176/ajp.128.10.1239. [DOI] [PubMed] [Google Scholar]
- McFarland K, Price DL, Bonhaus DW. Pimavanserin, a 5-HT2A inverse agonist, reverses psychosis-like behaviors in a rodent model of Parkinson’s disease. Behav Pharmacol. 2011;22:681–692. doi: 10.1097/FBP.0b013e32834aff98. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Brit J Med Psychology. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Meincke U, Light GA, Geyer MA, Braff DL, Gouzoulis-Mayfrank E. Sensitization and habituation of the acoustic startle reflex in patients with schizophrenia. Psychiatry Research. 2004;126:51–61. doi: 10.1016/j.psychres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17:263–287. doi: 10.1093/schbul/17.2.263. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Arvanitis L, Bauer D, Rein W Meta-Trial Study Group. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161:975–984. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Bastani B, Ramirez L, Matsubara S. Clozapine: new research on efficacy and mechanism of action. Eur Arch Psychiatry Neurol Sci. 1989;238:332–339. doi: 10.1007/BF00449814. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D, Friedman JH. Pimavanserin, a serotonin2A receptor inverse agonist, for the treatment of parkinson’s disease psychosis. Neuropsychopharmacology. 2010;35:881–892. doi: 10.1038/npp.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro G, Traficante A, Riozzi B, Di Menna L, Curto M, Pallottino S, Nicoletti F, Bruno V, Battaglia G. Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice. Mol Pharmacol. 2009;76:379–387. doi: 10.1124/mol.109.056580. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011a;493:76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Rayannavar V, Sealfon SC, González-Maeso J. Chronic treatment with LY341495 decreases 5-HT2A receptor binding and hallucinogenic effects of LSD in mice. Neurosci Lett. 2013;536:69–73. doi: 10.1016/j.neulet.2012.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Kurita M, Holloway T, López J, Cadagan R, Martínez-Sobrido L, García-Sastre A, González-Maeso J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J Neurosci. 2011b;31:1863–1872. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuéllar F, Mocci G, Seto J, Callado LF, Neve RL, Milligan G, Sealfon SC, López-Giménez JF, Meana JJ, Benson DL, González-Maeso J. Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A·mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem. 2012;287:44301–44319. doi: 10.1074/jbc.M112.413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruza C, Moreno JL, Umali A, Callado LF, Meana JJ, González-Maeso J. Dysregulated 5-HT(2A) receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.10.006. in press pii: S0924-977X(12)00285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan ET, Yatham LN, Ruth TJ, Liddle PF. Decreased serotonin 2A receptor densities in neuroleptic-naive patients with schizophrenia: a PET study using [(18)F]setoperone. Am J Psychiatry. 2000;157:1016–1018. doi: 10.1176/appi.ajp.157.6.1016. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Serotonin 5-HT2 receptors in schizophrenic patients studied by positron emission tomography. Life Sci. 2000;66:2455–2464. doi: 10.1016/s0024-3205(00)80005-3. [DOI] [PubMed] [Google Scholar]
- Osmond H, Smythies J. Schizophrenia: a new approach. J Ment Sci. 1952;98:309–315. doi: 10.1192/bjp.98.411.309. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A, Grottick AJ, Moreau J, Higgins GA. Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology. 2001;25:565–575. doi: 10.1016/S0893-133X(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Padich RA, McCloskey TC, Kehne JH. 5-HT modulation of auditory and visual sensorimotor gating: II. Effects of the 5-HT2A antagonist MDL 100,907 on disruption of sound and light prepulse inhibition produced by 5-HT agonists in Wistar rats. Psychopharmacology. 1996;124:107–116. doi: 10.1007/BF02245610. [DOI] [PubMed] [Google Scholar]
- Pálenícek T, Balíková M, Bubeníková-Valesová V, Horácek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology. 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Sanfilipo M, Chappell PB, Chakravorty S, Gonzenbach S, Ko GN, Rotrosen JP. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Penney TB, Gibbon J, Meck WH. Categorical scaling of duration bisection in pigeons (Columba livia), mice (Mus musculus), and humans (Homo sapiens) Psychol Sci. 2008;19:1103–1109. doi: 10.1111/j.1467-9280.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S. Keeping time: effects of focal frontal lesions. Neuropsychologia. 2006;441:1195–209. doi: 10.1016/j.neuropsychologia.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Pletcher A, Shore PA, Brodie BB. Serotonin release as a possible mechanism of reserpine action. Science. 1955;122:374–375. doi: 10.1126/science.122.3165.374. [DOI] [PubMed] [Google Scholar]
