Abstract
The segregation of thalamocortical inputs into eye-specific stripes in the developing cat or monkey visual cortex is prevented by manipulations that perturb or abolish neural activity in the visual pathway. Such findings show that proper development of the functional organization of visual cortex is dependent on normal patterns of neural activity. The generalisation of this conclusion to other sensory cortices has been questioned by findings that the segregation of thalamocortical afferents into a somatotopic barrel pattern in developing rodent primary somatosensory cortex (S1) is not prevented by activity blockade. We show that a temporary block of N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptors in rat S1 during the critical period for barrel development disrupts the topographic refinement of thalamocortical connectivity and columnar organization. These effects are evident well after the blockade is ineffective and thus may be permanent. Our findings show that neural activity and specifically the activation of postsynaptic cortical neurons has a prominent role in establishing the primary sensory map in S1, as well as the topographic organization of higher order synaptic connections.
Full text
PDF

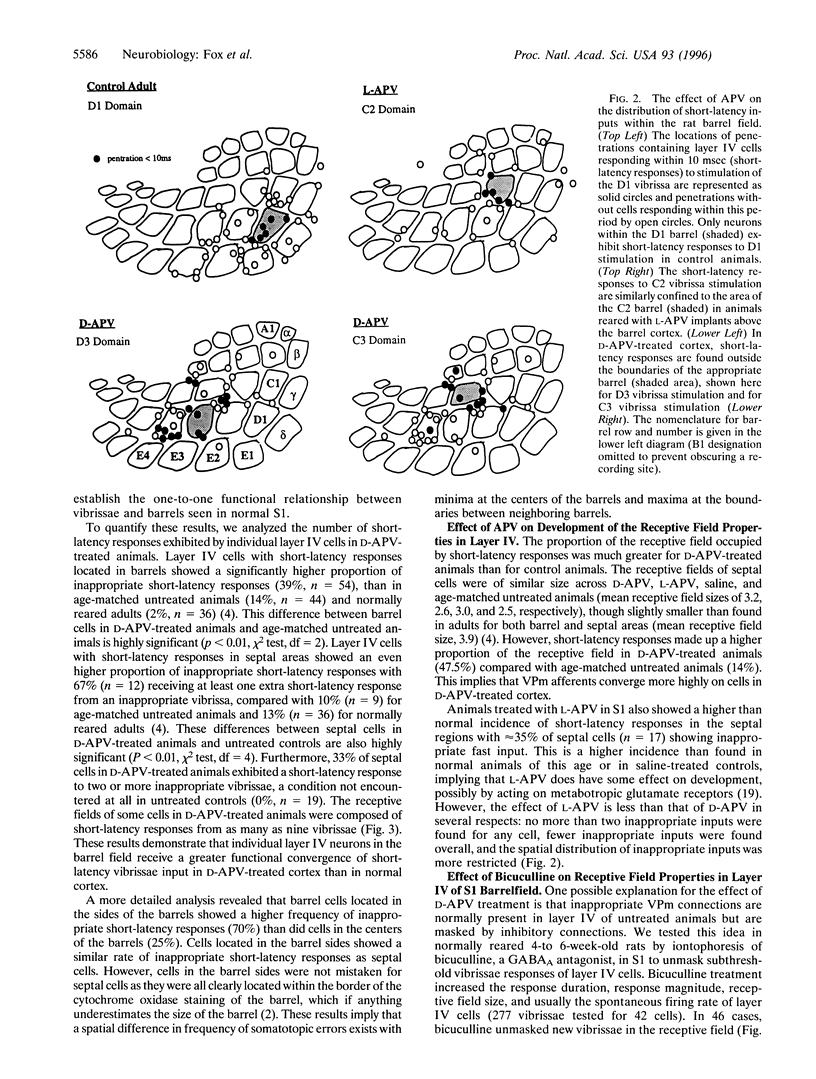
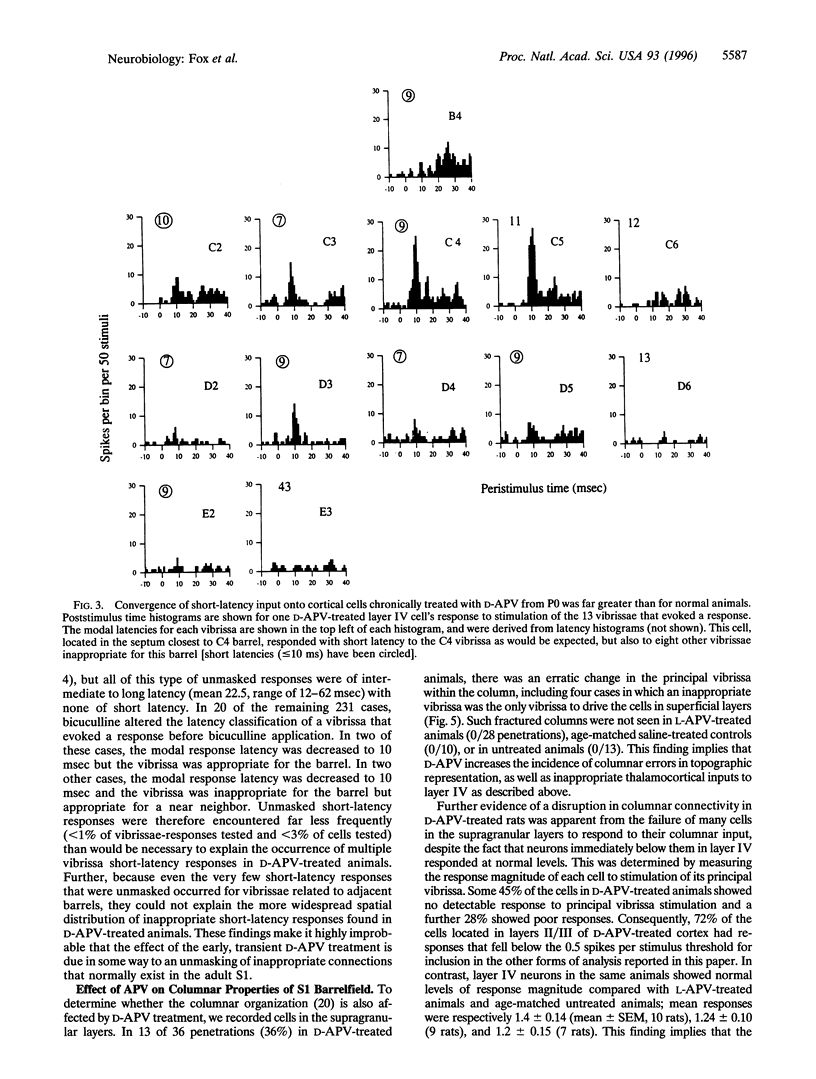
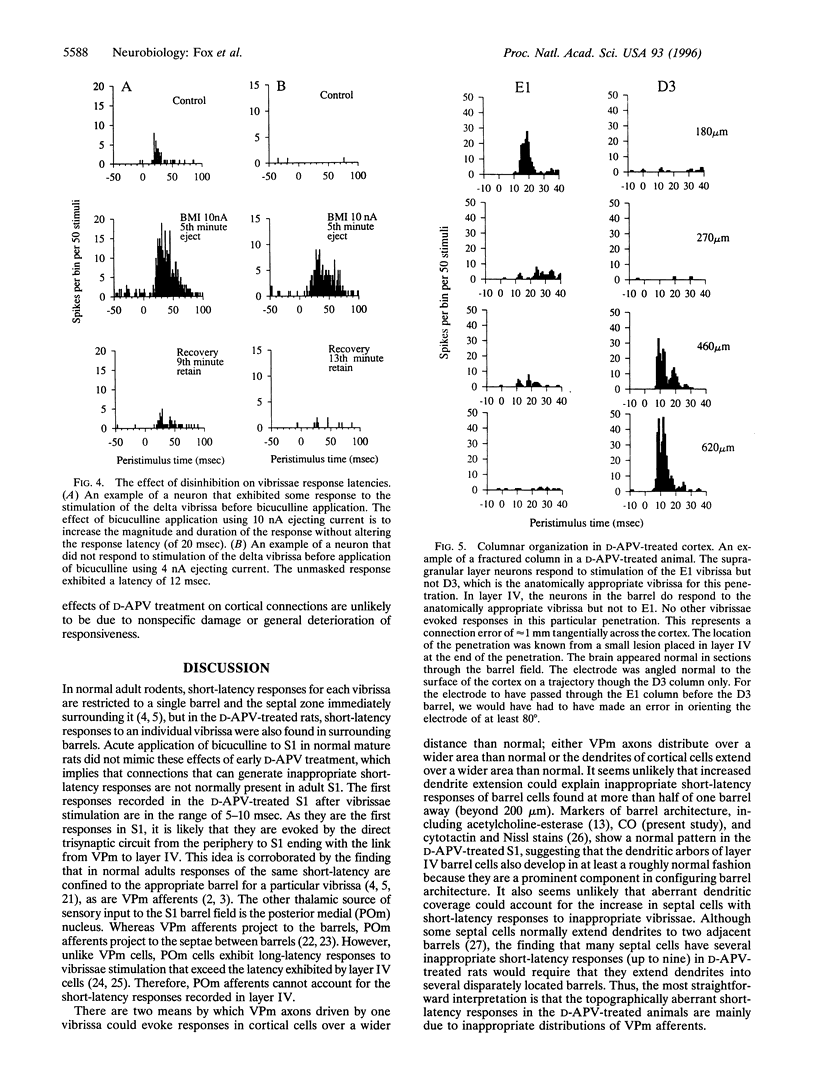

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agmon A., O'Dowd D. K. NMDA receptor-mediated currents are prominent in the thalamocortical synaptic response before maturation of inhibition. J Neurophysiol. 1992 Jul;68(1):345–349. doi: 10.1152/jn.1992.68.1.345. [DOI] [PubMed] [Google Scholar]
- Agmon A., Yang L. T., Jones E. G., O'Dowd D. K. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995 Jan;15(1 Pt 2):549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A., Yang L. T., O'Dowd D. K., Jones E. G. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993 Dec;13(12):5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammermüller J., Kolb H. The organization of the turtle inner retina. I. ON- and OFF-center pathways. J Comp Neurol. 1995 Jul 17;358(1):1–34. doi: 10.1002/cne.903580102. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K., Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992 Oct;68(4):1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K. Spatiotemporal convergence and divergence in the rat S1 "barrel" cortex. J Comp Neurol. 1987 Sep 8;263(2):265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Belford G. R., Killackey H. P. The sensitive period in the development of the trigeminal system of the neonatal rat. J Comp Neurol. 1980 Sep 15;193(2):335–350. doi: 10.1002/cne.901930203. [DOI] [PubMed] [Google Scholar]
- Blasdel G. G., Pettigrew J. D. Degree of interocular synchrony required for maintenance of binocularity in kitten's visual cortex. J Neurophysiol. 1979 Nov;42(6):1692–1710. doi: 10.1152/jn.1979.42.6.1692. [DOI] [PubMed] [Google Scholar]
- Burgard E. C., Hablitz J. J. Developmental changes in NMDA and non-NMDA receptor-mediated synaptic potentials in rat neocortex. J Neurophysiol. 1993 Jan;69(1):230–240. doi: 10.1152/jn.1993.69.1.230. [DOI] [PubMed] [Google Scholar]
- Chiaia N. L., Fish S. E., Bauer W. R., Bennett-Clarke C. A., Rhoades R. W. Postnatal blockade of cortical activity by tetrodotoxin does not disrupt the formation of vibrissa-related patterns in the rat's somatosensory cortex. Brain Res Dev Brain Res. 1992 Apr 24;66(2):244–250. doi: 10.1016/0165-3806(92)90086-c. [DOI] [PubMed] [Google Scholar]
- Cline H. T., Debski E. A., Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P. A., Miller R. F. Do N-methyl-D-aspartate receptors mediate synaptic responses in the mudpuppy retina? J Neurosci. 1988 Dec;8(12):4728–4733. doi: 10.1523/JNEUROSCI.08-12-04728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Crair M. C., Malenka R. C. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995 May 25;375(6529):325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Diamond M. E., Armstrong-James M., Budway M. J., Ebner F. F. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J Comp Neurol. 1992 May 1;319(1):66–84. doi: 10.1002/cne.903190108. [DOI] [PubMed] [Google Scholar]
