Abstract
We previously demonstrated with functional magnetic resonance imaging (fMRI) that religious belief depends upon three cognitive dimensions, which can be mapped to specific brain regions. In the present study, we considered these co-activated regions as nodes of three networks each one corresponding to a particular dimension, corresponding to each dimension and examined the causal flow within and between these networks to address two important hypotheses that remained untested in our previous work. First, we hypothesized that regions involved in theory of mind (ToM) are located upstream the causal flow and drive non-ToM regions, in line with theories attributing religion to the evolution of ToM. Second, we hypothesized that differences in directional connectivity are associated with differences in religiosity. To test these hypotheses, we performed a multivariate Granger causality-based directional connectivity analysis of fMRI data to demonstrate the causal flow within religious belief-related networks. Our results supported both hypotheses. Religious subjects preferentially activated a pathway from inferolateral to dorsomedial frontal cortex to monitor the intent and involvement of supernatural agents (SAs; intent-related ToM). Perception of SAs engaged pathways involved in fear regulation and affective ToM. Religious beliefs are founded both on propositional statements for doctrine, but also on episodic memory and imagery. Beliefs based on doctrine engaged a pathway from Broca's to Wernicke's language areas. Beliefs related to everyday life experiences engaged pathways involved in imagery. Beliefs implying less involved SAs and evoking imagery activated a pathway from right lateral temporal to occipital regions. This pathway was more active in non-religious compared to religious subjects, suggesting greater difficulty and procedural demands for imagining and processing the intent of SAs. Insights gained by Granger connectivity analysis inform us about the causal binding of individual regions activated during religious belief processing.
Key words: : belief formation, emotion, imagery, semantic memory, social cognition, theory of mind
Introduction
Religious behavior is a uniquely human trait, the cornerstone of which is religious belief (Boyer and Bergstrom, 2008). Religious beliefs refer to supernatural agents (SAs, exemplified by “God”) and to cosmological concepts and domains (such as “Heaven” and “Hell”). Belief representations involve multiple elemental cognitive and affective processes recruited in parallel. In a previous study on religious belief, we identified the three most readily demonstrable (and, arguably, most important) among these processes, which constitute “Dimensions” of religious belief (Baylor Institute for Studies of Religion, 2006; Kapogiannis et al., 2009b): Dimension 1 (D1) monitors the level of involvement and intent of perceived SAs; D2 monitors the love and anger of perceived SAs (D2); and D3 refers to the mixed foundation of religious beliefs on abstract semantic processing (i.e., processing of propositional statements for knowledge of religious doctrines) and episodic memory and imagery. Using a functional magnetic resonance imaging (fMRI) paradigm, in which subjects had to indicate whether they agreed or not to a range of religious beliefs, we demonstrated sets of brain regions that became active in association with these dimensions (Kapogiannis et al., 2009b).
The main regions activated by D1 were bilateral inferior frontal gyrus (IFG, BA 45), right (R) middle temporal gyrus (MTG, BA 21), R inferior temporal gyrus (ITG, BA 20), R precuneus (BA 7), and R superior medial frontal gyrus (SMFG), BA 8 (part of dorsomedial PFC) and 10 (frontopolar PFC). These areas play key roles in action understanding and intent-related theory of mind (ToM) (German et al., 2004; Han et al., 2008; Molnar-Szakacs et al., 2005). The main regions activated by D2 were R middle frontal gyrus (MFG, BA 11, part of ventrolateral PFC) with perception of SAs' love, and left (L) MTG (BA 21) with perception of SAs' anger; these areas play roles in affective ToM and emotional regulation. Regarding D3, bilateral calcarine (CaG) and L fusiform (FG) gyri (BA 17, 18, and 19); L precuneus (BA 7); and L IFG (BA 44, Broca's area) were activated with beliefs mainly founded on episodic memory and imagery (Desai et al., 2010; Szpunar et al., 2007). Beliefs mainly processed as propositional statements for doctrine activated lateral temporal areas, including the L superior temporal gyrus (STG, BA 22, Wernicke's area).
In the present study, we considered the coactivated regions as nodes of three networks, each one corresponding to a particular dimension and hypothesized that religious beliefs emerge from causal flow within and between these networks. According to modern theories that attribute the development of religion to the evolution of ToM (Boyer, 2003; Boyer and Bergstrom, 2008), we hypothesized that regions involved in intent-related (such as the IFG) and affective ToM are located upstream the causal flow, i.e., they are drivers of activity in non-ToM regions. In addition, the previous fMRI analysis generated the unexpected finding of common brain activation patterns in religious and non-religious subjects (Kapogiannis et al., 2009b). To address this paradox, we hypothesized that differences in the causal flow within and between networks is associated with differences in religiosity.
To address these hypotheses, the present study utilized a multivariate Granger causality (GC)-based directional connectivity analysis of our fMRI data. Techniques based on the principle of GC (Granger, 1969) have been successfully employed to demonstrate the effective connectivity of brain networks involved in sensory (Deshpande et al., 2008; Roebroeck et al., 2005; Stilla et al., 2007, 2008), motor (Abler et al., 2006; Deshpande et al., 2009), and cognitive processing (Hampstead et al., 2010; Krueger et al., 2011; Sridharan et al., 2008; Strenziok et al., 2011), but they have not been applied to the study of religious cognition.
