Abstract
Reactive oxygen species (ROS) are key signaling molecules that play an important role in the progression of inflammatory disorders. An enhanced ROS generation by polymorphonuclear neutrophils (PMNs) at the site of inflammation causes endothelial dysfunction and tissue injury. The vascular endothelium plays an important role in passage of macromolecules and inflammatory cells from the blood to tissue. Under the inflammatory conditions, oxidative stress produced by PMNs leads to the opening of inter-endothelial junctions and promotes the migration of inflammatory cells across the endothelial barrier. The migrated inflammatory cells not only help in the clearance of pathogens and foreign particles but also lead to tissue injury. The current review compiles the past and current research in the area of inflammation with particular emphasis on oxidative stress-mediated signaling mechanisms that are involved in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167.
I. Introduction
Inflammation is a defensive immune response that is conferred by the host against foreign pathogens. The immune system of vertebrates has evolved this survival strategy property to facilitate tissue repair. There are two complementary immune systems in vertebrates that recognize and eliminate pathogens: innate immune system and adaptive immune system (65). The innate immune system on encountering pathogens elicits the acute inflammatory response that is accompanied by systemic vasodilation, vascular leakage, and leukocyte emigration (65). While this is considered advantageous to the organism, it can if unchecked also lead to inflammation and disease. The four cardinal signs of localized acute inflammation described almost 2000 years ago by the Roman physician Celsus are as follows: Calor heat, Rubor redness, tumor swelling, and Dolor pain, leading to Functiolaesa loss (or impairment) of function. The innate immune system recognizes a wide range of pathogens such as viruses, bacteria, and fungi, by germline-encoded receptors known as pattern-recognition receptors (PRRs) (409). The family of PRRs includes both membrane-bound receptors such as Toll like receptors (TLRs) and c-type lectin receptors, as well as cytoplasmic nod-like receptors (NLR). These receptors recognize conserved domains known as pathogen-associated molecular patterns, including flagellin, sugars, and the cell wall components of various microbes such as peptidoglycan and lipopolysaccharide (LPS) as well as danger-associated molecular patterns that are released by injured cells such as mammalian dsDNA and uric acid crystals. PRRs are expressed by a variety of immune cells, including macrophages, monocytes, dendritic cells (DCs), and neutrophils, which enables early detection of pathogens (409).
Within a short period of activation of the innate immune system, the acute inflammatory response is started by immune cells enabling secretion of various cytokines and chemokines in order to recruit immune cells to the site of infection. Neutrophils are the first to adhere to endothelial cells, and they begin to migrate across the vascular wall at the site of infection to engulf the invading pathogens and also secrete vasoactive and pro-inflammatory mediators (222). Most of the early vascular changes observed in acute inflammation are due to inflammatory mediators that are released by inflammatory cells at the site of injury (65). These mediators, including histamine, platelet-activating factors (PAFs), bradykinin, and thrombin, increases vascular permeability followed by fluid accumulation (edema) and leukocyte extravasation. Acute inflammation can be caused by bacterial or viral infection (e.g., as in acute respiratory distress syndrome (ARDS), tissue necrosis (e.g., as in acute myocardial infarction), trauma, radiation, burns, or by any foreign body present in tissue. However, if the innate immune system exceeds its capacity or its defensive function becomes limited, it engages the adaptive immune system, activating specific T and B cells for pathogen clearance (65). If this process is prolonged or inefficient, it progresses to the chronic state of inflammation that is associated with many diseases such as of the heart and rheumatoid arthritis. Chronic inflammation is also associated with persistent bacterial infections such as tuberculosis, ARDS, autoimmune diseases, inflammatory bowel disease, atherosclerosis, and neurodegenerative and metabolic hormonal disorders.
Production of reactive oxygen species (ROS) is central to the progression of many inflammatory diseases. The ROS are produced by cells that are involved in the host-defense response, such as polymorphonuclear neutrophils (PMNs) and promote endothelial dysfunction by oxidation of crucial cellular signaling proteins such as tyrosine phosphatases. The ROS act as both a signaling molecule and a mediator of inflammation. The ROS such as superoxide can rapidly combine with NO at a diffusion limited rate (k=5 to 10×109 M−1s−1) to form reactive nitrogen species (RNS), such as peroxynitrite, and is three to four times faster than the dismutation of superoxide by the superoxide dismutase (SOD) (33). The RNS, in turn, induces nitrosative stress, which adds to the pro-inflammatory burden of ROS. While attempting to be inclusive, this review cannot deal with the role of RNS, and, thus, the reader is advised to refer to the excellent reviews written in this area (401). The major focus of this review is on the ROS-dependent mechanisms of inflammation with a bias toward endothelial mechanisms, and our view that the endothelial cell for good or ill is a dominant orchestrator of inflammation.
II. Sources of ROS and Their Regulation in Inflammation
ROS are classically defined as partially reduced metabolites of oxygen that possess strong oxidizing capabilities. They are deleterious to cells at high concentrations but at low concentrations (exact concentrations still remaining to be defined), they serve complex signaling functions. They are injurious, because they oxidize protein and lipid cellular constituents and damage the DNA. At “physiological concentrations,” ROS function as signaling molecules that regulate cell growth, the adhesion of cells toward other cells, differentiation, senescence, and apoptosis (102, 413). The concept of chronic or prolonged ROS production is considered central to the progression of inflammatory disease (155). What are the biologically relevant ROS? The widely studied and understood family members are the superoxide anion (O2•−), hydroxyl radical (OH•), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl) (413). Although others may be important in signaling and disease (155, 413), their functions remain poorly understood. ROS are generated as byproducts of cellular metabolism through the electron transport chain (ETC) in mitochondria as well as via the cytochrome P450. The other major source, where ROS are not produced as by products, are the NADPH oxidases that are present in a variety of cells, especially the professional phagocytes and endothelial cells (339), which are central to the genesis of the inflammatory response (155).
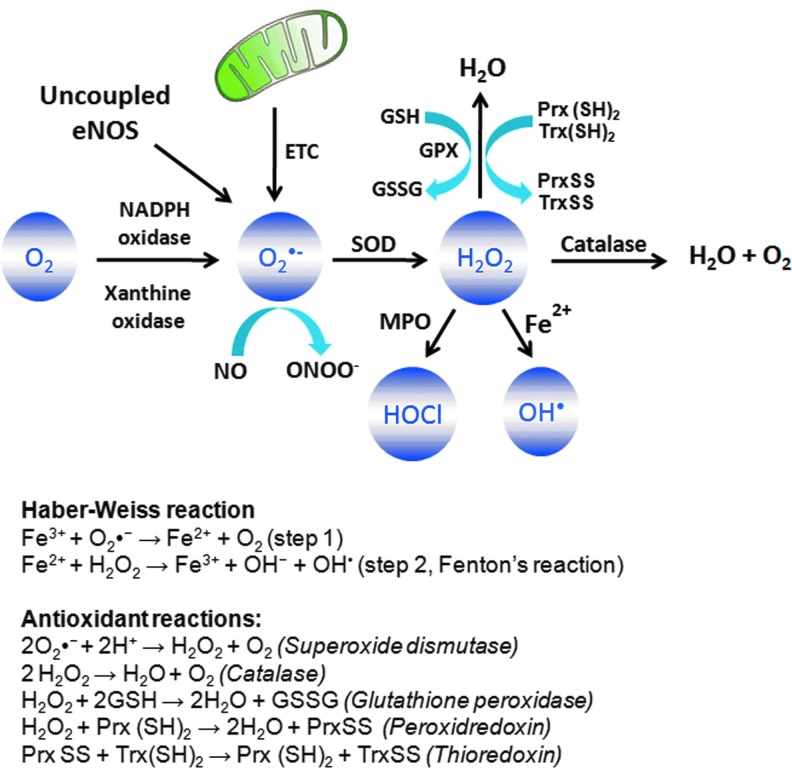
O2•− is generated by one-electron reduction of O2 through enzymatic catalysis by NADPH oxidase or xanthine oxidase (XO) or during electron transfer reactions in the ETC of mitochondria (Fig. 1) (163, 233, 413). O2•− has a half life of 106 ns (374), as it undergoes spontaneous dismutation to H2O2 (under physiological conditions k=2×105 M−1s−1). This reaction can be accelerated to 104-fold by the enzyme SOD (K=1.6×109 M−1s−1) (233, 413). In the presence of the transition metal iron, O2•− and H2O2, in turn, generate the highly reactive OH− and OH• (Fig. 1) (Haber–Weiss reaction). In the first step of this reaction, O2•− reacts with Fe3+ to form Fe2+ and O2. However, this reaction is thermodynamically unfavorable under the physiological conditions (209). The second step of this reaction is also known as Fenton's reaction and occurs under the biological conditions in which Fe2+ reacts with H2O2 to form both OH• and OH− .OH• is defined as the most potent oxidizing species of biological membrane proteins and lipids and has an extremely short half life (150, 374, 413). At inflammatory sites where PMN are abundant, H2O2 and chloride generate HOCl by the enzyme myeloperoxidase, generally considered as being a PMN-specific enzyme (Fig. 1) (161). The passage of O2•− across biological membranes is highly restricted because of its negative charge. However, certain transmembrane proteins, such as voltage-dependent anion channels (VDAC) found in mitochondria, allow trans-membrane passage of O2•− produced in ETC (162). H2O2, on the other hand, can cross biological membranes through aquaporin channels such as AQP3 and AQP8 which mediate membrane H2O2 uptake, raising the possibility that it can enter cells that are contacting one another (37, 165, 290).
