Summary
Eicosanoids, the metabolites of arachidonic acid, have diverse functions in the regulation of cancer including prostate cancer. This review will provide an overview of the roles of eicosanoids and endocannabinoids and their potential as therapeutic targets for prostate cancer treatment.
Keywords: Arachidonic acid, cyclooxygenase, cytochrome P450, eicosanoids, endocannabinoids, lipoxygenase, prostate cancer
Prostate cancer is among the most diagnosed malignancies and contributes to cancer-related mortality rate in men. The vast majority of morbidity and mortality results from spread of the tumor beyond the prostate gland and/or tumor becoming hormone refractory. The process of metastasis is a complex multistage process consisting of a series of sequential, interrelated steps that include growth, vascularization, adhesion, extravasation, and invasion. The discovery of new molecular targets to inhibit cell proliferation, growth, and invasion/migration is among the most important endeavors in prostate cancer therapy.
High fat diets, particularly ω-6 polyunsaturated fatty acids, are associated with prostate cancer development and progression [1, 2] and prostate cancer cell growth [3, 4]. On the other hand, diets rich in ω-3 fatty acids are associated with the lower incidents of the disease in some studies [1, 5], whereas other reports indicated no evidence of a significant reduced risk of prostate cancer [6] or even increased risk [7]. These opposing roles of fatty acids are consistent with the fact that diet is an important factor in the coincidence of prostate cancer. The high ratio of ω-3/ω-6 fatty acids in the diet has been suggested to prevent prostate cancer [8]. Although in one study showed that plant-based foods and fish diets do not reduce the prostate specific antigen (PSA) levels in prostate cancer patients, they significantly increase the PSA doubling times [9].
Arachidonic acid (AA, 20:4 ω-6) is one of the major polyunsaturated ω-6 fatty acids. AA is metabolized by three groups of oxygenases namely cyclooxygenases (COX), lipoxygenases (LOX) and cytochrome P450s (CYP) to a number of biologically active eicosanoids (Figure 1). This review will focus on the role of these AA metabolites in prostate cancer. Eicosanoids have diverse roles in the regulation of cellular functions and they have been extensively studied as important lipid mediators in cardiovascular systems. However, their roles in cancer, particularly prostate cancer are much less investigated.
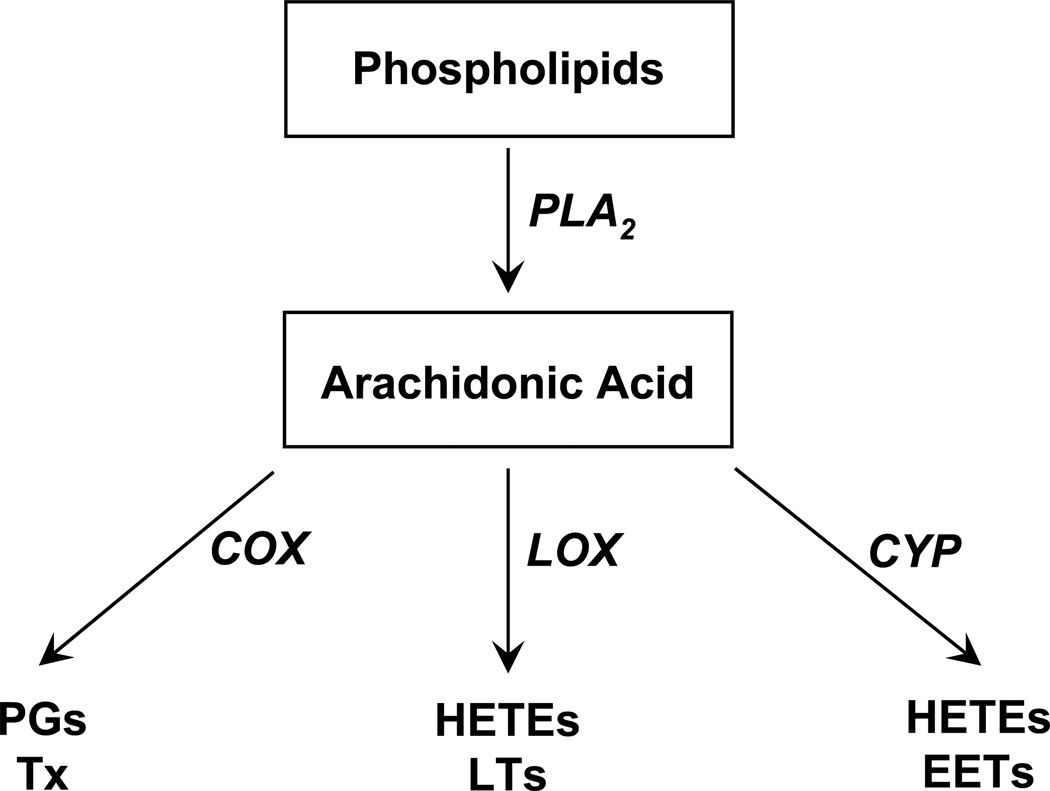
Figure 1.
Arachidonic acid metabolic pathways. Free arachidonic acid (AA) is formed mainly from the metabolism of membrane phospholipids by phospholipase A2 (PLA2). Free AA is metabolized by cyclooxygenases (COX) to prostaglandins (PGs) and thromboxane (Tx), by lipoxygenases (LOX) to hydroxyeicosatetraenoic acids (HETEs) and leukotrienes (LTs), and by cytochrome P450s (CYP) to HETEs and epoxyeicosatrienoic acids (EETs).
I. Cyclooxygenase (COX) Pathways
There are two well-characterized COX isoenzymes, COX-1 and COX-2. A splice variant of COX-1 which retains intron one and a frameshift mutation has been named COX-3, COX-1b, or COX-1 variant (COX-1v) [10]. COX-1 is constitutively expressed in most cells while COX-2 is an inducible enzyme. The COX pathway is the most studied in prostate cancer. COX-2 is overexpressed in prostate cancer tissues [11–19]. The immunohistochemistry staining of COX-2 increases with prostate tumor grade [20], suggesting that COX-2 has a role in prostate cancer development and progression [21]. Contrarily, several studies did not find a consistent and significant difference in COX-2 expression in normal and prostate cancer tissues [22–24], but the overexpression of COX-2 correlated with the chronic inflammatory lesions [19, 23]. A similar controversy exists concerning the expression of COX-2 in human prostate cancer cells compared with normal epithelial cells. Some studies demonstrated the high COX-2 expression in prostate cancer cells while others reported the low or absent COX-2 expression [21, 24–28]. Similarly, the effects of nonselective COX inhibitors such as nonsteroidal anti-inflammatory drugs (NSAIDs) and specific COX-2 inhibitors on prostate cancer cell proliferation and invasion are complex and not uniform, and their association with reduced risk of prostate cancer is not conclusive [28–44].
Within the cyclooxygenase active site, COX inserts molecular oxygen at C11 and C15 to form prostaglandin G2 (PGG2); then, its peroxidase active site reduces PGG2 to PGH2 [45]. Subsequently, specific synthases or isomerases convert PGH2 to various prostaglandins (PGs) and thromboxanes (TXs) (Figure 2). Common COX metabolites of AA are PGI2, PGD2, PGE2, PGF2α, PGJ2, and TxA2. PGI2 and TxA2 are unstable and are rapidly converted to the stable 6-keto-PGF1α and TxB2, respectively.
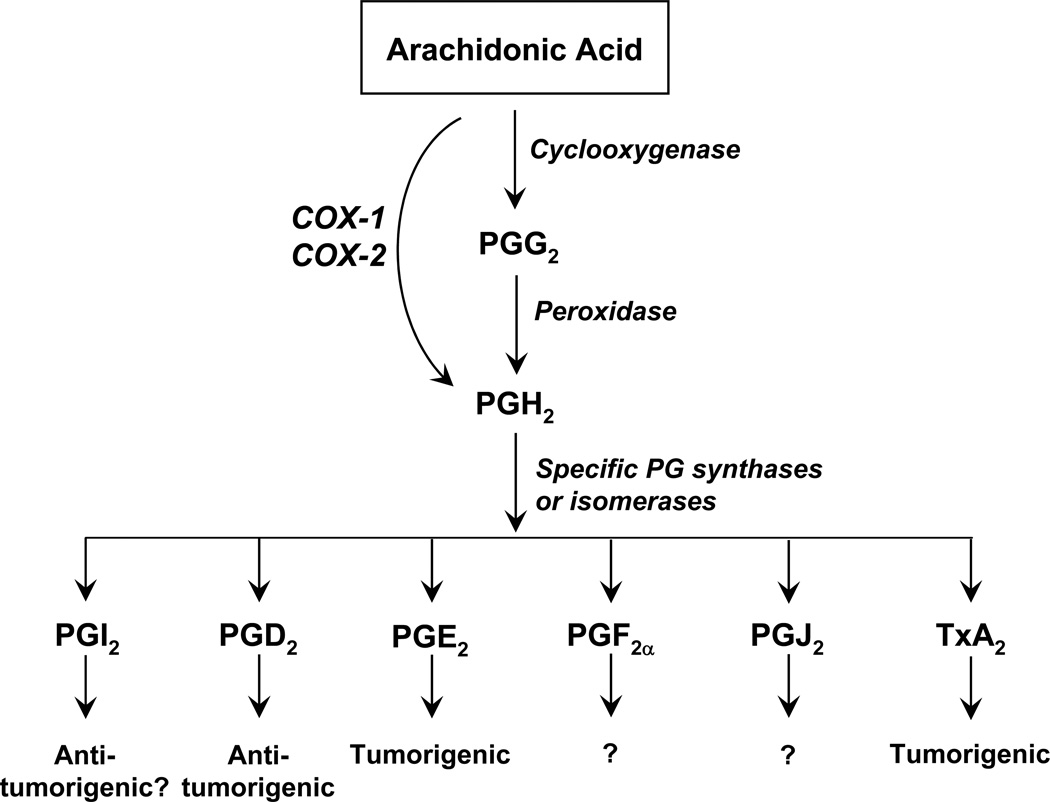
Figure 2.
Cyclooxygenase pathway of AA. Cyclooxygenase active site of COX converts AA to PPG2 and peroxidase active site of COX converts PGG2 to PGH2. Then, specific prostaglandin synthases or isomerases convert PGH2 to their corresponding PGI2, PGD2, PGE2, PGF2α, PGJ2, and TxA2. In prostate cancer, PGI2 and PGD2 are antitumorigenic while PGE2 and TxA2 are tumorigenic.
I.1 Prostaglandins and prostate cancer
The plasma PGI2 concentrations (detected as 6-keto-PGF1α) in normal men are similar to patients with benign prostatic hypertrophy (BPH) but the levels rise with advancing disease and varied with the degree of tumor differentiation [46]. Interestingly, PGI2 and its stable analogs inhibit metastasis of prostate cancer [47–49]. The role of endogenous PGI2 in prostate cancer is still unclear and has not been studied in recent years.
