Abstract
Significance: Steady electric fields (EFs) surround cells and tissues in vivo and may regulate cellular behavior during development, wound healing, or tissue regeneration. Application of exogenous EFs of similar magnitude as those found in vivo can direct migration, growth, and division in most cell types, ranging from bacteria to mammalian cells. These EF effects have therapeutic potential, for instance, in accelerating wound healing or improving nerve repair. EFs are thought to signal through the plasma membrane to locally activate or recruit components of the cytoskeleton and the polarity machinery. How EFs might function to steer polarity is, however, poorly understood at a molecular level.
Recent Advances: Here, we review recent work introducing genetically tractable systems, such as yeast and Dictyostelium cells, that begin to identify proteins and pathways involved in this response both at the level of ion transport at the membrane and at the level of cytoskeleton regulation.
Critical Issues: These studies highlight the complexity of these EF effects and bring important novel views on core polarity regulation.
Future Directions: Future work pursuing initial screening in model organisms should generate broad mechanistic understanding of electrotactic effects.

Nicolas Minc, PhD
Scope and Significance
This review will provide an overview of the recent advances made in understanding the molecular mechanisms of galvanotactic effects, which is the process by which cells sense and utilize small electric fields (EFs) to orient polarity, migration, or division. These effects have long been known to influence many physiological processes, including development and wound healing, and the discovery of gene products regulating these effects promises to open new avenues for medical applications in these contexts.
Translational Relevance
The study and molecular understanding of EF effects on cell polarity will aid in understanding many medically relevant in vivo tissue behaviors. The most important one is wound healing, which is known to be influenced by endogenous EFs in vivo. Other in vivo relevance also includes nerve regeneration and metastasis.
Clinical Relevance
The discovery of genes and proteins regulating electrotaxis will likely provide the driving knowledge to design chemical enhancers of wound healing in vivo. Additionally, the control over cellular behavior provided by exogenous EFs may serve as a potent tool to drive repair- or target-specific cells to sites of infections.
Background
Cell polarization describes the ability of a cell to use external and/or internal stimuli to decide in which direction to grow, migrate, or divide. It is a prerequisite for the development of a multicellular organism and is involved in numerous biological processes such as tissue repair, cancer metastasis, or cell–cell communication.1 Cell polarity is usually regulated by internal polarity effectors that promote the assembly of actin and microtubule cytoskeleton, which trigger cell movement and shape changes.2 Conserved polarity hubs include the one regulating the small guanosine triphosphate hydrolase enzyme (GTPase) cdc42p, or the one controlling the phosphorylation state of phosphatidylinositol lipids (phosphatidylinositol-phosphate [PIP]).3,4 In tissues, these internal polarity modules are usually biased and oriented by external cues, such as chemical gradients, mechanical signals, and electrical signals, which allow cells to organize spatially at the tissue level. Although the effects of extracellular cues on single-cell or tissue polarity have been described in many contexts, the molecular details of the cross-talk between external and internal cues remain unclear in most cases. Here, we review the molecular mechanisms underlying this cross-talk in the context of electrical signals.
Cells and tissues in our body are surrounded by organized electrical currents and ion flux, yet the role of such electrochemical signals in organizing cellular behavior remains poorly appreciated. Steady electrical currents and fields have been measured across epithelial layers and proposed to guide cellular behavior in wound healing, development, and metastasis.5–9 Even single cells may organize ion flux and electrical currents in large polarized single cells, such as developing eggs or pollen tubes; organized ionic currents around the cell have been measured and are implicated in helping to establish a global order to maintain polarized growth.10–13
It has been observed for decades that the exogenous application of an EF on the same order of magnitude as those measured in vivo (ranging typically from 0.1 to 10 V/cm) can direct cell polarity, migration, and division in many different cell types ranging from bacteria and fungi to neurons and neutrophils.9,14–17 This near-universal process is known as galvanotaxis when the cell migrates directionally in the EF, and galvanotropism when the cell reorients its growth axis with respect to the EF. EF effects may have important therapeutic and diagnostic values, for instance, in nerve repair, wound healing, or to control the orientation of cells within tissues. For instance, it has been widely appreciated that EFs may serve as prime directional cues to direct cell migration and division during wound healing, and that their manipulation affects wound closure in vivo.18 Although these effects have long been described and investigated, both molecular and biophysical mechanisms remain elusive. A deep understanding of these EF-sensing mechanisms should enable clinicians and engineers to develop new therapeutic methods for improving wound-healing treatment.
In this article, we review recent advances in the dissection of the molecular mechanisms underlying EF effects on cell polarity, with a particular emphasis on the introduction of genetically tractable organisms and quantitative approaches, which begin to bring understanding of these effects.
Discussion of Findings and Relevant Literature
Cathode, anode, or perpendicular: which way to polarize in an EF?