FIG. 1.
Sources of reactive oxygen species (ROS) and antioxidant defense system. The major ROS include superoxide (O2•−), hydrogen peroxide (H2O2), hydroxyl anions (OH−), hydroxyl radicals (OH•), and hypochlorous acid (HOCl). Superoxide is produced by NADPH oxidase/xanthine oxidase-derived reduction of molecular oxygen, uncoupled endothelial nitric oxide synthase (eNOS), or mitochondrial electron transport chain (ETC). Superoxide is rapidly dismutated to H2O2 by superoxide dismutase (SOD). However, in the presence of nitric oxide (NO), O2•− rapidly reacts with NO, resulting in the formation of highly reactive peroxynitrite (ONOO−), which is three to four times faster than dismutation of O2•− to H2O2. H2O2 can change to highly reactive HOCl at the inflammatory sites by an enzyme known as myeloperoxidase (MPO), which is abundantly expressed in neutrophils. H2O2 can also change to the highly toxic OH• in presence of Fe2+ by Fenton's reaction. H2O2 is scavenged to H2O and O2 by catalase, glutathione peroxidase (GPX), or peroxiredoxins (Prx) antioxidant enzymes. Prx uses thioredoxin (Trx) to detoxify H2O2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The role of oxidants in inducing inflammation has been vigorously investigated in all manner of experimental models. The consensus is they are fundamentally involved, but how they contribute to the response and whether antioxidant therapy is a valid means of arresting inflammation in patients remains largely unresolved. Among the commonly used inflammatory mediators used to simulate inflammation are included cytokines (e.g., TNF-α), the stress of hyperoxia, ischemia-reperfusion injury, bacterial toxins (LPS), and mediators that ligate cell surface receptors (PAF, thrombin, histamine, VEGF, and bradykinins). These and other mediators except LPS induce only a subset of changes that are associated with full-blown inflammation.
A. NADPH oxidase-derived ROS in inflammation
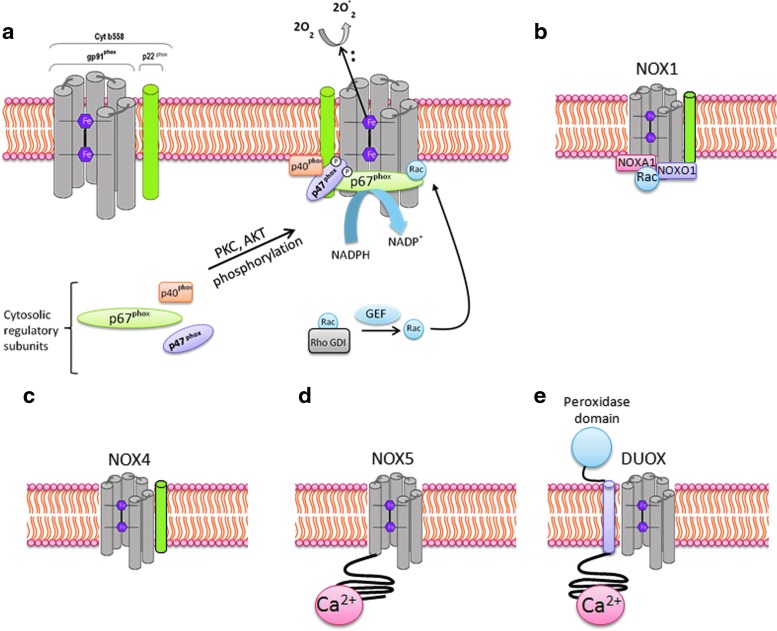
NADPH oxidases were first identified in phagocytes for their role in inducing respiratory burst and bacterial killing (19, 376). So far, there are seven described homologs of NADPH oxidase (NOX1–NOX5 and Duox1 and 2) (Fig. 2). NADPH oxidase homologs differ in their structure, expression levels in different tissue, and their activation mechanisms (227, 233). The catalytic core of classical phagocytic NADPH oxidase 2 (NOX2) consists of two membrane-bound subunits, gp91phox and p22phox, which form the flavocytochrome b558 complex. The gp91phox subunit consists of six transmembrane domains, and its C-terminal region contains the binding sites for flavin-adenine dinucleotide (FAD) and electron donor NADPH. All NADPH oxidases, with the exception of NOX5 and Duox1 and 2, share a similar topological structure of the catalytic core of gp91phox. NOX5 carries an additional intracellular N-terminal calcium-binding domain. However, Duox1 and 2, in addition to the catalytic NOX5 structure, carry another N-terminus transmembrane α-helix, which possesses a peroxidase homology domain (Fig. 2) (233). NOX1, NOX2, and NOX4 are the major isoforms of NADPH oxidase that are expressed in the vascular system, and each is strongly implicated in inflammation-induced vascular injury (340).
FIG. 2.
Activation mechanism and assembly of different NADPH oxidase homologs. (a) Two independent events are required for the activation of gp91phox, resulting in the assembly of the cytosolic regulatory proteins (p40phox, p47phox, and p67phox) with the flavocytochrome b558 (made up of the membrane-associated catalytic subunit gp91phox and p22phox). One of the two events is the activation of protein kinases such as protein kinase C (PKC) and AKT, which phosphorylate the autoinhibitory region (AIR) of p47phox, thus relieving its inhibition from the autoinhibitory loop and enabling p47phox to bind with p22phox. The second event results in the replacement of GDP residue with GTP by a guanine nucleotide exchange factor (GEF), resulting in a conformational change of Rac protein by relieving inhibition from Rho GDP-dissociation inhibitor (RhoGDI), promoting its binding with p67phox, and finally, resulting in the formation of active complex. The pink hexagon represents heme. (b–e) differential assembly of NADPH oxides homologs. NOX1, NOX3 (not shown), and NOX4 share a similar topological structure of the catalytic core of gp91phox. NOX5 carries an additional intracellular N-terminal calcium-binding domain, whereas Duox1 and 2 are built on NOX5 structure that carries another additional N-terminus transmembrane α-helix which possesses a peroxidase homology domain. ROS generation by NOX1 is induced by assembly with cytosolic subunits NOXO1, NOXA1, and Rac. NOX4 does not require cytosolic subunits for ROS generation but requires p22phox. ROS generation by NOX5 and DUOX can be induced by calcium and is independent of p22phox subunit. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
O2•− production by gp91phox (current designation NOX2) in leukocytes is essential for killing engulfed microbes within phagolysosomes, the amalgam of phagosomes and lysosomes (155). There have been at least 410 mutations identified in different constituents of the oxidase complex, gp91phox, p22phox, p67phox, p47phox, or Rac2. These mutations produce the genetic disorder chronic granulomatous disease, an immunodeficiency that is characterized by greatly increased susceptibility to bacterial and fungal infections due to defective oxidase assembly and production of O2−• (177). O2−•generated by NOX2 is induced by the translocation of cytosolic subunits p47phox, p40phox, p67phox, and Rac1 to the plasma membrane and the formation of a complex with cytochrome b558 (Fig. 2a). The assembly of cytosolic subunits with membrane-bound cyt b558 complex results in the transfer of electrons from cellular NADPH to molecular oxygen and the formation of O2− •. Once activated, neutrophils produce ∼10 nmol/min O2−• per million neutrophils during the oxidative burst (19). The phosphorylation of p47phox and the activation of Rac1 plays an essential role in the assembly of the oxidase complex. Pro-inflammatory cytokines (TNF-α, GM-CSF, and G-CSF), LPS, phorbol-12-myristate-13-acetate (PMA), and N-formylmethionyl leucyl phenylalanine (fMLP) are well-known kinase inducers of p47phox phosphorylation (81, 83, 88, 93, 241). p47phox is phosphorylated at multiple serine residues at C-terminus and is targeted by multiple pathways, such as protein kinase C-δ, -β and -ζ, protein kinase A, Akt, ERK1/2, and p38 mitogen-activated protein kinase (62, 82, 83, 87, 94, 106). The phosphorylation of p47phox relieves it from auto-inhibitory conformation, which subsequently leads to unmasking of N-terminal SH3 domain and enabling binding to the proline-rich target in the C-terminus of p22phox. Phosphorylation of p47phox thereby promotes the recruitment of p67phox by serving as an adapter and bridging p67phox with the cyt b558 complex (80).