PGE2 is the most characterized in prostate cancer. Since early 1980’s, the effects of PGE2 in prostate cancer has been recognized [50]. In BPH and prostate cancer tissues, PGE2 is the only major PG that is produced in significant amounts by the prostate and its levels are higher in prostate cancer tissues [51]. PGE2 stimulates growth of prostate cancer cells including PC-3 and LNCaP cells [52, 53]. Interestingly, in PC-3 ML cells, exogenously added PGE2 increases hypoxia inducible factor-1alpha (HIF-1α) protein and induces nuclear localization of HIF-1α [54], which in turn induces transcription of many genes associated with cell growth. Conversely, selective COX-2 inhibitors such as meloxicam and NS-398 decrease HIF-1α levels and nuclear translocation. This pathway is supported by the positive feedback mechanism that PGE2 upregulates the expression of COX-2 mRNA, its own synthesizing enzyme [52, 53]. These results suggest a cycle of stimulation of COX-2 and PGE2 that promotes prostate cancer progression. PGE2 stimulates growth and invasion of prostate cancer cells through multiple signaling pathways. For examples, PGE2 activates protein kinase A (PKA) pathway to induce the expression of c-fos mRNA and growth in PC-3 cells [55]. PGE2 increases the secretion of vascular endothelial growth factor (VEGF) and cyclic adenosine monophosphate (cAMP) in PC-3 cells but less in DU-145 and LNCaP cells [56]. PGE2 activates the interleukin-6 signaling pathway to promote prostatic intraepithelial neoplasia cell growth, providing evidence that overexpression of COX-2 and PGE2 mediate prostate cancer development and progression through the IL-6 signaling pathway [57]. NS-398, a specific COX-2 inhibitor, inhibits invasion of PC-3 and DU-145 cells by reducing the matrix metalloproteinases (proMMP-2, MMP-9 and proMMP-9) [58]. In this case, PGE2 alone does not increase cell invasion of PC-3 and DU-145 cells but reverses the NS-398-reduced cell invasion [58]. In a later study, PGE2 does not stimulate invasion of low invasive PC-3 cells but induces invasion of high invasive PC-3 cells [59]. These results suggest that the endogenous PGE2 may be necessary for rendering cell invasion; however, cells may require a high level of exogenous PGE2 or cooperation with other factors to induce cell invasion [59].
PGD2 is transformed to 15-deoxy-Δ12,14-PGD2 (15-d-PGD2) and 15-deoxy-Δ12,14-PGJ2 (15-d-PGJ2) in some cells. PGD2 produced by normal prostate stromal cells suppresses the growth of prostate cancer PC-3, DU-145, and LNCaP cells [60]. PGD2 and its metabolites inhibit prostate cancer cell growth by the activation of peroxisome proliferator-activated receptor γ (PPARγ) in the order of 15-d-PGJ2 > 15-d-PGD2 > PGD2 [60]. 15-d-PGJ2 is an effective ligand for PPARγ and it induces apoptosis and nonapoptotic cell death in prostate cancer cells dependent and independent of PPARγ activation [61–66]. Interestingly, 15-d-PGJ2 increases the expression of PPARγ and VEGF in PC-3 cells [67]. However, in a later study, 15-d-PGJ2 did not increase the expression of PPARγ but blocked the serum-induced COX-2 expression in PC-3 cells [65]. Exogenous PGD2 is an antiinvasive factor and inhibits invasion of PC-3 cells in a concentration-dependent manner [59]. The importance of endogenous PGD2 is not clear.
I.2 Thromboxane and prostate cancer
Only few studies addressed the association of thromboxane and prostate cancer. For example, UK 38485, a thromboxane synthase (TxS) inhibitor reduced the pulmonary metastasis in prostate cancer bearing rats [47] and oestrogen therapy reduced the urinary concentrations of 2,3-dinor TxB2 and increased the ratio of PGI2 to TxB2 in prostate cancer patients [68]. The expression of TxS and TxA2 receptor (TP) are elevated in less differentiated or advanced tumor tissues [69, 70], suggesting the roles of TxS, TP and TxA2 in prostate tumorigenesis. Differential expression of TxS was observed in different types of human prostate cancer cells with high expression in PC-3, PC-3M, and ML-2 cells and low expression in normal prostate epithelial, DU-145 and LNCaP cells [69]. TxS and TxA2 may regulate prostate cancer progression through the activation of cell motility as the inhibition of TxS reduces motility of PC-3 cells while overexpression of TxS increases motility of DU-145 cells [69]. In PC-3 cells, TxA2 activates extracellular signal-regulated kinases (ERKs), transactivates EGF receptor (EGFR), and stimulates the release of MMPs [71]. These signaling pathways are important in the regulation of cell proliferation and invasion.
I.3 Prostanoid receptors and prostate cancer
Prostanoid receptors are G-protein coupled receptors that are activated by prostanoids. Each prostanoid can activate its own receptor namely DP, EP, FP, IP and TP for PGD2, PGE2, PGF2α, PGI2, and TxA2, respectively. There are only one type of FP and IP receptors but two subtypes for DP (DP1 and DP2) and TP (TPα and TPβ) receptors and four subtypes for EP (EP1, EP2, EP3, and EP4) receptors [72]. Among these receptors, EP and TP receptors have been characterized in prostate cancer. The immunostaining of EP1 receptor is higher in cancer cells of prostate tissues than in nontumor containing glands and correlated with Gleason scores [73]. EP2 and EP4 are higher in cancer tissues than nontumor glands but neither receptor correlate with the pathologic features. These findings suggest that EP receptors, particularly EP1 receptor, may have an important role in prostate cancer progression. Interestingly, EP2 and EP4 receptors but not EP1 and EP3 receptors, are present in prostate cancer cell lines [55, 56]. Thus, the cell lines do not express the same EP receptor subtypes as prostate tumors. The activation of EP2, and possibly EP4, receptor in PC-3 cells by PGE2 activates PKA pathway to increase cell growth but are less effective in DU-145 and LNCaP cells [55, 56]. Calcitriol (1,25-dihydroxyvitamin D3) inhibits prostate cancer cell growth and differentiation by down regulating the expression of COX-2 and EP2 and FP receptors [74–80]. These studies further indicate that COX-2 and prostanoid receptors have important role in prostate cancer.
Activation of TP by thrombin-induced TxA2 release or U46619 enhances cell invasion of PC-3 and DU-145 cells by the activation of the small GTPase RhoA [81]. This observation was further confirmed by a recent study that RhoA is activated by TP activation leading to an increase of cell motility and cytoskeleton reorganization of prostate cancer cells [82]. These studies suggest that the function of TxA2 in prostate cancer progression is mainly through the regulation of RhoA-mediated cell motility.
II. Lipoxygenase (LOX) Pathways
Lipoxygenases (LOX) are a family of non-heme containing oxygenases that insert molecular oxygen into polyunsaturated fatty acids such as AA at specific double bond positions. There are four LOXs, namely 5-LOX, 8-LOX, 12-LOX, and 15-LOX that metabolize AA to corresponding hydroperoxides (HPETEs). The HPETEs are reduced to their corresponding hydroxyeicosatetraenoic acids (HETEs) (Figure 3). Recent studies have demonstrated that LOXs are diversified enzymes in different species or organs including platelet 12-LOX, leukocyte 12-LOX, epidermal 12-LOX, 12(R)-LOX, 15-LOX-1, and 15-LOX-2. In recent years, LOXs have gained more attention as regulators of cancer and strong evidence indicates that 5-LOX, platelet 12-LOX and 15-LOX-1 are tumorigenic while 8-LOX and 15-LOX-2 are potentially anti-tumoricgenic.
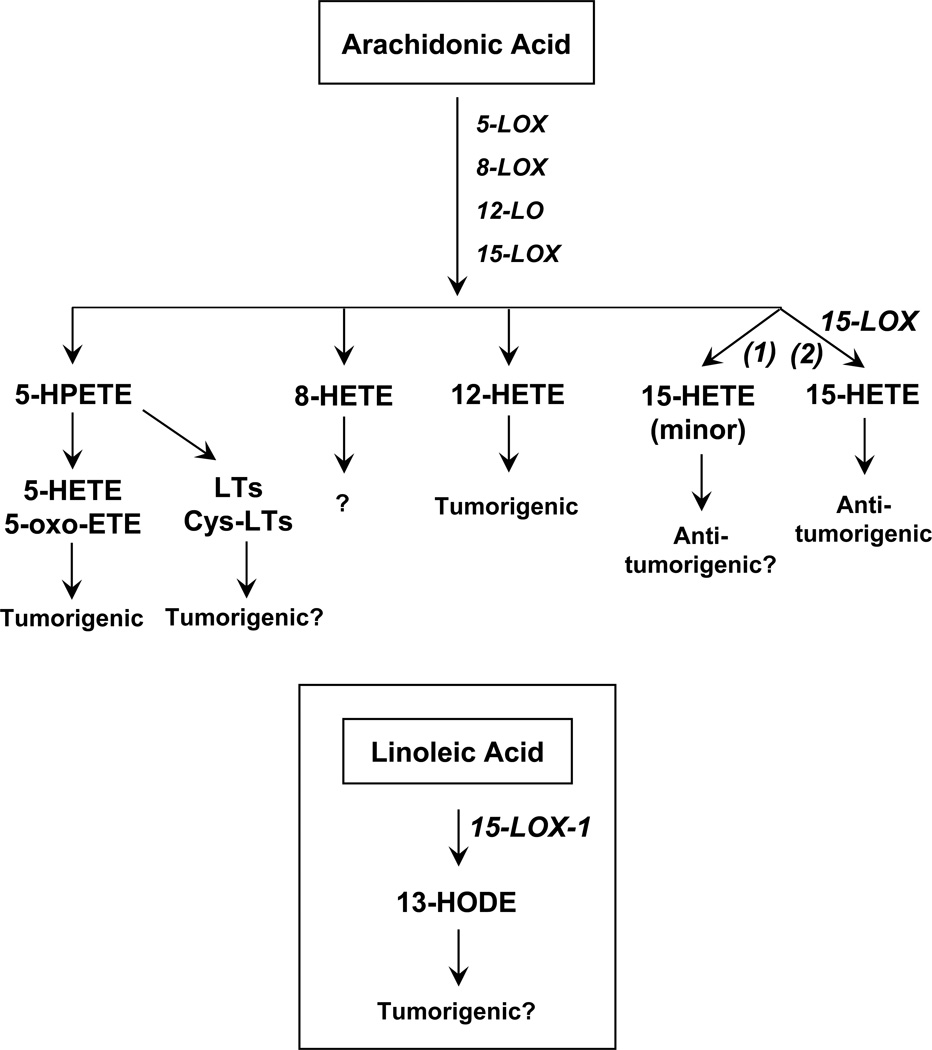
Figure 3.
Lipoxygenase pathway of AA. LOX metabolizes AA to their corresponding isomeric hydroperoxyeicosatetraenoic acids (HPETEs). 5-HPETE is reduced by peroxidase to 5-HETE, then, 5-HETE is converted to 5-oxo-eicosatetraenoic acid (5-oxo-ETE). 5-HETE and 5-ox-ETE are tumorigenic. Role of 8-HETE in prostate cancer is not known while 12-HETE is tumorigenic. 15-LOX-1 metabolizes the major substrate, linoleic acid (LA) to 13-hydroxyoctadecadienoic acid (13-HODE) (inset). 15-LOX-1 is tumorigenic. 15-LOX-2 metabolizes AA to 15-HETE, which is antitumorigenic in prostate cancer.