Materials and Methods
Detailed information on subjects and fMRI acquisition methods can be found in our original article (Kapogiannis et al., 2009b). All subjects provided written informed consent in compliance with the Institutional Review Board of the National Institute of Neurological Disorders and Stroke (Bethesda, MD). Briefly, first, we used a data-reduction approach (Multidimensional Scaling) to identify dimensions underlying religious belief in 13 religious and 13 non-religious healthy volunteers; this allowed us to create a three-dimensional cognitive space where a set of statements describing religious beliefs was represented. Then, fMRI was performed in a different cohort of 20 religious and 20 non-religious healthy volunteers (matched for age, sex, and education), using the same set of statements as stimuli; subjects had to read and indicate whether they agreed or not with each statement. We employed a General Linear Model analysis, in which the dimension coordinates of the statements (D1, D2, and D3) were treated as parametric modulators of the hemodynamic response function. Eight areas showed a positive linear association with D1; one area had a positive and another a negative linear association with D2; and six areas had a negative and five a positive linear association with D3.
To pursue GC analysis, we considered these 21 areas as regions of interest (ROIs) and extracted their representative time series (first eigenvariate), which were input to a single dynamic multivariate autoregressive model (dMVAR).
Dynamic correlation-purged Granger causality
GC analysis, in this context, attempts to determine whether there is a causal relationship between activity in different nodes of a neural network. Suppose xm, m=1.k correspond to the k selected ROI time series and 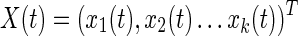 , then the dMVAR using X(t) was defined such that its coefficients are a function of time
, then the dMVAR using X(t) was defined such that its coefficients are a function of time
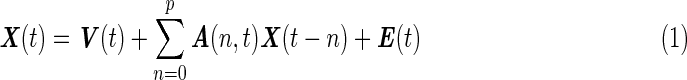 |
where V is the intercept vector, E(t) is the vector corresponding to the residuals, and t represents discrete time. The model order p was determined to be one, using the Bayesian information criterion (Deshpande et al., 2009). Being a multivariate model, the dMVAR is less sensitive to indirect causal relationships due to two regions being influenced from a third variable (Kus et al., 2004). In accordance with previous studies (Sato et al., 2006), the elements of A(n,t), that is, aij(n,t), can be expanded using a wavelet basis as follows
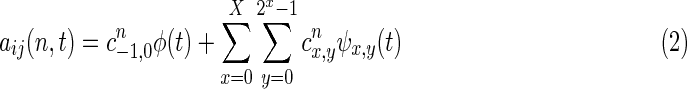 |
where 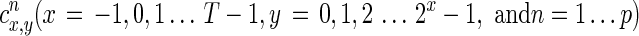 are the wavelet coefficients, φ(t) is the scaling function, and ψx,y(t) are orthonormal basis functions derived from a mother wavelet. We chose the Daubechies wavelet as the mother wavelet owing to its regularity and compact support (Daubechies, 1988). The choice of the specific Daubechies wavelet (D2−D20) is dictated by the expected order of polynomial behavior in the data, given the fact that the number of vanishing moments of DN is N/2. For example, D4 is most suited for modeling a constant and linear component in the data (polynomial with two coefficients) because it has four wavelet filter coefficients and two vanishing moments. In particular, we chose the D8 Daubechies wavelet as the mother wavelet in this study because previous studies have indicated that fMRI activation data may be appropriately modeled by polynomials of an order of 3 to 5 (Clark, 2002; Gibbons et al., 2004). Both parameter T and maximum resolution parameter X must be a power of two. An iterative generalized least squares estimation procedure (Sato et al., 2006) was adopted to solve for the wavelet coefficients to obtain A′(n,t) and V(t). As shown before (Sato et al., 2006), the number of temporal observations we have (i.e., 280 volumes per scan) is enough to reliably estimate the unknown parameters via this procedure. Dynamic correlation-purged Granger causality (CPGC) (Deshpande et al., 2010a, 2010b; Lacey et al., 2010b) was then obtained as shown in Equation (3). A custom implementation of the dMVAR model was performed using MATLAB.
are the wavelet coefficients, φ(t) is the scaling function, and ψx,y(t) are orthonormal basis functions derived from a mother wavelet. We chose the Daubechies wavelet as the mother wavelet owing to its regularity and compact support (Daubechies, 1988). The choice of the specific Daubechies wavelet (D2−D20) is dictated by the expected order of polynomial behavior in the data, given the fact that the number of vanishing moments of DN is N/2. For example, D4 is most suited for modeling a constant and linear component in the data (polynomial with two coefficients) because it has four wavelet filter coefficients and two vanishing moments. In particular, we chose the D8 Daubechies wavelet as the mother wavelet in this study because previous studies have indicated that fMRI activation data may be appropriately modeled by polynomials of an order of 3 to 5 (Clark, 2002; Gibbons et al., 2004). Both parameter T and maximum resolution parameter X must be a power of two. An iterative generalized least squares estimation procedure (Sato et al., 2006) was adopted to solve for the wavelet coefficients to obtain A′(n,t) and V(t). As shown before (Sato et al., 2006), the number of temporal observations we have (i.e., 280 volumes per scan) is enough to reliably estimate the unknown parameters via this procedure. Dynamic correlation-purged Granger causality (CPGC) (Deshpande et al., 2010a, 2010b; Lacey et al., 2010b) was then obtained as shown in Equation (3). A custom implementation of the dMVAR model was performed using MATLAB.