p47phox has been documented to be important in the progression of atherosclerosis and pulmonary fibrosis (26, 268). p47phox null mice were protected against formation of atherosclerotic lesions and development of pulmonary fibrosis (26, 268). Mice deficient in p47phox subunit or NOX2 were also protected against TNF-α-induced lung inflammation or sepsis-induced lung microvascular injury (126 469). However, there are also contradictory findings. Zhang et al. (468) reported no difference in LPS-induced acute inflammatory responses in NOX2−/− and p47phox−/− mice compared with the control mice. On the contrary, they observed enhanced gene expression of inflammatory mediators and increased neutrophil recruitment to the lung and heart, resulting in impaired resolution. This discrepancy in the results was attributed to LPS-mediated ROS generation via NADPH oxidases, which also instead contributed to the resolution of inflammatory response (468). The NOX2 expressed in immune cells such as T cells has also been implicated in angiotensin II (Ang II)-induced hypertension (158). Guzik et al. have shown on the basis of adoptive transfer experiments that T cells lacking the AT1-receptor or p47phox subunit resulted in decreased aortic O2•− production and blunted Ang II-dependent hypertension (158). Moreover, Ang II infusion induced the expression of NOX2, p47phox, and p22phox in the T cells and stimulated superoxide production (158). In a similar study, Wenzel et al. have shown that Ang II infusion stimulated the accumulation of both macrophages and neutrophils in the mouse aorta (450). The depletion of myelomonocytic cells by inducible diphtheria toxin receptor (LysMiDTR mice) attenuated Ang II-induced blood pressure increase and reduced vascular O2•− formation (450). Moreover, adoptive transfer of wild-type monocytes into depleted LysMiDTR mice re-established the Ang II-induced oxidative stress, and arterial hypertension, whereas adoptive transfer of neutrophils or monocytes lacking NOX2 did not (129). The NOX2 activity has also been implicated in cardiac inflammation and fibrosis. The mice deficient in NOX2 were protected against cardiac remodeling and contractile dysfunction induced by coronary artery ligation (252), aortic banding (153), Ang II infusion (36), or Doxorubicin treatment (471) compared with the wild-type mice. Moreover, in aortic-banding model, the treatment of wild-type mice with N-acetylcysteine resulted in recovery of contractile dysfunction (153). Altogether, these findings highlight the important role of NOX2-derived ROS in cardiac remodeling, which could be prevented by antioxidant treatment.
Rac1 is another important cytosolic subunit that is required for activation of NOX2. Rac1 activation is accompanied by the replacement of GDP residue with GTP by guanine nucleotide exchange factor (GEF), resulting in conformational change of Rac protein by relieving inhibition from Rho GDP-dissociation inhibitor. Rac1 activation promotes binding of p67phox with cyt b558 complex (79). Rac1 activation is induced by a variety of inflammatory stimuli such as TNF-α (453), interleukin-1β (IL-1β), thrombin, VEGF (299), histamine (321), Ang II (384), ischemia-reperfusion injury (216), and shear stress (463). The expression of the active Rac1 mutant V12Rac1 resulted in enhanced ROS production and loss of endothelial barrier integrity (427). Rac1 activation has also been demonstrated in trafficking of inflammatory cells across the endothelial barrier (428). Cross-linking of vascular cell adhesion molecule-1 (VCAM-1) on endothelial cell surface induced Rac1 activation and ROS generation, and resulted in loss of endothelial cell–cell adhesion and transendothelial leukocyte migration (428).
In contrast to NOX2, NOX1 is activated by homologues of p47phox and p67phox, known as NOXO1 (NOX organizer 1) and NOXA1 proteins (NOX activator 1), respectively (23). NOXO1 does not contain an autoinhibitory region and is constitutively active in the cells (410). Co-expression of NOXO1 and NOXA1 with NOX1 in HEK 293 cells was sufficient to generate ROS independent of any stimulus (23, 410). High expression of NOX1 was observed in colon epithelium, and LPS induced the expression of NOX1 and NOXO1 in gastric mucosal cells (208, 410). NOX1 activation was dependent on Rac1 activation, and Rac inhibitor LY-294002 (PI3-K inhibitor) blocked O2−• generation (208). Mice deficient in NOX1 were protected against hyperoxia-induced acute lung injury (54). Surprisingly, NOX2−/− mice were not protected against hyperoxia-induced lung injury in the same study (54), suggesting that hyperoxia may induce lung injury secondary to NOX1 activation, or NOX1 may compensate for the loss of NOX2. In addition, NOX1-deficient mice were less susceptible to Ang II-induced aortic dissection and aneurysm formation (131). NOX1 was also important in angiogenesis and tumor formation. Mice deficient in NOX1 exhibited impaired angiogenesis and tumor growth (129). Activity of NOX1 was increased in endothelial cells on angiogenic stimulation. However, angiogenesis remained unaffected in NOX2 or NOX4 knockout mice in this study.
NOX4 was first characterized in the kidney as an “oxygen sensor” regulating oxygen-dependent expression of erythropoietin and development of inflammatory processes in the kidney (132). NOX4 has also been termed renal NADPH oxidase (Renox). Activity of NOX4 is largely controlled by expression levels of NOX4 and is independent of the Rac activation or the presence of p47phox/p67phox or NOXO1/NOXA1 proteins (12, 132, 275). However, NOX4 activity required the presence of p22phox (275). The type of ROS generated by NOX4-expressing cells is primarily H2O2, whereas O2•− was almost undetectable in these cells (383). Recently, another mechanism of NOX4 activation has been reported. Lyle et al. have shown that polymerase (DNA-directed) delta-interacting protein 2 (Poldip2) associates with p22phox to activate NOX4, which regulates cytoskeletal reorganization and cellular migration in an Rho A-dependent manner (257). NOX4 is the predominant homolog expressed in human lung microvascular and human pulmonary arterial endothelial cells compared with NOX1, NOX2, NOX3, or NOX5 (338). Inflammatory stimuli LPS, TNF α, hyperoxia, TGF-β, and hypoxia were demonstrated to enhance ROS generation via NOX4 (29, 191, 242, 295, 335, 338). The expression of intercellular adhesion molecule-1 (ICAM-1), IL-8, and MCP-1 in endothelial cells in response to LPS was demonstrated to be dependent on NOX4 activation (335). Treatment of endothelial cells with NOX4 siRNA decreased LPS induced migration and adhesion of monocytes by 36% and 52%, respectively (335). Increased NOX4 activity is also a reported risk factor in the progression of type-2 diabetic nephropathy, stroke, pulmonary fibrosis, and atherosclerotic lesions (11, 145, 219, 240, 331, 382). However, the contradictory reports in the literature suggest that NOX4 is a vascular protective enzyme rather than a destructive one (415). NOX4 overexpression in the endothelium was found to enhance vasodilation and reduced basal blood pressure, which was related to increased H2O2 production and reduced NO inactivation (415). Moreover, in tamoxifen-inducible NOX4 knockout mice, Ang II-mediated aortic inflammation, vascular remodeling, and endothelial dysfunction were exaggerated, which correlated to a decrease in endothelial nitric oxide synthase (eNOS) expression (415). In addition, NOX4 overexpression was found to increase eNOS protein and promote recovery of ischemic tissue by enhanced angiogenesis and aortic sprouting (415). It is not easy to reconcile all these contradictory reports, and the part of the reason behind these discrepancies may be related to the tissue-specific abundance of different NADPH oxidase homologs and differential regulation of NOX4 in different models.
The expression of NOX5 has been reported in human vascular endothelial cells and smooth muscle cells. NOX5 is not expressed in rodents. Four variants of NOX5 have been identified so far, including NOX5α, NOX5β, NOX5γ, and NOX5δ (34). ROS generation by NOX5 is induced by calcium binding on its N-terminal domain, which has four Ca2+-binding sites (referred to as EF hands). The binding of Ca2+ to NOX5 induced conformational change that enhances ROS generation by NOX5. Unlike other NOX homologs, O2•− production by NOX5 does not require p22phox. NOX5 has been reported to be induced by thrombin (34), Ang II (300), and platelet-derived growth factor (197). An increased expression level of NOX5 has been implicated in the coronary artery disease patients, which correlates with the oxidative damage observed in atherosclerosis (157). NOX5 has also been reported to be essential for PDGF-stimulated proliferation of vascular smooth muscle cells and proliferation of endothelial cells and angiogenesis (34, 197). Thus, while NOX5 may be important, it is still relatively understudied.