II.1 5-LOX and prostate cancer
In 5-LOX pathway, 5-LOX metabolizes AA to 5-HPETE and then to 5(S)-HETE. 5-HPETE is also converted to the unstable epoxide leukotriene A4 (LTA4), which is further hydrolyzed to LTB4, and other leukotriene metabolites. LTA4 is also metabolized to cysteinyl leukotrienes (Cys-LTs) such as LTC4, LTD4, and LTE4, and these Cys-LTs can be further metabolized to numerous leukotriene derivatives [83].
5-LOX protein is slightly detected in benign prostatic hyperplasia (BPH) and normal prostate tissues but it is markedly increased in prostatic intraepithelial neoplasia (PIN) and prostate cancer tissues [84]. 5-LOX is also present in human prostate cancer cell lines and 5-LOX inhibitors effectively inhibit cell growth through the induction of apoptosis [84–87]. Specific 5-LOX inhibitors, AA861 and 5-lipoxygenase activating protein (FLAP) inhibitor, MK886, strongly inhibit the AA-stimulated growth of PC-3 and LNCaP cells [88, 89]. Conversely, exogenously added 5-HETE enhances cell growth similar to AA. 5-HETE also reverses the inhibitory effect of MK886, suggesting that the AA-induced prostate cancer cell growth is from the 5-LOX metabolism of AA to 5-HETE. The activation of apoptosis by MK886 is from its inhibition of 5-HETE production, stimulation of the mitochondrial permeability, externalization of phosphatidylserine, and degradation of DNA [89]. In PC-3 cells, 5-HETE activates extracellular signal-regulated kinases and Akt, and these responses are blocked by pertussis toxin, suggesting that 5-HETE stimulates cell growth by a receptor-dependent mechanism [90].
5-HETE can undergo dehydrogenated to 5-oxoeicosatetraenoic acid (5-ox-ETE). 5-oxo-ETE and its precursor, 5-HETE, reverse the selenium-induced apoptosis in human prostate cancer cells [91]. A G-protein coupled receptor for 5-oxo-ETE with less affinity for 5-HETE has been recently identified [92–94]. This 5-oxo-ETE receptor is expressed in PC-3 cells in higher amounts than in LNCaP cells [95]. Inhibiting expression of 5-oxo-ETE receptor by siRNA significantly reduces cell viability of PC-3 cells, suggesting that 5-oxo-ETE and its receptor have a critical role in prostate cancer cell survival [95].
Among the Cys-LTs, LTD4 is the most potent agonist for cysteinyl LT receptor type 1 (CysLT1R) and type 2 (CysLT2R) [96]. LTD4 activates different intracellular signaling pathways through these receptors to promote cell growth and survival. Interestingly, CysLT1R is present in prostate tissues and prostate cancer cells [97]. Low expression of CysLT1R is detected in BPH and normal prostate tissues but highly expressed in PIN and prostate cancer tissues with a correlation with grades of tumor. Furthermore, CysLT1R antagonist induces early apoptosis in prostate cancer cells, indicating its antiproliferative potential in prostate cancer [97].
II.2 12-LOX and prostate cancer
Platelet-type (P-), leukocyte-type (L-) and epidermal-type (E-) 12-LOX metabolize AA to 12(S)-HETE [98, 99]. 12(R)-LOX has been cloned from human keratinocytes [100], and produces 12(R)-HETE from AA. P-12-LOX mRNA levels are elevated in prostate cancer cells and in about 40% prostate cancer tissues compared to the matching normal prostate tissues from the same patients [101, 102], suggesting that it may be a diagnostic marker for prostate cancer advancement. 12-LOX protein is slightly detected in BPH and normal prostate tissues but it is markedly increased in PIN and prostate cancer tissues [84]. P-12-LOX may regulate prostate cancer development and progression by promoting angiogenesis [103–108], and it increases the metastatic potential of prostate cancer cells [109, 110]. On the other hand, L-12-LOX is expressed in human cancer tissues and cells [111] including PC-3 cells [112]; however, its role in prostate cancer is not clear.
The role of 12(S)-HETE in promoting prostate cancer growth and metastasis is well-established [106, 107] while the role of 12(R)-HETE in prostate cancer is not known. Urinary levels of 12(S)-HETE are elevated in prostate cancer and BPH patients but decreased to the levels in normal men following radical prostatectomy. These findings suggest that 12(S)-HETE may have a potential role as a marker in prostatic diseases [113]. 12-LOX inhibitors, baicalein and N-benzyl-N-hydroxy-5-phenylpentamide (BHPP) decrease proliferation of PC-3 and DU-145 cells, suppress cyclin D1 and D3, and arrest cell cycle at G0/G1 phase and exogenous 12(S)-HETE blocks the baicalein-induced apoptosis in DU-145 cells [105]. The activity of 12(S)-HETE in proliferation, motility and angiogenesis in PC-3 cells is mediated by the activation of nuclear factor-kappa B (NF-κB) via the degradation of IkappaB and NF-κB translocation to the nucleus initiating NF-κB-induced transcription [106]. Overexpression of 12-LOX in PC-3 cells or treatment of cells with 12(S)-HETE also increases expression of ανβ3 and ανβ5 integrins [107], increases the PI-3K activity and VEGF expression. These effects of 12-LOX are blocked by either baicalein or LY294002, a PI-3K inhibitor [114]. These treatments lead to prolonged cell survival of prostate cancer cells. Role of other 12-LOX metabolites of AA such as hepoxilins in prostate cancer is not known.
II.3 15-LOX and prostate cancer
There are two isoforms of 15-LOX, 15-LOX-1 and 15-LOX-2 [115, 116]. Both isoforms metabolize AA to 15(S)-HETE, although AA is the preferred substrate for 15-LOX-2 and linoleic acid (LA) is the preferred substrate for 15-LOX-1. 15-LOX-1 is present in PC-3 cells and human normal prostate tissues and its expression is significantly higher in prostate cancer tissues [117]. 15-LOX-1 is localized in secretory cells of peripheral zone of glands, prostatic ducts and seminal vessels, but not in the basal layer or stroma. These findings suggest that 15-LOX-1 is tumorigenic. Overexpression of 15-LOX-1 increases cell proliferation in PC-3 cells which is reversed by the specific 15-LOX-1 inhibitor, PD146176 [118]. In 15-LOX-1 transfected PC-3 cells, insulin-like growth factor-1 (IGF-1) and insulin-like growth factor-1 receptor (IGF-1R) are increased [119]. IGF-1R in normal and prostate cancer tissues is correlated well with expression of 15-LOX-1, suggesting that 15-LOX-1 promotes prostate tumorigenesis by mediating IGF-1R expression and activation. A recent study identified methylation of a CpG island in 15-LOX-1 promoter in prostate cancer cells and prostate cancer tissues but much less methylation in normal tissues [120], suggesting the requirement for promoter methylation for 15-LOX-1 expression in prostate cancer.
In benign prostate tissues, 15-LOX-2 protein is located mainly in secretory cells of peripheral zone glands and large prostatic ducts, and is not detected in the basal cell layer, stroma, ejaculatory ducts, seminal vesicles, or transitional epithelium [121]. Thus, its distribution differs from 15-LOX-1. Interestingly, 15-LOX-2 is absent in the majority of prostate cancer tissues, and the reduction of staining is greater in higher Gleason scores [122]. This leads to the implication that 15-LOX-2 may function as a prostate cancer suppressor [123]. The expression of 15-LOX-2 correlates well with the concentration of 15-HETE, which is high in normal tissues and markedly reduced or undetectable in prostate cancer tissues [121]. In PC-3 cells, exogenous 15-HETE inhibits cell proliferation and reduces PPARγ expression but upregulates the PPAR response element [124]. These findings indicate that 15(S)-HETE is a ligand for PPARγ and the loss of 15-LOX-2 may contribute to an increase of cell proliferation and a decrease of differentiation in prostate cancer. The cross talk between 15-LOX-2 gene and PPARγ has been identified, which is mediated by 15(S)-HETE and it may contribute to the diverse effects in normal and prostate cancer cells [125, 126].
Factors that contribute to the opposing effects of 15-LOX-1 and 15-LOX-2 are not well understood. One possibility is that 15-LOX-1 metabolizes the preferred substrate, LA, to 13(S)-hydroxyoctadecadienoic acid (13(S)-HODE). 13-HODE has been shown at higher amounts in prostate cancer tissues than adjacent normal tissues [117, 127]. More compelling evidence is from the study that demonstrated exogenous 13(S)-HODE increases the PPARγ phosphorylation while 15(S)-HETE decreases the PPARγ phosphorylation in PC-3 cells [115].
Whether other 15-LOX metabolites of AA such as lipoxins have role in prostate cancer is not known.
III. Cytochrome P450 Pathways
Cytochrome P450s (CYPs) are a large class of heme-containing enzymes that metabolize a variety of endogenous and exogenous substrates. CYP hydroxylases and CYP epoxygenases metabolize free AA to biologically active eicosanoids (Figure 4). CYP hydroxylases including CYP2E, 3A, 4A, and 4F convert AA to 20-, 19-, 18-, 17-, or 16-HETE [128–130]. Among these HETEs, 20-HETE (the metabolite of CYP ω-hydroxylases) has been intensely studied and it activates many signaling pathways to regulate vascular functions. CYP epoxygenases including CYP1A, 1B, 2C, 2D, and 2J convert AA to 14,15-, 11,12-, 8,9-, and 5,6-epoxyeicosatrienoic acids (EETs) [131–134]. EETs can be further metabolized by soluble epoxide hydrolase to their corresponding regioisomeric dihydroxyeicosatrienoic acids (DHETs). In human, CYP2C8, 2C9 and 2J2 have prominent roles in AA metabolism [135]. Only recently roles of these HETEs and EETs in cancer received attention.
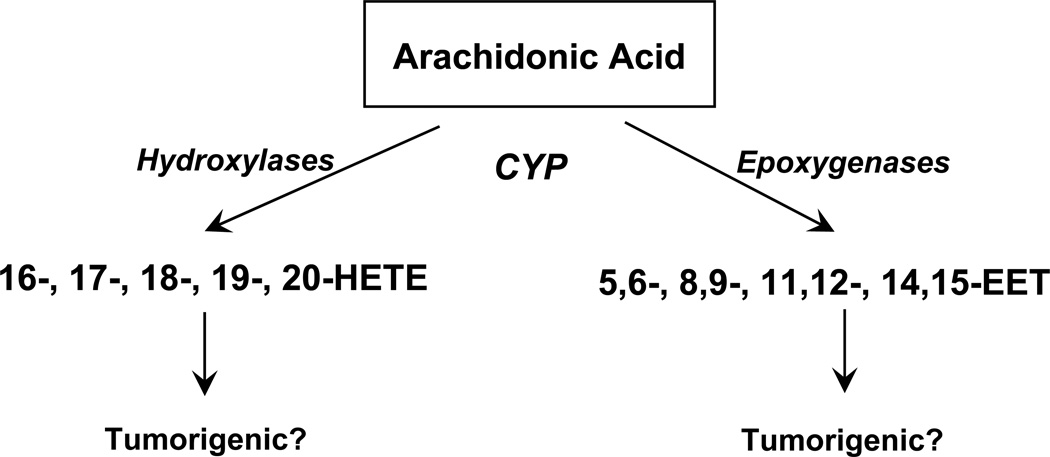
Figure 4.