 |
Stimulus entrained dynamic CPGC analysis
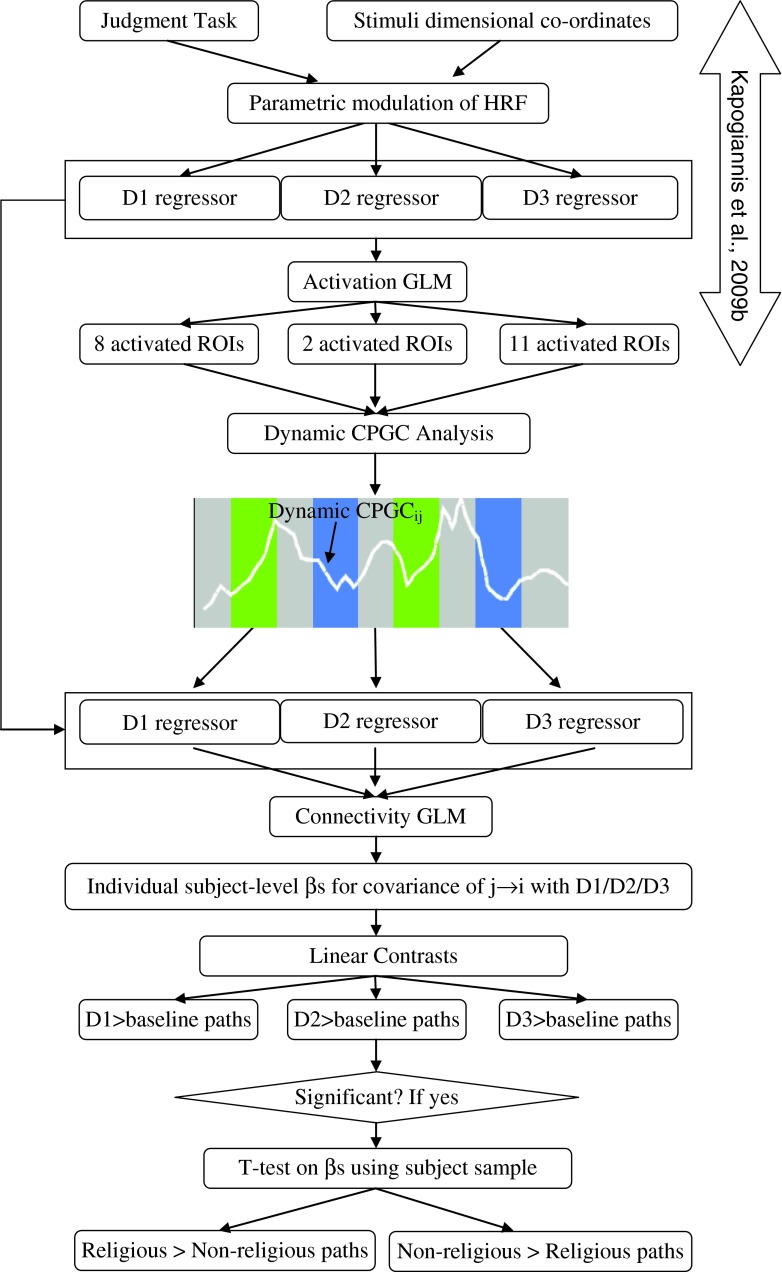
Dynamic CPGC between all 21 ROIs was obtained for every run and every subject using a first order dMVAR model using wavelets as described above. Using the GLM design matrix used in the previous study (Kapogiannis et al., 2009b), the beta values indicating the strength of covariance between CPGC pathways (as opposed to fMRI time series in activation analysis) and the experimental paradigm, specifically D1, D2, and D3, were determined for each individual subject. Linear contrasts were computed to assess the effect of each dimensional regressor compared to the baseline, as in (Kapogiannis et al., 2009b). This yielded pathways significant for contrasts D1>baseline, D2>baseline, and D3>baseline. Such paths had connectivity that covaried significantly more with the effect of interest than with the baseline. For such paths, a t-test was carried out on the betas to find out connections that were significantly different between the religious and non-religious groups. Significance was set to p<0.001 (FDR corrected) in all cases. A schematic of this procedure is shown in Figure 1.
FIG. 1.
A schematic illustrating the analysis methods adopted in this article. Specifically, the activation analysis performed (Kapogiannis et al., 2009b) is summarized first. The time series from the activated regions of interest (ROIs) are input to the dynamic connectivity model following which the covariance of the obtained dynamic connectivities with dimensional regressions is modeled using a GLM. Individual subject-level βs obtained from the GLM are subject to dimension>baseline contrasts. The significant paths resulting from this are again tested for significant differences between religious and non-religious groups.
The motivation for using the stimulus entrained dynamic connectivity analysis in place of other simpler techniques, which do not model the dynamics, is as follows. First, our approach formulates connectivity investigation within the methodological framework of “activity detection,” which makes it easier to interpret the relationship between activity and connectivity (Lacey et al., 2010a). Second, intrinsic causality that is not entrained to the external stimulus, though interesting, is not relevant to the specific brain mechanism being investigated when using a task to evoke brain activity. Our method allows the characterization of stimulus-evoked connectivity changes. Third, hemodynamic variability arising due to non-neuronal sources is structural, rather than functional, in nature and hence does not change with time. Therefore, as shown previously (Deshpande and Hu, 2012), the results obtained from this model are not influenced by the variability of the hemodynamic response across regions and subjects.
Results
The effective connectivity analysis demonstrates the causal flow between the 21 ROIs. The results are graphically depicted as causal pathways linking network nodes in Figure 2 (Fig. 2a for D1 network nodes and Fig. 2b for pathways linking D1 with D2 and D3 nodes), Figure 3 (for pathways linking D2 with D1 and D3 nodes, given that we did not find a significant pathway between the two D2 nodes) and Figure 4 (Fig. 4a for D3 network nodes and Fig. 4b for pathways linking it with D1 and D2 nodes). Our analysis identified pathways for which the directional connectivity significantly covaried with the corresponding dimension (Table 1). Moreover, for some causal pathways this covariance was significantly greater for religious or non-religious subjects. Note that splitting the pathways whose connectivity covaried with a given dimension into inter-network paths and intra-network paths is only for convenience of interpretation and visual display; all the paths were computed using a single model with all 21 ROI time series.
