Abstract
Significance: A range of studies point to the efficacy of electrical stimulation (ES) in wound treatment, but the methodology of its application has not been determined to date. This article provides a critical review of the results of clinical trials published by researchers using high-voltage pulsed current (HVPC) to treat chronic wounds. In describing the methodology of the trials, the article gives special attention to electric stimulus parameters, the frequency of procedures and total treatment duration.
Recent Advances: HVPC is a monophasic pulsed electric current that consists of double-peaked impulses (5–200 μs), at very high peak-current amplitude (2–2.5 A), and high voltage (up to 500 V), at a frequency of 1–125 pulses per second. HVPC can activate “skin battery” and cellular galvanotaxis, and improves blood flow and capillary density.
Critical Issues: HVPC efficacy was evaluated in conservatively treated patients with diabetic foot, venous leg and pressure ulcers (PUs), and in some patients with surgically treated venous insufficiency.
Future Directions: The efficacy of HVPC as one of several biophysical energies promoting venous leg ulcer (VLU) and PU healing has been confirmed. Additional studies are needed to investigate its effect on the healing of other types of soft tissue defects. Other areas that require more research include the identification of the therapeutic effect of HVPC on infected wounds, the determination of the efficacy of cathodal versus anodal stimulation, and the minimal daily/weekly duration of HVPC required to ensure optimal promotion of wound healing.

Anna Polak, PT, PhD
Scope and Significance
A range of studies point to the efficacy of ES in wound treatment, but its methodology has not been determined to date. This article provides a critical review of the results of clinical trials published by researchers using high-voltage pulsed current (HVPC) to treat chronic wounds. In describing the methodology of the trials, the article gives special attention to electric stimulus parameters, the frequency of procedures, and total treatment duration.
Translational Relevance
Healthy epidermis has a negative charge (−23.4 mV) as opposed to the dermis that is electropositive.1 This difference between charges causes a flow of a natural monophasic electric current that ostensibly stimulates wound healing. An exogenous current applied to the wound surface is believed to further enhance healing processes.2,3 Another mechanism that may promote wound healing is cellular electrotaxis2,3 of macrophages,4,5 neutrophils,4,6 and fibroblasts7–10 to the wound. Electrical stimulation (ES) activates the production of ATP and DNA,4,11 makes fibroblasts generate more collagen,7,12,13 and increases blood flow and capillary density.14–16
Clinical Relevance
The treatment of chronic wounds takes time and involves the application of various therapies. If the wound fails to respond to standard care, various physical therapeutic energies are available for treatment. Gudelines17 on pressure ulcer (PU) treatment give a special role to ES, recommending it for the management of recalcitrant Stage II-IV PUs. However, a wider use of ES in wound treatment may be hampered by the lack of studies precisely defining the type of therapeutic electric current to be used, its pulse parameters, electrode polarity, and the duration and frequency of treatments.
Discussion of Findings and Relevant Literature
This article critically reviews the results of clinical trials whose authors used HVPC to treat chronic wounds. The relevant studies were identified using a computer-based literature search of the following databases: EBSCOhost, MEDLINE, Academic Search Complete, and Health Source: Nursing/Academic Edition. There was no restriction because of language of the article; the only requirement was that the publication had an abstract in English. The main keywords used for selection purposes were wound healing, PU, leg ulcer, venous ulcer, diabetic foot, chronic wound, chronic ulcer, ES, high-voltage stimulation, and HVPC. In all cases, the analysis was made based on full articles.
In the review, randomized controlled trials (RCT) or clinical trials involving human subjects were considered. Eleven clinical trials18–28 published between 1988 and 2012 were accepted for analysis, including 10 RCTs18,19,21–28 and one casuistic case study20 selected for its extremely high rate of ulcer healing. HVPC efficacy was evaluated in conservatively treated patients with pressure,18,19,27,28 chronic leg20–22,24,26 and diabetic foot23 ulcers, and in some patients with surgically treated venous insufficiency.25,26
In describing the methodology of the trials, the electric stimulus parameters, the frequency of procedures, and the total treatment times will be presented first, and then the risk of bias in the reviewed studies will be assessed. The authors of almost all analyzed trials18,19,21–28 designed them with control groups that received standard wound care (SWC) alone21,22,25–28 or SWC plus sham HVPC.18,19,23,24
The experimental groups were treated with single 45–60 min sessions of active HVPC in a 24-h period. During the other 23 h of the research protocol, the investigators were ethically bound to provide treatment to the wound. Although the treatment was not always described in the published HVPC studies, it was almost always SWC selected to match the type of the wound and includes debridement and dressings to maintain a moist wound environment. SWC measures applied to PUs also include a pressure-reducing or relieving bed and/or a wheelchair, and patient turning/repositioning at 2-h intervals. Venous leg ulcers (VLUs) are treated with compression therapy and diabetic foot ulcers (DFU) may be treated with offloading casts or boots.
Kloth and Feedar18 (1988) in their controlled study randomly assigned 16 patients with stage IV dermal ulcers to an experimental group (active HVPC; nine subjects) and a control group (sham HVPC; seven subjects). A person not involved in the study tossed a coin to assign patients to the ES or control groups. Both groups received 23 h of SWC per day. In addition, 45 min of HVPC was delivered 5 days per week directly into the 9 wounds of the experimental group at 105 pulses per second (pps) and the current amplitude set to just below that which produced a visible muscle contraction. ES always started with anodal stimulation directly to the wound; in five patients, it was continued for the whole period of treatment. Four patients in the experimental group reached an initial healing plateau; the cathode was then moved over the wound. When the same patients reached a second healing plateau, electrode polarity on the wound was alternated daily. Seven patients in the control group received 45 min of sham HVPC plus SWC. The wounds of patients in the treatment group healed completely in a mean of 7.3 weeks at a rate of 45% per week. In the control group wound size increased a mean of 29% during a mean period of 7.4 weeks.
Treatment results that the authors18 observed in the control group are also interesting. In three patients that initially received SWC, wound area increased by 1.2 percent over 8.7 weeks. After they were transferred to the treatment crossover group, their wounds healed at an average rate of 38% per week, closing completely in an average of 8.3 weeks.
Kloth and Feedar18 always placed the anode cephalad closer to the neuraxis than the cathode to amplify the injury potential, as suggested by Becker (as quoted in Kloth and Feedar18). The authors of the other studies did not adhere to this rule.
In a second randomized, controlled study, Griffin et al.19 (1991) assessed HVPC efficacy for healing stage II, III, or IV PUs in male patients with spinal cord injury. Of 17 patients with PU in the pelvic region, 8 were randomly assigned to the HVPC group and 9 to the control group (sham HVPC). All wounds were daily treated with SWC. In addition, the HVPC group received ES for 60 min on 20 consecutive days, with the cathode applied to deliver current directly into the wound. The stimulator was set to deliver 100 pps and 200 V, which produced 500 μC/s at the treatment electrode. Ulcer surface area was measured before and during ES treatment on days 5, 10, 15, and 20. Percentage of change from pretreatment ulcer size was calculated for each measurement interval.
After only 5 days of treatment, ulcers in the HVPC group had significantly greater mean wound area reductions compared with their pretreatment size than ulcers in the control group (p=0.03). Subsequently, in the next days, PUs treated with ES continued to heal faster than in the placebo group (p=0.05 on days 15 and 20). Griffin et al.19 established that after 20 days of applying HVPC to 8 patients (initial mean wound size of 2.34 cm2) their wounds healed 80% compared with an average of 52% in the placebo group (p=0.05).