Cytochrome P450 pathway of AA. CYP hydroxylases metabolize AA to HETEs and CYP epoxygenases metabolize AA to four regioisomeric epoxyeicosatrienoic acids (EETs). At present, roles of HETEs and EETs in prostate cancer are not known.
Recent studies have demonstrated that CYPs and particularly the EETs have roles in many types of cancer. CYP2C and 3A are variably expressed in normal and neoplastic tissues of different human organs [136]. CYP2C9 has been associated with an increased risk of colorectal cancer [137] and lung cancer [138, 139]. CYP2J2 expression is detected in many types of human cancer tissues and cancer cell lines while it is not detected in adjacent normal tissues and normal cells [140]. Overexpression of CYP2J2 or addition of exogenous EETs in cancer cells increases cell proliferation and inhibits apoptosis by the activation of MAPK and PI-3 kinase-Akt signaling pathways and the EGFR phosphorylation [140]. CYP2J2 and EETs also promotes cancer cell migration and invasion through the upregulation of MMPs and CD44 and down regulation of the antimetastatic genes CD82 and nm-23 [141]. A comprehensive analysis of CYP2C8, 2C9, and 2J2 in human cancer tissues and their surrounding normal tissues using tissue microarrays has been reported [142]. Interestingly, these enzymes exhibit diverse expression patterns in different organ tissues.
Numerous studies have demonstrated the roles of CYPs in prostate cancer but not in the association with the synthesis of eicosanoids. CYP1A, 2C, and 3A are present in 63, 25, and 61% of human prostate cancer tissues, respectively [143]. A genetic polymorphism of CYP1A1 has been proposed to associate with prostate cancer susceptibility [144]. Interestingly, CYP2C8 and 2C9 are detected in prostate cancer tissues but CYP2J2 is not detected in those tissues [142]. In prostate cancer cells, PC-3, DU-145, and LNCaP cells express CYP1A1 and 1A2 while normal prostate cells do not express CYP1A1 [145]. Although roles of these CYPs in prostate cancer are not currently understood, their presence in prostate cancer cells and tissues suggest that they may play roles in AA metabolism and regulation of prostate cancer. A clinical implication of CYP eicosanoids in prostate cancer has been demonstrated by higher urinary levels of 20-HETE in BPH and prostate cancer patients than normal men [113]. Radical prostatectomy reduces the urinary levels of 20-HETE to the values in normal men, suggesting that 20-HETE may be involved in prostatic diseases. The CYP pathway may prove to be important in prostate cancer progression and deserves further investigation.
IV. Endocannabinoids and Prostate Cancer
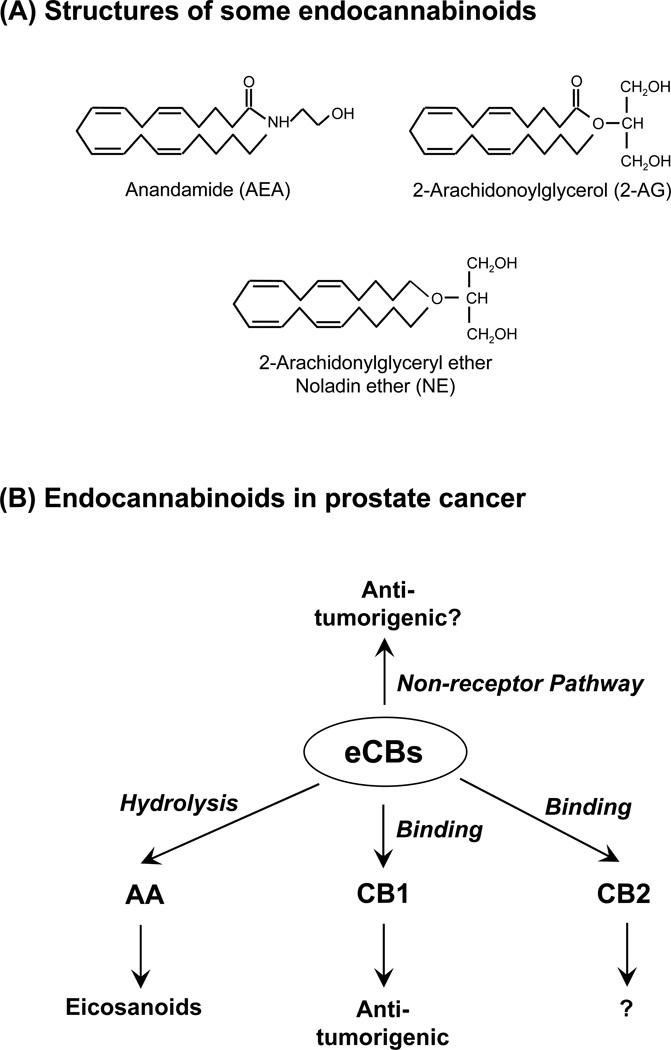
Endocannabinoids (eCBs) are endogenously produced lipids and bind to cannabinoid (CB) receptors to initiate signaling pathways. These lipid mediators are derivatives of fatty acids. Two well characterized AA-containing eCBs are arachidonylethanolamide (anandamide, AEA) [146] and 2-arachidonoylglycerol (2-AG) [147, 148] (Figure 5). A putative endocannabinoid, 2-arachidonylglyceryl ether (noladin ether, NE) is more enzymatically stable [149–151] and it has been used for investigating pharmacological functions of eCBs. Although NE was first identified in porcine brain [152] and rat brain regions [153], it was not detected in the brains of various mammalian species [154, 155].
Figure 5.
Endocannabinoids (eCBs) in prostate cancer. (A) Structures of some of well-characterized endocannabinoids (shown are AA-derived eCBs), anandamide (AEA), 2-arachidonoylglycerol (2-AG), and noladin ether (NE). (B) Mechanistic pathways for eCBs. Hydrolysis of eCBs produces free AA which is further metabolized by oxygenases to eicosanoids in the AA pathways. The eCBs bind to cannabinoid receptor type 1 (CB1 receptor) to inhibit prostate caner cell proliferation and invasion/migration; however, the role of CB2 receptor is not clear. The eCBs can inhibit prostate cancer proliferation by a non-receptor-mediated pathway.
Endocannabinoids function through the activation of CB receptors. However, several biological effects of eCBs occur through non-CB receptor mechanisms [156, 157]. In either case, the balance between synthesis and metabolism of eCB is critical to maintaining the endogenous concentrations of eCBs. Hydrolysis of eCBs is an important regulatory step that limits the actions of eCBs in many physiological and pathological processes. AEA is hydrolyzed by fatty acid amide hydrolase (FAAH) [158–160] and 2-AG is hydrolyzed by FAAH and monoacylglycerol lipase (MGL) [161–163] to free AA. Hydrolysis of AEA and 2-AG will decrease the activity of eCBs and will liberate free AA as the substrate for the enzymes in the AA cascade discussed above. It is now recognized that the inhibition of hydrolysis is a potential therapeutic target for cancer treatment [160, 164, 165].
Endocannabinoids regulate growth and migration of a variety of cancer cells and have therapeutic potential in cancer treatment [160, 164–177]. In prostate cancer, AEA, acting through the CB1 receptor, inhibits the EGF-induced proliferation of prostate carcinoma cells [178] by decreasing EGFR expression and increasing ceramide production. LNCaP cells are the least sensitive to AEA and PC-3 cells are the most sensitive to AEA in the inhibition of proliferation. These observations correlated well with the high expression of FAAH (enzyme hydrolyzing AEA) in LNCaP cells and extremely low expression of FAAH in PC-3 cells [179]. AEA, 2-AG and HU-210 (a synthetic CB receptor agonist) inhibit prolactin-induced proliferation of DU-145 cells through the activation of CB1 receptors [180]. However, (R)-methanandamide (a synthetic AEA analog) increases proliferation of androgen-dependent LNCaP cells through CB1 and CB2 receptors and the PI3K pathway to increase androgen receptor expression [181]. NE and CB1 receptor agonists inhibit invasion of PC-3 and DU-145 cells [182] and AEA inhibits invasion of PC-3 cells [179]. Inhibition of eCB hydrolysis by either pharmacological inhibitors or siRNA knockdown of the FAAH inhibits invasion of these cells [179, 183]. The combination of eCB hydrolysis inhibition and treatment with exogenous eCBs further inhibit cell invasion [179]. LNCaP cells, with high eCB hydrolytic activity, are not sensitive to treatments with eCBs or CB1 receptor agonists unless the eCB hydrolysis is blocked [179]. Furthermore, 2-AG can increase cell invasion due to its hydrolysis to AA and subsequent metabolism to 12-HETE [112], but it will inhibit invasion in the presence of the hydrolysis inhibitors [179]. These studies demonstrated that eCBs regulate prostate cancer proliferation and invasion, and their hydrolysis can dictate the differential effects of eCBs and responses.
Conclusions
The ω-6 polyunsaturated fatty acid, AA, plays various important roles in prostate cancer. AA can be metabolized to numerous metabolites and act as endogenous lipid signaling molecules that may have diverse effects on prostate cancer cells. The balance and/or specific pathways of synthesis, degradation, and signal transduction activation will dictate prostate cancer cell fate and cancer development and progression.
Future perspective
The AA metabolism is complex and consists of multiple pathways to produce numerous eicosanoids by specific oxygenases. In prostate cancer, some of these enzymes and eicosanoids are tumorigenic and some are antitumorigenic. Several of them are potential targets in clinical applications. Although the AA pathways have been demonstrated as regulators in prostate cancer for several decades, their functions are not fully understood and merit further study. In particular, a better understanding of signaling pathways by these lipid mediators is needed to advance the field for cancer prevention and treatment. A new pathway of AA metabolism by CYP in cancer may be important in prostate cancer. Lastly, endocannabinoids, endogenous AA-containing signaling molecules have significant roles in proliferation and migration/invasion of prostate cancer cells. Thus, further studies will elucidate their function as therapeutic targets for prostate cancer.
Executive summary.
Introduction
Prostate cancer cell growth and invasion/migration leads to significant morbidity and mortality.
Arachidonic acid (AA) has been recognized as protumorigenic.
AA is metabolized by COX, LOX, and CYP to biologically active eicosanoids. These enzymes and eicosanoids have diverse cellular functions.
Cyclooxygenase pathway
COX-2 is a prostate cancer promoter and it is expressed in prostate cancer tissues and cells. PGE2 is the major COX metabolite of AA in prostate cancer cells and tissues.
PGI2 and PGD2 are antitumorigenic while PGE2 and TxA2 are tumorigenic by acting through their prostanoid receptors.
Lipoxygenase pathway
5-LOX and its metabolites such as 5-HETE, 5-oxo-ETE and Cys-LTs are tumorigenic. 5-HETE and 5-oxo-ETE activate 5-oxo-ETE receptor and Cys-LTs are ligands for Cys-LT1R and Cys-LT2R.
12-LOX is highly expressed in prostate cancer tissues and cells. 12-LOX and it metabolite, 12-HETE are tumorigenic.