B. Mitochondrial-derived ROS in inflammation
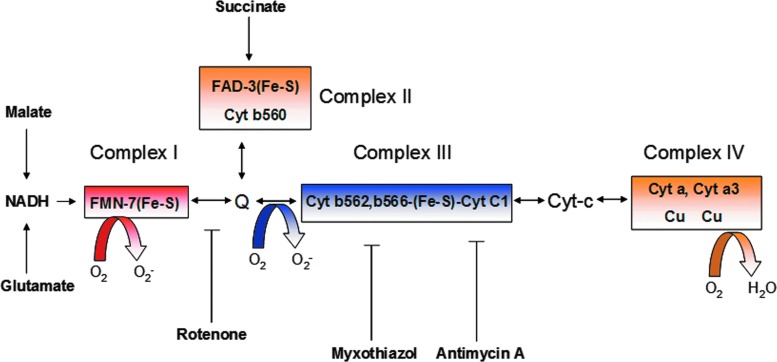
Mitochondria generate high-energy phosphate bonds of ATP by electrochemical proton gradient created by the transfer of electrons through a series of electron carriers embedded in the mitochondrial membrane. There are four electron transport carriers that are spatially organized in order of their increasing redox potential, complex I (NADH-ubiquinone oxidoreductase), complex II (succinate-ubiquinone oxidoreductase), complex III (ubiquinol-cytochrome c reductase), and complex IV (cytochrome c oxidase) (Fig. 3) (163). Transfer of electrons to molecular O2 is a tightly controlled process, and only 1%–2% of electrons that leaked out in this process react with O2, resulting in O2•− (163). The main sites of O2•− production in the ETC are complex I and III, with complex I being more predominant in skeletal muscle and neural cells; whereas complex III is more predominant for endothelial cells (Fig. 3) (323, 416). O2•− generated by mitochondria reacts with manganese SOD (MnSOD) in the mitochondrial matrix to generate H2O2, which can cross the mitochondrial outer membrane to access cytosolic targets. This can lead to multiple functional outcomes such as activation of redox-sensitive transcription factors (such as HIF-1α and NF-κB) (60, 269, 435), activation of pro-inflammatory cytokines, and activation of inflammasomes (185, 308).
FIG. 3.
Schematic presentation of mitochondrial ETC and site of ROS generation. The Mitochondrial ETC consists of a series of electron carriers that are arranged spatially in the order of their increasing redox potential and organized into four complexes. Arrows in the region of complexes I-IV show pathways of electron transfer between flavins (FMN-H2, FADH2), iron-sulfur centers (Fe-S), coenzyme Q (Q-QH2), cytochromes (c1, c a, and a3), and molecular oxygen (O2), resulting in the formation of H2O. The main sites of ROS generation are complex I and III. Pharmacological inhibitors of complex I (such as rotenone) and complex III (myxothiazol and antimycin A) are known to enhance mitochondrial ROS generation because of uncoupling of ETC. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The genetic mutations in mitochondrial respiratory chain can result in a variety of neurological disorders, including Leigh's syndrome, leukodystrophy, paragangliomas, and pheochromocytomas (97). Mitochondrial-derived oxidative stress has also been implicated in chronic inflammation, cancer progression (206), diabetes mellitus (148, 152, 317–319, 378), and atherosclerosis (21, 258). Mitochondrial-derived ROS (MtROS) also contributes to LPS-mediated production of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α (48). Notably, MtROS have been implicated in ectodomain shedding of cytokine receptor TNF receptor–1 (TNFR1) in endothelial cells, which is important for regulation of inflammatory progression (364). TNF-α-converting enzyme, which mediates cleavage of TNFR1, is activated by ROS (440). Ectodomain shedding of cytokine receptors facilitates diffusion of soluble receptors into the extracellular space, which dampens the inflammatory response by binding and neutralizing the cytokine ligands. Missense mutations in the extracellular domain of the gene encoding TNFR1 lead to inheritable autosomal dominant disorder known as tumor necrosis factor receptor-associated periodic syndrome (TRAPS) (48). TRAPS is an auto-inflammatory disease that is associated with recurrent fevers, peritonitis, migratory rash, myalgia, and arthralgia. Monocytes and neutrophils isolated from TRAPS patients showed elevated baseline levels of mitochondrial ROS compared with healthy donors and also had constitutive and prolonged activation of JNK and p38 MAPK on LPS challenge (48). Consequently, these cells showed enhanced production of cytokines IL-6 and TNF-α on LPS challenge. Inhibition of mitochondrial ROS with MitoQ suppressed p38MAPK activation and production of IL-6 and TNF-α (48). Although the basis by which TNFR1 mutations leads to increased mitochondrial ROS is not clear, possible mechanisms include retention of the mutated protein in the endoplasmic reticulum, which can induce unfolded protein response, triggering calcium release from ER and depolarizing mitochondria by disruption of the mitochondrial ETC (467).
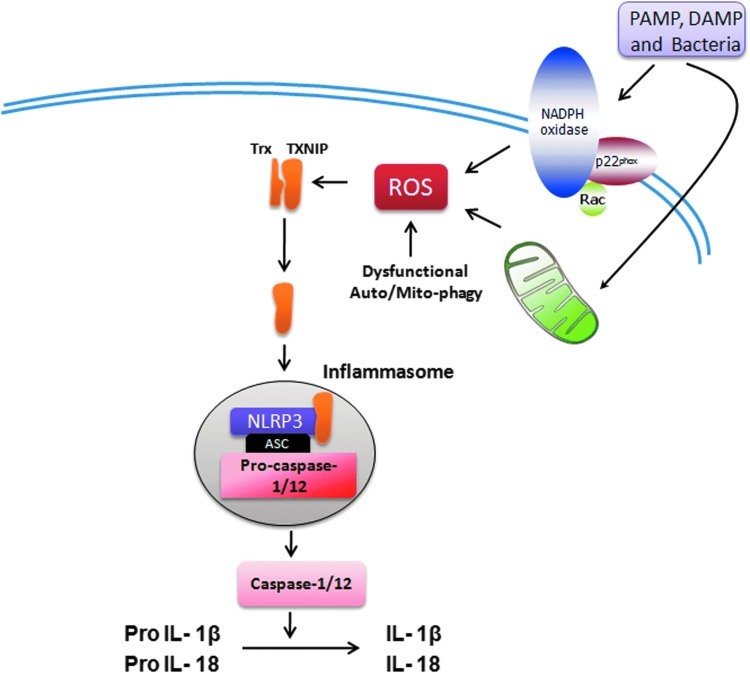
Another important role of MtROS has been recognized in the regulation of inflammasome, the high-molecular-weight complexes that activate inflammatory caspases (caspase-1 and -12) and cytokines (IL-1β and IL-18) in macrophages (274). Three different prototypes of inflammasomes have been recognized: NALP1, NALP3 (also known as NLRP3), and IPAF. NLRP3 inflammasome, the most well-characterized form, is redox sensitive (99, 379). The key components of NALP3 are NLRP3, apoptosis-associated speck-like protein (ASC), and caspase-1. NLRP3 is a cytoplasmic receptor that interacts with ASC and recruits procaspase-1 (379). The NLRP3 inflammasome has been shown to interact with redox-sensitive protein thioredoxin (Trx)-binding protein-2 (TBP-2, also known as vitamin D3 up-regulated protein 1 [VDUP1]). Increased intracellular ROS generation mediates dissociation of TBP-2 from Trx, enabling association with NLRP3 inflammasome and resulting in its activation (Fig. 4) (473). Mice deficient in NLRP3 or ASC on LPS treatment have reduced serum levels of IL-1β and IL-18 and are resistant to LPS-induced lethality (403). ASC-deficient mice were also protected against LPS challenge (403). On activation by ROS, NLRP3 recruited ASC and procaspase-1, which mediated proteolytic cleavage, leading to activation of caspase-1 and IL-1β and IL-18 (473). The MtROS is important in the activation of NLRP3 inflammasome. Inhibition of mitochondrial function by depleting VDAC with shRNA impaired the activation of NLRP3 and cleavage of IL-1β and caspase-1 (474). In addition to direct activation by ROS, the activity of NLRP3 inflammasome is negatively regulated by autophagy/mitophagy (310, 367). Autophagy/mitophagy indirectly regulates intracellular oxidative stress by clearance of dysfunctional mitochondria and damaged proteins (170). Macrophages isolated from mice deficient in the autophagy proteins LC3B and ATG16L1 have enhanced NLRP3 inflammasome activation and produced higher IL-1β and IL-18 on LPS challenge (310, 367). MtROS has also been implicated in increasing oxidative stress through cross-talk with nitric oxide synthases (NOSs) (293). Arginase II, which activates NOS, was shown to promote the macrophage inflammatory response that contributes to insulin resistance and atherogenesis (293).