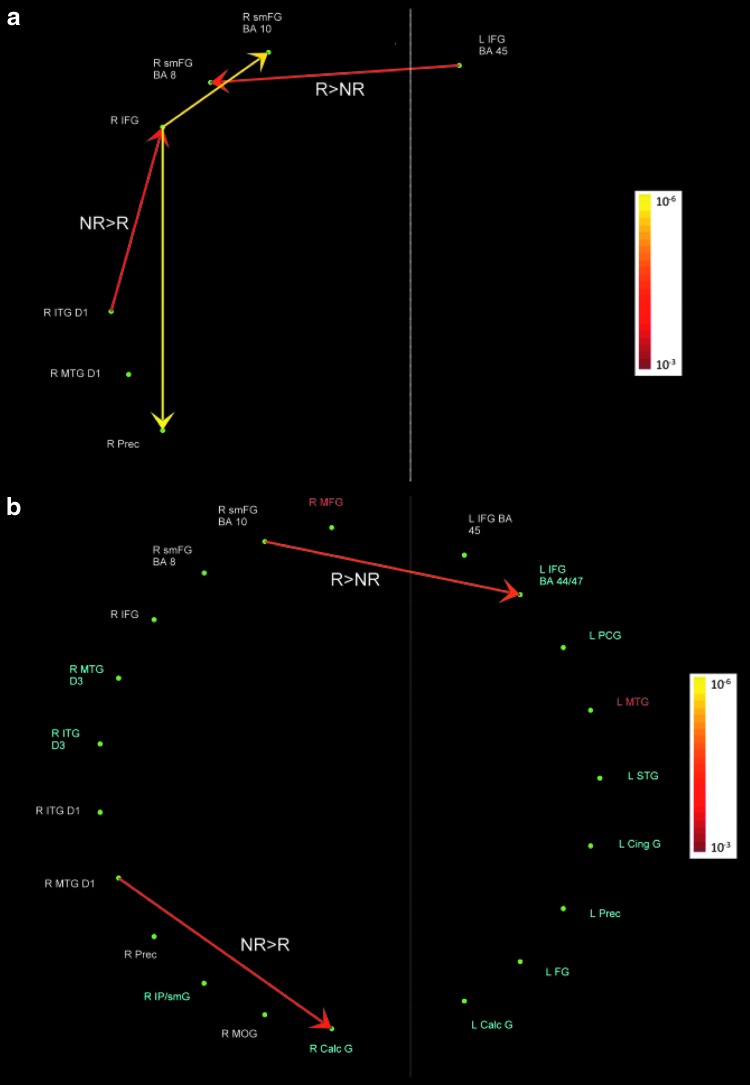
FIG. 2.
(a) Pathways between dimension 1 (D1) network nodes; (b) Pathways between D1 and other networks. Red-Yellow: pathway strength positively covaries with D1. All covariances were significant at p<0.001, FDR corrected. The color bar represents the p-values obtained in the D1>baseline contrast. The pathways labeled R>NR and NR>R were stronger in religious compared to non-religious subjects and vice versa, respectively.
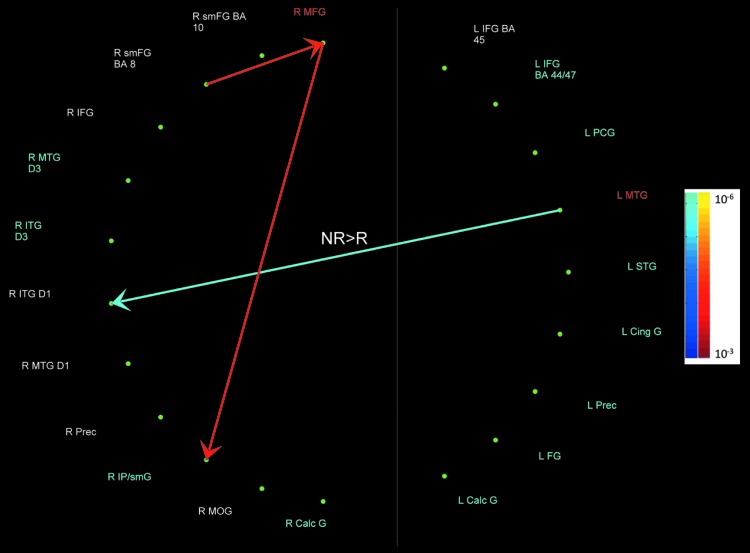
FIG. 3.
Pathways between D2 and other networks. Red-Yellow: pathway strength positively covaries with D2; Blue-Aquamarine: pathway strength negatively covaries with D2. All covariances were significant at p<0.001, FDR corrected. The color bar represents the p-values obtained in the D2>baseline contrast. The pathways labeled R>NR and NR>R were stronger in religious compared to non-religious subjects and vice versa, respectively.