A drawback of the Kloth and Feedar18 and Griffin et al.19 studies was that their samples of subjects were relatively small (respectively 9 and 8 in their treatment groups and 7 and 9 in the control groups).
Polak and Franek20 (1999) have described a case of a particularly fast healing VLU treated with HVPC. An 85-year-old woman was admitted to the hospital on November 10, 1997 to be treated for an ulcer that had developed in 1995 and kept increasing in size, despite the application of various therapies. The patient was administered HVPC (double-peak, monophasic impulses; 100 μs; 100 pps; 100 V) 50 min a day, six times a week. Treatment continued for 14 weeks during which a total of 92 procedures were provided. The first 12 procedures utilized cathodal stimulation, which was replaced by anodal stimulation until treatment ended on February 2, 1998. The baseline wound surface area (WSA) was 166.4 cm2. HVPC helped reduce it to 4 cm2. The mean rate of decrease was 11.6 cm2 a week. The decrease was the fastest in the first 7 weeks, its average rate being 17.1 cm2 a week. Over the next 7 weeks, the rate declined to 6.1 cm2 a week, which was still a good result. In the period of treatment, only physiological saline dressings were additionally applied to prevent desiccation between ES sessions.
In two studies conducted in 2000, Polak et al.21 and Franek et al.22 evaluated HVPC for its healing effect on chronic VLUs and found it to be very promising.
The first study21 involved two groups of a total of 44 patients with VLUs, of whom 22 received HVPC and the remaining 22 served as the control group that received SWC. Although 7 weeks of therapy produced positive results in both groups, the surface area of wounds in the HVPC group decreased significantly more than in the control group—the respective rates were 73.4% and 46.9% (p<0.05).
In the second investigation,22 three groups totaling 79 patients were studied. One group had 33 patients that received HVPC (the HVPC group) and the second had 32 patients treated with SWC (the SWC group). Fourteen patients in the SWC group were treated with Unna's Boot (the Unna's Boot-group). The referring physician alternated referrals to each hospital as patients came, so their assignment to one or the other ward (i.e., to the ES or control group) was largely random.
In both studies,21,22 patients in HVPC and SWC groups received compression therapy involving two layers of short-stretch bandages and SWC. The Unna's Boot patients23 were treated on an outpatient basis, with the Unna's boot usually replaced once a week.
The average periods of treatment were 7, 6, and 5.5 weeks in the HVPC, SWC, and the Unna's Boot groups, respectively. In all three groups wound size significantly decreased against the baseline (p<0.001). Although the weekly wound area change was the greatest in the HVPC group, the groups were relatively similar in that respect. The granulation areas compared before and after treatment were not statistically significantly different; however, differences were noted after 2 weeks of treatment—the amount of granulation tissue in the HVPC group was statistically greater than in the other groups (p<0.003). In this group, granulation tissue accounted for 84.9% of wound surface (53.9% in the SWC group and 63.7% in the Unna's Boot group), a likely indication of cathodal stimulation having a positive effect on the growth of granulation tissue.
The authors of both studies21,22 used the same stimulus parameters to apply ES. HVPC (100 μs, 100 pps, 100 V, 50 min, 6 days in a week) delivered to wounds only induced a comfortable sub-motor sensory perception by the patient. Each session started with cathodal stimulation, which continued for 1–3 weeks until granulation tissue was observed. Then polarity was changed to positive for the remaining time of treatment.
In a double-blind, placebo-controlled, 12-week trial Peters et al.23 (2001) studied the effect of HVPC as an adjunct to healing DFUs in 40 patients randomized to active HVPC (20 patients) and sham HVPC (20 patients) groups. Placebo and ES devices were randomly distributed among the study patients by the investigators who were unaware of which device was a placebo and which was a “live” device. The manufacturer of the devices was not informed on what devices the particular patients were given. Each device had a unique identification number; the manufacturer of the devices revealed their status (whether placebo or ES) only after they were collected from all patients. This made it possible for the investigators to match the identification numbers with the corresponding devices and to start analyzing the data. After 12 weeks, 65% of wounds closed in the active HVPC group compared with 35% in the sham HVPC group (p=0.058). After stratification by compliance, a significant difference was found between the compliant patients (71% of wounds closed) and the noncompliant patients (50% of wounds closed) in the treatment group. In the placebo group, 39% of wounds closed in the compliant patients compared with 29% in the noncompliant patient wounds (p=0.038).
The stimulus parameters that Peters et al.23 applied differed from the studies previously described. Active and sub-sensory HVPC (50 V, pulse duration of 100 μs) was delivered via a Dacron-mesh silver nylon stocking worn nightly for 8 h. An 8-h cycle consisted of 20 min on (first 80 pps and then 8 pps, each for 10 min) and 40 min off. ES was applied 160 min a day, 7 days a week, for a total of 18.7 h per week. Differences in the healing rates of DFU of the compliant and noncompliant HVCP patients observed by the authors23 showed that ES of duration shorter than 18.7 h per week was therapeutically less effective.
In another randomized, double-blind prospective clinical trial Houghton et al.24 (2003) treated 27 patients with 42 chronic leg ulcers of various etiologies (diabetic, arterial, venous, and mixed). The subjects were randomly assigned to group A or B. Both groups were identically treated with HVPC ES devices marked either “A” or “B,” as appropriate. The equipment used on the subjects who received the sham treatment had been deactivated by the manufacturer in an inconspicuous manner, so that neither the subjects nor the researchers were aware which group of subjects had received real or sham treatment. Fourteen patients received SWC in tandem with active HVPC (100 μs, 100 pps, 150 V), while the other 13 received SWC and sham HVPC. Active (negative) or sham electrodes were applied to all wounds. Because 45-min sessions were held once a day 3 times a week, the total treatment time was only 2.25 h per week.
Interestingly, ES outcomes in the Houghton et al.24 study were positive despite the short time of its application. By the 4th week WSA in the HVPC group decreased by 44.3% compared with only 16% in the control group (p<0.05). The limitation of the Houghton et al.24 study was that the ulcers they treated were of different origin—diabetic (2), arterial (2), venous (7), and mixed (3).
In two other prospective, randomized, controlled trials Franek et al.25,26 (2005, 2006) reported that even though HVPC (100 μs; 100 V; 100 pps) was efficacious in the conservative treatment (CT) of chronic VLUs, it failed to accelerate the healing of ulcers in patients after venous surgery (VS).
The first study25 involved 60 patients after VS conducted with the modified Babcock's29–31 method. Postsurgery patients were randomly divided into an HVPC group and control group of 30 subjects each. Patients in both groups received SWC that included, leg compression therapy with compression stockings that exerted 30 mmHg pressure at the ankles, local wet dressings of 0.9% NaCl and drug treatment consisting of two tablets of 450 mg diosmin and 50 mg hesperidin daily (Diosmin strengthens blood vessel walls and normalizes vascular permeability; Hesperidin has an anti-inflammatory effect, increases the strength and elasticity of vascular walls, and decreases vascular permeability). The experimental group also received HVPC (100 μs; 100 V; 100 pps).