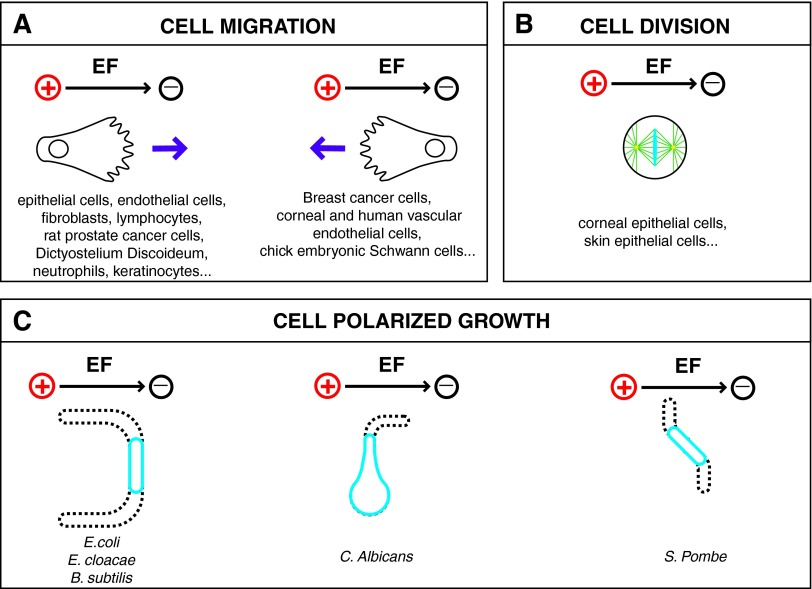
Whereas most cell types respond to EFs by reorienting their internal polarity to guide migration, growth, or division, a puzzling result obtained over the years is that different cell types respond by orienting to different directions (Fig. 1A). Most migrating cells, including epithelial cells, fibroblasts, or neutrophils, respond to EFs by migrating to the cathode of the EF (negative electrode).18 In contrast, breast cancer cells and some endothelial cells migrate to the opposite direction, which is toward the anode of the field.19–22 Some cells also display additional atypical shape changes that accompany the directional migration phenotype. Mouse fibroblasts depict, for instance, a striking shape elongation perpendicular to the EF and start migrating to the cathode of the EF.23
Figure 1.
Polarity reorientation of different cell types to exogenous EF. (A) Different cell types that show directional migration to the cathode or anode of the EF. (B) Cells that depict a perpendicular orientation of the metaphase plate with respect to the EF during division. (C) Different cell types that orient their growth axis toward the anode (left), the cathode (center), or perpendicular to the EF (right). EF, electric field. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
EFs may also orient cellular growth in a nonmotile walled cell that displays polarized growth, such as rod-shape bacteria, filamentous fungi, and the rod-shape fission yeast.16,24,25 In this situation, the cells reorient their growth axis by bending or branching with respect to the EF direction (Fig. 1C). There again, different cell types appear to reorient differently. Most bacteria grow and bend toward the anode, while some fungi such as Candida albicans elongate its hyphal tip toward the cathode. Other mycelia fungi and the fission yeast Schizosaccharomyces pombe, reorient their polarity and grow perpendicular to the EF.25,26
These different orientations, as well as the multiple effects caused by the EF on certain cells, highlight the complexity of these responses and reveal the putative existence of dominant modes that may have a prevalence to steer cells to the cathode versus anode versus perpendicular.
The biophysics of galvanotactic effects
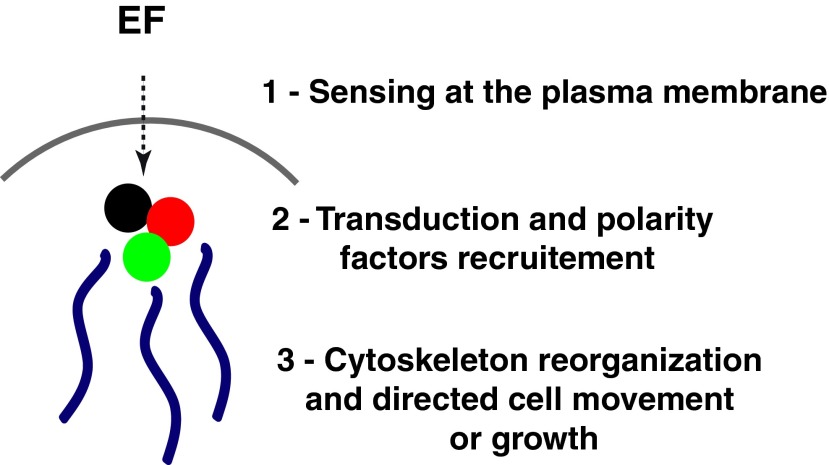
To dig into the understanding of galvanotactic effects, one needs to start asking questions on the biophysical effects that EFs may cause to cells. One well-accepted view is that EFs signal at or through the plasma membrane of cells, which serves as an electrical insulator. In other words, the cell response to EFs is not caused by the movement or direct rearrangement of certain proteins or organelles inside the cytoplasm. Rather, this response may involve a complex signal transduction, which eventually leads to the reorganization of the cytoskeleton and polarity machinery with respect to the EF direction (Fig. 2).
Figure 2.