- Erzurumlu R. S., Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Res Dev Brain Res. 1990 Nov 1;56(2):229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992 May;12(5):1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazewski S., Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996 Apr;75(4):1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Shatz C. J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993 Jan;72 (Suppl):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Harris R. M., Woolsey T. A. Dendritic plasticity in mouse barrel cortex following postnatal vibrissa follicle damage. J Comp Neurol. 1981 Mar 1;196(3):357–376. doi: 10.1002/cne.901960302. [DOI] [PubMed] [Google Scholar]
- Henderson T. A., Woolsey T. A., Jacquin M. F. Infraorbital nerve blockade from birth does not disrupt central trigeminal pattern formation in the rat. Brain Res Dev Brain Res. 1992 Mar 20;66(1):146–152. doi: 10.1016/0165-3806(92)90152-m. [DOI] [PubMed] [Google Scholar]
- Hoogland P. V., Welker E., Van der Loos H. Organization of the projections from barrel cortex to thalamus in mice studied with Phaseolus vulgaris-leucoagglutinin and HRP. Exp Brain Res. 1987;68(1):73–87. doi: 10.1007/BF00255235. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Killackey H. P. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. I. The normal morphology of specific thalamocortical afferents. J Neurosci. 1987 Nov;7(11):3529–3543. doi: 10.1523/JNEUROSCI.07-11-03529.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. G., Fox K., Connors B. W. Properties of excitatory synaptic events in neurons of primary somatosensory cortex of neonatal rats. Cereb Cortex. 1995 Mar-Apr;5(2):148–157. doi: 10.1093/cercor/5.2.148. [DOI] [PubMed] [Google Scholar]
- Koralek K. A., Jensen K. F., Killackey H. P. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res. 1988 Nov 1;463(2):346–351. doi: 10.1016/0006-8993(88)90408-8. [DOI] [PubMed] [Google Scholar]
- Langer R., Brown L., Edelman E. Controlled release and magnetically modulated release systems for macromolecules. Methods Enzymol. 1985;112:399–422. doi: 10.1016/s0076-6879(85)12032-x. [DOI] [PubMed] [Google Scholar]