The two groups had statistically significant differences only in the growth of granulation tissue. Granulation tissue growth was greater in the HVPC group both after 2 weeks of treatment (p=0.0002) and at the end of treatment (p=0.0006) than in the control group. Wound sizes decreased in both groups but the differences were not statistically significant. The authors concluded that HVPC did not expedite the healing of VLUs in postsurgical patients.26
In the second study, Franek et al.26 compared the effects of treatment of 110 patients with VLUs divided into four comparable groups. Fifty-five patients divided into two groups had undergone VS with the modified Babcock's29–31 method. One group (27 patients) received only SWC (the VS group) and the other group (28 patients) received SWC plus HVPC (the VS+HVPC group). Patients in the other two groups were treated conservatively. One group (27 patients) received only SWC (the CT group) and the other group (28 patients) was treated with SWC combined with HVPC (the CT+HVPC group). SWC administered to all four comparable groups was the same as in the aforementioned study.25 VS+HVPC and CT+HVPC groups had ES daily (100 V; 100 μs; 100 pps) for 50 min, six times a week.
The study26 confirmed the earlier results.25 Although treatment was effective in all studied groups, HVPC produced better results only in the conservatively treated patients. The rate of healed ulcers in the CT+HVPC group was statistically greater than in the CT group (21.42% versus 7.40%; p=0.03). Percentage of healed ulcers in the VS+HVPC group (21.4%) was not statistically greater than VS+SWC group (25.92%; p>0.05).
HVPC effect on granulation tissue growth was confirmed again. After the first 2 weeks of cathodal stimulation the amount of granulation tissue in the CT+HVPC group was statistically greater than in the CT and VS groups (p=0.01 and p=0.001, respectively), and in the VS+HVPC group compared with the CT and VS groups (p=0.01 and p=0.01). This tendency continued after treatment (p=0.01 in all cases). In the CT+HVPC and VS+HVPC groups, the amounts of granulation tissue were similar.26
Franek et al.25,26 contend that a well-performed surgery can lead to wound closure, without the healing process having to be enhanced by HVPC. However, in conservatively treated patients HVPC accelerates granulation and re-epithelialization of wounds, thereby reducing total WSA and volume.
Two recent studies point to the efficacy of HVPC applied to treat stage II-IV PUs.27,28
Houghton et al.27 (2010) conducted a single-blind, parallel-group, randomized, controlled clinical trial with 34 patients whose stage II-IV PUs developed after spinal cord injuries. All patients received SWC and 16 were additionally treated with active HVPC in a community setting. The eligible subjects were assigned to receive either SWC or HVPC+SWC using a concealed, random process that involved the opening of an opaque envelope with a random number prepared by an independent person.
In a treatment period of 12 weeks, all stage II PUs closed in both the HVPC group and the control group. In the HVPC group, 33.3% of stage III-IV PUs closed compared with 7.1% in the control group (p=0.550). In the HVPC group, 80% of PUs decreased in surface area by at least 50%. This result was significantly better than in the control group, where the wound area reduction was 36% (p=0.02). The average percentage decrease in WSA at treatment end was significantly greater in the HVPC group (70.0%) than in the control group (36%; p=0.048).
A major achievement of this study was proving that ES could be effectively delivered in the community, or at home, without a direct oversight by healthcare providers. HVPC (50 μs; 50–150 V) was applied about 5.3 h a day (typically overnight). The stimulator device was programmed to provide 20 min at a pulse frequency of 100 pps followed by 20 min at 10 pps and then 20 min off cycle for 8 h each day for a period of at least 3 months (a total of 443 h of stimulation time), or until the ulcer closed. The current intensity was set below the level of muscle contraction. The polarity of the treatment electrode was initially negative and then alternated weekly.
One drawback of the Houghton et al.27 study was the inconsistent application of the therapeutic protocol. Three patients used the ES device for a total average of 67 h over 3 months (which was much shorter than the intended 443 h).
The other randomized, controlled clinical study was carried out by Franek et al.28 (2012) with fifty patients with stage II-III PUs located on the lower extremities. The patients were randomly divided into two comparative groups. Measures to prevent the development of new PUs were taken on all patients. All wounds received SWC to promote moist wound healing. In one group, HVPC (100 pps; 100 μs; 100 V) was additionally applied for 50 min a day, 5 days a week (the HVPC group). Cathodal stimulation applied for the first 1–2 weeks was replaced by anodal stimulation for the remainder of the treatment period.
After 6 weeks wound areas decreased significantly in both groups (p=0.000008 in the HVPC group and p=0.0009 in the control group). Granulation tissue grew against the baseline in both groups, but the difference was statistically significant only in the HVPC group (p=0.0006 in the HVPC group and p=0.845 in the control group).
A mean decrease in surface wound area was 88.9% in the HVPC group and 44.4% in the control group (p=0.00003). The healing rate was greater in the HVPC group after 2 weeks of treatment and did not change until the end of treatment (p=0.0008).
Innovation
The ES literature extensively covers HVPC. Studies are sufficiently numerous to attempt a summary of how HVPC influences wound healing and to propose some practical solutions for its application. The methodologies and the results of the reviewed studies are summarized in Tables 1 and 2.
Table 1.
Methods of HVPC electrical stimulations used in controlled randomized studies
| Kloth and Feedar18 | Griffin et al.19 | Polak et al.21 | Franek et al.22 | Peters et al.23 | |
|---|---|---|---|---|---|
| Type of wound | Pressure ulcers (IV stage) | Pressure ulcers (II-IV stage) | Venous leg ulcers | Venous leg ulcers | Diabetic foot ulcers |
| Total number of patients | 16 | 17 (only males) | 42 | 79 | 40 |
| Number of groups receiving ES | 1 group | 1 group | 1 group | 1 group | 1 group |
| Number of patients in the ES group(s) | 9 patients | 8 patients | 22 patients | 33 patients | 20 patients |
| Average patient age in the ES group(s) | 71 years | 32.5 years | 67 years | 68.1 years | 54.4 years |
| Treatment in the ES group(s) | HVPC | HVPC | HVPC+wet dressings | HVPC+wet dressings | HVPC+SWC |
| Treatment in the control group(s) | Sham HVPC | Sham HVPC | SWC | SWC | Sham HVPC+SWC |
| Unna's boot | |||||
| Characteristics of current | 100–175 V; 100 μs; 105 pps; 342 μC/s; | 200 V; 100 pps; 500 μC/s; | 100 V; 100 μs; 100 pps | 50 V; 100 μs; 80 pps per 10 min followed by 10 min of 8 pps | |