Schematic representation of how EFs may signal to reorganize cell polarity and the cytoskeleton. The EF signal is transduced at or through the plasma membrane, which acts as an electrical insulator. This initial effect may trigger a complex signaling cascade, which eventually leads to the reorganization of the cytoskeleton and polarity machinery with respect to the EF direction. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
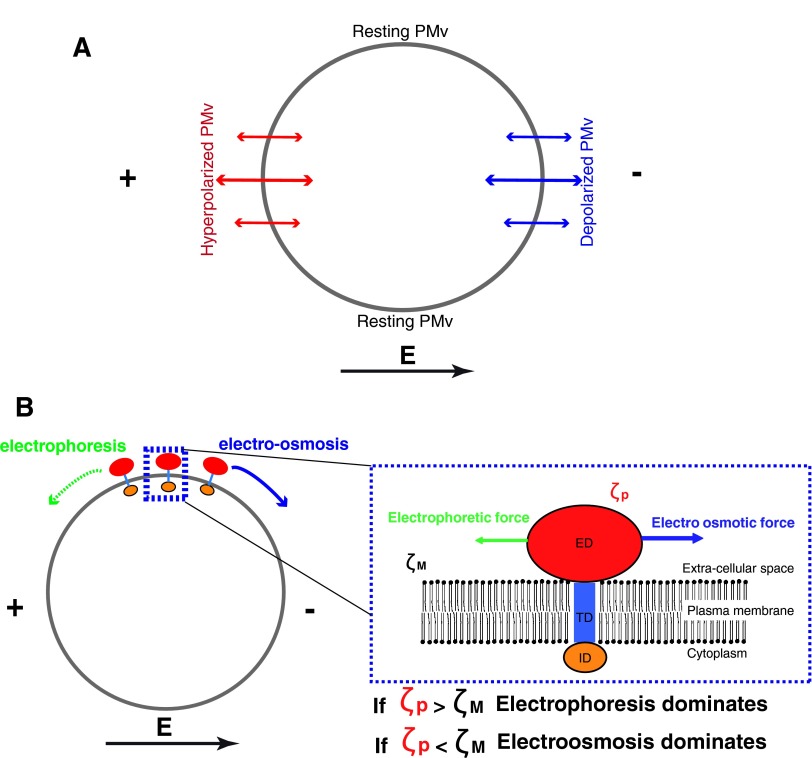
Several biophysical mechanisms for EF effects have been proposed throughout the years and are supported by experimental evidence (Fig. 3). A prevalent model is that the EF causes local inhomogeneity in transmembrane potential values (PMv) around the cell: the cathode-facing side would be depolarized (reduced PMv) and the anode-facing side would be hyperpolarized (increased PMv), while the parts of the cell facing the perpendicular axis would stay at their resting PMv (Fig. 3). These changes in PMv may yield local imbalances in ion fluxes, or turn on or off voltage-gated channels, or have other yet uncharacterized effects that would initiate a signaling cascade recruiting polarity component. Quantitatively, the extra-transmembrane potential caused by the EF scales with the intensity of the EF multiplied by the typical size of the cell, and thus, if this effect is dominant in EF experiments, larger cells are expected to respond at smaller EFs, which is most likely true from inspecting values in the literature.6 These effects on PMv have been directly highlighted using membrane potential dyes27 and genetically encoded proteins.28 Best supports for the role of PMv in EF responses come from experiments in which PMv is altered, from changing specific ion concentrations (H+ or K+) in or out the cell, or genetically inhibiting membrane potential regulators.14,25,29
Figure 3.
Biophysics of galvanotactic effects. (A) EF can cause local inhomogeneity in PMv around the cell, leading to depolarization (reduced PMv) at the cathode-facing side and hyperpolarization (increased PMv) of the anode-facing side. (B) EF can cause movements of membrane proteins along the plasma membrane through electrophoresis or electro-osmosis of membrane proteins with a charged extracellular domain. This effect involves competitive forces on the extracellular domain of membrane proteins, and the dominance of steering electrophorectic versus electro-osmotic forces may depend on the surface charge of the domain. PMv, transmembrane potential value. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
A second important view is that the EF may cause movements of membrane proteins, along the plasma membrane. These movements may result from the electrophoresis or electro-osmosis of membrane proteins, which have charged extracellular domains protruding the plasma membrane. If the Zeta potential (effective surface charge) of the extracellular domain of the protein is more negative than the local Zeta potential of the surrounding membrane, then the prediction is that the protein should move toward the anode; in the opposite case, the protein will be moved by electro-osmosis toward the cathode. Several models coupled with experimental data depicting movements of different membrane proteins, provide support for this view,30–32 although very little functional data linking protein movement and polarity re-orientation have been reported so far. It is plausible that in any given cell type, some extracellular domains of some proteins may display enough surface charge to yield movements, but the question is whether these movements really drive polarity downstream. Modeling considerations provide arguments for how this effect would depend on cell size, protein charge, and diffusion constant in the membrane.31 Trafficking and recycling of these membrane proteins is also likely to bias these modeling predictions, and should be taken into account in future extensions of these models.
How might cells sense and transduce EF?
The hidden side of galvanotactic effects is found in the molecular machinery transducing an EF into a defined internal cell polarity. Until recently, there has not been a complete picture in a single cell type that provides a pathway linking biophysical effects of EFs at the membrane down to cytoskeletal organization. In Table 1, we summarize some of the most important proteins or types of proteins that have been suggested to sense and transduce EF effects and be involved in reorganizing polarity in response.9,14,21,25,33–38
Table 1.
Examples of gene products and putative pathways identified in electric field reponses in different cells
| Cell Type | Sensing at the Membrane | Sensing in the Cytosol | References |
|---|---|---|---|
| Xenopus neuron growth cones | Unknown | Cdc42/Rho/Rac | 34,36 |
| Keratinocytes | Integrin | Rac/cAMP | 21,33 |
| Dictyostelium discoideum | NHE2/Ca2+ | PI3K/PTEN/cGMP | 9,35,37 |
| Candida albicans | Cch1 | Rsr1/cdc42 | 14,38 |
| Fission yeast | Pma1 | Cdc42/for3 | 25 |
cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; PI3K, phosphatidylinositide 3-kinases; PTEN, phosphatase and tensin homolog.
