Abstract
Mucin-related carbohydrates are overexpressed on the surface of cancer cells, providing a disease-specific target for cancer immunotherapy. Here, we describe the design and construction of peptide-free multivalent glycosylated nanoscale constructs as potential synthetic cancer vaccines that generate significant titers of antibodies selective for aberrant mucin glycans. A polymerizable version of the Tn-antigen glycan was prepared and converted into well-defined glycopolymers by Reversible Addition–Fragmentation chain Transfer (RAFT) polymerization. The polymers were then conjugated to gold nanoparticles, yielding ‘multicopy-multivalent’ nanoscale glycoconjugates. Immunological studies indicated that these nanomaterials generated strong and long-lasting production of antibodies that are selective to the Tn-antigen glycan and cross-reactive toward mucin proteins displaying Tn. The results demonstrate proof-of-concept of a simple and modular approach toward synthetic anticancer vaccines based on multivalent glycosylated nanomaterials without the need for a typical vaccine protein component.
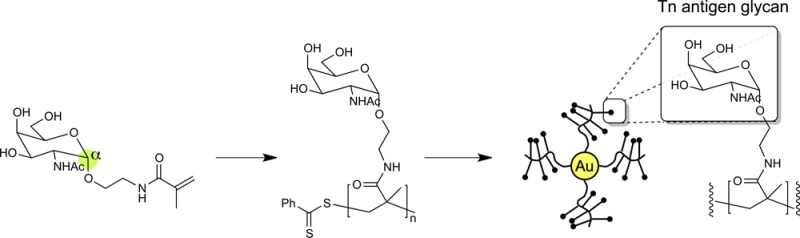
Healthy cells of the mammary gland are characterized by the surface presentation of branched, O-linked core 2 glycans containing high levels of N-acetyl-d-glucosamine (GlcNAc). However, proteins on the surface of breast cancer cells instead present mainly linear, truncated core 1 mucin-type glycans such as α-N-acetyl-d-galactosamine (αGalNAc, the Tn-antigen glycan) (Figure 1), with complete or near-complete absence of core 2 residues.1 These differences have been targeted as a strategy for cancer immunotherapy.2 Accordingly, multivalent glycoconjugates have been prepared in which mucin glycans are presented on a variety of scaffolds, including peptides,3 lipopeptides,4 dendrimers5 and proteins.6 Some of these approaches have developed as far as clinical trials.7
Figure 1.
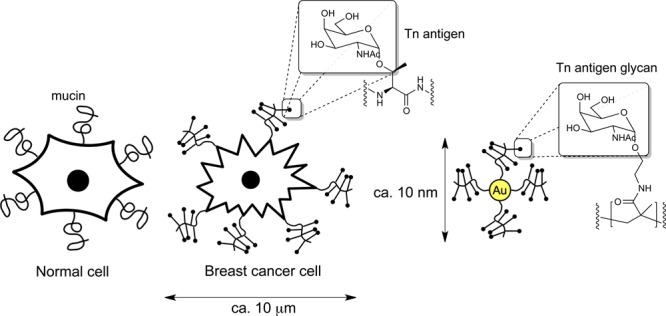
Overview of approach to develop gold nanoparticle-based synthetic anticancer vaccines. Breast cancer cells express aberrant mucins displaying Core 1 glycans such as the Tn-antigen glycan. This ‘multicopy-multivalent’ presentation is mimicked by displaying Tn-antigen glycan glycopolymers on the surface of nanoparticles.
Nanomaterials represent an alternative platform for the presentation of glycans8 that allow greater synthetic control and higher density than on current protein scaffolds. Carbohydrate-presenting gold nanoparticles (AuNPs)9 decorated with small molecule thiolated glycans have been used as tools to study carbohydrate–carbohydrate interactions,10 in antiadhesive therapy,11 and as anti-HIV12 and cancer vaccine candidates.13 However, these typically monomolecular sugar coatings do not represent well the structure of mucin glycoproteins, which feature a dense presentation of glycans attached to a protein backbone. We hypothesized that presenting core 1 glycans such as αGalNAc in a ‘multicopy-multivalent’ manner14 might produce a nanoparticle with a surface that mimics much more closely the surface of cancer cells which engage the surface receptors of cells of the immune system, and thus produce an effective synthetic vaccine (Figure 1).