| Arrangement of the electrodes | Active on the PU; passive located proximally 15 cm from the PU | Active on the PU; passive located distally from the PU | Active on the wound and passive above the knee | Dacron-mesh silver nylon stocking on foot | |
| Polarity of the active electrode | Positive (polarity reversed if healing progress not observed) | Negative | Negative 1–3 weeks then positive | n/a | |
| Duration of the procedure | 45 min | 60 min | 50 min | 20 min | |
| Frequency of procedures | Once a day, 5 days a week | Once a day, 7 days a week | Once a day, 6 days a week | 8 times a day | |
| 7 days a week | |||||
| Period of treatment | 7.3 weeks | 20 successive days | 7 weeks | 12 weeks or until healing, whichever occurred first | |
| Number of hours per week | 3.75 h/a week | 7 h/a week | 5 h/a week | 18.7 h/a week | |
| Total ES time | 27.4 h | 20 h | 35 h | 224 h | |
| Houghton et al.24 | Franek et al.25 | Franek et al.26 | Houghton et al.27 | Franek et al.28 | |
|---|---|---|---|---|---|
| Type of wound | Chronic leg ulcers | Venous leg ulcers | Venous leg ulcers | Pressure ulcers (II-IV stage) | Pressure ulcers (II-IV stage) |
| Total number of patients | 27 | 60 | 110 | 34 | 50 |
| Number of groups receiving ES | 1 group | 1 group | 2 groups | 1 group | 1 group |
| Number of patients in the ES group(s) | 14 patients | 30 patients | 28 patients | 16 patients | 26 patients |
| 28 patients | |||||
| Average patient age in the ES group(s) | 66.3 years | 60 years | 68 years | 50.3 years | 59.0 years |
| 61 years | |||||
| Treatment in the ES group(s) | HVPC+SWC | VS+HVPC | VS+HVPC | HVPC+SWC | HVPC+SWC |
| CT+HVPC | |||||
| Treatment in the control group(s) | Sham HVPC+SWC | VS+SWC | VS+SWC | SWC | SWC |
| CT+SWC | |||||
| Characteristics of current | 150 V; 100 μs; 100 pps | 100 V; 100 μs; 100 pps | 100 V; 100 μs; 100 pps | 50–150 V; 50 μs; 100 pps per 20 min followed by 20 min of 10 pps | 100 V; 100 μs; 100 pps |
| Arrangement of the electrodes | Active on the PU; passive located proximally 20 cm from the PU | Active on the wound and passive above the knee | Active on the PU; passive located at least 20 cm from the PU | ||
| Polarity of the active electrode | Negative | Negative 2 weeks, then positive | Initially negative, then reverse weekly | Negative 1–2 weeks, then positive | |
| Duration of the procedure | 45 min | 50 min | 50 min | 40 min | 50 min |
| Frequency of procedures | Once a day | Once a day | Once a day | 8 times a day | Once a day |
| 3 days a week | 6 days a week | 6 days a week | 7 days a week | 5 days a week | |
| Period of treatment | 4 weeks | 7 weeks | 7 weeks | 12 weeks or until healing, whichever occurred first | 6 weeks |
| Number of hours per week | 2.25 h/a week | 5 h/a week | 5 h/a week | Around 37 h/a week | 4.16 h/a week |
| Total ES time | 9 h | 35 h | 35 h | Around 443 h | 25 h |
HVPC, high-voltage pulsed current; ES, electrical stimulation; SWC, standard wound care; pps, pulses per second; VS, venous surgery; PU, pressure ulcer.
Table 2.
Results of treatment with HVPC electrical stimulation in controlled clinical studies
| Reference | Wound Type | Groups | Number of Patients | Average Wound Area Before Therapy (cm2) | Healed Area (%) | Closed Ulcers (%) |
|---|---|---|---|---|---|---|
| Kloth et al.18 | PU | HVPC | 9 | 4.08 | 100 | 100 |
| Sham HVPC | 7 | 5.2 | 0 (ulcers increased by 8.9%) | 0 | ||
| Griffin et al.19 | PU | HVPC | 8 | 2.34 | 80 | 25 |
| Sham HVPC | 9 | 2.71 | 52 | 22 | ||
| Polak et al.21 | VLU | HVPC+wet dressing | 22 | 15.5 | 73.4 | n/a |
| SWC | 20 | 12.2 | 46.9 | |||
| Franek et al.22 | VLU | HVPC+wet dressing | 33 | 22.7 | 59.03 | n/a |
| SWC | 32 | 23.9 | 34.73 | |||
| Unna's boot | 14 | 10.5 | 24.76 | |||
| Peters et al.23 | DFU | HVPC+SWC | 20 | n/a | 86.2 | 65 |
| Sham HVPC+SWC | 20 | 71.4 | 35 | |||
| Houghton et al.24 | Leg ulcers | HVPC+SWC | 14 | 6.39 | 44.3 | n/a |
| Sham HVPC+SWC | 13 | 5.53 | 16.0 | |||
| Franek et al.25 | VLU | VS+HVPC | 30 | 25.85 | 59.63 | 20.00 |
| VS+SWC | 30 | 25.27 | 60.01 | 23.33 | ||
| Franek et al.26 | VLU | VS+HVPC | 28 | 21.4 | 87.11 | 21.42 |
| VS+SWC | 27 | 19.7 | 85.67 | 25.92 | ||
| CT+HVPC | 28 | 18.6 | 61.54 | 21.42 | ||
| CT+SWC | 27 | 19.3 | 44.11 | 7.40 | ||
| Houghton et al.27 | PU | HVPC+SWC | 16 | 3.38 | 70.0 | II stage—100% |
| III–IV stage—33.3% | ||||||
| At least 50% smaller—80% | ||||||
| SWC | 18 | 2.73 | 36.0 | II stage—100% | ||
| III–IV stage—7.1% | ||||||
| At least 50% smaller—36% | ||||||
| Franek et al.28 | PU | HVPC+SWC | 26 | 4.54 | 88.90 | n/a |
| SWC | 24 | 3.97 | 44.40 | n/a |
VLU, venous leg ulcer; DFU, diabetic foot ulcer.
HVPC performs well in the CT of various types of chronic wounds, such as stage II-IV PUs, venous ulcers, and DFU.
However, for clinical efficacy of HVPC ES in treating chronic wounds to be fully determined, the assessment of risk of bias in the included studies must be performed.
Effects of treatment
Stage II, III, and IV PUs treated with HVPC decreased in the analyzed studies by an average of 66–88.9% after 3–12 weeks (their initial sizes ranged from 2.34 to 4.54 cm2).19,27,28 Kloth and Feedar18 even found that stage IV PUs averaging 4.08 cm2 when therapy commenced closed after 7.3 weeks.
HVPC applied to chronic leg ulcers produced similar results. After 3–7 weeks, ulcers that initially ranged in area from 6.39 to 22.7 cm2 closed in 44.3–73.4%.21,22,24–26
HVPC promotes wound healing in the early stages of treatment.19,28 HPVC applied by Griffin19 for 5 days contributed to a significantly greater rate of PU healing in the HVPC group (p=0.03). The authors of another study28 on PUs found after 2 weeks that in the HVPC group wounds advanced toward closure more than control wounds (p=0.0008).
Electrical stimulus parameters
All reviewed studies18–28 used HVPC with monophasic double-peaked pulses. The pulse duration was set at 100 μs,18,20–26,28 excluding one study where it was 50 μs.27 Voltage always ranged from 50 to 200 V. The stimulation intensity was at a sensory level to prevent the occurrence of motor reactions.
In most studies, HVPC frequency ranged between 100 and 105 pps.18–22,24–26,28 However, Peters et al.23 used 80 pps for the first 10 min of stimulation and then 8 pps for the next 10 min. Houghton27 chose 100 pps for the first 20 min and 10 pps for the remaining 20 min to stimulate wounds.
Treatment duration
The review of clinical research studies showed that HVPC applied for only 2.25 h a week (45 min/day, 3 days in a week)24 effectively promotes wound healing. However, a typical treatment time is 45–60 min/day, 5 to 7 days a week, yielding a total of 3.75–7 h of treatment in a week.18–22,25,26,28
In two studies,23,27 the total weekly time of HPVC was 18.7 and 37.1 h a week; these treatment times were therapeutically effective without exposing the patients to any side effects.