Connections between EF effects and downstream cytoskeletal regulators, including the small GTPase cdc42p, the Rho/Rac pathways, integrin signaling, and phosphatidylinositol (PIP) signaling, have been suggested in different cellular systems.9,25,33–35 A pioneering work, performed in the context of mammalian wound healing, showed that neutrophils and keratinocytes wound-directed migration depended on phosphatidylinositide 3-kinases (PI3K) and on the phosphatase tensin homolog (PTEN) which, respectively, positively and negatively regulate phosphatidylinositol bisphosphate (PIP2) homeostasis.9,39 Wound-healing relies, in part, on endogenous EFs in the wound, and can be inhibited or accelerated by exogenous application of EFs pointing toward or away from the wound, respectively.9 In this electrotactic assay, exogenous EFs induce the activation of signaling kinases, including ERK, p38, Src, and Akt. In mouse models lacking the catalytic γ-subunit of PI3K, neutrophils and keratinocytes displayed reduced activation of these kinases, reduced electrotactic migration, and defective wound closure. Conversely, PTEN deletion enhanced EF-induced Akt and Src phosphorylation and directional migration, and accelerated wound healing. Thus, PIP signaling regulates electrotactic migration of cells in the wounded tissue and supports proper healing.18 It is interesting to note that PIP signaling also regulates chemotaxis in neutrophils.40 The downstream machinery required for directional migration is thus likely to be similar regardless of the nature of the spatial cue in this situation.
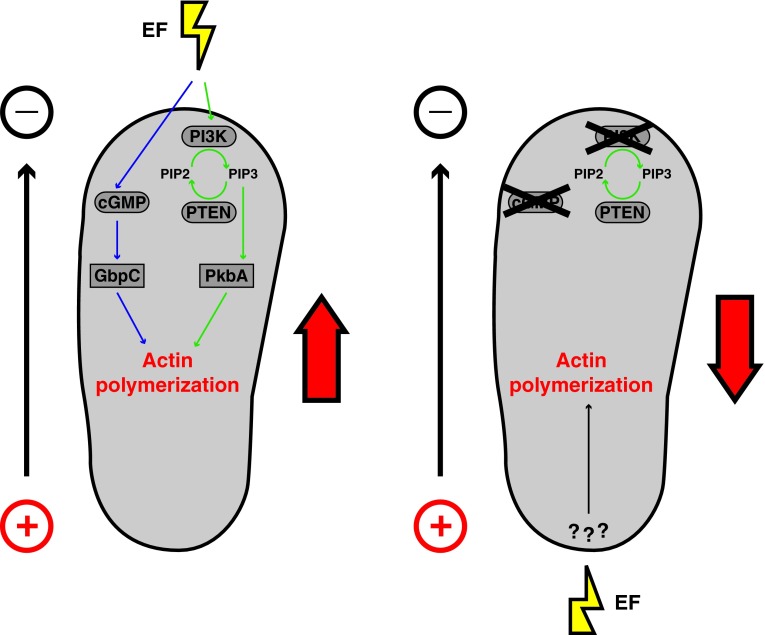
The amoebae Dictyostelium discoideum has long served as a genetic model to dissect molecular mechanisms of directional cell migration and chemotaxis.41,42 When exposed to homogeneous concentrations of cyclic adenosine monophosphate and small EFs, these cells depict striking galvanotaxis, orienting their migration to the cathode of the EF within minutes.37 This EF response is independent of chemotactic receptors.43 Downstream signaling modules regulating directional cell migration during chemotaxis include PIP and cyclic guanosine monophosphate (cGMP) signaling. These effectors promote actin polymerization at the leading edge for directed migration.44,45 In a recent work, Sato et al. tested the role of these signaling modules in galvanotaxis.35 Intracellular cGMP is produced mainly by two enzymes, soluble guanilyl cyclase (sGC) and guanilyl cyclase A (GCA). Mutants lacking the sGC and GCA (gca−/sgc−) and mutants lacking the cGMP-binding protein C (gbpC−), which display reduced levels of cGMP, exhibited attenuated cathodal electrotactic migration. Similar phenotypes were obtained when PIP signaling was repressed through PI3-kinase inhibition (Fig. 4). Strikingly, when both PIP2 synthesis and cGMP pathways were knocked down, cells migrated to the opposite direction, to the anode of the EF. These results suggest the existence of parallel pathways participating in regulating electrotaxis and point to the existence of a third pathway promoting anodal migration.35 These studies support the role of PIP signaling for electrotaxis in another cell type, and provide detailed genetic characterization of the molecular mechanisms involved. Cross-talk between EFs and polarity in these systems have been proposed to be mediated by calcium transport and membrane potential,23,36,37,46 yet the details of this transduction remain to be studied.
Figure 4.