- LeVay S., Stryker M. P., Shatz C. J. Ocular dominance columns and their development in layer IV of the cat's visual cortex: a quantitative study. J Comp Neurol. 1978 May 1;179(1):223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- Lu S. M., Lin R. C. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res. 1993;10(1):1–16. doi: 10.3109/08990229309028819. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- McCandlish C. A., Li C. X., Waters R. S. Early development of the SI cortical barrel field representation in neonatal rats follows a lateral-to-medial gradient: an electrophysiological study. Exp Brain Res. 1993;92(3):369–374. doi: 10.1007/BF00229024. [DOI] [PubMed] [Google Scholar]
- McCasland J. S., Bernardo K. L., Probst K. L., Woolsey T. A. Cortical local circuit axons do not mature after early deafferentation. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1832–1836. doi: 10.1073/pnas.89.5.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B. L., Fox K., O'Leary D. D. Postsynaptic control of plasticity in developing somatosensory cortex. Nature. 1993 Aug 12;364(6438):623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- Schlaggar B. L., O'Leary D. D. Early development of the somatotopic map and barrel patterning in rat somatosensory cortex. J Comp Neurol. 1994 Aug 1;346(1):80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- Senft S. L., Woolsey T. A. Growth of thalamic afferents into mouse barrel cortex. Cereb Cortex. 1991 Jul-Aug;1(4):308–335. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Lindström S., Wiesel T. N. The distribution of afferents representing the right and left eyes in the cat's visual cortex. Brain Res. 1977 Aug 5;131(1):103–116. doi: 10.1016/0006-8993(77)90031-2. [DOI] [PubMed] [Google Scholar]
- Stent G. S. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci U S A. 1973 Apr;70(4):997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker M. P., Harris W. A. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986 Aug;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Woolsey T. A., Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970 Jan 20;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]