FIG. 4.
Inflammasome activation by ROS. Increased ROS production inside the cell either through NADPH oxidase or mitochondrial ETC is sensed by a complex of Trx and thioredoxin interacting protein (TXNIP), which dissociates and enables the binding of TXINP with NLRP3. This is followed by activation of NLRP3 and recruitment of Asc and Pro-caspase1/12 proteins, leading to formation of active inflammasome. Active NLRP3 inflammasome cleaves pro-interleukin-1 (IL-1) beta and pro-IL-18 to active IL1 beta and IL-18, which are subsequently secreted by the inflammatory cells. PAMP, pathogen-associated molecular patterns; DAMP, danger-associated molecular patterns; ASC, apoptosis-associated speck-like protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Recent reports have highlighted an interesting concept of cross-talk between MtROS and NADPH oxidase, which can drive both feed-forward and feedback regulations of NADPH oxidases (73, 96). Ang II-stimulated mitochondrial H2O2 was blocked by NADPH oxidase inhibitor apocynin and protein kinase C inhibitor chelerythrine (96). Moreover, depletion of p22phox with siRNA also inhibited Ang II-mediated MtROS production (100). In contrast, ROS produced by the mitochondria on serum withdrawal has been shown to trigger NOX1 activation (96). Moreover, Ang II-mediated mitochondrial dysfunction has been shown to increase the expression and activity of NOX1 and NOX4 in vascular smooth muscle cells (96). Block et al. have reported the existence of NOX4 in the mitochondria of rat kidney cortex that was induced in rat model of diabetes (42). In an independent study, Ago et al. have similarly reported the localization of NOX4 in cardiac mitochondria with F1F0-ATP synthase as well as the p22phox subunit of NADPH oxidases (3). However, no such localization of NOX4 or any other NADPH oxidase was observed in mitochondria in other reports (100), whereas Gorlach group localized NOX4 to endoplasmic reticulum in endothelial cells and was shown to be essential for endothelial ROS production and proliferation (346). Thus, further investigations are needed to identify the localization of NOX4 and the potential cross-talk between mitochondria and NADPH oxidase. A dysregulation in this relationship may drive the vicious feed-forward cycle of ROS accumulation that can enhance inflammatory response in different diseases (96, 380).
C. Uncoupled NOS-derived ROS in inflammation
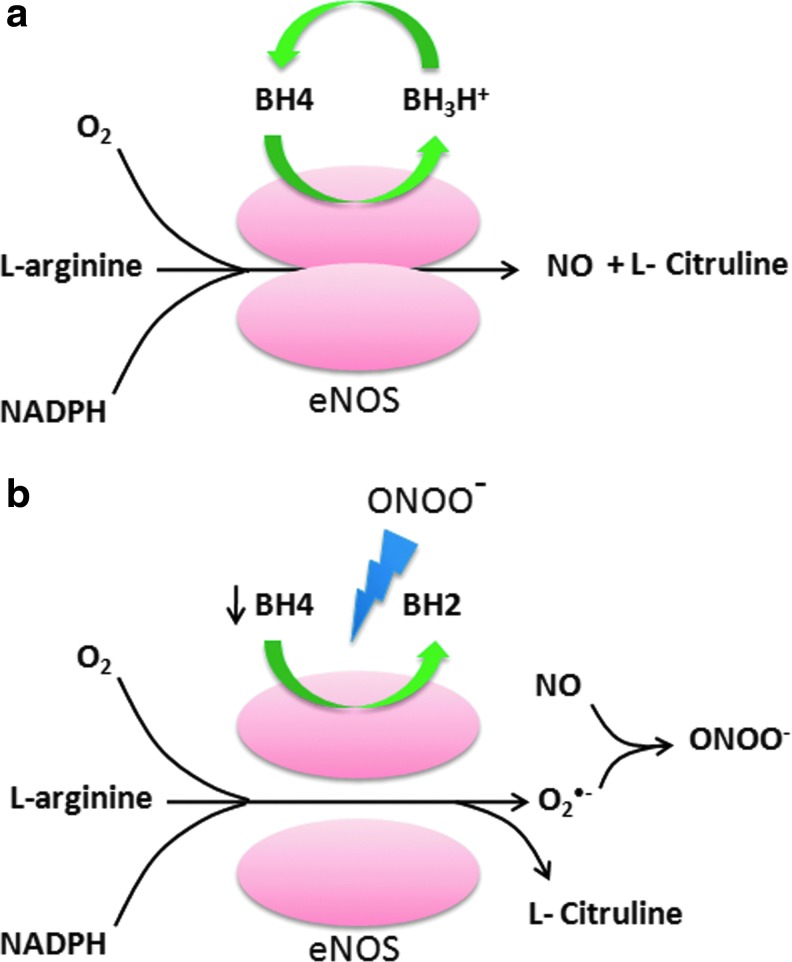
NOSs are a family of enzymes that catalyze the production of nitric oxide (NO) from L-arginine. There are three different isoforms of NOS, including neuronal NOS (nNOS), inducible NOS (iNOS), and eNOS. Of these isoforms, only eNOS is the membrane-associated protein and is the predominant source of NO in vascular endothelial cells (121). iNOS and nNOS are soluble isoforms and are present in the cytosol. eNOS exists as a dimer consisting of a c-terminus reductase domain of one monomer connected with the oxygenase domain of the other monomer at N-terminus (Fig. 5a). The reductase domain binds to NADPH, FMN, and FAD, and the oxygenase domain binds to prosthetic heme group as well as the cofactor tetrahydrobiopterin (BH4) and molecular oxygen. The prosthetic heme group connects the two monomers. The electrons are transferred from the bound NADPH in the reductase domain to the heme prosthetic group on oxygenase domain of eNOS. NO is produced by eNOS in two successive mono-oxygenation reactions of arginine, leading to formation of L-citruline (Fig. 5a) (121). The electron transfer in eNOS is a tightly regulated process; however, eNOS uncoupling may lead to transfer of electrons to molecular oxygen rather than arginine, resulting in O2•− production (121). The superoxide generated by eNOS uncoupling has been implicated in a variety of inflammatory conditions, including acute lung injury (385), diabetes mellitus (178), and Ang II-induced hypertension (298). NO possesses strong anti-inflammatory properties. Dal et al. have shown that iNOS−/− mice exhibit enhanced neutrophil migration compared with the wild-type mice on LPS challenge (75). Moreover, the mice that have been treated with NOS inhibitors such as NG-nitro-l-arginine, or with a soluble guanylate cyclase inhibitor, ODQ exhibited enhanced LPS-induced neutrophil migration that was accompanied by enhanced expression of ICAM-1 on endothelial cells (74). The anti-inflammatory effects of NO are mediated by suppressing LPS-induced increase in ICAM-1 expression and decreasing the rolling and adhesion of the neutrophils on the endothelium (74).
FIG. 5.
Schematic presentation of ROS generation by uncoupling of eNOS. (a) eNOS is a homodimeric enzyme consisting of a reductase and an oxygenase domain. The dimer interface of eNOS carries the binding sites for the cofactor tetrahydrobiopterin (BH4) and the substrate L-arginine. eNOS carries out two successive monoxygenation of L-arginine, resulting in formation of L-citruline and NO as a by product. In this reaction, BH4 is oxidized to form trihydropterin radicals protonated at N5 (BH3H+), which are recycled to BH4 by endothelial NO synthase itself (using an electron supplied by the flavins). (b) Increased levels of peroxynitrite as a result of oxidative stress leads to oxidation of BH4 to biologically inactive products BH2, which cannot be recycled back by cellular machinery. This creates an “uncoupled” enzymatic state that reduces oxygen to superoxide and no longer synthesizes NO. ONOO−, Peroxynitrite; O2, molecular oxygen; O2•−, superoxide anion. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The cofactor BH4 plays a crucial role in maintaining the integrity of the eNOS system by stabilizing eNOS dimers (10). BH4 donates the second electron to the first mono-oxygenation reaction of L-arginine, generating N-hydroxy-arginine as the intermediate The deficiency of BH4 leads to eNOS uncoupling, as the intermediate reacts with molecular oxygen, resulting in O2•− production (Fig. 5b) (10). The reduced bio-availability of BH4 has been reported to be due to oxidation by peroxynitrite, resulting in the formation of inactive BH2 (237). The oxidative inactivation of BH4 is sufficient to produce eNOS uncoupling. The mice deficient in eNOS did not exhibit an increase in vascular superoxide production when compared with the wild-type mice in the mouse model of desoxycorticosterone acetate (DOCA)-salt hypertension, suggesting that eNOS uncoupling is the major source of endothelial-generated O2•− (235). In addition, p47phox knockout mice were relatively protected from BH4 oxidation and eNOS uncoupling, suggesting that NADPH oxidase-mediated O2•− production is an important contributor to oxidative loss of BH4 and eNOS uncoupling (330). Interestingly, the administration of BH4 in eNOS over-expressing mice was found to significantly decrease O2•− production and reduce the formation of atherosclerotic plaques by preventing eNOS uncoupling (330). Thus, the stoichiometric ration of BH4 to eNOS is critical in eNOS uncoupling.