15-LOX-1 is highly expressed in prostate cancer tissues but 15-LOX-2 is not detected in prostate cancer tissues. 15-LOX-1 is tumorigenic while 15-LOX-2 is antitumorigenic. The major 15-LOX-1 metabolite from LA is 13(S)-HODE and the major 15-LOX-2 metabolite form AA is 15(S)-HETE. 15(S)-HETE is a ligand for PPARγ.
Cytochrome P450 pathway
AA is metabolized by CYP hydroxylases to HETEs and by CYP epoxygenases to EETs.
Some isoforms of CYP epoxygenases and EETs have been shown to stimulate cancer progression. Their roles in prostate cancer are not known.
Endocannabinoids
Endocannabinoids, acting through CB1 receptor inhibit proliferation and invasion of prostate cancer cells.
Hydrolysis of endocannabinoids affects the activity of endocannabinoids in the regulation of prostate cancer cells because it reduces the ligand concentrations and liberates the free AA.
Endocannabinoids may regulate prostate cancer cells by non-receptor-mediated mechanisms.
Acknowledgements
This work was supported by the grants from the Breast Cancer Showhouse, Medical College of Wisconsin Cancer Center, and the National Institute of Health, HL-51055, HL-37981, and DA-09155.
References
- 1.Leitzmann MF, Stampfer MJ, Michaud DS, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 2.Ritch CR, Wan RL, Stephens LB, et al. Dietary fatty acids correlate with prostate cancer biopsy grade and volume in Jamaican men. J Urol. 2007;177:97–101. doi: 10.1016/j.juro.2006.08.105. discussion 101. [DOI] [PubMed] [Google Scholar]
- 3.Hughes-Fulford M, Chen Y, Tjandrawinata RR. Fatty acid regulates gene expression and growth of human prostate cancer PC-3 cells. Carcinogenesis. 2001;22:701–707. doi: 10.1093/carcin/22.5.701. [DOI] [PubMed] [Google Scholar]
- 4.Hughes-Fulford M, Li CF, Boonyaratanakornkit J, Sayyah S. Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Res. 2006;66:1427–1433. doi: 10.1158/0008-5472.CAN-05-0914. [DOI] [PubMed] [Google Scholar]
- 5.Chavarro JE, Stampfer MJ, Li H, Campos H, Kurth T, Ma J. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1364–1370. doi: 10.1158/1055-9965.EPI-06-1033. [DOI] [PubMed] [Google Scholar]
- 6.MacLean CH, Newberry SJ, Mojica WA, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. Jama. 2006;295:403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. 2004;134:919–922. doi: 10.1093/jn/134.4.919. [DOI] [PubMed] [Google Scholar]
- 8.Aronson WJ, Glaspy JA, Reddy ST, Reese D, Heber D, Bagga D. Modulation of omega-3/omega-6 polyunsaturated ratios with dietary fish oils in men with prostate cancer. Urology. 2001;58:283–288. doi: 10.1016/s0090-4295(01)01116-5. [DOI] [PubMed] [Google Scholar]
- 9.Carmody J, Olendzki B, Reed G, Andersen V, Rosenzweig P. A Dietary Intervention for Recurrent Prostate Cancer After Definitive Primary Treatment: Results of a Randomized Pilot Trial. Urology. 2008 doi: 10.1016/j.urology.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekharan NV, Dai H, Roos KL, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Madaan S, Abel PD, Chaudhary KS, et al. Cytoplasmic induction and overexpression of cyclooxygenase-2 in human prostate cancer: implications for prevention and treatment. BJU Int. 2000;86:736–741. doi: 10.1046/j.1464-410x.2000.00867.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura R, Sano H, Masuda C, et al. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89:589–596. [PubMed] [Google Scholar]
- 14.Kirschenbaum A, Klausner AP, Lee R, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56:671–676. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 15.Kirschenbaum A, Liu X, Yao S, Levine AC. The role of cyclooxygenase-2 in prostate cancer. Urology. 2001;58:127–131. doi: 10.1016/s0090-4295(01)01255-9. [DOI] [PubMed] [Google Scholar]
- 16.Uotila P, Valve E, Martikainen P, Nevalainen M, Nurmi M, Harkonen P. Increased expression of cyclooxygenase-2 and nitric oxide synthase-2 in human prostate cancer. Urol Res. 2001;29:23–28. doi: 10.1007/s002400000148. [DOI] [PubMed] [Google Scholar]
- 17.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191:125–135. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 18.Edwards J, Mukherjee R, Munro AF, Wells AC, Almushatat A, Bartlett JM. HER2 and COX2 expression in human prostate cancer. Eur J Cancer. 2004;40:50–55. doi: 10.1016/j.ejca.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Bergh A, Damber JE. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res. 2005;11:3250–3256. doi: 10.1158/1078-0432.CCR-04-2405. [DOI] [PubMed] [Google Scholar]
- 20.Lee LM, Pan CC, Cheng CJ, Chi CW, Liu TY. Expression of cyclooxygenase-2 in prostate adenocarcinoma and benign prostatic hyperplasia. Anticancer Res. 2001;21:1291–1294. [PubMed] [Google Scholar]
- 21.Fujita H, Koshida K, Keller ET, et al. Cyclooxygenase-2 promotes prostate cancer progression. Prostate. 2002;53:232–240. doi: 10.1002/pros.10152. [DOI] [PubMed] [Google Scholar]
- 22.Shappell SB, Manning S, Boeglin WE, et al. Alterations in lipoxygenase and cyclooxygenase-2 catalytic activity and mRNA expression in prostate carcinoma. Neoplasia. 2001;3:287–303. doi: 10.1038/sj.neo.7900166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zha S, Gage WR, Sauvageot J, et al. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–8623. [PubMed] [Google Scholar]
- 24.Wagner M, Loos J, Weksler N, et al. Resistance of prostate cancer cell lines to COX-2 inhibitor treatment. Biochem Biophys Res Commun. 2005;332:800–807. doi: 10.1016/j.bbrc.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Lim JT, Piazza GA, Han EK, et al. Sulindac derivatives inhibit growth and induce apoptosis in human prostate cancer cell lines. Biochem Pharmacol. 1999;58:1097–1107. doi: 10.1016/s0006-2952(99)00200-2. [DOI] [PubMed] [Google Scholar]
- 26.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 27.Subbarayan V, Sabichi AL, Llansa N, Lippman SM, Menter DG. Differential expression of cyclooxygenase-2 and its regulation by tumor necrosis factor-alpha in normal and malignant prostate cells. Cancer Res. 2001;61:2720–2726. [PubMed] [Google Scholar]
- 28.Patel MI, Subbaramaiah K, Du B, et al. Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clin Cancer Res. 2005;11:1999–2007. doi: 10.1158/1078-0432.CCR-04-1877. [DOI] [PubMed] [Google Scholar]
- 29.Kakizoe T. Chemoprevention of cancer--focusing on clinical trials. Jpn J Clin Oncol. 2003;33:421–442. doi: 10.1093/jjco/hyg090. [DOI] [PubMed] [Google Scholar]
- 30.Wen B, Deutsch E, Eschwege P, et al. Cyclooxygenase-2 inhibitor NS398 enhances antitumor effect of irradiation on hormone refractory human prostate carcinoma cells. J Urol. 2003;170:2036–2039. doi: 10.1097/01.ju.0000092239.98832.52. [DOI] [PubMed] [Google Scholar]
- 31.Sabichi AL, Lippman SM. COX-2 inhibitors and other NSAIDs in bladder and prostate cancer. Prog Exp Tumor Res. 2003;37:163–178. doi: 10.1159/000071372. [DOI] [PubMed] [Google Scholar]
- 32.Andrews J, Djakiew D, Krygier S, Andrews P. Superior effectiveness of ibuprofen compared with other NSAIDs for reducing the survival of human prostate cancer cells. Cancer Chemother Pharmacol. 2002;50:277–284. doi: 10.1007/s00280-002-0485-8. [DOI] [PubMed] [Google Scholar]
- 33.Kamijo T, Sato T, Nagatomi Y, Kitamura T. Induction of apoptosis by cyclooxygenase-2 inhibitors in prostate cancer cell lines. Int J Urol. 2001;8:S35–S39. doi: 10.1046/j.1442-2042.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC. Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J Urol. 2000;164:820–825. doi: 10.1097/00005392-200009010-00056. [DOI] [PubMed] [Google Scholar]
- 35.Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398 a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 36.Norrish AE, Jackson RT, McRae CU. Non-steroidal anti-inflammatory drugs and prostate cancer progression. Int J Cancer. 1998;77:511–515. doi: 10.1002/(sici)1097-0215(19980812)77:4<511::aid-ijc6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 37.Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. Bmj. 2000;320:1642–1646. doi: 10.1136/bmj.320.7250.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fosslien E. Review: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann Clin Lab Sci. 2001;31:325–348. [PubMed] [Google Scholar]
- 39.Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000;37:431–502. doi: 10.1080/10408360091174286. [DOI] [PubMed] [Google Scholar]
- 40.Jain S, Chakraborty G, Kundu GC. The crucial role of cyclooxygenase-2 in osteopontin-induced protein kinase C alpha/c-Src/IkappaB kinase alpha/beta-dependent prostate tumor progression and angiogenesis. Cancer Res. 2006;66:6638–6648. doi: 10.1158/0008-5472.CAN-06-0661. [DOI] [PubMed] [Google Scholar]
- 41.Aparicio Gallego G, Diaz Prado S, Jimenez Fonseca P, Garcia Campelo R, Cassinello Espinosa J, Anton Aparicio LM. Cyclooxygenase-2 (COX-2): a molecular target in prostate cancer. Clin Transl Oncol. 2007;9:694–702. doi: 10.1007/s12094-007-0126-0. [DOI] [PubMed] [Google Scholar]
- 42.Adachi M, Sakamoto H, Kawamura R, Wang W, Imai K, Shinomura Y. Nonsteroidal anti-inflammatory drugs and oxidative stress in cancer cells. Histol Histopathol. 2007;22:437–442. doi: 10.14670/HH-22.437. [DOI] [PubMed] [Google Scholar]
- 43.Cai Y, Lee YF, Li G, et al. A new prostate cancer therapeutic approach: Combination of androgen ablation with COX-2 inhibitor. Int J Cancer. 2008 doi: 10.1002/ijc.23481. [DOI] [PubMed] [Google Scholar]
- 44.Mehar A, Macanas-Pirard P, Mizokami A, Takahashi Y, Kass GE, Coley HM. The effects of cyclooxygenase-2 expression in prostate cancer cells: modulation of response to cytotoxic agents. J Pharmacol Exp Ther. 2008;324:1181–1187. doi: 10.1124/jpet.107.131383. [DOI] [PubMed] [Google Scholar]
- 45.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 46.Khan O, Hensby CN, Williams G. Prostacyclin in prostatic cancer: a better marker than bone scan or serum acid phosphatase? Br J Urol. 1982;54:26–31. doi: 10.1111/j.1464-410x.1982.tb13506.x. [DOI] [PubMed] [Google Scholar]
- 47.Drago JR, Al-Mondhiry HA. The effect of prostaglandin modulators on prostate tumor growth and metastasis. Anticancer Res. 1984;4:391–394. [PubMed] [Google Scholar]