Novel Tn glycan monomer 2 was synthesized using a nonparticipatory glycosyl donor sugar reactant to create the desired α-anomeric stereochemistry (Figure 2).15 α-Glycosylation of the linker moiety with glycosyl donor 1 in diethyl ether/DCM gave the azido-glycoside in 87%. Following successful attachment of the sugar precursor, the azide functionality was converted to acetamide using a one-pot Staudinger reduction16-acetylation procedure. The unwanted β-anomer was readily removed to give linked pure α-glycoside. Selective methanolysis followed by careful neutralization allowed removal of the acetate protecting groups to yield the pure Tn α-anomer 2. In this way, gram quantities of the polymerizable antigen building block were readily generated.
Figure 2.
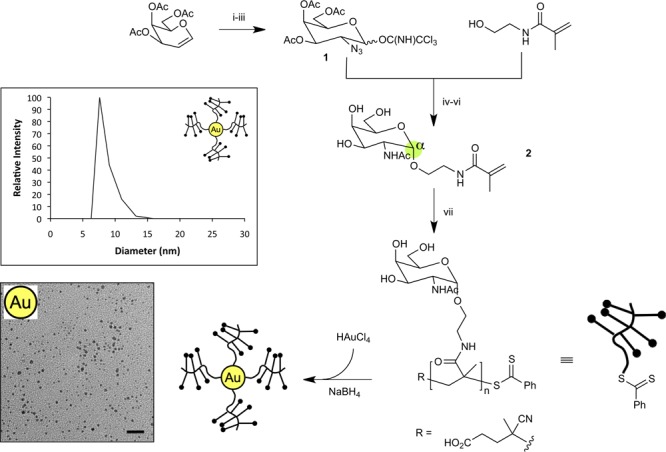
Preparation and characterization of Tn-antigen gold nanoparticles (for abbreviations see Supporting Information). Reagents, conditions and yields: (i) NaN3, CAN, CH3CN, −20 °C, 30 h, 80%; (ii) PhSH, DiPEA, CH3CN, 1 h, 72%; (iii) K2CO3, CCl3CN, dichloromethane (DCM), 8 h, 62%; (iv) HEMAm, TMSOTf, Et3N, Et2O/DCM (2:1), −20 °C, 30 min, 80%; (v) DPPE, DCM, 1 h then Ac2O, DMAP, Et3N, H+ resin, 55% α-anomer; (vi) K2CO3, MeOH, 65%; (vii) PEGMA, CPADB, ACVA, 70 °C, 48 h, 51–75%. Insets show representative dynamic light scattering data (top) and TEM image (bottom) for glyconanoparticles (scale bar = 20 nm).
Controlled glycopolymers were prepared by Reversible Addition–Fragmentation chain Transfer (RAFT) polymerization.17 Polymers of varied length and composition could be prepared (Table 1) by varying feedstock composition, ratios and conditions. First, a Tn-antigen glycan monocomponent homopolymer was created in an optimum yield of 65% in a solvent mixture of DMF:H2O (7:3). The resulting polymer was analyzed by SEC and possessed a number-average molecular weight (Mn) of 14.2 kDa and a polydispersity index (PDI) of 1.16 (see Table 1). Next, the Tn-antigen glycan monomer 2 and poly(ethylene glycol) methyl ether methacrylate (PEGMA; Mn = 300 Da) were copolymerized at varying comonomer feedstock ratios, with [total monomer]0/[CTA]0 ratios of 100/1 and 50/1, and after 48 h all proceeded with excellent overall conversion (see Table 1). The number- and weight-average molecular weights (Mn and Mw) determined by SEC were in good to excellent agreement with those predicted and the PDIs ranged from 1.12 to 1.23.