Electrode placement
In all studies the treatment electrode was placed on the wound18–28 with return electrode on healthy skin at least 15–20 cm from the treatment electrode.18–22,24–28
Polarity of the wound treatment electrode
The polarity of the treatment electrode differed between studies, but most researchers first used the negative electrode to stimulate the wound surface.19–22,24–28
In Franek22,25,26,28 and Polak et al.20,21 studies, the cathode was the treatment electrode for the first 1–3 weeks and the stimulation time was coordinated with the growth of granulation tissue. Then the cathode was replaced by the anode for the remainder of treatment.
In Houghton's study,27 wounds were stimulated by the cathode in the 1st week and then polarity was reversed weekly over the next 11 weeks of treatment.
Griffin19 and Houghton24 used the cathode for the whole period of treatment to stimulate wounds. Kloth and Feedar18 stimulated wounds with the anode, switching polarity to negative only if healing progress was not observed.
It is difficult today to provide clear guidelines on how anode and cathode should be used to treat wounds in humans. However, the results of in vitro and in vivo studies suggest that both the electrodes promote wound healing, and that the type of the treatment electrode should correspond to the wound healing stage.
The authors of in vitro studies have established that the anode facilitates electrotaxis of macrophages4,5 and neutrophils6 for autolysis and reactivation of the inflammatory phase of healing.2,3 The efficacy of anodal stimulation in the early stages of wound healing has also been confirmed by recent in vivo studies with animals.14,32,33
Mehmandoust et al.32 (2007) investigated the effects of anodal and cathodal ES on acute wound healing. In an RCT 42 male albino guinea pigs were divided into two control groups (C1 and C2) and four experimental groups (E1–E4). A linear incision was made at the dorsal skin of all guinea pigs. A unidirectional pulse current of 300 to 600 μA, 80 pps, and 0.3 ms pulse duration was administrated for 1 h a day, for 14 or 21 days depending on the group. In groups E1 and E2, positive polarity was applied for the first 3 days and then negative polarity for the remaining days. In groups E2 and E4, negative polarity was applied for the first 3 days and positive polarity for the rest of treatment. Groups E1, E2, and C1 were killed on day 14 and E3, E4, and C2 on day 21. The authors measured the percentage decrease in WSA (daily tracing) and tensile strength (on days 14 and 21). According to the results, both anodal and cathodal stimulation increased the rate of wound closure. Beginning with day 12, the percentage decrease in wound surface was found to significantly differ between all treatments groups and control groups (p<0.05), but the ultimate tensile strength and stress increased only in the anodal group compared with the cathodal and control groups. At the end of day 14, the ultimate tensile stress in E1 was significantly greater in relation to C1 (p<0.05). The authors have concluded that regardless of the polarity ES can significantly decrease WSA and make wounds close faster, but anodal stimulation applied for the first 3 days and then replaced with cathodal stimulation for the rest of treatment may lead to stronger repaired tissue.
Talebi et al.33 (2008) studied the effect of anodal and cathodal ES on injury potential and wound size in guinea pigs. Injury potential may have a regulatory role in the wound healing process and exogenous ES may mimic natural bioelectric current that may improve wound healing. Thirty-nine male guinea pigs were randomly divided into a control group (sham treatment) and two experimental groups of which one received anodal and the other cathodal direct current (600 μA; 1 h/day; thrice a week for 3 weeks). A 2.5 cm-long full-thickness skin incision was made on each animal's dorsal region. The authors measured the differential skin surface potential of the wound site relative to the adjacent intact skin at a distance of 2 cm before and immediately after the injury and once a day throughout the healing period (21 days). WSA was also measured throughout the 21-day healing period. Immediately after injury, injury potential significantly increased in all three groups, reaching a maximum on day 1 for the control and cathodal groups, and on day 3 for the anodal group (p<0.05), and then decreasing through the healing period. In the anodal group, injury potential returned to preinjury levels by the end of the healing period. By days 19 and 21, wound potential decreased more for the anodal group than for the control group (p<0.05). By day 15 for the anodal group and by day 17 for the cathodal group, WSA decreased more compared with the control group (p<0.05). The authors concluded that anodal ES is appropriate for improving the healing of acute skin wounds because it causes the wound surface to close and the wound potential to return faster to the preinjury level.
The randomized in vivo study that Borba et al.14 (2011) conducted with rats showed that anodal stimulation had positive effect on neoangiogenesis in the early stage of acute experimental wound healing. In the stimulation group, the anode (treatment electrode) was placed on the animals' backs and a pulsed current was applied for 30 min delivering a rectangular pulse current of 8 mA at a frequency of 7.7 pps (the negative electrode was placed on the abdominal wall). After ES, an incision was made on the animal's back. The animals in the control and stimulation groups were subdivided into two subgroups of 10 animals each on postoperative days 7 and 14. In both the control and stimulation groups, the number of newly formed vessels was determined with histomorphometric analysis based on morphologic characteristics, such as the presence of thinner endothelial walls than those of other vessels. In the stimulation group, the number of blood vessels on postoperative day 7 was greater than in the control group (p<0.0091). No significant differences (p<0.3375) were found in the number of blood vessels between the groups on postoperative day 14. The anodal group in Borba's14 trial also showed a greater number of fibroblasts on postoperative day 7 than the control group (p<0.0060), whereas on postoperative day 14 no significant differences (p<0.1267) were observed in the number of fibroblasts between the groups. Borba's14 trial shows that preoperative anodal stimulation can be useful in treating early/acute stages of wound healing.
Mertz et al.34 in 1993 reported a study of monophasic pulsed-current ES to assess its effect on wound healing in pigs. After two 30-minute sessions of monophasic pulsed-current ES, they assessed epidermal migration macroscopically for 7 days. They observed that wounds treated with negative polarity on day 0 followed by positive polarity on days 1–7 demonstrated enhanced epithelialization by 20% compared to those receiving treatment with either positive (+9%) or negative (−9%) alone. In addition, they observed that alternating positive and negative polarity daily inhibited epithelialization by 45%. The results of the Mertz study35 have provided grounds for assuming that ES starting initially with cathode stimulation and then using anode stimulation may promote wound epithelialization. In recent years, however, Zhao and colleagues35 concluded based on their in vitro study that the epithelial cells are drawn to the cathode. Thus, in the opinion of the authors, it is the negative electrode that can effectively stimulate the epithelialization of wounds.
A literature review by Kloth,3 based on in vitro research suggests that cathode stimulation may be appropriate for treating infection and inflammation. When inflammation and exudation appear in the wound, macrophages and polymorphonucleaur granulocytes autolytically remove the nonviable tissue. Fibrin and the products of its degradation have chemotactic properties toward neutrophil and monocytes that phagocytose bacteria and necrotic tissue in the wound. Applying the cathode to a wound at this stage may attract positively polarized macrophages and neutrophil granulocytes to the wound to enhance the bactericidal process.2–6,35
Cathodal stimulation can also enhance the proliferative phase of healing. In vitro studies also suggest that granulation tissue formation may be affected by cathode stimulation.7–10
Further, the results of Bourguignon7 in vitro study with human fibroblasts indicated that HVPC utilizing monophasic twin-spike pulses (100 μs) with a frequency of 100 pps could significantly increase the rates of both protein and DNA synthesis. The maximum stimulation of protein and DNA synthesis was obtained at, respectively, 50 and 75 V at cells located to the negative electrode.