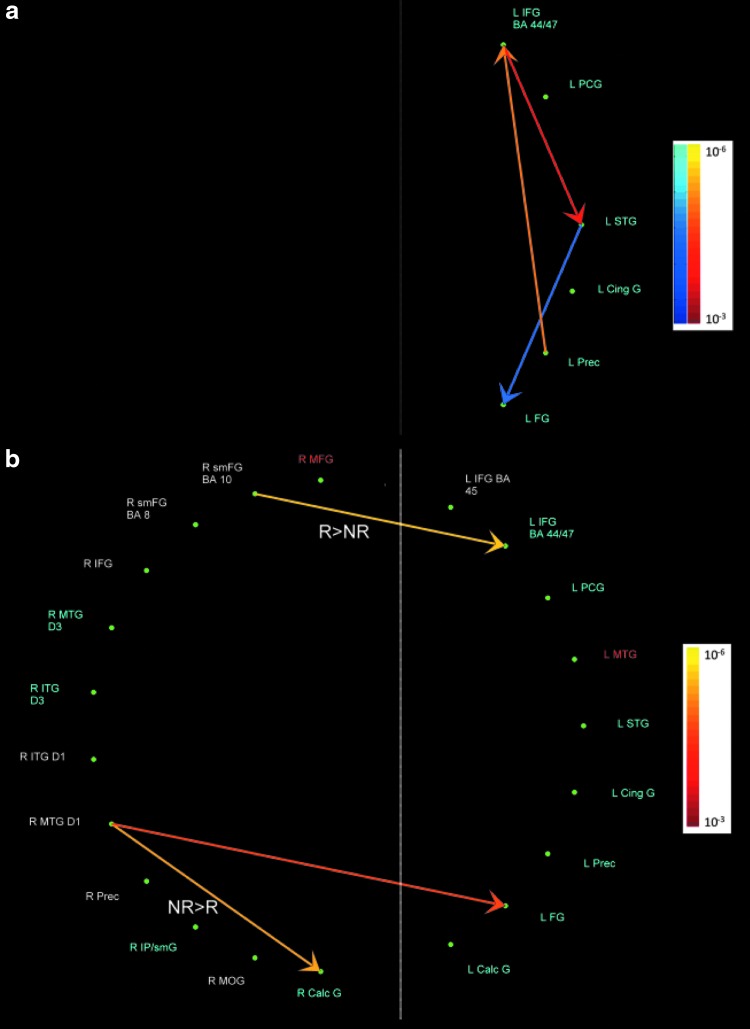
FIG. 4.
(a) Pathways between D3 network nodes; (b) Pathways between D3 and other networks. Red-Yellow: pathway strength positively covaries with D3; Blue-Aquamarine: pathway strength negatively covaries with D3. All covariances were significant at p<0.001, FDR corrected. The color bar represents the p-values obtained in the D3>baseline contrast. The pathways labeled R>NR and NR>R were stronger in religious compared to non-religious subjects and vice versa, respectively.
Table 1.
Beta Values for the Positive or Negative Covariance of Each Pathway with the Dimensions (p<0.001)
| Within-network pathways | Between-network pathways | ||||||
|---|---|---|---|---|---|---|---|
| Dimension | Covariance of the pathway with the dimension (positive or negative) | All | Religious>non-religious | Non-religious>religious | All | Religious>non-religious | Non-religious>religious |
| D1 (SAs perceived involvement) | +D1 (less involved SA) | R IFG→R PREC=0.38 | L IFG→R SMFG (BA 8)=0.09 | R ITG→R IFG=0.02 | R SMFG (10)→L IFG=0.25 | R MTG→R CalcG=0.21 | |
| R IFG→R SMFG (BA 10)=0.34 | |||||||
| −D1 (more involved SA) | – | – | – | – | – | – | |
| D2 (SAs perceived love/anger) | +D2 (more loving SA) | – | – | – | R SMFG (BA 8)→R MFG=0.29 | – | – |
| R MFG→R IP/smG=0.16 | |||||||
| −D2 (more angry SA) | – | – | – | – | L MTG→R ITG=− 0.30 | ||
| D3 (foundation of religious belief) | +D3 (founded on doctrine/semantics) | L STG→L FG=− 0.17 | – | – | – | – | – |
| −D3 (founded on episodic memory/imagery) | L PREC→L IFG=0.29 | – | – | R MTG→L FG=0.21 | R SMFG→L IFG=0.37 | R MTG→R CalcG=0.34 | |
| L IFG→L STG=0.08 | |||||||
SA, supernatural agent; IFG, inferior frontal gyrus; MTG, middle temporal gyrus; ITG, inferior temporal gyrus; SMFG, superior medial frontal gyrus; MFG, middle frontal gyrus; STG, superior temporal gyrus.
D1 network, perceived SAs' level of involvement
D1 network nodes showed higher activation in association with perception of SAs' lack of involvement (+D1). The pathways from R IFG, BA 45, to R precuneus, BA 7, and R SMFG, BA 10, had a positive association with D1 for all subjects. In other words, the connectivity from R IFG to R precuneus and R SMFG were stronger with processing statements describing relatively less involved SAs. Moreover, the pathway from R ITG, BA 20 to R IFG was stronger in non-religious compared to religious subjects. Religious subjects compared to non-religious subjects showed greater connectivity from L IFG, BA 45 to R SMFG, BA 8 (Fig. 2a). Examining pathways between D1, D2, and D3 network nodes revealed that, in religious compared to non-religious subjects, the pathway from R SMFG, BA 10, to L IFG, BA 44 (a D3 node that activates with religious beliefs based on episodic memory/imagery) had a greater positive association with perception of SAs' lack of involvement. Conversely, in non-religious compared to religious subjects, the pathway from R MTG, BA 21, to R CaG, BA 18 (a D3 node that also activates with religious beliefs based on episodic memory/imagery) had a greater positive association with perception of SAs' lack of involvement (Fig. 2b).