Table 1. Data for the Synthesis and Characterization of Glyconanoparticles.
| polymera | conv | yield | Mn, Th | Mn | PDId | Dh | FAu | [Pol] | [Tn] |
|---|---|---|---|---|---|---|---|---|---|
| (%)b | (%) | (kDa)c | (kDa)d | (nm)e | (%)f | (mmol)g | (mmol)h | ||
| Tn50 | 65 | 52 | 11.1 | 14.2 | 1.16 | 16 | 34 | 4.7 × 10–5 | 2.3 × 10–3 |
| PEG40Tn10 | 99, 95i | 73 | 15.3 | 15.0 | 1.12 | 25 | 61 | 4.1 × 10–5 | 2.0 × 10–4 |
| PEG25Tn25 | 90, 70i | 59 | 12.9 | 16.9 | 1.18 | 13 | 45 | 3.3 × 10–5 | 2.0 × 10–4 |
| PEG80Tn20 | 99, 75i | 68 | 29.0 | 40.4 | 1.15 | 9 | 6 | 2.3 × 10–5 | 3.0 × 10–4 |
| PEG50Tn50 | 99, 75i | 75 | 27.7 | 30.4 | 1.18 | 7 | 21 | 2.6 × 10–5 | 1.0 × 10–3 |
PEG = poly(ethyleneglycol) methyl ether methacrylate, Tn = Tn-antigen glycan monomer 2, subscript = target degree of polymerization.
Determined by 1H NMR spectroscopy by comparison of the integrals of the monomer alkene peaks to a selected polymer peak in the spectrum of the crude polymer.
Theoretical Mn, at observed conversion, determined from [monomer]0:[CTA]0;
.
Determined by SEC.
Mean hydrodynamic diameter determined by dynamic light scattering.
Mass fraction of Au per mg of nanoparticle, determined by thermogravimetric analysis.
Quantity of polymer per mg of nanoparticle, determined by thermogravimetric analysis.
Quantity of Tn-antigen glycan per mg of nanoparticle, determined by thermogravimetric analysis.
Values refer to copolymer first and second block, respectively.
Sodium borohydride was then used to reduce simultaneously HAuCl4 to Au0 and the dithioester end groups of the RAFT polymers to thiol,18 thereby forming a range of Tn-antigen glycan nanoparticles (polyTn-NPs) in situ (Figure 2 and Table 1). The polymer coronas of these particles varied in Tn glycan density (20, 50, and 100 mol %) and polymer DPn (50 or 100 units). The size of the nanoparticles was confirmed by dynamic light scattering (DLS) and transmission electron microscopy (TEM) (Figures 2, S1 and S2). All samples contained a narrow size distribution of particles of diameter between 5 and 20 nm, except for PEG40Tn10 which resulted in a polydisperse sample (Figures S1 and S2). Determination of antigen and polymer loading using thermogravimetric analysis (TGA) indicated that not only could loading be tuned, but also that the use of longer polymer chains allowed the creation of smaller nanoparticles (Table 1, entries 4 and 5) with a lower mass fraction of gold core.