Molecular mechanisms regulating Dictyostelium discoideum galvanotaxis. WT cells migrate to the cathode of an applied EF. This polarized migration involves at least two different pathways: The PI3 kinase/PTEN pathway that lead to a polarized distribution of PIP (green arrows); and the cGMP pathway (purple arrows). Mutant cells deficient in the PI3K and cGMP pathway migrate to the anode, suggesting the existence of a third pathway for EF sensing and directional migration. cGMP, cyclic guanosine monophosphate; PI3K, phosphatidylinositide 3-kinases; PIP, phosphatidylinositol-phosphate; PTEN, phosphatase and tensin homolog; WT, wild-type. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Fungal cells and yeasts are model systems to dissect molecular mechanisms of cell polarity. These nonmigrating cells exhibit polarized growth, which involves similar regulatory modules and conserved effectors as many higher eukaryotes.47 They usually possess a self-sustained internal polarity, which allows them to grow in a highly polarized manner even in the absence of external guiding cues. This polarity machinery may be biased and redirected by chemical gradients, for instance, during mating, or by mechanical signals in processes such as thigmotropism that may have relevance to infection in some hyphal species.14,38 Fungi and yeast also depict strong galvanotropism.25,26,48 The physiological relevance of the EF response in fungi is not well-established, but EFs are most likely present in the natural fungal habitat, such as on the surface of plants or in humid soils. Some fungi and molds have further been suggested to target wounds by following ion currents and EFs, and thus, the EF response could also have relevance in infection.49 Ion transporters and membrane potential regulators are widely shared between fungi and higher organisms, and thus, fungal and yeast cells will likely serve as excellent prototype genetic systems to dissect molecular mechanisms of the EF response at different levels.
One such example can be highlighted from work on the pathogenic fungi C. albicans. This single-celled organism grows by budding and switches to highly polarized hyphal growth in certain conditions. EFs can direct both the site of bud emergence and the hyphal polarized growth toward the cathode.24 Using forward genetic and chemical inhibitors, Brand et al. recently demonstrated that the galvanotactic response of C. albicans involved the conserved calcium transporter CaCch1p.14 This voltage-gated calcium channel shares high homology with mammalian homologues and with many other eukaryotes, and may serve to transduce membrane potential changes into calcium transport. Further work should reveal whether its function in EF sensing is conserved in other species. Other work from the same group further implicates the role of the Ras-like GTPase Rsr1 that serves as an internal landmark regulating Cdc42 activation in C. albicans.38 This set of studies begins to identify important regulatory nodes at the membrane and in the cytoplasm, and further work should reveal how these different modules are connected to drive galvanotropism.
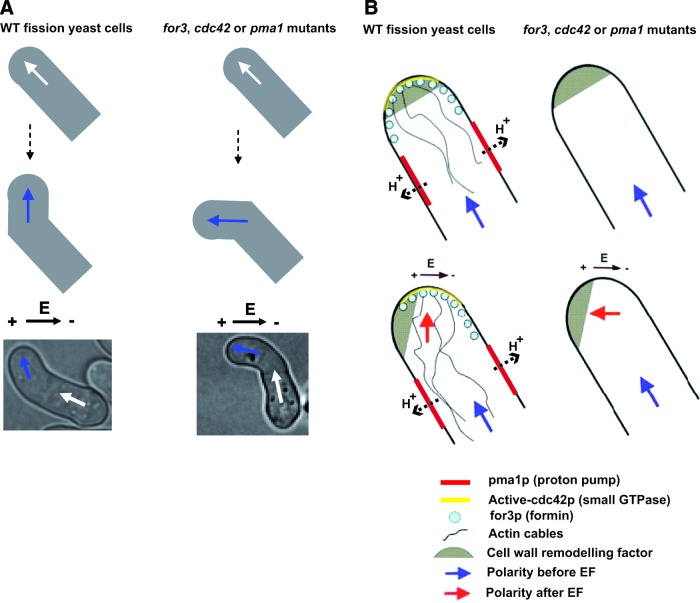
The fission yeast S. pombe serves as an excellent system to dissect the molecular mechanisms of eukaryotic polarized cell growth and cell form.50 These cells depict a constant and quantitative rod-shape and grow exclusively at cell tips. Genetic libraries of individual knockout strains are available, and provide a very powerful tool to perform systematic genetic studies of basic biological processes. We recently introduced the use of this model to study EF effects on cell polarity.25 The EF caused the fission yeast cells to reorient their growth axis by bending to a direction perpendicular to the EF, creating cells with a bent morphology.25 Candidate genetic screens of mutants in major polarity regulators and in ion transporters suggested that this response depended on the conserved formin for3p and the small GTPase cdc42p, which regulate actin cable polymerization for cell polarity.51,52 This screen identified a conserved plasma membrane ion pump, the proton ATPase pma1p, as a major regulator of EF effects. One interesting result was that mutants in these different genes still oriented to the EF, but to the wrong direction, toward the anode of the EF (Fig. 5). Coupling simulation of biophysical EF effects with detailed localization of these identified components suggested that the main mode orienting cell growth perpendicular to the EF involved membrane potential and local pH effects that may promote formin activation to nucleate actin cables. In turn, the anodal orientation in pma1 for3 or cdc42 mutants appeared to rely on the anodal electrophoresis of cell wall enzymes, beta-glucan synthases that possess highly charged extracellular domains. The role of cdc42 and formin are consistent with findings in neuronal growth cone migration, which implicate function for the Cdc42/Rho/Rac pathway.34
Figure 5.