In addition to BH4, increased levels of asymmetrical dimethyl arginines (ADMA), an endogenous inhibitor of eNOS, reportedly lead to eNOS uncoupling and enhanced O2•− production (404). Increased levels of ADMA have been reported in endothelial cultures treated with low-density lipoprotein, mouse lung exposed to endotoxin LPS, and septic shock patients (44, 322, 385). ADMA are generated by S-adenosylmethionine–dependent protein arginine methyltransferases that themselves are induced by enhanced oxidative stress (404).
D. XO-derived ROS in inflammation
Xanthine oxidoreductase is a soluble high-molecular-weight (270 kD) enzyme that catalyzes the oxidation of hypoxanthine to xanthine and uric acid. It exists in two forms: xanthine dehydrogenase (XDH) and XO (332). XDH reduces NAD+, whereas the XO is the main superoxide-producing enzyme. An irreversible proteolytic conversion of XDH to XO can be triggered by ischemic conditions (282). The activity of XDH/XO is specifically induced by IFN-γ in lung microvascular endothelial cells and the pulmonary artery endothelial cells. In contrast, IFN-α/β, TNF-α, IL-1 or -6, LPS, and PMA have no effect on the activity of XDH/XO (104). The activity of XO is up-regulated in the airway inflammatory disorders, ischemia reperfusion injury, atherosclerosis, diabetes, and autoimmune disorders such as rheumatoid arthritis (332). The serum samples from rheumatic patients may have approximately 50-fold higher levels of XO compared with healthy donors (289). Moreover, in diabetic rats, the administration of XO inhibitor allopurinol suppressed the NF-κB activation, neutrophil infiltration, and expression of inflammatory cytokines in the liver, suggesting a key role of XO-derived ROS in type I diabetes (363). XO-derived ROS has also been implicated in pulmonary vascular remodeling induced by chronic hypoxia in neonatal rats (196). The serum activity of XO is up-regulated by hypoxia, and administration of allopurinol limited the hypoxia-induced oxidative stress in the lung and pulmonary vascular remodeling; highlighting the critical role of XO-derived ROS in the chronic hypoxia induced pulmonary hypertension (196). Altogether, the XO-derived ROS seems to be important in a variety of inflammatory disorders; however, the therapeutic advantage of XO inhibitors such as allopurinol for other inflammatory diseases and other forms of organ injury remains to be investigated.
E. Regulation of antioxidant defense systems in inflammation
To prevent the damaging effects of oxidants, vertebrate cells have evolved an array of antioxidant defense systems that functions to remove ROS. The antioxidant enzymes SOD (dismutates O2•− to H2O2), catalase, glutathione peroxidase (GPx) (converts H2O2 to H2O), peroxidredoxins, and Trxs are classified as ROS scavengers (Fig. 1) (163). Thus, cells experience an oxidative stress when the capacity of antioxidant enzymes is overcome by enhanced oxidant production. There are three different isoforms of SOD expressed in mammalian cells: SOD1, also known as CuZn SOD, a 32 kD homodimer expressed in the cytosol and nucleus; SOD2 or MnSOD, a 93 kD homotetramer expressed in mitochondrial matrix and extracellular (EC-SOD); and a plasma membrane-associated enzyme, a tetramer with a molecular weight of 135 kD, which has four Cu atoms per molecule. The EC-SOD isoform, although plasma-membrane associated, is a secreted glycoprotein that scavenges extracellular O2•− (163). All SODs are highly expressed in lung tissue, vessels, and airways (272). However, when their relative distribution is compared, the activities of CuZnSOD and MnSOD are lower in the lung compared with the other organs such as the liver, kidney, heart, and brain; whereas the EC-SOD activity has been reported to be much higher in the lung (217). The knockout mice deficient in CuZn SOD and EC-SOD are viable and show no abnormality associated with oxidative damage, indicating that other antioxidant enzymes compensate for their deficiency under normal physiological conditions (51, 475). However, the knockout mice deficient in MnSOD die in the neonatal stage from dilated cardiomyopathy and impaired neural development (238, 245). The expression of CuZn SOD has been reported to be induced by shear stress and hyperoxia in cultured endothelial cells (188, 223); EC-SOD, in contrast, is induced by a variety of inflammatory cytokines, including TNFα, IFNγ, and IL4 in arterial smooth muscle cells (47, 399). Similar to EC-SOD, the expression and activity of MnSOD is induced by TNF-α and IL-1 as well as by LPS, ROS, and VEGF (1, 201, 292, 431). However, SOD1 knockout mice showed no enhancement of LPS-induced hepatotoxicity or hyperoxia-induced lung injury (180, 475). In contrast, EC-SOD knockout mice exhibited enhanced sensitivity to LPS-induced neutrophilic lung inflammation and hyperoxia-induced lung injury (46, 51). All these observations suggest that tissue-specific distribution of the SOD isoforms and their relative amounts in different tissues is important in determining their role in inflammation.
Catalase is a cytoplasmic 240 kD homotetrameric protein and is an important intracellular antioxidant enzyme detoxifying the H2O2 to oxygen and water. The expression of catalase has been reported in alveolar type II cells and macrophages, and highest expression has been reported in the liver and erythrocytes (355). Arita et al. have shown that targeting of catalase directly to the mitochondria in lung epithelial cells protects them from H2O2-induced apoptosis (14). Despite this important physiological link, no increase in the activity of catalase was reported after hyperoxia in endothelial cells (202) and bronchial epithelial cells, which made them more susceptible to hyperoxia-induced injury (110). Moreover, the LPS treatment decreased the expression and activity of catalase in mouse lung, a response preceding NF-κB activation (67). The congenital deficiency of catalase known as acatalasia is benign; however, in certain conditions, increased susceptibility to diabetes has been reported (355). Whether catalase is important in the pathogenesis of inflammation remains an open question.
The family of GPx enzymes serves the similar function of detoxifying H2O2 as catalase. There are four selenium-dependent GPx enzymes (GPx1–4) in mammalian tissue with a wide tissue distribution (270). GPx enzymes are tetrameric 85 kDa protein and carry four atoms of selenium bound in their catalytic core. These enzymes detoxify H2O2 by oxidizing monomeric glutathione (GSH) into dimeric glutathione disulfide (GSSG). Oxidized GSSG is converted back to its monomeric GSH form by glutathione reductase. The expression and activity of GPx is induced by hyperoxia in endothelial cells (202). However, GPx knockout mice showed no hypersensitivity to hyperoxia-induced lung injury, indicating the compensation from other antioxidant enzymes (180). Reduced levels of GSH have been reported in a variety of inflammatory conditions (356), indicative of its important role in the inflammatory response through enhancing oxidative stress.
Peroxiredoxins are a group of related antioxidant enzymes that catalyze the degradation of H2O2 to water. There are six different (Prx1–6) identified so far with molecular weights ranging between 17—and 31 kD (190). All subtypes of Peroxiredoxins are expressed in lung tissue (190). The Prx6 knockout mice exhibit enhanced hypersensitivity to hyperoxia-induced lung injury (439), whereas Prx6 overexpressed mice were resistant to such damage (441). Prx1 knockout mice were also reported to be prone to bleomycin-induced lung inflammation (214) and allergic airway inflammation (187). Further, administration by N-acetyl-cysteine in Prx1 knockout mice was found to protect them against bleomycin-induced acute lung injury, indicating a protective role of Prx1 against oxidative damage in inflammation (214).
Trxs are 10–12 kDa redox-sensitive antioxidant enzymes that maintain the proteins in their reduced state by catalyzing the reduction of proteins disulfides to their corresponding sulfhydryls utilizing the reducing equivalents NADPH (311). Three Trx enzymes have been identified thus far (Trx1, 2, and 3). Oxidized Trx is recycled back to its reduced state by Trx reductase. The protective effects of Trxs as an antioxidant enzyme was reported in a variety of oxidative stress-related diseases such as ishchemia reperfusion injury, inflammation resulting from activation of neutrohils, blemomycin-induced lung injury, and inflammation induced by pro-inflammatory cytokines (311). There are two Trxs interacting proteins discovered so far; TBP-1 or p40phox and TBP-2, also known as VDUP1 (311). The interaction of Trx with p40phox subunit of NADPH indicates that it may also regulate the ROS generation via NADPH oxidases.