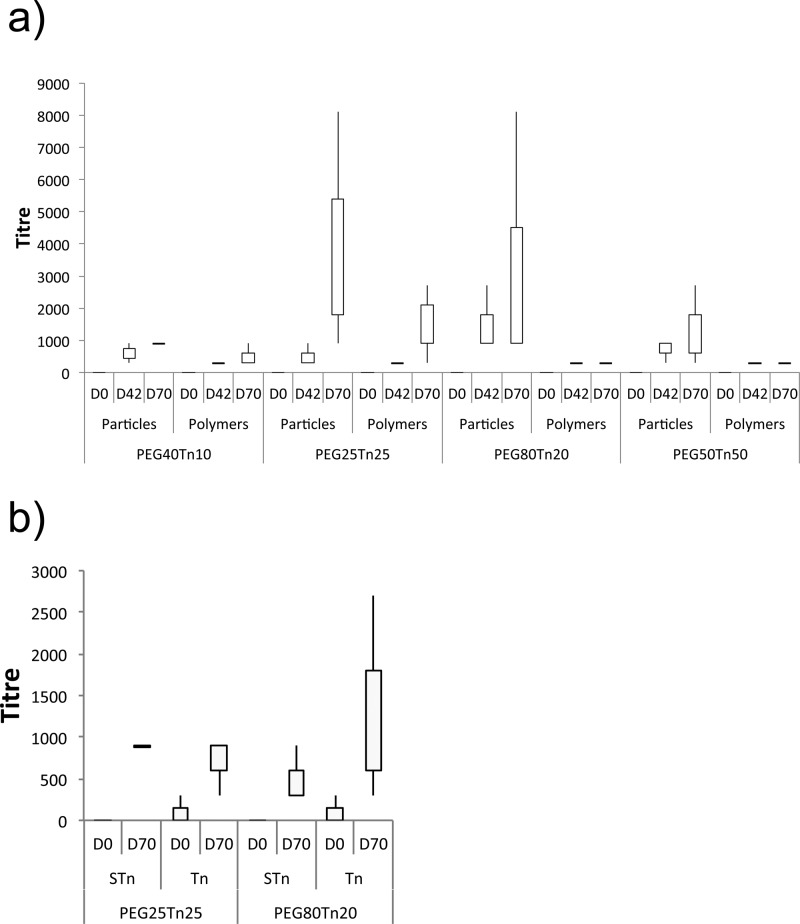
The Tn-antigen glycan presenting nanoparticles were analyzed for their efficacy and ability to induce an immune response in vivo. New Zealand White rabbits (n = 3) were immunized at days 0, 14, 28, and 56 with either polyTn-NP or free polymer solution. Serum samples were taken at day 0 (preimmune bleed), 42 and 70 and antibodies present were quantified using an ELISA assay against the synthetic antigens. The results are presented in Figure 3a. The free polymers gave low or negligible response, whereas all glyconanoparticles generated a higher response that increased over time as judged by antibody (IgG) titers. Examination of the data in Figure 3a reveals a strong influence of nanoparticle composition on immunological properties. The highest titers were observed for AuNPs prepared with the polymers PEG25Tn25 and PEG80Tn20, with weaker responses observed for PEG40Tn10 and PEG50Tn50 (subscript denotes number average block length). The polydispersity of PEG40Tn10 may reduce its stability in vivo and hence produce lower titers than the other particles.
Figure 3.
Box plots showing results of immunological experiments with glyconanoparticles and glycopolymers. (a) Serum antibody (IgG) titers (ELISA); (b) cross-reactivity of serum antibodies (ELISA) with mucins. Tn = Tn-antigen glycan (α-GalNAc), sTn = sialylated Tn; polymer key as in Table 1.
The relationship observed between carbohydrate density and immune response is notable. It appears that the optimum Tn-antigen glycan density is 20–25 units per polymer chain, regardless of chain length. Antigen induced cross-linking of B cell receptors leads to B cell activation and antibody production, whereas cross-linking with coreceptors can either increase or suppress B cell response.19 It is likely that the glycoconjugate carbohydrate density has a strong influence on the subtle interplay between these factors and therefore on the production of antibodies.
Barchi et al. have prepared glycosylated gold nanoparticles bearing the Thomson-Friedenreich (TF) antigen and demonstrated moderate antibody responses.13b Direct comparison with their data is difficult since optical density values rather than serial dilution titers were reported; nonetheless, it seems that the maximum response of our nanoparticles is of the same order of magnitude.
To probe the ability of the nanoparticle-generated antibodies to recognize naturally occurring antigens, cross-reactivity with different mucin glycoproteins bearing the Tn-antigen was investigated. Bovine submaxillary mucin (BSM) is known to contain significant levels of sialylated Tn-residues (sTn),20 which can be desialylated readily to expose Tn.21 Serum samples (days 0 and 70) from immunization with glyconanoparticles presenting very different polymers PEG25Tn25 and PEG80Tn20 were reacted with mucins bearing Tn-antigen glycans in different forms (Figure 3b). While no or little detectable cross-reactivity was seen in day 0 samples, all experiments with 70 day samples indicated the presence of detectable levels of antibodies specific for naturally occurring mucin glycans. Interestingly, serum generated in the presence of each nanoparticle type showed the ability to bind the Tn-antigen glycan in both terminal and nonterminal context.