Molecular pathways regulating Schizosaccharomyces pombe galvanotropism. (A) In fission yeast cells that normally grow in perfect rod-shaped morphology; EF causes WT cells to reorient growth perpendicular to the EF direction. This reorientation depends on the proton ATPase pump pma1p, the small GTPase cdc42p, and the formin for3p. Pma1 functions as a pH regulator and is located on the side of the cell, which may establish putative transcellular proton currents and cortical pH gradients that transduce EF effects to for3p and cdc42p regulation. (B) The anodal orientation in pma1 for3 or cdc42 mutants may rely on the anodal electrophoresis of cell wall enzymes, which possess highly charged extracellular domains, and are important regulators of polarized cell growth in these cells. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
A very novel aspect of these studies is to promote the role of pH and/or membrane potential in mediating the cross talk between EFs and actin.25 These layers of regulation may be important even in normal cells as pma1 mutants depict strong morphogenesis and polarity defects. These studies thus bring important fresh views on general cell polarization mechanisms.
All together, these genetic dissections in different organisms suggest downstream transduction of EF effects by small GTPase, lipid signaling, and actin regulation factors. The cross-talk between EFs and some of these polarity modules remains to be clearly defined, although some transporters and ions have been specifically identified in these different systems. Membrane potential, calcium, and pH regulation may play key roles in mediating EF effects into polarized reorganization of cytoskeletal regulators. Genetic studies in Dictyostelium and yeast begin to reveal why different cells may polarize to different directions, and suggest that directionality in response to the EF may be sensitive to the expression of a single protein, or a cellular component. These different directional responses found in different mutants support the existence of competing pathways steering the cell in different directions with one dominant mode. When the dominant mode is knocked down, the second pathway takes over to drive polarity in another direction.
Biological significance of galvanotactic effects
These EF effects are likely to reflect physiological events in wound healing, neuron organization, and development. As EFs are present around tissues, studying these effects in isolated cells in vitro will reveal important mechanisms of tissue organization and cell behavior in vivo. These effects offer one unique manner to control the orientation and shapes of many different cells, and have the potential to open new avenues in bioengineering and medicine.
Beyond their significance in basic biological processes, these galvanotactic experiments bring fundamental understanding in core polarity mechanisms.53 The fact that most cell types can sense and orient to EFs suggest that galvanotactic effects involve an evolutionarily conserved layer of spatial organization. We speculate that EF effects could reflect the natural electrochemical regulation of polarity and cytoskeletal elements. If this is the case, the EF effect may bias or exacerbate an existing electrical organization, leading to the polarized reorientation in the EF. A specific cytoskeletal regulator may, for instance, naturally bind to portions of the plasma membrane with specific charges,54,55 or be activated within a narrow pH window; the EF-induced perturbation on the membrane potential, membrane charge, or pH would cause the relocation or reactivation of this element to redirect polarity. It has long been a puzzle to understand how such small EFs, which perturb only 1–5% of the resting membrane potential, could orient polarity in such a striking manner.6 Positive feedback regulating polarization modules, the cytoskeletons and ion transport may begin to provide answers to these long-standing questions. There are many recent reports that highlight the role of membrane potential, pH gradients, and membrane inner leaflet charges as fundamental regulators of polarity processes in single cells, tissues, and whole organisms (for a recent review, see Campetelli et al.53). Galvanotactic experiments will thus continue revealing important aspects of general polarization mechanisms, and may provide novel approaches to develop suitable therapeutic alternatives in the context of wound healing, development, and regeneration.
Take-Home Messages.
• EFs may influence the spatial behavior of cells and tissues in vivo, during processes, such as wound healing, development, and cancer
• Exogenous EFs can direct cell migration, growth, and division in many different cell types, such as bacteria, neutrophils, and neurons.
• Biophysical and molecular mechanisms of EFs are poorly characterized, but may involve complex signal transductions at the plasma membrane.
• Recent work using genetic models such as Dictyostelium and yeast cells, begin to identify key molecular players at the level of membrane signaling and in the regulation of the cytoskeleton to direct migration and growth in response to EFs.
Conclusions and Future Directions
In sum, the road to understanding the molecular mechanisms regulating galvanotactic effects is still long, before one can provide a system-level detailed understanding of such fascinating effects. Model organisms which allow reliable forward genetic studies, such as yeast or Dictyostelium, will help to rigorously identify and characterize gene products that may be involved in the electric response. It will then be possible to test these hits, either in mammalian cells using RNA silencing in cultured cell lines, or in animal models, and to discern relevant signal transduction mechanisms directly relevant to human care. The identification of specific proteins also promises to pave the way for the synthesis of specific chemical inhibitors, which may be used to enhance the galvanotactic effect to improve healing or nerve repair and to develop accurate therapeutic methods for treating chronic wounds and spinal injury. Besides the genetic investigation of these sensing mechanisms, efforts at the biophysical level need to be made to generate a detailed understanding of the processes at play, and modeling together with detailed dynamic microscopy should help research move in this direction.
Abbreviations and Acronyms
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- EF
electric field
- GCA
guanilyl cyclase A
- GTPase
guanosine triphosphate hydrolase enzyme
- PI3K
phosphatidylinositide 3-kinases
- PIP
phosphatidylinositol-phosphate
- PIP2
phosphatidylinositol bisphosphate
- PMv
transmembrane potential value
- PTEN
phosphatase and tensin homolog
- sGC
soluble guanilyl cyclase
- WT
wild-type
Acknowledgments
N.M. acknowledges financial support from an Agence Nationale de la Recherche (ANR) ‘retour post-doctorants’ grant ANR-10PDOC-003-01 and a European FP7-People-CIG grant. D.B. is supported by an Institut Curie PhD fellowship.
Author Disclosure and Ghostwriting
The authors declare no competing financial interests. No ghostwriters were used.