The Asadi et al.36 study (2011) showed that cathodal sensory ES increases the release of vascular endothelial growth factor (VEGF) in skin. The authors evaluated the effect of sensory direct current (600 μA) and motor monophasic current (pulse duration 300 μs; 100 pps, 2.5–3.0 mA) of cathodal ES on VEGF release in muscle and skin in the wound site. They randomly assigned 48 male Sprague-Dawley rats into one control and two experimental groups (sensory and motor ESs). A full-thickness skin incision was made on each animal's dorsal region. The experimental groups received ES for 1 h every day, for 3 or 7 days. In the control group, no current was applied. VEGF expression was measured in muscle and skin on the 3rd and 7th days after surgical incision. The outcomes showed no difference in the groups' VEGF levels on the 3rd day. On the 7th day, the skin VEGF levels in the sensory group were significantly higher than in the other groups (p<0.05). In the muscle VEGF, no differences were found. According to the results, sensory cathodal ES increases the release of VEGF in skin. This mechanism may be one through which a sensory negative current is effective in promoting wound healing.
Assessment of risk of bias in included studies
We independently assessed the risk of bias in the included studies. As recommended by the Cochrane Collaboration Back Review Group published by van Tulder et al. (as quoted in McGaughey37), we addressed the methodological domains presented in Table 3, points A–K, and additionally introduced criteria from Table 3, points L–P.
Table 3.
Methodological quality of studies37
| A | Was the method of randomization adequate? | Y/N/DK |
| B | Was the treatment allocation concealed? | Y/N/DK |
| C | Were the groups similar at baseline regarding most prognostic indicators? | Y/N/DK |
| D | Was the patient blinded to the intervention? | Y/N/DK |
| E | Was the care provider blinded to the intervention? | Y/N/DK |
| F | Was the outcome assessor blinded to the intervention? | Y/N/DK |
| G | Were co-interventions avoided or comparable? | Y/N/DK |
| H | Was the compliance acceptable in all groups? | Y/N/DK |
| I | Was the dropout rate described and acceptable? | Y/N/DK |
| J | Was the timing of the outcome assessment in all groups similar? | Y/N/DK |
| K | Did the analysis include an intention-to-treat analysis? | Y/N/DK |
| L | Were there at least 10 participants? | Y/N/DK |
| M | Was there only one type of wound? | Y/N/DK |
| N | Was the duration of study at least 10 weeks? | Y/N/DK |
| O | Was the duration of study at least 4 weeks? | Y/N/DK |
| P | Was complete closure of all wounds achieved? | Y/N/DK |
DK, don't know; N, no; Y, yes.
The methodological quality of the reviewed studies (see Table 4) ranged from 7 to 12 points, with a mean of 9.7 (standard deviation 1.76; median 9.7) out of 16. The studies performed best in having comparable timing of outcome assessments (J),18,19,21–28 and in avoiding the use of co-interventions (G).18,19,21–28 They also adequately performed an intention-to-treat analysis (K).18,19,21–28 The groups of subjects seem to have been appropriately selected and were similar at baseline regarding most prognostic indicators (C).19,22–28 Unfortunately, only six authors out of ten adequately provided an acceptable dropout rate (I).18,19,23,24,27,28
Table 4.
Methodological quality scores
| Study | Kloth and Feedar18 | Griffin et al.19 | Polak et al. 21 | Franek et al.22 | Peters et al. 23 | Houghton et al.24 | Franek et al.25 | Franek et al.26 | Houghton et al27 | Franek et al.28 |
|---|---|---|---|---|---|---|---|---|---|---|
| Type of study | RCT, PlacC | RCT, PlacC | RCT | RCT | RCT | RCT | RCT | RCT | RCT | RCT |
| PlacC | PlacC | SB | ||||||||
| DB | DB | |||||||||
| A | Y | DK | DK | Y | DK | DK | DK | DK | Y | Y |
| B | Y | DK | DK | Y | DK | DK | DK | DK | Y | Y |
| C | DK | Y | DK | Y | Y | Y | Y | Y | Y | Y |
| D | DK | Y | DK | DK | Y | Y | N | N | N | N |
| E | DK | N | N | N | Y | Y | N | N | N | N |
| F | N | DK | N | N | Y | Y | DK | DK | DK | N |
| G | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| H | Y | Y | Y | Y | N | Y | Y | Y | DK | Y |
| I | Y | Y | N | N | Y | Y | N | N | Y | Y |
| J | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| K | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| L | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| M | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| N | Y | N | N | N | Y | N | N | N | Y | N |
| O | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| P | Y | N | N | N | N | N | N | N | N | N |
| Total score | 11 | 8 | 7 | 10 | 12 | 11 | 8 | 8 | 11 | 11 |
SB, single blind; DB, double blind; PlacC, placebo controlled; DK, don't know; N, no; Y, yes; RCT, randomized controlled trial.
A noteworthy fact is that all studies described treatment methods in the experimental and control groups correctly and specifically, particularly the methodology of HVPC ES (G).18,19,21–28 Further, the studies were generally organized well enough to recognize them as ensuring appropriate treatment of patients in both types of groups. The researchers made sure that the preventive measures and SWC matched the needs of particular patients and used similar methods in both the HVPC group and the control groups (H).18,19,21–28 In most studies, efforts were taken to ensure that the HVPC ES procedure was consistent with the accepted methodology of research.18,19,21,22,24–26,28 The authors of only two studies23,27 were not certain whether ES was always applied as planned, but these researchers evaluated wound healing rates outside hospital wards. Treatment was effective in both cases. The results of the studies are significant in that they show that ES with HVPC delivered through miniature ES devices can be successfully employed in the community setting (in patients' homes) without the direct supervision from trained medical personnel.
The randomization aspect of the reviewed studies stirs some reservations. Although all the studies are considered RCTs, only four of them provide information proving that the method of randomization was adequate (A)18,22,27,28 and only four studies can be considered to have fully concealed treatment allocation (B).18,22,27,28
Sham ES treatment in control groups was only used in four studies,18,19,23,24 three of which give information showing that the patients were blinded to the intervention (D).19,23,24 In the other studies,21,22,25–28 the experimental groups received HVPC+SWC and the control groups were treated with SWC; it is quite probable that the patients were aware of what therapy they were administered.
There were only two double-blinded studies23,24 where care providers and outcome assessors were blinded to the intervention (E, F).
Attention should to be given to the fact that the randomization and blinding questions received many “don't know” scores, which shows that the studies frequently omit key information or give incomplete information.
It can be presumed that the authors of the reviewed studies used correct methods for assessing wound healing progress. In most studies, HVPC was applied to at least 10 patients (L).21–28 In almost all studies (but one24), the same type of wounds was assessed for healing progress (M), that is, PUs, VLUs, and DFUs.18,19,21–23,25–28 As regards PUs, the authors of one study18 treated only stage IV PUs. The other studies treated PUs ranging from stage II to stage IV, but some researchers assessed19,27 healing progress separately for PUs of different stages.