- 48.Schneider MR, Schillinger E, Schirner M, Skuballa W, Sturzebecher S, Witt W. Effects of prostacyclin analogues in in vivo tumor models. Adv Prostaglandin Thromboxane Leukot Res. 1991;21B:901–908. [PubMed] [Google Scholar]
- 49.Schirner M, Schneider MR. The prostacyclin analogue cicaprost inhibits metastasis of tumours of R 3327 MAT Lu prostate carcinoma and SMT 2A mammary carcinoma. J Cancer Res Clin Oncol. 1992;118:497–501. doi: 10.1007/BF01225263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ablin RJ. Prostaglandin E2 affects the tumor immune response in prostatic carcinoma. J Urol. 1982;127:997–998. doi: 10.1016/s0022-5347(17)54173-7. [DOI] [PubMed] [Google Scholar]
- 51.Chaudry AA, Wahle KW, McClinton S, Moffat LE. Arachidonic acid metabolism in benign and malignant prostatic tissue in vitro: effects of fatty acids and cyclooxygenase inhibitors. Int J Cancer. 1994;57:176–180. doi: 10.1002/ijc.2910570208. [DOI] [PubMed] [Google Scholar]
- 52.Tjandrawinata RR, Hughes-Fulford M. Up-regulation of cyclooxygenase-2 by product-prostaglandin E2. Adv Exp Med Biol. 1997;407:163–170. doi: 10.1007/978-1-4899-1813-0_25. [DOI] [PubMed] [Google Scholar]
- 53.Tjandrawinata RR, Dahiya R, Hughes-Fulford M. Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Br J Cancer. 1997;75:1111–1118. doi: 10.1038/bjc.1997.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu XH, Kirschenbaum A, Lu M, et al. Prostaglandin E2 induces hypoxia-inducible factor-1alpha stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem. 2002;277:50081–50086. doi: 10.1074/jbc.M201095200. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, Hughes-Fulford M. Prostaglandin E2 and the protein kinase A pathway mediate arachidonic acid induction of c-fos in human prostate cancer cells. Br J Cancer. 2000;82:2000–2006. doi: 10.1054/bjoc.2000.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Klein RD. Prostaglandin E2 induces vascular endothelial growth factor secretion in prostate cancer cells through EP2 receptor-mediated cAMP pathway. Mol Carcinog. 2007;46:912–923. doi: 10.1002/mc.20320. [DOI] [PubMed] [Google Scholar]
- 57.Liu XH, Kirschenbaum A, Lu M, et al. Prostaglandin E(2) stimulates prostatic intraepithelial neoplasia cell growth through activation of the interleukin-6/GP130/STAT-3 signaling pathway. Biochem Biophys Res Commun. 2002;290:249–255. doi: 10.1006/bbrc.2001.6188. [DOI] [PubMed] [Google Scholar]
- 58.Attiga FA, Fernandez PM, Weeraratna AT, Manyak MJ, Patierno SR. Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Res. 2000;60:4629–4637. [PubMed] [Google Scholar]
- 59.Nithipatikom K, Isbell MA, Lindholm PF, Kajdacsy-Balla A, Kaul S, Campell WB. Requirement of cyclooxygenase-2 expression and prostaglandins for human prostate cancer cell invasion. Clin Exp Metastasis. 2002;19:593–601. doi: 10.1023/a:1020915914376. [DOI] [PubMed] [Google Scholar]
- 60.Kim J, Yang P, Suraokar M, et al. Suppression of prostate tumor cell growth by stromal cell prostaglandin D synthase-derived products. Cancer Res. 2005;65:6189–6198. doi: 10.1158/0008-5472.CAN-04-4439. [DOI] [PubMed] [Google Scholar]
- 61.Nakata S, Yoshida T, Shiraishi T, et al. 15-Deoxy-Delta12,14-prostaglandin J(2) induces death receptor 5 expression through mRNA stabilization independently of PPARgamma and potentiates TRAIL-induced apoptosis. Mol Cancer Ther. 2006;5:1827–1835. doi: 10.1158/1535-7163.MCT-06-0023. [DOI] [PubMed] [Google Scholar]
- 62.Chaffer CL, Thomas DM, Thompson EW, Williams ED. PPARgamma-independent induction of growth arrest and apoptosis in prostate and bladder carcinoma. BMC Cancer. 2006;6:53. doi: 10.1186/1471-2407-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nunez NP, Liu H, Meadows GG. PPAR-gamma ligands and amino acid deprivation promote apoptosis of melanoma, prostate, and breast cancer cells. Cancer Lett. 2006;236:133–141. doi: 10.1016/j.canlet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Subbarayan V, Sabichi AL, Kim J, et al. Differential peroxisome proliferator-activated receptor-gamma isoform expression and agonist effects in normal and malignant prostate cells. Cancer Epidemiol Biomarkers Prev. 2004;13:1710–1716. [PubMed] [Google Scholar]
- 65.Sabichi AL, Subbarayan V, Llansa N, Lippman SM, Menter DG. Peroxisome proliferator-activated receptor-gamma suppresses cyclooxygenase-2 expression in human prostate cells. Cancer Epidemiol Biomarkers Prev. 2004;13:1704–1709. [PubMed] [Google Scholar]
- 66.Butler R, Mitchell SH, Tindall DJ, Young CY. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta12,14-prostaglandin J2. Cell Growth Differ. 2000;11:49–61. [PubMed] [Google Scholar]
- 67.Haslmayer P, Thalhammer T, Jager W, et al. The peroxisome proliferator-activated receptor gamma ligand 15-deoxy-Delta12,14-prostaglandin J2 induces vascular endothelial growth factor in the hormone-independent prostate cancer cell line PC 3 and the urinary bladder carcinoma cell line 5637. Int J Oncol. 2002;21:915–920. [PubMed] [Google Scholar]
- 68.Henriksson P, Stege R, Green K. Profound decrease of in vivo formation of thromboxane during oestrogen therapy. Eur J Clin Invest. 1996;26:1186–1188. doi: 10.1046/j.1365-2362.1996.950606.x. [DOI] [PubMed] [Google Scholar]
- 69.Nie D, Che M, Zacharek A, et al. Differential expression of thromboxane synthase in prostate carcinoma: role in tumor cell motility. Am J Pathol. 2004;164:429–439. doi: 10.1016/S0002-9440(10)63133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dassesse T, de Leval X, de Leval L, Pirotte B, Castronovo V, Waltregny D. Activation of the thromboxane A2 pathway in human prostate cancer correlates with tumor Gleason score and pathologic stage. Eur Urol. 2006;50:1021–1031. doi: 10.1016/j.eururo.2006.01.036. discussion 1031. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Wei J, Tai HH. Activation of extracellular signal-regulated kinase by 12-hydroxyheptadecatrienoic acid in prostate cancer PC3 cells. Arch Biochem Biophys. 2007;467:20–30. doi: 10.1016/j.abb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Fujino H, Regan JW. Prostanoid receptors and phosphatidylinositol 3-kinase: a pathway to cancer? Trends Pharmacol Sci. 2003;24:335–340. doi: 10.1016/S0165-6147(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 73.Miyata Y, Kanda S, Maruta S, et al. Relationship between prostaglandin E2 receptors and clinicopathologic features in human prostate cancer tissue. Urology. 2006;68:1360–1365. doi: 10.1016/j.urology.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 74.Moreno J, Krishnan AV, Peehl DM, Feldman D. Mechanisms of vitamin D-mediated growth inhibition in prostate cancer cells: inhibition of the prostaglandin pathway. Anticancer Res. 2006;26:2525–2530. [PubMed] [Google Scholar]
- 75.Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65:7917–7925. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 76.Moreno J, Krishnan AV, Feldman D. Molecular mechanisms mediating the anti-proliferative effects of Vitamin D in prostate cancer. J Steroid Biochem Mol Biol. 2005;97:31–36. doi: 10.1016/j.jsbmb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Swami S, Krishnan AV, Moreno J, Bhattacharyya RB, Peehl DM, Feldman D. Calcitriol and genistein actions to inhibit the prostaglandin pathway: potential combination therapy to treat prostate cancer. J Nutr. 2007;137:205S–210S. doi: 10.1093/jn/137.1.205S. [DOI] [PubMed] [Google Scholar]
- 78.Krishnan AV, Moreno J, Nonn L, et al. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol. 2007;103:694–702. doi: 10.1016/j.jsbmb.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 79.Feldman D, Krishnan A, Moreno J, Swami S, Peehl DM, Srinivas S. Vitamin D inhibition of the prostaglandin pathway as therapy for prostate cancer. Nutr Rev. 2007;65:S113–S115. doi: 10.1111/j.1753-4887.2007.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 80.Krishnan AV, Moreno J, Nonn L, Swami S, Peehl DM, Feldman D. Calcitriol as a chemopreventive and therapeutic agent in prostate cancer: role of anti-inflammatory activity. J Bone Miner Res. 2007;22(Suppl 2):V74–V80. doi: 10.1359/jbmr.07s213. [DOI] [PubMed] [Google Scholar]
- 81.Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J Biol Chem. 2006;281:26483–26490. doi: 10.1074/jbc.M604376200. [DOI] [PubMed] [Google Scholar]
- 82.Nie D, Guo Y, Yang D, et al. Thromboxane A2 receptors in prostate carcinoma: expression and its role in regulating cell motility via small GTPase Rho. Cancer Res. 2008;68:115–121. doi: 10.1158/0008-5472.CAN-07-1018. [DOI] [PubMed] [Google Scholar]
- 83.Flamand N, Mancuso P, Serezani CH, Brock TG. Leukotrienes: mediators that have been typecast as villains. Cell Mol Life Sci. 2007;64:2657–2670. doi: 10.1007/s00018-007-7228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuyama M, Yoshimura R, Mitsuhashi M, et al. Expression of lipoxygenase in human prostate cancer and growth reduction by its inhibitors. Int J Oncol. 2004;24:821–827. [PubMed] [Google Scholar]
- 85.Myers CE, Ghosh J. Lipoxygenase inhibition in prostate cancer. Eur Urol. 1999;35:395–398. doi: 10.1159/000019915. [DOI] [PubMed] [Google Scholar]
- 86.Hong SH, Avis I, Vos MD, Martinez A, Treston AM, Mulshine JL. Relationship of arachidonic acid metabolizing enzyme expression in epithelial cancer cell lines to the growth effect of selective biochemical inhibitors. Cancer Res. 1999;59:2223–2228. [PubMed] [Google Scholar]
- 87.Matsuyama M, Yoshimura R, Tsuchida K, et al. Lipoxygenase inhibitors prevent urological cancer cell growth. Int J Mol Med. 2004;13:665–668. [PubMed] [Google Scholar]
- 88.Ghosh J, Myers CE. Arachidonic acid stimulates prostate cancer cell growth: critical role of 5-lipoxygenase. Biochem Biophys Res Commun. 1997;235:418–423. doi: 10.1006/bbrc.1997.6799. [DOI] [PubMed] [Google Scholar]
- 89.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O'Flaherty JT, Rogers LC, Chadwell BA, et al. 5(S)-Hydroxy-6,8,11,14-E, Z, Z, Z-eicosatetraenoate stimulates PC3 cell signaling and growth by a receptor-dependent mechanism. Cancer Res. 2002;62:6817–6819. [PubMed] [Google Scholar]
- 91.Ghosh J. Rapid induction of apoptosis in prostate cancer cells by selenium: reversal by metabolites of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun. 2004;315:624–635. doi: 10.1016/j.bbrc.2004.01.100. [DOI] [PubMed] [Google Scholar]