About the Authors
Daria Bonazzi studied chemistry at the University of Bologna, discovering a particular interest for bioelectrochemistry. She then moved to the Institut Curie (Paris, France), where she focused on a cell biology research topic. She now is a PhD student at the Institut Curie and Institut Jacques Monod (Paris, France) under the supervision of Nicolas Minc, working on biophysical aspects of cell polarity in yeast. Nicolas Minc initially trained in physics and completed his initial training in cell biology during his post-doctorate at Columbia University. He is a CNRS researcher and a group leader at the Institut Jacques Monod (Paris, France). His group focuses on the biophysical studies of cell shape and cell polarity, and conduct these investigations in yeast and early embryos.
References
- 1.Drubin DG. and Nelson WJ: Origins of cell polarity. Cell 1996; 84:335. [DOI] [PubMed] [Google Scholar]
- 2.Li R. and Gundersen GG: Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol 2008; 9:860. [DOI] [PubMed] [Google Scholar]
- 3.Cain RJ. and Ridley AJ: Phosphoinositide 3-kinases in cell migration. Biol Cell 2009; 101:13. [DOI] [PubMed] [Google Scholar]
- 4.Iden S. and Collard JG: Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 2008; 9:846. [DOI] [PubMed] [Google Scholar]
- 5.Djamgoz MBA, Mycielska M, Madeja Z, Fraser SP, and Korohoda W: Directional movement of rat prostate cancer cells in direct-current electric field: involvement of voltagegated Na+ channel activity. J Cell Sci 2001; 114:2697. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe LF. and Nuccitelli R: Electrical controls of development. Annu Rev Biophys Bioeng 1977; 6:445. [DOI] [PubMed] [Google Scholar]
- 7.Mycielska ME. and Djamgoz MB: Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J Cell Sci 2004; 117:1631. [DOI] [PubMed] [Google Scholar]
- 8.Reid B, Nuccitelli R, and Zhao M: Non-invasive measurement of bioelectric currents with a vibrating probe. Nat Protoc 2007; 2:661. [DOI] [PubMed] [Google Scholar]
- 9.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, and Penninger JM: Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006; 442:457. [DOI] [PubMed] [Google Scholar]
- 10.Kropf D, Lupa M, Caldwell J, and Harold FM H: Cell polarity: endogenous ion currents precede and predict branching in the water mold Achyla. Science 1983; 220:1385. [DOI] [PubMed] [Google Scholar]
- 11.Levin M, Thorlin T, Robinson KR, Nogi T, and Mercola M: Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell 2002; 111:77. [DOI] [PubMed] [Google Scholar]
- 12.Nuccitelli R, Poo MM, and Jaffe LF: Relations between ameboid movement and membrane-controlled electrical currents. J Gen Physiol 1977; 69:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisenseel MH, Nuccitelli R, and Jaffe LF: Large electrical currents traverse growing pollen tubes. J Cell Biol 1975; 66:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand A, Shanks S, Duncan VM, Yang M, Mackenzie K, and Gow NA: Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr Biol 2007; 17:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaig CD, Rajnicek AM, Song B, and Zhao M: Controlling cell behavior electrically: current views and future potential. Physiol Rev 2005; 85:943. [DOI] [PubMed] [Google Scholar]
- 16.Rajnicek AM, McCaig CD, and Gow NA: Electric fields induce curved growth of Enterobacter cloacae, Escherichia coli, and Bacillus subtilis cells: implications for mechanisms of galvanotropism and bacterial growth. J Bacteriol 1994; 176:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson KR: The responses of cells to electrical fields: a review. J Cell Biol 1985; 101:2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M: Electrical fields in wound healing—an overriding signal that directs cell migration. Semin Cell Dev Biol 2009; 20:674. [DOI] [PubMed] [Google Scholar]
- 19.Chang PC, Sulik GI, Soong HK, and Parkinson WC P: Galvanotropic and galvanotaxic responses of corneal endothelial cells. J Formos Med Assoc 1996; 95:623. [PubMed] [Google Scholar]
- 20.McKasson MJ, Huang L, and Robinson KR: Chick embryonic Schwann cells migrate anodally in small electrical fields. Exp Neurol 2008; 211:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pu J, McCaig CD, Cao L, Zhao Z, Segall JE, and Zhao M: EGF receptor signalling is essential for electric-field-directed migration of breast cancer cells. J Cell Sci 2007; 120:3395. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Bai H, Wang E, Forrester JV, and McCaig CD: Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci 2004; 117:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onuma EK. and Hui SW: Electric field-directed cell shape changes, displacement, and cytoskeletal reorganization are calcium dependent. J Cell Biol 1988; 106:2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crombie T, Gow NA, and Gooday GW: Influence of applied electrical fields on yeast and hyphal growth of Candida albicans. J Gen Microbiol 1990; 136:311. [DOI] [PubMed] [Google Scholar]
- 25.Minc N. and Chang F: Electrical control of cell polarization in the fission yeast Schizosaccharomyces pombe. Curr Biol 2010; 20:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGillivray A. and Gow NAR: Applied electrical fileds polarize the growth of mycelial fungi. J Gen Microbiol 1986; 132:2515 [Google Scholar]