In all studies (except one19), wound healing progress was observed for at least 4 weeks (O),18,21–28 but only three studies went on longer than 10 weeks (N)18,23,27 and, unfortunately, there was only one study18 when wounds were treated until they closed completely (P).18 In all the other studies, some wounds did not heal completely, but all authors provided the percentage of healed wound area and most of them stated the rates of completely healed wounds (Table 3).18,19,23,25–27
Overall, six studies18,22–24,27,28 in this review were of high quality scoring at least 10 points in internal validity criteria, and four19,21,25,26 were of low quality.
Half of the six high-quality studies concerned PUs.18,27,28 In these studies, PUs were treated for at least 6 weeks and the healed area exceeded 70% in relation compared with their pretreatment sizes. These results are promising indications of the efficacy of HVPC ES as a PU treatment modality.
The authors of the two other high-quality studies treated DFUs23 and VLUs,22 achieving healing rates of, respectively, 86% after 12 weeks and of 59% after 6 weeks. These results too show that HVPC can be effectively used for treating these types of wounds.
The last of the high-quality studies concerned vascular leg ulcers (venous, arterial, diabetic, and mixed).24 Its authors did not analyze wound healing progress by ulcer etiology, but only provided an aggregate healing rate of 44% after 4-week therapy.
Future Developments of Interest
While the shortcomings of the reviewed studies hinder reliable predictions as to the efficacy of HVPC ES in wound treatment, their findings suggest that HVPC can be successfully used in treating PUs.
Additional studies are necessary to investigate its effect on the healing of other types of soft tissue defects and infected wounds, to determine the efficacy of cathodal versus anodal stimulation, and the minimal daily/weekly duration of HVPC needed to ensure optimal wound healing.
The authors of future studies should also make sure that their randomization methods are precise and that the rules for blinding the patients, care providers, and outcome assessors to the intervention are observed. Studies should be designed to cover longer periods, preferably until the wounds are closed.
Caution, Critical Remarks, and Recommendations
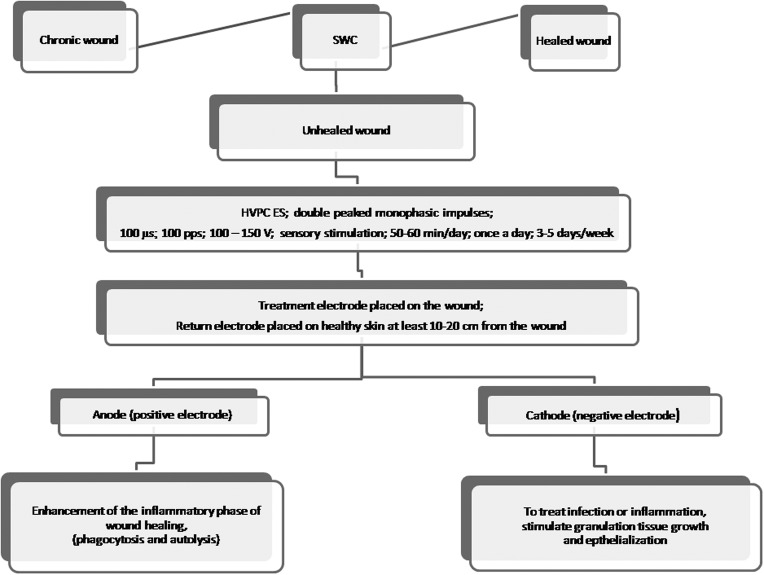
HVPC is a promising therapy. It is relatively inexpensive, noninvasive, painless and safe from measurable side effects, and suitable for application in clinical and nonclinical setting and in patients' homes. An outline of HVPC application for wound healing is presented in Fig. 1.
Figure 1.
Application of high-voltage pulsed current electrical stimulation in wound healing.
Take-Home Messages.
Basic science advances
• ES promotes wound healing at acute and chronic stages.
• “Skin battery” produces an electrical current that activates the healing process.
• A monophasic pulsed current applied to a wound simulates or reinforces bioelectrical current and thereby wound healing.
• Cellular electrotaxis of macrophages, neutrophils, and fibroblasts to the wound also promotes healing.
• ES improves protein and collagen synthesis and the production of ATP and DNA.
• ES can increase blood flow and capillary density.
Clinical science advances
• HVPC is effective in CT of chronic wounds, venous ulcers, stage II-IV PUs, and DFU.
• HVPC using the cathode as the treatment electrode promotes the growth of granulation tissue.
• HVPC applied 2.25–7 h a week effectively promotes the healing of chronic wounds.
Relevance to clinical care
ES and other biophysical energies are recommended for treating hard to heal chronic wounds. More in vitro, animal and clinical studies are needed to develop specific guidelines for effective application of ES in wound treatment.
Abbreviations and Acronyms
- CT
conservative treatment
- DB
double blind
- DFU
diabetic foot ulcer
- ES
electrical stimulation
- HVPC
high-voltage pulsed current
- NaCL
sodium chloride
- PlacC
placebo controlled
- pps
pulses per second
- PU
pressure ulcer
- RCT
randomized controlled trial
- SB
single blind
- SWC
standard wound care
- VEGF
vascular endothelial growth factor
- VLU
venous leg ulcer
- VS
venous surgery
- WSA
wound surface area
Acknowledgment and Funding Sources
The authors wish to acknowledge their gratitude for Prof. Luther C. Kloth's extensive editorial input and friendly encouragement in writing this article. No funding sources to acknowledge.
Author Disclosure and Ghostwriting
The authors have no competing financial interests. The content of this article was expressly written by the authors listed. No ghostwriters were involved in writing of this article.
About the Authors
Anna Polak, PT, PhD, is an Assistant Professor, Department of Physical Therapy, Academy of Physical Education, Katowice and an Assistant Professor, Institute of Medical Science, Katowice School of Economics, Poland. Prof. Andrzej Franek is a Professor of Medical Sciences and Head of the Department of Biophysics, Medical University of Silesia, Katowice, Poland. Jakub Taradaj, PT, PhD, is a Professor and Head of the Department of Physical Therapy, Academy of Physical Education, Katowice, Poland.