We have described the synthesis of ‘multicopy-multivalent’ nanoparticles decorated with tumor-associated (Tn) antigen glycans and have shown that these generate a significant immune response in vivo, with promising indications that the antibodies generated are capable of recognizing natural Tn-antigen glycans and mammalian-mucin glycoproteins. While the absolute titers reported here are lower than those obtained with glycoconjugates based on protein toxin platforms,22 the ability to create fully synthetic protein- and peptide-free glycoconjugate vaccines through layered multivalent display is the first of its kind.
Acknowledgments
The authors thank the MRC and EPSRC (joint grant reference G0700080) for funding. N.R.C. acknowledges the P2M RNP programme of the European Science Foundation. B.G.D. is a recipient of a Royal Society Wolfson Merit Award. Professor Quentin Sattentau (Sir William Dunn School of Pathology, University of Oxford) is thanked for helpful discussions.
Supporting Information Available
Procedures for glycomonmer, glycopolymer and nanoparticle preparation, immunization experiments and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- a Picco G.; Julien S.; Brockhausen I.; Beatson R.; Antonopoulos A.; Haslam S.; Mandel U.; Dell A.; Pinder S.; Taylor-Papadimitriou J.; Burchell J. Glycobiology 2010, 20, 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Beatson R. E.; Taylor-Papadimitriou J.; Burchell J. M. Immunotherapy 2010, 2, 305. [DOI] [PubMed] [Google Scholar]
- a Danishefsky S. J.; Allen J. R. Angew. Chem., Int. Ed. 2000, 39, 836. [DOI] [PubMed] [Google Scholar]; b Buskas T.; Thompson P.; Boons G. J. Chem. Commun. 2009, 5335. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Guo Z. W.; Wang Q. L. Curr. Opin. Chem. Biol. 2009, 13, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jeon I.; Lee D.; Krauss I. J.; Danishefsky S. J. J. Am. Chem. Soc. 2009, 131, 14337. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ragupathi G.; Koide F.; Livingston P. O.; Cho Y. S.; Endo A.; Wan Q.; Spassova M. K.; Keding S. J.; Allen J.; Ouerfelli O.; Wilson R. M.; Danishefsky S. J. J. Am. Chem. Soc. 2006, 128, 2715. [DOI] [PubMed] [Google Scholar]; c Westerlind U.; Hobel A.; Gaidzik N.; Schmitt E.; Kunz H. Angew. Chem., Int. Ed. 2008, 47, 7551. [DOI] [PubMed] [Google Scholar]
- a Buskas T.; Ingale S.; Boons G. J. Angew. Chem., Int. Ed. 2005, 44, 5985. [DOI] [PubMed] [Google Scholar]; b Kaiser A.; Gaidzik N.; Becker T.; Menge C.; Groh K.; Cai H.; Li Y. M.; Gerlitzki B.; Schmitt E.; Kunz H. Angew. Chem., Int. Ed. 2010, 49, 3688. [DOI] [PubMed] [Google Scholar]; c Cai H.; Huang Z. H.; Shi L.; Zhao Y. F.; Kunz H.; Li Y. M. Chem.—Eur. J. 2011, 17, 6396. [DOI] [PubMed] [Google Scholar]; d Ingale S.; Awolfert M.; Gaekwad J.; Buskas T.; Boons G. J. Nat. Chem. Biol. 2007, 3, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil S.; Kaiser A.; Syed F.; Kunz H. Synthesis 2009, 1355. [Google Scholar]