- 27.Gross D, Loew LM, and Webb WW: Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J 1986; 50:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kralj JM, Hochbaum DR, Douglass AD, and Cohen AE: Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science 2011; 333:345. [DOI] [PubMed] [Google Scholar]
- 29.Pantazopoulos P, Kwong K, Lillycrop W, Wong L, Gao Y, Chalouh S, Samadhin M, Ratnayake WM, Krenosky S, Dumais L, and L'Abbe MR: Trans and saturated fat on food labels in Canada: fact or fiction? Can J Public Health 2011; 102:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaffe LF: Electrophoresis along cell membranes. Nature 1977; 265:600. [DOI] [PubMed] [Google Scholar]
- 31.Poo M: In situ electrophoresis of membrane components. Annu Rev Biophys Bioeng 1981; 10:245. [DOI] [PubMed] [Google Scholar]
- 32.Poo M. and Robinson KR: Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature 1977; 265:602. [DOI] [PubMed] [Google Scholar]
- 33.Pullar CE, Baier BS, Kariya Y, Russell AJ, Horst BA, Marinkovich MP, and Isseroff RR: Beta4 integrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol Biol Cell 2006; 17:4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajnicek AM, Foubister LE, and McCaig CD: Temporally and spatially coordinated roles for Rho, Rac, Cdc42 and their effectors in growth cone guidance by a physiological electric field. J Cell Sci 2006; 119:1723. [DOI] [PubMed] [Google Scholar]
- 35.Sato MJ, Kuwayama H, van Egmond WN, Takayama AL, Takagi H, van Haastert PJ, Yanagida T, and Ueda M: Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling. Proc Natl Acad Sci USA 2009; 106:6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pullar CE. and Isseroff RR: Cyclic AMP mediates keratinocyte directional migration in an electric field. J Cell Sci 2005; 118:2023. [DOI] [PubMed] [Google Scholar]
- 37.Shanley LJ, Walczysko P, Bain M, MacEwan DJ, and Zhao M: Influx of extracellular Ca2+ is necessary for electrotaxis in Dictyostelium. J Cell Sci 2006; 119:4741. [DOI] [PubMed] [Google Scholar]
- 38.Brand A, Vacharaksa A, Bendel C, Norton J, Haynes P, Henry-Stanley M, Wells C, Ross K, Gow NA, and Gale CA: An internal polarity landmark is important for externally induced hyphal behaviors in Candida albicans. Eukaryot Cell 2008; 7:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao M, Pu J, Forrester JV, and McCaig CD: Membrane lipids, EGF receptors, and intracellular signals colocalize and are polarized in epithelial cells moving directionally in a physiological electric field. FASEB J 2002; 16:857. [DOI] [PubMed] [Google Scholar]
- 40.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, and Bourne HR: Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 2000; 287:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devreotes PN. and Zigmond SH: Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol 1988; 4:649. [DOI] [PubMed] [Google Scholar]
- 42.Parent CA. and Devreotes PN: Molecular genetics of signal transduction in Dictyostelium. Annu Rev Biochem 1996; 65:411. [DOI] [PubMed] [Google Scholar]
- 43.Song B, Zhao M, Forrester JV, and McCaig CD: Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci USA 2002; 99:13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veltman DM, Keizer-Gunnik I, and Van Haastert PJ: Four key signaling pathways mediating chemotaxis in Dictyostelium discoideum. J Cell Biol 2008; 180:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veltman DM. and Van Haastert PJ: Guanylyl cyclase protein and cGMP product independently control front and back of chemotaxing Dictyostelium cells. Mol Biol Cell 2006; 17:3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao RC, Zhang XD, Sun YH, Kamimura Y, Mogilner A, Devreotes PN, and Zhao M: Different roles of membrane potentials in electrotaxis and chemotaxis of dictyostelium cells. Eukaryot Cell 2011; 10:1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang F. and Peter M: Yeasts make their mark. Nat Cell Biol 2003; 5:294. [DOI] [PubMed] [Google Scholar]
- 48.Harold FM, Schreurs WJ, Harold RL, and Caldwell JH: Electrobiology of fungal hyphae. Microbiol Sci 1985; 2:363. [PubMed] [Google Scholar]
- 49.van West P, Morris BM, Reid B, Appiah AA, Osborne MC, Campbell TA, and Shepherd SJ: Oomycete plant pathogens use electric fields to target roots. Mol Plant Microbe Interact 2002; 15:790. [DOI] [PubMed] [Google Scholar]
- 50.Chang F. and Martin SG: Shaping fission yeast with microtubules. Cold Spring Harb Perspect Biol 2009; 1:a001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin SG, Rincon SA, Basu R, Perez P, and Chang F: Regulation of the formin for3p by cdc42p and bud6p. Mol Biol Cell 2007; 18:4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minc N, Bratman SV, Basu R, and Chang F: Establishing new sites of polarization by microtubules. Curr Biol 2009; 19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campetelli A, Bonazzi D, and Minc N: Electrochemical regulation of cell polarity and the cytoskeleton. Cytoskeleton (Hoboken) 2012; 69:601. [DOI] [PubMed] [Google Scholar]
- 54.Das A, Slaughter BD, Unruh JR, Bradford WD, Alexander R, Rubinstein B, and Li R: Flippase-mediated phospholipid asymmetry promotes fast Cdc42 recycling in dynamic maintenance of cell polarity. Nat Cell Biol 2012; 14:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fairn GD, Hermansson M, Somerharju P, and Grinstein S: Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nat Cell Biol 2011; 13:1424. [DOI] [PubMed] [Google Scholar]