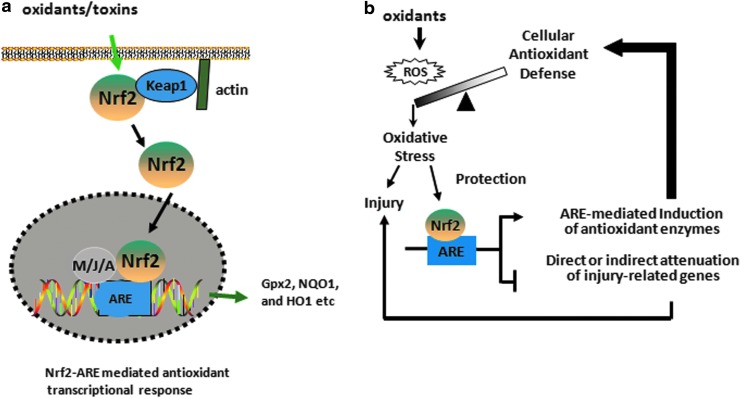
The induction of several antioxidant and cytoprotective genes is mainly regulated by the transcription factor NF-E2-related factor 2 (Nrf2), which is a cap'n’collar basic leucine zipper protein. The Kelch-like ECH-associated protein 1 (Keap1) retains Nrf2 in the cytoplasm and promotes its proteosomal degradation under basal condition (192, 193). In response to oxidative stress, Nrf2 dissociates from Keap1 and translocates into the nucleus, where it dimerizes mainly with the MAF (Maf-G, Maf-F, and Maf-K), JUN (c-Jun, Jun-B, and Jun-D), and ATF (ATF-4) families of bZIP proteins and transactivates a network of genes encoding cytoprotective and antioxidative enzymes containing the antioxidant response element (ARE), 5′-TGAG/CnnnGC-3′ in their promoters such as Gpx, NAD(P)H:quinone oxidoreductase (NQO1), and heme oxygenase-1 (HO1) (Fig. 6). The deficiency of Nrf2 enhances the susceptibility to experimental acute lung injury and impairs the resolution of lung inflammation in mice. Nrf2 activators such as triterpenoids (CDDO-Im) that target cysteine residues of Keap1 have been used to specifically disrupt Keap1:Nrf2 interactions, thereby promoting Nrf2 nuclear accumulation and leading to Nrf2-target gene induction (247) in Nrf2-sufficient but not in Nrf2-deficient cells in vitro and in vivo, suggesting that specific targeting of Nrf2-ARE signaling may provide a novel therapeutic strategy for treating human diseases. We and others have shown reduced levels of acute lung injury and inflammation (360) and emphysema in mice treated with CDDO-Im (402). Recently, triterpenoid analogue, bardoxolone methyl, showed improved renal function in early-stage chronic kidney disease in type 2 diabetes; however, Phase III clinical trial with this compound for very severe-stage patients was halted due to undisclosed safety issues (Reata Phamaceuticals) (ClinicalTrials.gov; NCT01351675). Moreover, the recent study by Zoja et al. has shown that bardoxolone analogues are ineffective in curing diabetic nephropathy in Zucker diabetic fatty rats but instead, the rats receiving such analogues worsen the outcome of the disease (476). While the CDDO-Im potently activates Nrf2 target genes in multiple tissues, proteomic analysis recently revealed that this CDDO-Im interacts with ∼600 different proteins, including many different transcription factors (464). Since Nrf2 confers protection against oxidation-related pathologies and Nrf-2 activation may be a useful antioxidant strategy, it is likely that the unwarranted effects of chronic CDDO treatment may be related to nonspecific off-target effects. Alternatively, it is possible that prolonged activation of Nrf2 signaling may be more complicated because of the possibility of activation compensatory pro-oxidative stress pathways.
FIG. 6.
NF-E2-related factor 2 (Nrf2)-dependent antioxidant mechanism. (a) Nrf2 dissociates from Kelch-like ECH-associated protein 1 (Keap1) on exposure to oxidants and translocates to the nucleus where it binds to the promoter region of antioxidant enzymes containing antioxidant response element (ARE) such as Gpx2, NQO1, and GCLC. M/J/A: Maf, Jun, or ATF family of proteins. (b) Nrf2-dependent effector mechanism involves transcription of antioxidant enzymes and attenuation of injury-related genes that provide protection against oxidant-induced acute lung injury. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
III. Cell Adhesion Molecules and Leukocyte Migration Across Transendothelial Barrier
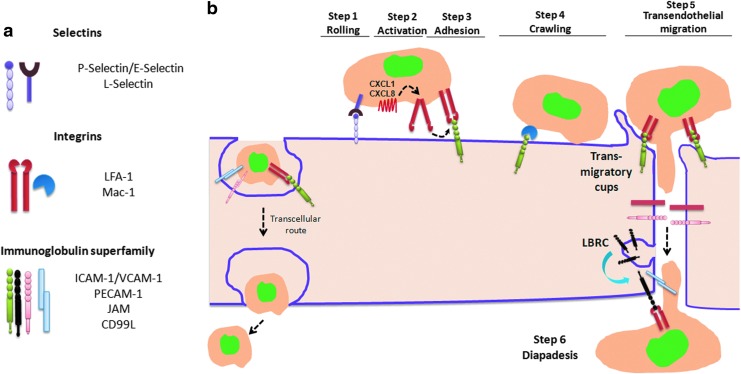
The migration of inflammatory cells across the endothelium of postcapillary venules is mediated by the adhesive interactions between the cell adhesion molecules (CAMs) expressed on activated endothelial and leukocytes. The neutrophils are the first cells to arrive at the inflamed location. The extravasation of neutrophils across the endothelial barrier occurs through endothelial junctions (paracellular pathway) and more rarely through the endothelial cell body (transcellular pathway) (459). The transcellular route is used by 10%–15% of the migrating neutrophils, but it varies greatly among tissues (366, 462). The three families of adhesion molecules that are crucial for leukocyte transmigration are selectins, integrins, and ICAM members of immunoglobulin (Ig) superfamily (Fig. 7a). The process of neutrophil transmigration is mediated by four sequential steps: (i) rolling, (ii) activation directed migration by setting up a chemoattractant gradient, (iii) adhesion to the endothelial cells, and (iv) transendothelial migration (Fig. 7b).
FIG. 7.
Schematic representation of transendothelial migration of leukocytes. (a) Different classes of cell adhesion molecules (CAMs) expressed in endothelial and inflammatory immune cells. There are three main classes of CAMs: selectin family, integrin family, and immunoglobulin superfamily. (b) The initial binding of leukocytes on endothelial surface is mediated by low-affinity binding of selectins, which enables rolling of neutrophils on the endothelium. The selectin-mediated bonds are formed between P- and E-selectin on endothelial cells and L-selectin, E-selectin ligand-1 (ESL-1) and P-selectin glycoprotein ligand-1 (PSGL-1) expressed on leukocytes. The affinity of PSGL-1 to P-selectin is much higher compared with E-selectin; whereas E-selectin binds predominantly to ESL-1. The secretion of chemokine CXCL8 from WPB binds to chemokine receptor on neutrophils and activates integrin by changing its conformation from bend to a fully extended form. The activated integrins then subsequently bind to intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) expressed in endothelial cells and cause neutrophil arrest on the endothelial surface. leukocyte function–associated antigen 1 (LFA-1 or αLβ2) and αMβ2 (Mac1) are the principal integrins that are expressed in neutrophils and bind to ICAM-1 in endothelial cells. The Mac-1 integrins enable slow crawling of neutrophils toward the endothelial junctions. At the site of endothelial junctions, ICAM-1 and VCAM-1 are gathered in transmigratory cups that hold neutrophils and facilitate their transmigration. The binding of JAM molecules further enables the deep penetration of neutrophils between endothelial cells. Platelet endothelial cell adhesion molecule (PECAM1) is recruited to sites of transmigration from the lateral border recycling compartment (LBRC), which stores 30% of the total PECAM-1. CD99L plays a similar role to PECAM-1 in enabling neutrophil exit across endothelial junctional compartments. The transcellular route of migration is adopted by 10%–15% of neutrophils that can greatly increase in the absence of Mac-1 integrin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
A. Selectins
They are membrane glycoproteins that are expressed in both endothelial cells and leukocytes and are of three different types: P- (expressed in platelets and endothelial cells), L- (expressed in all leukocytes), and E- selectins (expressed in endothelial cells) (27, 466). The initial rolling of neutrophils on endothelial cells in response to inflammatory stimuli is mediated by selectins (Fig. 7b). Selectins are characterized by the presence of a carbohydrate recognition domain known as lectin that allows a low-affinity binding to sialylated carbohydrate moieties of mucin-like CAMs on leukocytes. These residues, known as sialyl Lewis-x (SLex) antigen, play an important role in leukocyte tethering and rolling (466). Inherited defects in intracellular fucose transport impairs the formation of Lewis-x, resulting in leukocyte adhesion deficiency type II (LAD II) that is characterized by recurrent bacterial infection and impaired ability of neutrophils to bind endothelial cells expressing E-selectin (113, 255, 348). Neutrophil rolling on vascular endothelium is the result of sequential formation and the detachment of selectin-mediated bonds between neutrophils and endothelial cells (466). Selectin mediated rolling of leukocytes on endothelial cells greatly reduced velocity of leukocytes from hydrodynamic velocity of 200 to <5 μm/s (137, 466).