- 92.Hosoi T, Koguchi Y, Sugikawa E, et al. Identification of a novel human eicosanoid receptor coupled to G(i/o) J Biol Chem. 2002;277:31459–31465. doi: 10.1074/jbc.M203194200. [DOI] [PubMed] [Google Scholar]
- 93.Hosoi T, Sugikawa E, Chikada A, Koguchi Y, Ohnuki T. TG1019/OXE, a Galpha(i/o)-protein-coupled receptor, mediates 5-oxo-eicosatetraenoic acid-induced chemotaxis. Biochem Biophys Res Commun. 2005;334:987–995. doi: 10.1016/j.bbrc.2005.06.191. [DOI] [PubMed] [Google Scholar]
- 94.Jones CE, Holden S, Tenaillon L, et al. Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol Pharmacol. 2003;63:471–477. doi: 10.1124/mol.63.3.471. [DOI] [PubMed] [Google Scholar]
- 95.Sundaram S, Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem Biophys Res Commun. 2006;339:93–98. doi: 10.1016/j.bbrc.2005.10.189. [DOI] [PubMed] [Google Scholar]
- 96.Rovati GE, Capra V. Cysteinyl-leukotriene receptors and cellular signals. ScientificWorldJournal. 2007;7:1375–1392. doi: 10.1100/tsw.2007.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsuyama M, Hayama T, Funao K, et al. Overexpression of cysteinyl LT1 receptor in prostate cancer and CysLT1R antagonist inhibits prostate cancer cell growth through apoptosis. Oncol Rep. 2007;18:99–104. [PubMed] [Google Scholar]
- 98.Funk CD, Furci L, FitzGerald GA. Molecular cloning, primary structure, and expression of the human platelet/erythroleukemia cell 12-lipoxygenase. Proc Natl Acad Sci U S A. 1990;87:5638–5642. doi: 10.1073/pnas.87.15.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoshimoto T, Suzuki H, Yamamoto S, Takai T, Yokoyama C, Tanabe T. Cloning and sequence analysis of the cDNA for arachidonate 12-lipoxygenase of porcine leukocytes. Proc Natl Acad Sci U S A. 1990;87:2142–2146. doi: 10.1073/pnas.87.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boeglin WE, Kim RB, Brash AR. A 12R-lipoxygenase in human skin: mechanistic evidence, molecular cloning, and expression. Proc Natl Acad Sci U S A. 1998;95:6744–6749. doi: 10.1073/pnas.95.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao X, Grignon DJ, Chbihi T, et al. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995;46:227–237. doi: 10.1016/s0090-4295(99)80198-8. [DOI] [PubMed] [Google Scholar]
- 102.Gao X, Porter AT, Honn KV. Involvement of the multiple tumor suppressor genes and 12-lipoxygenase in human prostate cancer. Therapeutic implications. Adv Exp Med Biol. 1997;407:41–53. doi: 10.1007/978-1-4899-1813-0_7. [DOI] [PubMed] [Google Scholar]
- 103.Nie D, Hillman GG, Geddes T, et al. Platelet-type 12-lipoxygenase in a human prostate carcinoma stimulates angiogenesis and tumor growth. Cancer Res. 1998;58:4047–4051. [PubMed] [Google Scholar]
- 104.Nie D, Hillman GG, Geddes T, et al. Platelet-type 12-lipoxygenase regulates angiogenesis in human prostate carcinoma. Adv Exp Med Biol. 1999;469:623–630. doi: 10.1007/978-1-4615-4793-8_90. [DOI] [PubMed] [Google Scholar]
- 105.Pidgeon GP, Kandouz M, Meram A, Honn KV. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002;62:2721–2727. [PubMed] [Google Scholar]
- 106.Kandouz M, Nie D, Pidgeon GP, Krishnamoorthy S, Maddipati KR, Honn KV. Platelet-type 12-lipoxygenase activates NF-kappaB in prostate cancer cells. Prostaglandins Other Lipid Mediat. 2003;71:189–204. doi: 10.1016/s1098-8823(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 107.Pidgeon GP, Tang K, Cai YL, Piasentin E, Honn KV. Overexpression of platelet-type 12-lipoxygenase promotes tumor cell survival by enhancing alpha(v)beta(3) and alpha(v)beta(5) integrin expression. Cancer Res. 2003;63:4258–4267. [PubMed] [Google Scholar]
- 108.Gu JL, Natarajan R, Ben-Ezra J, et al. Evidence that a leukocyte type of 12-lipoxygenase is expressed and regulated by angiotensin II in human adrenal glomerulosa cells. Endocrinology. 1994;134:70–77. doi: 10.1210/endo.134.1.8275971. [DOI] [PubMed] [Google Scholar]
- 109.Timar J, Raso E, Dome B, et al. Expression, subcellular localization and putative function of platelet-type 12-lipoxygenase in human prostate cancer cell lines of different metastatic potential. Int J Cancer. 2000;87:37–43. doi: 10.1002/1097-0215(20000701)87:1<37::aid-ijc6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 110.Nie D, Nemeth J, Qiao Y, et al. Increased metastatic potential in human prostate carcinoma cells by overexpression of arachidonate 12-lipoxygenase. Clin Exp Metastasis. 2003;20:657–663. doi: 10.1023/a:1027302408187. [DOI] [PubMed] [Google Scholar]
- 111.Natarajan R, Esworthy R, Bai W, Gu JL, Wilczynski S, Nadler J. Increased 12-lipoxygenase expression in breast cancer tissues and cells. Regulation by epidermal growth factor. J Clin Endocrinol Metab. 1997;82:1790–1798. doi: 10.1210/jcem.82.6.3990. [DOI] [PubMed] [Google Scholar]
- 112.Endsley MP, Aggarwal N, Isbell MA, et al. Diverse roles of 2-arachidonoylglycerol in invasion of prostate carcinoma cells: Location, hydrolysis and 12-lipoxygenase metabolism. Int J Cancer. 2007;121:984–991. doi: 10.1002/ijc.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nithipatikom K, Isbell MA, See WA, Campbell WB. Elevated 12- and 20-hydroxyeicosatetraenoic acid in urine of patients with prostatic diseases. Cancer Lett. 2006;233:219–225. doi: 10.1016/j.canlet.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 114.Nie D, Krishnamoorthy S, Jin R, et al. Mechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cells. J Biol Chem. 2006;281:18601–18609. doi: 10.1074/jbc.M601887200. [DOI] [PubMed] [Google Scholar]
- 115.Hsi LC, Wilson LC, Eling TE. Opposing effects of 15-lipoxygenase-1 and -2 metabolites on MAPK signaling in prostate. Alteration in peroxisome proliferator-activated receptor gamma. J Biol Chem. 2002;277:40549–40556. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- 116.Kelavkar U, Lin Y, Landsittel D, Chandran U, Dhir R. The yin and yang of 15-lipoxygenase-1 and delta-desaturases: dietary omega-6 linoleic acid metabolic pathway in prostate. J Carcinog. 2006;5:9. doi: 10.1186/1477-3163-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kelavkar UP, Cohen C, Kamitani H, Eling TE, Badr KF. Concordant induction of 15-lipoxygenase-1 and mutant p53 expression in human prostate adenocarcinoma: correlation with Gleason staging. Carcinogenesis. 2000;21:1777–1787. doi: 10.1093/carcin/21.10.1777. [DOI] [PubMed] [Google Scholar]
- 118.Kelavkar UP, Nixon JB, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis. 2001;22:1765–1773. doi: 10.1093/carcin/22.11.1765. [DOI] [PubMed] [Google Scholar]
- 119.Kelavkar UP, Cohen C. 15-lipoxygenase-1 expression upregulates and activates insulin-like growth factor-1 receptor in prostate cancer cells. Neoplasia. 2004;6:41–52. doi: 10.1016/s1476-5586(04)80052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kelavkar UP, Harya NS, Hutzley J, et al. DNA methylation paradigm shift: 15-lipoxygenase-1 upregulation in prostatic intraepithelial neoplasia and prostate cancer by atypical promoter hypermethylation. Prostaglandins Other Lipid Mediat. 2007;82:185–197. doi: 10.1016/j.prostaglandins.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 121.Shappell SB, Boeglin WE, Olson SJ, Kasper S, Brash AR. 15-lipoxygenase-2 (15-LOX-2) is expressed in benign prostatic epithelium and reduced in prostate adenocarcinoma. Am J Pathol. 1999;155:235–245. doi: 10.1016/S0002-9440(10)65117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jack GS, Brash AR, Olson SJ, et al. Reduced 15-lipoxygenase-2 immunostaining in prostate adenocarcinoma: correlation with grade and expression in high-grade prostatic intraepithelial neoplasia. Hum Pathol. 2000;31:1146–1154. doi: 10.1053/hupa.2000.16670. [DOI] [PubMed] [Google Scholar]
- 123.Tang DG, Bhatia B, Tang S, Schneider-Broussard R. 15-lipoxygenase 2 (15-LOX2) is a functional tumor suppressor that regulates human prostate epithelial cell differentiation, senescence, and growth (size) Prostaglandins Other Lipid Mediat. 2007;82:135–146. doi: 10.1016/j.prostaglandins.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 124.Shappell SB, Gupta RA, Manning S, et al. 15S-Hydroxyeicosatetraenoic acid activates peroxisome proliferator-activated receptor gamma and inhibits proliferation in PC3 prostate carcinoma cells. Cancer Res. 2001;61:497–503. [PubMed] [Google Scholar]
- 125.Subbarayan V, Krieg P, Hsi LC, et al. 15-Lipoxygenase-2 gene regulation by its product 15-(S)-hydroxyeicosatetraenoic acid through a negative feedback mechanism that involves peroxisome proliferator-activated receptor gamma. Oncogene. 2006;25:6015–6025. doi: 10.1038/sj.onc.1209617. [DOI] [PubMed] [Google Scholar]
- 126.Subbarayan V, Xu XC, Kim J, et al. Inverse relationship between 15-lipoxygenase-2 and PPAR-gamma gene expression in normal epithelia compared with tumor epithelia. Neoplasia. 2005;7:280–293. doi: 10.1593/neo.04457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spindler SA, Sarkar FH, Sakr WA, et al. Production of 13-hydroxyoctadecadienoic acid (13-HODE) by prostate tumors and cell lines. Biochem Biophys Res Commun. 1997;239:775–781. doi: 10.1006/bbrc.1997.7471. [DOI] [PubMed] [Google Scholar]
- 128.Wu S, Chen W, Murphy E, et al. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J Biol Chem. 1997;272:12551–12559. doi: 10.1074/jbc.272.19.12551. [DOI] [PubMed] [Google Scholar]
- 129.Wang MH, Brand-Schieber E, Zand BA, et al. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- 130.Powell PK, Wolf I, Jin R, Lasker JM. Metabolism of arachidonic acid to 20-hydroxy-5,8,11, 14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. J Pharmacol Exp Ther. 1998;285:1327–1336. [PubMed] [Google Scholar]
- 131.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 132.Falck JR, Schueler VJ, Jacobson HR, Siddhanta AK, Pramanik B, Capdevila J. Arachidonate epoxygenase: identification of epoxyeicosatrienoic acids in rabbit kidney. J Lipid Res. 1987;28:840–846. [PubMed] [Google Scholar]