D2 network, perceived SAs' love and anger
The strength of the pathways from R SMFG, BA 8 (dorsomedial PFC), to R MFG, BA 11 (ventrolateral PFC), and from there to R SMG, BA 40 (IPL), was greater with greater perception of SAs' love (+D2) for all subjects. In addition, in non-religious compared to religious subjects, the pathway from L MTG, BA 21, to R ITG, BA 21, had a greater negative association with perception of SAs' anger (−D2) (Fig. 3).
D3 network, content of religious belief
Beliefs founded on doctrine (+D3) had a negative association with activation of the pathway from L STG, BA 22, to L FG, BA 19, while beliefs founded on episodic memory/imagery (−D3) had a positive association with activation of the pathway from L precuneus, BA 7, to L IFG, BA 45, and from there to L STG, BA 22, in all subjects (Fig. 4a). Further, pathways linking D3 and other networks, and covarying with D3, were observed (Fig. 4b). The pathway from R MTG, BA 21, to L FG, BA 18, had a positive association with+D3, in all subjects. Religious compared to non-religious subjects showed a greater positive association of the pathway from R SMFG, BA 10, to L IFG, BA 44/47, with+D3. Non-religious compared to religious subjects showed greater positive association of the pathway from R MTG, BA 21, to R CaG, BA 17, with −D3.
Discussion
Our findings that D1 regions involved in action understanding and intent-related ToM (German et al., 2004; Han et al., 2008; Molnar-Szakacs et al., 2005) are located upstream the causal flow and drive non-ToM regions support theories attributing religion to evolution of ToM for SAs (Boyer, 2003; Boyer and Bergstrom, 2008). Connectivity within the D1 network originated in the R IFG, a key area of the mirror-neuron system, consistently activated by intent-related, and affective, ToM (Mason and Just, 2011; Mier et al., 2010). The right sided predominance of the D1 network suggests that it is monitoring intent over simple action understanding (Ortigue et al., 2010). Pathways from IFG modulated the precuneus and dmPFC (BAs 8 and 10), areas heavily interconnected with each other, which play key roles in processing of self versus other (Cavanna and Trimble, 2006; Margulies et al., 2009; Nahab et al., 2011). The pathway to dmPFC was more active with perception of less involved SAs, perhaps, because of increased uncertainty about their intent (Jenkins and Mitchell, 2010). Strikingly similar networks monitor the protagonist's intent during discourse processing (Mason and Just, 2011) and self-agency during movement (Nahab et al., 2011).
Interestingly, all subjects shared the R-sided IFG to dmPFC pathway, but religious subjects also possessed a similar L-sided network, therefore, providing bilateral IFG input to this self-referential area. This double input may support a more anthropomorphic representation of SAs, since the more human-like an observed agent is, the higher medial frontal activation it evokes (Steinbeis and Koelsch, 2009).
Moreover, in non-religious subjects, a pathway from R ITG to R IFG positively covaried with +D1. White matter connections between these regions [especially the arcuate fasciculus, which connects the IFG, pars triangularis, with the lateral temporal lobe (Kaplan et al., 2010)] have been strengthened in recent phylogenesis, presumably, in association with the evolution of language (to the L) and other symbolic representation systems (to the R) (Rilling et al., 2008). Preferential recruitment of this pathway by non-religious subjects suggests that processing of symbolic representations may have driven their understanding of SAs' intent. By contrast, with higher +D1 (and +D3), religious subjects recruited a pathway that assesses plausibility and resolves conceptual ambiguities (Ye and Zhou, 2009): this pathway originates at R frontopolar PFC (BA 10) and terminates at Broca's area, a key area for action understanding and verbal representation of intended behaviors (Mason and Just, 2011; Ortigue et al., 2010).
With increased detection of perceived SAs' love over anger (+D2), both religious and non-religious subjects recruited a pathway running from R dmPFC (BA 8) to vlPFC (BA 11) and from there to the R IPL (BA 40). We speculate that the role of this pathway during religious belief consideration is reappraisal and suppression of fear induced by SAs. The dmPFC participates in emotional regulation by guiding attention to resolve emotional conflicts and orchestrate emotional reappraisal (Mitchell, 2011). This reappraisal, in turn, may be carried out by vlPFC (Blair et al., 2007; Levesque et al., 2003). In line with the above interpretation of this pathway, its third node, the IPL, is involved in suppression of fear, such as fear induced by faces (Amting et al., 2010; Bayle and Taylor, 2010). Failure in emotional regulation through this pathway may result in susceptibility to fear, including fear of SAs; as we have already shown, subjects with decreased dlPFC volume are more prone to experience fear of SAs (Kapogiannis et al., 2009a). In addition, vlPFC is a key region for detection of punishment cues that require a change in behavior (Kringelbach and Rolls, 2003; Mitchell, 2011), therefore this pathway may also play a role in linking emotion-inducing aspects of religious belief with behavioral guidance.
Previously, we saw that perceived SAs' anger over love (−D2) correlated with activity at the L MTG, BA 21 (Kapogiannis et al., 2009b). Although the lateral temporal lobes do not generate fear responses, they play a role in fear modulation (Goldin et al., 2008; Meletti et al., 2006). The present pathway analysis demonstrated that, with −D2, religious subjects preferentially activate a pathway from the L MTG to the contralateral lateral temporal lobe (R ITG, BA 20), an area important for accurate characterization of emotions (Rosen et al., 2006).