- Wittrock S.; Becker T.; Kunz H. Angew. Chem., Int. Ed. 2007, 46, 5226. [DOI] [PubMed] [Google Scholar]
- a Slovin S. F.; Ragupathi G.; Adluri S.; Ungers G.; Terry K.; Kim S.; Spassova M.; Bornmann W. G.; Fazzari M.; Dantis L.; Olkiewicz K.; Lloyd K. O.; Livingston P. O.; Danishefsky S. J.; Scher H. I. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 5710. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gilewski T.; Ragupathi G.; Bhuta S.; Williams L. J.; Musselli C.; Zhang X. F.; Bencsath K. P.; Panageas K. S.; Chin J.; Hudis C. A.; Norton L.; Houghton A. N.; Livingston P. O.; Danishefsky S. J. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 3270. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Krug L. M.; Ragupathi G.; Hood C.; Kris M. G.; Miller V. A.; Allen J. R.; Keding S. J.; Danishefsky S. J.; Gomez J.; Tyson L.; Pizzo B.; Baez V.; Livingston P. O. Clin. Cancer Res. 2004, 10, 6094. [DOI] [PubMed] [Google Scholar]; d Sabbatini P. J.; Kudryashov V.; Ragupathi G.; Danishefsky S. J.; Livingston P. O.; Bornmann W.; Spassova M.; Zatorski A.; Spriggs D.; Aghajanian C.; Soignet S.; Peyton M.; O’Flaherty C.; Curtin J.; Lloyd K. O. Int. J. Cancer 2000, 87, 79. [PubMed] [Google Scholar]; e Slovin S. F.; Ragupathi G.; Musselli C.; Fernandez C.; Diani M.; Verbel D.; Danishefsky S.; Livingston P.; Scher H. I. Cancer Immunol. Immunother. 2005, 54, 694. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Sabbatini P. J.; Ragupathi G.; Hood C.; Aghajanian C. A.; Juertzka M.; Lasonos A.; Hensley M. L.; Spassova M. K.; Ouerfelli O.; Spriggs D. R.; Tew W. P.; Konner J.; Clausen H.; Abu Rustum N.; Dansihefsky S. J.; Livingston P. O. Clin. Cancer Res. 2007, 13, 4170. [DOI] [PubMed] [Google Scholar]
- a Hong S. Y.; Tobias G.; Al-Jamal K. T.; Ballesteros B.; Ali-Boucetta H.; Lozano-Perez S.; Nellist P. D.; Sim R. B.; Finucane C.; Mather S. J.; Green M. L. H.; Kostarelos K.; Davis B. G. Nat. Mater. 2010, 9, 485. [DOI] [PubMed] [Google Scholar]; b van Kasteren S. I.; Campbell S. J.; Serres S.; Anthony D. C.; Sibson N. R.; Davis B. G. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Barrientos A. G.; de la Fuente J. M.; Rojas T. C.; Fernandez A.; Penades S. Chem.—Eur. J. 2003, 9, 1909. [DOI] [PubMed] [Google Scholar]; b Garcia I.; Marradi M.; Penades S. Nanomedicine 2010, 5, 777. [DOI] [PubMed] [Google Scholar]; c De la Fuente J. M.; Penades S. Biochim. Biophys. Acta 2006, 1760, 636. [DOI] [PubMed] [Google Scholar]; d Sundgren A.; Barchi J. J. Carbohydr. Res. 2008, 343, 1594. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Svarovsky S. A.; Szekely Z.; Barchi J. J. Tetrahedron: Asymmetry 2005, 16, 587. [Google Scholar]
- a de la Fuente J. M.; Barrientos A. G.; Rojas T. C.; Rojo J.; Canada J.; Fernandez A.; Penades S. Angew. Chem., Int. Ed. 2001, 40, 2258. [PubMed] [Google Scholar]; b Hernaiz M. J.; de la Fuente J. M.; Barrientos A. G.; Penades S. Angew. Chem., Int. Ed. 2002, 41, 1554. [DOI] [PubMed] [Google Scholar]; c de la Fuente J. M.; Eaton P.; Barrientos A. G.; Menendez M.; Penades S. J. Am. Chem. Soc. 2005, 127, 6192. [DOI] [PubMed] [Google Scholar]