- 133.Michaelis UR, Fleming I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: Epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther. 2006;111:584–595. doi: 10.1016/j.pharmthera.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 134.Rifkind AB, Lee C, Chang TK, Waxman DJ. Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Arch Biochem Biophys. 1995;320:380–389. doi: 10.1016/0003-9861(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 135.Guengerich F, editor. Human cytochrome P450 enzymes. New York: Plenum; 1995. Cytochrome P450: structure, mechanism, and biochemistry, ed. P.R.O.d. Montellano; pp. 473–535. [Google Scholar]
- 136.Yokose T, Doy M, Taniguchi T, et al. Immunohistochemical study of cytochrome P450 2C and 3A in human non-neoplastic and neoplastic tissues. Virchows Arch. 1999;434:401–411. doi: 10.1007/s004280050359. [DOI] [PubMed] [Google Scholar]
- 137.Chan AT, Tranah GJ, Giovannucci EL, Hunter DJ, Fuchs CS. A prospective study of genetic polymorphisms in the cytochrome P-450 2C9 enzyme and the risk for distal colorectal adenoma. Clin Gastroenterol Hepatol. 2004;2:704–712. doi: 10.1016/s1542-3565(04)00294-0. [DOI] [PubMed] [Google Scholar]
- 138.London SJ, Daly AK, Leathart JB, Navidi WC, Idle JR. Lung cancer risk in relation to the CYP2C9*1/CYP2C9*2 genetic polymorphism among African-Americans and Caucasians in Los Angeles County, California. Pharmacogenetics. 1996;6:527–533. doi: 10.1097/00008571-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 139.London SJ, Sullivan-Klose T, Daly AK, Idle JR. Lung cancer risk in relation to the CYP2C9 genetic polymorphism among Caucasians in Los Angeles County. Pharmacogenetics. 1997;7:401–404. doi: 10.1097/00008571-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 140.Jiang JG, Chen CL, Card JW, et al. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65:4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- 141.Jiang JG, Ning YG, Chen C, et al. Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res. 2007;67:6665–6674. doi: 10.1158/0008-5472.CAN-06-3643. [DOI] [PubMed] [Google Scholar]
- 142.Enayetallah AE, French RA, Grant DF. Distribution of soluble epoxide hydrolase, cytochrome P450 2C8, 2C9 and 2J2 in human malignant neoplasms. J Mol Histol. 2006;37:133–141. doi: 10.1007/s10735-006-9050-9. [DOI] [PubMed] [Google Scholar]
- 143.Murray GI, Taylor VE, McKay JA, et al. The immunohistochemical localization of drug-metabolizing enzymes in prostate cancer. J Pathol. 1995;177:147–152. doi: 10.1002/path.1711770208. [DOI] [PubMed] [Google Scholar]
- 144.Murata M, Watanabe M, Yamanaka M, et al. Genetic polymorphisms in cytochrome P450 (CYP) 1A1, CYP1A2, CYP2E1, glutathione S-transferase (GST) M1 and GSTT1 and susceptibility to prostate cancer in the Japanese population. Cancer Lett. 2001;165:171–177. doi: 10.1016/s0304-3835(01)00398-6. [DOI] [PubMed] [Google Scholar]
- 145.Sterling KM, Jr, Cutroneo KR. Constitutive and inducible expression of cytochromes P4501A (CYP1A1 and CYP1A2) in normal prostate and prostate cancer cells. J Cell Biochem. 2004;91:423–429. doi: 10.1002/jcb.10753. [DOI] [PubMed] [Google Scholar]
- 146.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 147.Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 148.Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 149.Laine K, Jarvinen K, Mechoulam R, Breuer A, Jarvinen T. Comparison of the enzymatic stability and intraocular pressure effects of 2-arachidonylglycerol and noladin ether, a novel putative endocannabinoid. Invest Ophthalmol Vis Sci. 2002;43:3216–3222. [PubMed] [Google Scholar]
- 150.Suhara Y, Takayama H, Nakane S, Miyashita T, Waku K, Sugiura T. Synthesis and biological activities of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, and its metabolically stable ether-linked analogues. Chem Pharm Bull (Tokyo) 2000;48:903–907. doi: 10.1248/cpb.48.903. [DOI] [PubMed] [Google Scholar]
- 151.Steffens M, Zentner J, Honegger J, Feuerstein TJ. Binding affinity and agonist activity of putative endogenous cannabinoids at the human neocortical CB1 receptor. Biochem Pharmacol. 2005;69:169–178. doi: 10.1016/j.bcp.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 152.Hanus L, Abu-Lafi S, Fride E, et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fezza F, Bisogno T, Minassi A, Appendino G, Mechoulam R, Di Marzo V. Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002;513:294–298. doi: 10.1016/s0014-5793(02)02341-4. [DOI] [PubMed] [Google Scholar]
- 154.Oka S, Tsuchie A, Tokumura A, et al. Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J Neurochem. 2003;85:1374–1381. doi: 10.1046/j.1471-4159.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 155.Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem. 2007;360:216–226. doi: 10.1016/j.ab.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 156.Di Marzo V, Breivogel CS, Tao Q, et al. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- 157.Maccarrone M, Lorenzon T, Bari M, Melino G, Finazzi-Agro A. Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J Biol Chem. 2000;275:31938–31945. doi: 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- 158.Ueda N, Puffenbarger RA, Yamamoto S, Deutsch DG. The fatty acid amide hydrolase (FAAH) Chem Phys Lipids. 2000;108:107–121. doi: 10.1016/s0009-3084(00)00190-0. [DOI] [PubMed] [Google Scholar]
- 159.Di Marzo V, Bisogno T, De Petrocellis L, et al. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem. 1999;264:258–267. doi: 10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 160.Bari M, Battista N, Fezza F, Gasperi V, Maccarrone M. New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev Med Chem. 2006;6:257–268. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- 161.Dinh TP, Carpenter D, Leslie FM, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- 163.Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–1264. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- 164.Bifulco M, Laezza C, Valenti M, Ligresti A, Portella G. DIM V: A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. Faseb J. 2004;18:1606–1608. doi: 10.1096/fj.04-1754fje. [DOI] [PubMed] [Google Scholar]
- 165.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Di Marzo V, Bisogno T, De Petrocellis L. Endocannabinoids: new targets for drug development. Curr Pharm Des. 2000;6:1361–1380. doi: 10.2174/1381612003399365. [DOI] [PubMed] [Google Scholar]
- 167.Ligresti A, Bisogno T, Matias I, et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology. 2003;125:677–687. doi: 10.1016/s0016-5085(03)00881-3. [DOI] [PubMed] [Google Scholar]
- 168.De Petrocellis L, Melck D, Bisogno T, Di Marzo V. Endocannabinoids and fatty acid amides in cancer, inflammation and related disorders. Chem Phys Lipids. 2000;108:191–209. doi: 10.1016/s0009-3084(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 169.Parolaro D, Massi P, Rubino T, Monti E. Endocannabinoids in the immune system and cancer. Prostaglandins Leukot Essent Fatty Acids. 2002;66:319–332. doi: 10.1054/plef.2001.0355. [DOI] [PubMed] [Google Scholar]
- 170.Bifulco M, Di Marzo V. Targeting the endocannabinoid system in cancer therapy: a call for further research. Nat Med. 2002;8:547–550. doi: 10.1038/nm0602-547. [DOI] [PubMed] [Google Scholar]
- 171.Portella G, Laezza C, Laccetti P, De Petrocellis L, Di Marzo V, Bifulco M. Inhibitory effects of cannabinoid CB1 receptor stimulation on tumor growth and metastatic spreading: actions on signals involved in angiogenesis and metastasis. Faseb J. 2003;17:1771–1773. doi: 10.1096/fj.02-1129fje. [DOI] [PubMed] [Google Scholar]
- 172.Jones S, Howl J. Cannabinoid receptor systems: therapeutic targets for tumour intervention. Expert Opin Ther Targets. 2003;7:749–758. doi: 10.1517/14728222.7.6.749. [DOI] [PubMed] [Google Scholar]
- 173.Grimaldi C, Pisanti S, Laezza C, et al. Anandamide inhibits adhesion and migration of breast cancer cells. Exp Cell Res. 2006;312:363–373. doi: 10.1016/j.yexcr.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 174.Bifulco M, Laezza C, Pisanti S, Gazzerro P. Cannabinoids and cancer: pros and cons of an antitumour strategy. Br J Pharmacol. 2006;148:123–135. doi: 10.1038/sj.bjp.0706632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bifulco M, Laezza C, Gazzerro P, Pentimalli F. Endocannabinoids as emerging suppressors of angiogenesis and tumor invasion (review) Oncol Rep. 2007;17:813–816. [PubMed] [Google Scholar]
- 176.Flygare J, Sander B. The endocannabinoid system in cancer-potential therapeutic target? Semin Cancer Biol. 2008;18:176–189. doi: 10.1016/j.semcancer.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 177.Izzo AA, Aviello G, Petrosino S, et al. Increased endocannabinoid levels reduce the development of precancerous lesions in the mouse colon. J Mol Med. 2008;86:89–98. doi: 10.1007/s00109-007-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mimeault M, Pommery N, Wattez N, Bailly C, Henichart JP. Anti-proliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: implication of epidermal growth factor receptor down-regulation and ceramide production. Prostate. 2003;56:1–12. doi: 10.1002/pros.10190. [DOI] [PubMed] [Google Scholar]
- 179.Endsley MP, Thill R, Choudhry I, et al. Expression and function of fatty acid amide hydrolase in prostate cancer. Int J Cancer. 2008 doi: 10.1002/ijc.23674. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Melck D, De Petrocellis L, Orlando P, et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141:118–126. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- 181.Sanchez MG, Sanchez AM, Ruiz-Llorente L, Diaz-Laviada I. Enhancement of androgen receptor expression induced by (R)-methanandamide in prostate LNCaP cells. FEBS Lett. 2003;555:561–566. doi: 10.1016/s0014-5793(03)01349-8. [DOI] [PubMed] [Google Scholar]
- 182.Nithipatikom K, Endsley MP, Isbell MA, et al. 2-Arachidonoylglycerol: A novel inhibitor of androgen-independent prostate cancer cell invasion. Cancer Res. 2004;64:8826–8830. doi: 10.1158/0008-5472.CAN-04-3136. [DOI] [PubMed] [Google Scholar]
- 183.Nithipatikom K, Endsley MP, Isbell MA, Wheelock CE, Hammock BD, Campbell WB. A new class of inhibitors of 2-arachidonoylglycerol hydrolysis and invasion of prostate cancer cells. Biochem Biophys Res Commun. 2005;332:1028–1033. doi: 10.1016/j.bbrc.2005.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]