Dimension 3, of doctrine/semantics versus episodic memory/imagery recruited a pathway running from L precuneus to L IFG (Broca's area) and from there to L STG (Wernicke's area), nodes of the semantic processing network (Binder et al., 2009), in both religious and non-religious subjects. In particular, the activation of the pathway from Broca's to Wernicke's language areas may signify decoding of abstract content (Chen et al., 2008; Pobric et al., 2008). In addition, the strength of a pathway running from Wernicke's area to L FG involved in script-driven imagery (Esterman and Yantis, 2010) decreased with higher doctrinal semantic content (+D3), in both religious and non-religious subjects. These findings suggest a broader systems-level function for the D3 network, in establishing a balance between episodic memory and semantic processing systems (Battaglia and Pennartz, 2011). Activation of the R MTG to R CaG pathway differentiated non-religious from religious subjects. This pathway was preferentially activated, in non-religious compared to religious subjects, with −D3 (i.e., beliefs evoking imagery) and, +D1 (i.e., less involved SAs), suggesting greater difficulty and procedural demands for imagining and processing the intent of SAs among non-religious subjects.
It is worth noting a few limitations of this study. The methodology employed in this study was only applied to predefined functional ROIs. Therefore, it is possible that other regions modulate the activity and causal flow in the networks under consideration. It is also possible that the networks and pathways engaged during religious belief processing may change depending on the subjects' state, situational context, and the task at hand [such as activation of analytical thinking (Gervais and Norenzayan, 2012)].
Conclusions
This study demonstrated how insights gained by Granger connectivity analysis inform us about the causal binding of individual regions activated during religious belief processing. More broadly, this study enriches our understanding of the cognitive processes and networks involved in religious belief. It demonstrated that intent-related ToM for SAs is causally upstream in religious belief processing, perception of SAs engages pathways involved in fear regulation and affective ToM, and both semantic processing and imagery are foundations of religious beliefs. These processes are dynamic and constantly inform each other at multiple levels; this cross feeding of information varies among individuals in association with their religiosity.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS/NIH) and in part by the Intramural Research Program of the National Institute on Aging (NIA/NIH).
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- Abler B, Roebroeck A, Goebel R, Hose A, Schonfeldt-Lecuona C, Hole G, Walter H.2006. Investigating directed influences between activated brain areas in a motor-response task using fMRI. Magn Reson Imaging 24:181–185 [DOI] [PubMed] [Google Scholar]
- Amting JM, Greening SG, Mitchell DG. 2010. Multiple mechanisms of consciousness: the neural correlates of emotional awareness. J Neurosci 30:10039–10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Pennartz CM. 2011. The construction of semantic memory: grammar-based representations learned from relational episodic information. Front Comput Neurosci 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle DJ, Taylor MJ. 2010. Attention inhibition of early cortical activation to fearful faces. Brain Res 1313:113–123 [DOI] [PubMed] [Google Scholar]
- Baylor Institute for Studies of Religion 2006. American Piety in the 21st Century; New Insights to the Depth and Complexity of Religion in the US. Houston, TX: Baylor Institute for Studies of Religion [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. 2009. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. 2007. Modulation of emotion by cognition and cognition by emotion. Neuroimage 35:430–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. 2003. Religious thought and behaviour as by-products of brain function. Trends Cogn Sci 7:119–124 [DOI] [PubMed] [Google Scholar]
- Boyer P, Bergstrom B. 2008. Evolutionary perspectives on religion. Annu RevAnthropol 37:111–130 [Google Scholar]
- Cavanna AE, Trimble MR. 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583 [DOI] [PubMed] [Google Scholar]
- Chen E, Widick P, Chatterjee A. 2008. Functional-anatomical organization of predicate metaphor processing. Brain Lang 107:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP. 2002. Orthogonal polynomial regression for the detection of response variability in event-related MRI. Neuroimage 17:344–363 [DOI] [PubMed] [Google Scholar]
- Daubechies I. 1988. Orthonormal bases of compactly supported wavelets. Commun Pure Appl Math 41:909–996 [Google Scholar]
- Desai RH, Binder JR, Conant LL, Seidenberg MS. 2010. Activation of sensory-motor areas in sentence comprehension. Cereb Cortex 20:468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Hu X, 2012. Investigating effective brain connectivity from FMRI data: past findings and current issues with reference to granger causality analysis. Brain Connect 2:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Hu XP, Lacey S, Stilla R, Sathian K. 2010a. Object familiarity modulates effective connectivity during haptic shape perception. Neuroimage 49:1991–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Hu XP, Stilla R, Sathian K. 2008. Effective connectivity during haptic perception: a study using Granger causality analysis of functional magnetic resonance imaging data. Neuroimage 40:1807–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, LaConte S, James GA, Peltier S, Hu XP. 2009. Multivariate Granger causality analysis of fMRI data. Hum Brain Mapp 30:1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Sathian K, Hu XP. 2010b. Assessing and compensating for Zero-Lag correlation effects in time-lagged Granger causality analysis of fMRI. IEEE Trans Biomed Eng 57:1446–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Yantis S. 2010. Perceptual expectation evokes category-selective cortical activity. Cereb Cortex 20:1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German TP, Niehaus JL, Roarty MP, Giesbrecht B, Miller MB. 2004. Neural correlates of detecting pretense: automatic engagement of the intentional stance under covert conditions. J Cogn Neurosci 16:1805–1817 [DOI] [PubMed] [Google Scholar]