References
- 1.Foulds IS. and Barker AT: Human skin battery potentials and their possible role in wound healing. Br J Dermatol 1983; 109:515. [DOI] [PubMed] [Google Scholar]
- 2.Kloth LC: Electrical stimulation for wound healing. In: Wound Healing: Alternatives in Management. 3rd edition, edited by Kloth LC. and McCulloch JM. Philadelpia, PA: Davis Comp, 2002, pp. 271–315 [Google Scholar]
- 3.Kloth LC: Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 2005; 4:23. [DOI] [PubMed] [Google Scholar]
- 4.Lampe K: Electrotherapy in tissue repair. J Hand Ther 1998; 12:131. [DOI] [PubMed] [Google Scholar]
- 5.Orida N. and Feldman J: Directional protrusive pseudopodial activity and motility in macrophages induced by extra-cellular electric fields. Cell Motil 1982; 2:243. [DOI] [PubMed] [Google Scholar]
- 6.Eberhardt A, Szczypiorski P, and Korytowski G: Effect of transcutaneous electrostimulation on the cell composition of skin exudate. Acta Physiol Pol 1986; 37:41. [PubMed] [Google Scholar]
- 7.Bourguignon GJ. and Bourguignon LY: Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB J 1987; 1:398. [DOI] [PubMed] [Google Scholar]
- 8.Bourguignon GJ, Jy W, and Bourguignon LY: Electric stimulation of human fibroblasts causes an increase in Ca2+ influx and the exposure of additional insulin receptors. J Cell Physiol 1989; 140:379. [DOI] [PubMed] [Google Scholar]
- 9.Erickson CA. and Nuccitelli R: Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J Cell Biol 1984; 98:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young W, Onuma EK, and Hui SW: Response of C3H/10T1/2 fibroblasts to an external steady electric field stimulation. Exp Cell Res 1984; 155:92. [DOI] [PubMed] [Google Scholar]
- 11.Weiss DS, Kirsner R, and Eaglstein WH: Electrical stimulation and wound healing. Arch Dermatol 1990; 126:222. [PubMed] [Google Scholar]
- 12.Reger S, Hyodo A, Negami S, Kambic H, and Sahgal V: Experimental wound healing with electrical stimulation. Artif Organs 1999; 23:460. [DOI] [PubMed] [Google Scholar]
- 13.Wood J, Evans P, Schallreuter K.Jacobson WE, Sufit R, Newman J, White C, and Jacobson M: A multicenter study on the use of pulsed low-intensity direct current for healing chronic stage II and stage III decubitus ulcers. Arch Dermatol 1993; 129:999. [PubMed] [Google Scholar]
- 14.Borba GC, Hochman B, Liebano RE, Enokihara MM, and Ferreia LM: Does preoperative electrical stimulation of skin alter the healing process? J Surg Res 2011; 166:324. [DOI] [PubMed] [Google Scholar]
- 15.Junger M, Zuder D, Steins A, Hahn M, and Klyscz T: Treatment of venous ulcers with low frequency pulsem current (Dermapulse): effects on cutaneous microcirculation. Hautartz 1997; 48:879. [DOI] [PubMed] [Google Scholar]
- 16.Mohr T, Akers TK, and Wessman HC: Effect of high voltage stimulation on blood flow in the rat hind limb. Phys Ther 1987; 67:526. [DOI] [PubMed] [Google Scholar]
- 17.National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel: Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Washington DC: National Pressure Ulcer Advisory Panel, 2009 [Google Scholar]
- 18.Kloth LC. and Feedar JA: Acceleration of wound healing with high voltage monophasic, pulsed current. Phys Ther 1988; 68:503. [DOI] [PubMed] [Google Scholar]
- 19.Griffin JW, Tooms RE, Mendius RA, Clifft JK, Vander-Zwang R, and El-Zeky F: Efficacy of high voltage pulsed current for healing of pressure ulcers in patients with spinal cord injury. Phys Ther 1991; 71:433. [DOI] [PubMed] [Google Scholar]
- 20.Polak A, Franek A, Hunka-Zurawinska W, Swist D, and Krol P: Skuteczne leczenie owrzodzen zylnych podudzi [An efficient treatment of venous leg ulcers]. Przegl Dermatol 1999; 86:383 [Google Scholar]
- 21.Polak A, Franek A, Hunka-Zurawinska W, Bendkowski W, Kucharzewski M, and Swist D: Elektrostymulacja wysokonapieciowa w leczeniu owrzodzen zylnych podudzi [High voltage electrical stimulation in leg ulcer treatment]. Wiad Lek 2000; 53:417. [PubMed] [Google Scholar]
- 22.Franek A, Polak A, and Kucharzewski M: Modern application of high voltage for enhanced healing of venous crural ulceration. Med Eng Phys 2000; 22:647. [DOI] [PubMed] [Google Scholar]
- 23.Peters EJ, Lavery LA, Armstrong DG, and Fleischli JG: Electric stimulation as an adjunct to heal diabetic foot ulcers: A randomized clinical trial. Arch Phys Med Rehabil 2001; 82:721. [DOI] [PubMed] [Google Scholar]
- 24.Houghton PE, Kincaid CB, Lovell M, Campbell KE, Keast DH, Woodbury MG, and Harris KA: Effect of electrical stimulation on chronic leg ulcer size and appearance. Phys Ther 2003; 83:17. [PubMed] [Google Scholar]
- 25.Franek A, Taradaj J, Cierpka L, and Blaszczak E: High voltage stimulation for healing acceleration of venous leg ulcers. Usefulness after surgical treatment. Phlebologie 2005; 34:255 [Google Scholar]
- 26.Franek A, Taradaj J, Polak A, Cierpka L, and Blaszczak E: Efficacy of high voltage stimulation for healing of venous leg ulcers in surgically and conservatively treated patients. Phlebologie 2006; 35:127 [Google Scholar]
- 27.Houghton PE, Campbell KE, Fraser CH, Harris C, Keast DH, Potter PJ, Hayes KC, and Woodbury MG: Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal cord injury. Arch Phys Med Rehabil 2010; 91:669. [DOI] [PubMed] [Google Scholar]
- 28.Franek A, Kostur R, Polak A, Taradaj J, Szlachta Z, Blaszczak E, Dolibog P, Dolibog P, Koczy B, and Kucio C: Using high voltage electrical stimulation in the treatment of recalcitrant pressure ulcers: results of a randomized, controlled trial. Ostomy Wound Manag 2012; 58:30. [PubMed] [Google Scholar]
- 29.Perrin M, Guidicelli H, and Rastel D: Surgical techniques used for the treatment of varicose veins: survey of practice in France. J Mal Vasc 2003; 28:277. [PubMed] [Google Scholar]
- 30.Abela R, Liamis A, Prionidis I, Mathai J, Gorton L, Browne T, and Panayiotopoulos Y: Reverse foam sclerotherapy of the great saphenous vein with sapheno-femoral ligation compared to standard and invagination stripping: a prospective clinical series. J Vasc Endovasc Surg 2008; 36:485. [DOI] [PubMed] [Google Scholar]
- 31.Stenger D. and Hartmann M: High ligation and vein stripping. The classic procedure. Hautarzt 2012; 63:616. [DOI] [PubMed] [Google Scholar]
- 32.Mehmandoust FG, Torkaman G, Firoozabadi M, and Talebi G: Anodal and cathodal pulsed electrical stimulation on skin wound healing in guinea pigs. JRRD 2007; 44:611. [DOI] [PubMed] [Google Scholar]
- 33.Talebi G, Torkaman G, Firoozabadi M, and Shariat S: Effect of anodal and cathodal microamperage direct current electrical stimulation on injury potential and wound size in guinea pigs. JRRD 2008; 45:153. [DOI] [PubMed] [Google Scholar]
- 34.Mertz P, Davis S, Cazzaniga A, Cheng K, Reich JD, and Eaglstein VM: Electrical stimulation acceleration of soft tissue repair by varying the polarity. Wounds 1993; 5:153 [Google Scholar]
- 35.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM: Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006; 442:457. [DOI] [PubMed] [Google Scholar]
- 36.Asadi MR, Torkaman G, and Hedayati M: Effect of sensory and motor electrical stimulation in vascular endothelial growth factor expression of muscle and skin in full-hickness wound. JRRD 2011; 48:195. [DOI] [PubMed] [Google Scholar]
- 37.McGaughey H, Dhamija S, Oliver L, Porter-Armstrong A, and McDonough S: Pulsed electromagnetic energy in management of chronic wounds: a systematic review. Phys Ther Rev 2009; 14:132 [Google Scholar]