- Pouthas V, George N, Poline JB, Pfeuty M, Vandemoorteele PF, Hugueville L, Ferrandez AM, Lehéricy S, Lebihan D, Renault B. Neural network involved in time perception: an fMRI study comparing long and short interval estimation. Hum Brain Mapp. 2005;25:433–441. doi: 10.1002/hbm.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Geyer MA. Overview of animal models of schizophrenia. Curr Protoc Neurosci. 2007;Chapter 9(Unit 9):24. doi: 10.1002/0471142301.ns0924s39. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Geyer MA, Halberstadt AL. Serotonin and schizophrenia. In: Muller CP, Jacobs BL, editors. Handbook of the Behavioral Neurobiology of Serotonin. Amsterdam: Academic Press; 2010. pp. 585–620. [Google Scholar]
- Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology. 2012;37:630–640. doi: 10.1038/npp.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Kühn KU, Mössner R, Schwab SG, Schuhmacher A, Maier W, Wagner M. Sensorimotor gating of schizophrenia patients is influenced by 5-HT2A receptor polymorphisms. Biol Psychiatry. 2008;64:434–437. doi: 10.1016/j.biopsych.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Schmechtig A, Ettinger U, Petrovsky N, Collier DA, Vollenweider FX, Wagner M, Kumari V. Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study. Biol Psychiatry. 2009;66:614–620. doi: 10.1016/j.biopsych.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammsayer T. Temporal discrimination in schizophrenic and affective disorders: evidence for a dopamine-dependent internal clock. Int J Neurosci. 1990;53:111–120. doi: 10.3109/00207459008986593. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu C-F, Thompson RF. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Memory. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport MM. Serum vasoconstrictor (serotonin) the presence of creatinine in the complex; a proposed structure of the vasoconstrictor principle. J Biol Chem. 1949;180:961–969. [PubMed] [Google Scholar]
- Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176:1243–1251. [PubMed] [Google Scholar]
- Rasmussen H, Erritzoe D, Andersen R, Ebdrup BH, Aggernaes B, Oranje B, Kalbitzer J, Madsen J, Pinborg LH, Baaré W, Svarer C, Lublin H, Knudsen GM, Glenthoj B. Decreased frontal serotonin2A receptor binding in antipsychotic-naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 2010;67:9–16. doi: 10.1001/archgenpsychiatry.2009.176. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, Seiden LS. Fluoxetine prevents the disruptive effcts of fenfluramine on differential-reinforcement-of-low-rate 72-second schedule performance. J Pharmacol Exp Ther. 1993;267:1256–1263. [PubMed] [Google Scholar]
- Rigdon GC, Weatherspoon JK. 5-Hydroxytryptamine1a receptor agonists block prepulse inhibition of acoustic startle reflex. J Pharmacol Exp Ther. 1992;263:486–493. [PubMed] [Google Scholar]
- Rinkel M, De SH, Hyde RW, Solomon HC. Experimental schizophrenia-like symptoms. Am J Psychiatry. 1952;108:572–578. doi: 10.1176/ajp.108.8.572. [DOI] [PubMed] [Google Scholar]
- Rinkel M, Hyde RW, Solomon HC, Hoagland H. Experimental psychiatry. II. Clinical and physio-chemical observations in experimental psychosis. Am J Psychiatry. 1955;111:881–895. doi: 10.1176/ajp.111.12.881. [DOI] [PubMed] [Google Scholar]
- Roth B, Meltzer HY. The role of serotonin in schizophrenia. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. American College of Neuropsychopharmacology; Nashville TN: 2000. pp. ACNP website. [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Andrew C, Bullmore E. Prefrontal involvement in “temporal bridging” and timing movement. Neuropsychologia. 1998;36:1283–1293. doi: 10.1016/s0028-3932(98)00038-4. [DOI] [PubMed] [Google Scholar]
- Schmidt H, McFarland J, Ahmed M, McDonald C, Elliott MA. Low-level temporal coding impairments in psychosis: preliminary findings and recommendations for further studies. J Abnorm Psychol. 2011;120:476–482. doi: 10.1037/a0023387. [DOI] [PubMed] [Google Scholar]
- Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- Serko A. Im Mescalinrausch. Jahrbücher fur Psychiatrie Neurologie. 1913;31:355–366. [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. DOI disruption of prepulse inhibition in the rat is mediated by 5-HT2A and not by 5-HT2C receptors. Behav Pharmacol. 1995;6:839–842. [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits for mental timekeeping. Hum Brain Mapp. 2007;28:394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll WA. Lysergsäure-diäthylamid, ein Phantastikum aus der Mutterkorngruppe. Schweiz Arch Neurol Psychiatr. 1947;60:279–323. [Google Scholar]
- Stoll A, Hofmann A. Partialsynthese von Alkaloiden vom Typus des Ergobasins (6. Mitteilung iiber Mutterkornalkaloide) Helv Chimica Acta. 1943;26:944–966. [Google Scholar]
- Stubbs DA. Temporal differentiation and a free-operant psychophysical procedure. J Exp Anal Behav. 1980;33:167–185. doi: 10.1901/jeab.1980.33-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Sysoeva OV, Tonevitsky AG, Wackermann J. Genetic determinants of time perception mediated by the serotonergic system. PLoS One. 2010;5:e12650. doi: 10.1371/journal.pone.0012650. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Kolta P. Transitory increase of the acoustic startle reaction during habituation. Acta Physiologica Academiae Scientiarum Hungaricae. 1967;31:51–56. [PubMed] [Google Scholar]