- Gervais WM, Norenzayan A. 2012. Analytic thinking promotes religious disbelief. Science 336:493–496 [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Lazar NA, Bhaumik DK, Sclove SL, Chen HY, Thulborn KR, Sweeney JA, Hur K, Patterson D. 2004. Estimation and classification of fMRI hemodynamic response patterns. Neuroimage 22:804–814 [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. 2008. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 63:577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CWJ. 1969. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 37:424–438 [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Deshpande G, Hu X, Moore AB, Sathian K. 2010. Activation and effective connectivity changes following explicit-memory training for face-name pairs in patients with mild cognitive impairment: a pilot study. Neurorehabil Neural Repair 25:210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Mao L, Gu X, Zhu Y, Ge J, Ma Y. 2008. Neural consequences of religious belief on self-referential processing. Soc Neurosci 3:1–15 [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. 2010. Mentalizing under uncertainty: dissociated neural responses to ambiguous and unambiguous mental state inferences. Cereb Cortex 20:404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Naeser MA, Martin PI, Ho M, Wang Y, Baker E, Pascual-Leone A. 2010. Horizontal portion of arcuate fasciculus fibers track to pars opercularis, not pars triangularis, in right and left hemispheres: a DTI study. Neuroimage 52:436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Barbey AK, Su M, Krueger F, Grafman J. 2009a. Neuroanatomical variability of religiosity. PLoS One 4:e7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Barbey AK, Su M, Zamboni G, Krueger F, Grafman J. 2009b. Cognitive and neural foundations of religious belief. Proc Natl Acad Sci U S A 106:4876–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. 2003. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage 20:1371–1383 [DOI] [PubMed] [Google Scholar]
- Krueger F, Landgraf S, van der Meer E, Deshpande G, Hu X. 2011. Effective connectivity of the multiplication network: a functional MRI and multivariate Granger Causality Mapping study. Hum Brain Mapp 32:1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kus R, Kaminski M, Blinowska KJ. 2004. Determination of EEG activity propagation: pair-wise versus multichannel estimate. IEEE Trans Biomed Eng 51:1501–1510 [DOI] [PubMed] [Google Scholar]
- Lacey S, Flueckiger P, Stilla R, Lava M, Sathian K. 2010a. Object familiarity modulates the relationship between visual object imagery and haptic shape perception. Neuroimage 49:1977–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey S, Hagtvedt H, Patrick VM, Anderson A, Stilla R, Deshpande G, Hu X, Sato JR, Reddy S, Sathian K. 2010b. Art for reward's sake: visual art recruits the ventral striatum. Neuroimage 55:420–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. 2003. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry 53:502–510 [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. 2009. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A 106:20069–20074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Just MA. 2011. Differentiable cortical networks for inferences concerning people's intentions versus physical causality. Hum Brain Mapp 32:313–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletti S, Tassi L, Mai R, Fini N, Tassinari CA, Russo GL. 2006. Emotions induced by intracerebral electrical stimulation of the temporal lobe. Epilepsia 47Suppl 5:47–51 [DOI] [PubMed] [Google Scholar]
- Mier D, Lis S, Neuthe K, Sauer C, Esslinger C, Gallhofer B, Kirsch P. 2010. The involvement of emotion recognition in affective theory of mind. Psychophysiology 47:1028–1039 [DOI] [PubMed] [Google Scholar]
- Mitchell DG. 2011. The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behav Brain Res 217:215–231 [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. 2005. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cereb Cortex 15:986–994 [DOI] [PubMed] [Google Scholar]
- Nahab FB, Kundu P, Gallea C, Kakareka J, Pursley R, Pohida T, Miletta N, Friedman J, Hallett M. 2011. The neural processes underlying self-agency. Cereb Cortex 21:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortigue S, Sinigaglia C, Rizzolatti G, Grafton ST. 2010. Understanding actions of others: the electrodynamics of the left and right hemispheres. A high-density EEG neuroimaging study. PLoS One 5:e12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Mashal N, Faust M, Lavidor M. 2008. The role of the right cerebral hemisphere in processing novel metaphoric expressions: a transcranial magnetic stimulation study. J Cogn Neurosci 20:170–181 [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TE. 2008. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci 11:426–428 [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. 2005. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage 25:230–242 [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Wilson MR, Schauer GF, Allison S, Gorno-Tempini ML, Pace-Savitsky C, Kramer JH, Levenson RW, Weiner M, Miller BL. 2006. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia 44:365–373 [DOI] [PubMed] [Google Scholar]
- Sato JR, Amaro E, Takahashi DY, Felix MD, Brammer MJ, Morettin PA. 2006. A method to produce evolving functional connectivity maps during the course of an fMRI experiment using wavelet-based time-varying Granger causality. Neuroimage 31:187–196 [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 105:12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeis N, Koelsch S. 2009. Understanding the intentions behind man-made products elicits neural activity in areas dedicated to mental state attribution. Cereb Cortex 19:619–623 [DOI] [PubMed] [Google Scholar]
- Stilla R, Deshpande G, LaConte S, Hu XP, Sathian K. 2007. Posteromedial parietal cortical activity and inputs predict tactile spatial acuity. J Neurosci 27:11091–11102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilla R, Hanna R, Hu XP, Mariola E, Deshpande G, Sathian K. 2008. Neural processing underlying tactile microspatial discrimination in the blind: A functional magnetic resonance imaging study. J Vis 8:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenziok M, Krueger F, Deshpande G, Lenroot RK, van der Meer E, Grafman J. 2011. Fronto-parietal regulation of media violence exposure in adolescents: a multi-method study. Soc Cogn Affect Neurosci 6:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. 2007. Neural substrates of envisioning the future. Proc Natl Acad Sci U S A 104:642–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Zhou X. 2009. Conflict control during sentence comprehension: fMRI evidence. Neuroimage 48:280–290 [DOI] [PubMed] [Google Scholar]