- Rojo J.; Diaz V.; de la Fuente J. M.; Segura I.; Barrientos A. G.; Riese H. H.; Bernade A.; Penades S. ChemBioChem 2004, 5, 291. [DOI] [PubMed] [Google Scholar]
- Marradi M.; Di Gianvincenzo P.; Enriquez-Navas P. M.; Martinez-Avila O. M.; Chiodo F.; Yuste E.; Angulo J.; Penades S. J. Mol. Biol. 2011, 410, 798. [DOI] [PubMed] [Google Scholar]
- a Ojeda R.; de Paz J. L.; Barrientos A. G.; Martin-Lomas M.; Penades S. Carbohydr. Res. 2007, 342, 448. [DOI] [PubMed] [Google Scholar]; b Brinas R. P.; Sundgren A.; Sahoo P.; Morey S.; Rittenhouse-Olson K.; Wilding G. E.; Deng W.; Barchi J. J. Bioconjugate Chem. 2012, 23, 1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Spain S. G.; Cameron N. R. Polym. Chem. 2011, 2, 60. [Google Scholar]; b Ting S. R. S.; Chen G. J.; Stenzel M. H. Polym. Chem. 2010, 1, 1392. [Google Scholar]; c Spain S. G.; Gibson M. I.; Cameron N. R. J. Polym. Sci., Part A: Polym. Chem. 2007, 45, 2059. [Google Scholar]; d Ladmiral V.; Melia E.; Haddleton D. M. Eur. Polym. J. 2004, 40, 431. [Google Scholar]
- Kuduk S. D.; Schwarz J. B.; Chen X. T.; Glunz P. W.; Sames D.; Ragupathi G.; Livingston P. O.; Danishefsky S. J. J. Am. Chem. Soc. 1998, 120, 12474. [Google Scholar]
- O’Neil I. A.; Thompson S.; Murray C. L.; Barret Kalindjian B. Tetrahedron Lett. 1998, 39, 7787. [Google Scholar]
- a Chiefari J.; Chong Y. K.; Ercole F.; Krstina J.; Jeffery J.; Le T. P. T.; Mayadunne R. T. A.; Meijs G. F.; Moad C. L.; Moad G.; Rizzardo E.; Thang S. H. Macromolecules 1998, 31, 5559. [Google Scholar]; b Moad G.; Rizzardo E.; Thang S. H. Acc. Chem. Res. 2008, 41, 1133. [DOI] [PubMed] [Google Scholar]; c Moad G.; Rizzardo E.; Thang S. H. Polymer 2008, 49, 1079. [Google Scholar]
- a Lowe A. B.; Sumerlin B. S.; Donovan M. S.; McCormick C. L. J. Am. Chem. Soc. 2002, 124, 11562. [DOI] [PubMed] [Google Scholar]; b Raula J.; Shan J.; Nuopponen M.; Niskanen A.; Jiang H.; Kauppinen E. I.; Tenhu H. Langmuir 2003, 19, 3499. [Google Scholar]; c Shan J.; Nuopponen M.; Jiang H.; Viitala T.; Kauppinen E.; Kontturi K.; Tenhu H. Macromolecules 2005, 38, 2918. [Google Scholar]
- a Kurosaki T. Curr. Opin. Immunol. 2002, 14, 341. [DOI] [PubMed] [Google Scholar]; b Dal Porto J. M.; Gauld S. B.; Merrell K. T.; Mills D.; Pugh-Bernard A. E.; Cambier J. Mol. Immunol. 2004, 41, 599. [DOI] [PubMed] [Google Scholar]
- Yu G.-L.; Zhang Y.-B.; Zhang Z.-Q.; Song L.-T.; Wang P.-P.; Chai W.-A. Anal. Chem. 2010, 82, 9534. [DOI] [PubMed] [Google Scholar]
- O’Boyle K. P.; Coatsworth S.; Anthony G.; Ramirez M.; Greenwald E.; Kaleya R.; Steinberg J. J.; Dutcher J. P.; Wiernik P. H. Cancer Immun. 2006, 6, 5. [PubMed] [Google Scholar]
- Hoffmann-Roder A.; Kaiser A.; Wagner S.; Gaidzik N.; Kowalczyk D.; Westerlind U.; Gerlitzki B.; Schmitt E.; Kunz H. Angew. Chem., Int. Ed. 2010, 49, 8498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.