- Tadano T, Hozumi M, Satoh N, Oka R, Hishinuma T, Mizugaki M, Arai Y, Yasuhara H, Kinemuchi H, Niijima F, Nakagawasai O, Tan-no K, Kisara K. Central serotonergic mechanisms on head twitch response induced by benzodiazepine receptor agonists. Pharmacology. 2001;62:157–162. doi: 10.1159/000056089. [DOI] [PubMed] [Google Scholar]
- Taiminen T, Jääskeläinen S, Ilonen T, Meyer H, Karlsson H, Lauerma H, Leinonen KM, Wallenius E, Kaljonen A, Salokangas RK. Habituation of the blink reflex in first-episode schizophrenia, psychotic depression and non-psychotic depression. Schizophrenia Res. 2000;44:69–79. doi: 10.1016/s0920-9964(99)00140-1. [DOI] [PubMed] [Google Scholar]
- Tenckhoff A, Tost H, Braus DF. Altered perception of temporal relations in schizophrenic psychoses. Nervenarzt. 2002;73:428–433. doi: 10.1007/s00115-001-1254-3. [DOI] [PubMed] [Google Scholar]
- Todd J. Impaired detection of silent interval change in schizophrenia. Neuroreport. 2006;17:785–789. doi: 10.1097/01.wnr.0000220126.93973.31. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Monaco C, McMichael H, Tyson K, Chambliss C, Christensen HL, Celenza MA. Information-processing characteristics of explicit time estimation by patients with schizophrenia and normal controls. Percept Mot Skills. 1998;86:515–526. doi: 10.2466/pms.1998.86.2.515. [DOI] [PubMed] [Google Scholar]
- Trichard C, Paillere-Martinot ML, Attar-Levy D, Blin J, Feline A, Martinot JL. No serotonin 5-HT2A receptor density abnormality in the cortex of schizophrenic patients studied with PET. Schizophr Res. 1998;31:13–17. doi: 10.1016/s0920-9964(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Turgeon M, Giersch A, Delevoye-Turrell Y, Wing AM. Impaired predictive timing with spared time interval production in individual with schizophrenia. Psychiatry Res. 2012;197:13–8. doi: 10.1016/j.psychres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Twarog BM, Page IH. Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol. 1953;175:157–161. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- Tysk L. Estimation of time and the subclassification of schizophrenic disorders. Percept Motor Skills. 1983;57:911–918. doi: 10.2466/pms.1983.57.3.911. [DOI] [PubMed] [Google Scholar]
- Tysk L. A longitudinal study of time estimation in psychotic disorders. Percept Motor Skills. 1984;59:779–789. doi: 10.2466/pms.1984.59.3.779. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Ruimschotel E, Martin S, Risbrough VB, Halberstadt AL. Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT1A receptor knockout mice: implications for schizophrenia. Neuropharmacology. 2011;61:209–216. doi: 10.1016/j.neuropharm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Bakshi VP, Geyer MA. M100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague-Dawley and Wistar rats. Neuropsychopharmacology. 1999;20:311–321. doi: 10.1016/S0893-133X(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Examination of drug-induced and isolation-induced disruptions of prepulse inhibition as models to screen antipsychotic drugs. Psychopharmacology. 1995;122:15–26. doi: 10.1007/BF02246437. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- Volz HP, Nenadic I, Gaser C, Rammsayer T, Hager F, Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. NeuroReport. 2001;12:313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Wittmann M, Hasler F, Vollenweider FX. Effects of varied doses of psilocybin on time interval reproduction in human subjects. Neurosci Lett. 2008;435:51–55. doi: 10.1016/j.neulet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Wahl OF, Sieg D. Time estimation among schizophrenics. Percept Motor Skills. 1980;50:535–541. doi: 10.1177/003151258005000232. [DOI] [PubMed] [Google Scholar]
- Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2012;62:1175–1181. doi: 10.1016/j.neuropharm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters F, Jablensky A. Time discrimination in schizophrenia patients with first-rank (passivity) symptoms. Psychiatry Res. 2009;167:12–20. doi: 10.1016/j.psychres.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology. 2004;172:233–240. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Hollensteiner KJ, Koch M. Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav Pharmacol. 2011;22:805–813. doi: 10.1097/FBP.0b013e32834d6279. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Koch M. Pre-treatment with the mGlu2/3 receptor agonist LY379268 attenuates DOI-induced impulsive responding and regional c-Fos protein expression. Psychopharmacology. 2012;219:387–400. doi: 10.1007/s00213-011-2441-y. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Carter O, Hasler F, Cahn BR, Grimberg U, Spring P, Hell D, Flohr H, Vollenweider FX. Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol. 2007;21:50–64. doi: 10.1177/0269881106065859. [DOI] [PubMed] [Google Scholar]
- Woolley DW, Shaw E. Some neurophysiological aspects of serotonin. Br Med J. 1954;2:122–126. doi: 10.1136/bmj.2.4880.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Behavioral effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rats and mice. Eur J Pharmacol. 1975;32:156–162. doi: 10.1016/0014-2999(75)90278-2. [DOI] [PubMed] [Google Scholar]