In endothelial cells and platelets, P-selectin (also known as granule membrane protein-140 [GMP-140]) is stored within Weibel–Palade bodies from which it is rapidly recruited to the cell surface during neutrophil migration; whereas E-selectin is up-regulated due to protein synthesis induced by activation of E-selectin gene in response to inflammatory stimuli such as IL-1β, LPS, or TNF-α (154, 166). P-selectin glycoprotein ligand-1 (PSGL-1) is constitutively expressed in neutrophils and T lymphocytes and binds to both P and E-selectin; however, affinity of E-selectin for PSGL-1 is 50 times lower compared with P-selectin (301). E-selectin binds to ligand E-selectin ligand-1 (ESL-1) that is expressed in neutrophils and plays a key role in neutrophil rolling (395, 466). The ligands for L-selectin include PSGL1, P-selectin, and E-selectin as well as Glycam-1, CD34, and MAdCAM-1, which are highly expressed in high endothelial venules (HEVs) and help in the homing of T lymphocytes in lymph nodes (466). On leukocyte activation, L-selectin is rapidly shed from the cell surface via a protease-dependent mechanism, which is important in the mechanism of transmigration and has also been implicated in mediating inflammation (159).
B. Integrins
They are heterodimeric proteins, consisting of alpha and beta chains, and are expressed in leukocytes and many other cell types (114). They facilitate firm adherence to the vascular endothelium. β2-integrins are the primary adhesion molecules that are expressed in leukocytes, and they bind to Ig superfamily member ICAM-1 which is expressed on the endothelial cell surface (114). β2-integrins are involved in firm adhesion strengthening and transendothelial migration of leukocytes (114). In contrast to selectin-mediated adhesion, which leads to the rolling of neutrophils, adhesion of leukocytes by β2 integrins is firm and is regarded an essential step for trans-endothelial migration of inflammatory cells. β2-integrins are activated by chemokines such as CXCL1 (stored in small cytoplasmic granules) and CXCL8 (stored in Weibel–Palade bodies), which are secreted by endothelial cells (456, 465). The binding of chemokines to chemokine receptors (CXCR1 and CXCR2) changes the conformation of integrins from bent to fully extended high-affinity configuration, enabling neutrophil arrest by binding to ICAM-1 that is expressed in endothelial cells (Fig. 7b) (465).
β2 integrins are grouped into the categories according to the β subunit they contain. The β2 integrins include leukocyte function-associated antigen 1 (LFA-1 or αLβ2) and Mac-1 (αMβ2) and β1 integrins such as very late antigen 4 (VLA4 or α4β1), α9β1, and α5β1 are common antigens that are expressed in leukocytes (114, 181). LFA-1 is the principal adhesion molecule in neutrophils that binds to ICAM-1 in endothelial cells and mediates transition from rolling to adhesion on the endothelial cell surface (350). Mac-1 also binds to ICAM-1 and mediates crawling of neutrophils along endothelial cells to the entry of endothelial junctions for paracellular migration (350). There is a 10-fold decrease in crawling displacement in Mac-1 null neutrophils, which forces them to transmigrate close to the initial site of adhesion and leads to transmigration (350). The activation of LFA-1 is induced by the activation of chemokine receptors on neutrophils by ligands that are secreted by endothelial cells such as CXCL1 (465). Fully activated LFA-1 mediates the stop of rolling and makes firm adhesion along with Mac-1 (175). Deficiency of β2 integrin chain causes Leukocyte Adhesion Deficiency Type 1, an inherited disorder that is characterized by inability of leukocytes to undergo adhesion-dependent migration (49). These patients suffer from recurrent bacterial infections. β2 integrin Mac-1 favors paracellular migration of leucocytes and in Mac-1−/− (CD11b) mice, neutrophils predominantly emigrate transcellularly (through endothelial cells) and were delayed by 20–30 min compared with the more rapid paracellular emigration route (351). In contrast to β2 integrins, the β1 integrin family mediates neutrophil adhesion to extracellular matrix proteins such as fibronectin and VLA4 or α4β1 integrin. The coating of fibronectin in an in vitro migration assay can greatly enhance the migration of human PMNs (115). VLA4 or α4β1 integrin in murine neutrophils mediates leukocyte adhesion to endothelial cells by interacting with VCAM-1 (9). However, VLA4 is not expressed in human neutrophils, but its function instead is served by α9β1 integrin. α9β1 integrin is a major β1 integrin that is induced on neutrophil activation which mediates binding to VCAM-1 (265). However, β1-integrin-mediated adhesion has a limited role in adhesion and transendothelial migration of neutrophils compared with β2 integrins (265). After β2-integrin-mediated neutrophil arrest, there is a polarization of neutrophils at the leading edge referred to as lamellipodium that orchestrates receptors for chemokines and phagocytosis. Polarization of neutrophils, which is important for efficient transendothelial migration, is mediated by the organization of filamentous actin (F-actin) cytoskeleton at the leading edge (375, 461).
C. Ig superfamily
It consists of CAMs that have an Ig-like domain and are expressed in endothelial cells, platelets, and leukocytes. Included in this family are members of inter-cellular adhesion molecules (ICAM-1 and -2), vascular cell adhesion molecule (VCAM-1), platelet endothelial cell adhesion molecule (PECAM-1 or CD31), junctional adhesion molecules (JAM-A, -B and -C), CD99 antigen-like 2 (CD99L2), and endothelial cell-selective adhesion molecule (ESAM) (45). The expression of ICAM-1 and VCAM-1 is increased on activated endothelium (166). At the time of neutrophil transmigration, ICAM-1 and VCAM-1 are recruited in specialized preformed tetraspanin-enriched microdomains (TEMs) and promote nano-clustering of adhesion receptors to increase leukocyte binding to endothelium (25). These specialized TEM domains have been termed endothelial adhesive platforms and are formed independent of ligand binding and actin cytoskeleton anchorage (25). The binding of LFA-1 integrin on neutrophils with ICAM-1 on endothelial cells generate signals that activate Rho A, leading to actin polymerization and formation of transmigratory cups (423). Transmigratory cups are microvilli-like projections that surround leukocytes and help in efficient transendothelial migration of leukocytes (24, 52). The adaptor proteins of ERM family (ezrin, radixin, and moesin) link ICAM-1 and VCAM-1 with actin cytoskeleton and help in reorganization of actin cytoskeleton and formation of transmigratory cups (25). The deep penetration of neutrophils between endothelial cells is mediated by JAM-A (Fig. 7b), as neutrophils accumulate deeper down the junctions in JAM-A−/− mice treated with IL-1β (458). In the resting endothelium, PECAM-1 is stored along the cell borders in the intracellular compartment known as lateral border recycling compartment (LBRC) and translocates to the site of diapedesis when leukocyte migration occurs (Fig. 7b). The pool of PECAM-1 in LBRC is approximately 30% of the total (266). Neutrophils in PECAM-1−/− mice become impacted between endothelial cells and basement membrane in IL-1β-stimulated mice, indicating that PECAM-1 is essential for the neutrophil to traverse the junctions (458). Interestingly, the role of JAM-A, PECAM1, and ICAM-2 in neutrophil migration appears to be stimulus dependent. Neutrophil migration was suppressed in PECAM1, ICAM-2, or JAM-1 knockout mice on treatment of IL-1β and ischemia reperfusion injury; whereas TNF-α-induced neutrophil migration was unaffected in these knockout models (458). The mechanism behind stimulus-dependent specificity of these junctional proteins is not clear. CD99L2, also known as leukocyte antigen, is a small membrane protein of 100 amino acids that is expressed in endothelial cells. It is essential for neutrophil transmigration but not for lymphocytes (40). CD99 L2 is not relevant for the adhesion between leukocytes and endothelium but rather for the transmigration step. The blocking of antibodies against CD99L2 arrested the neutrophils between endothelial cells and basement membrane similar to the phenotype observed in PECAM1−/− neutrophils (39). The expression of ESAM, another endothelial adhesion molecule, is restricted to endothelial tight junctions (TJs) and plays a role in Rho A activation and junctional opening. The migration of leukocytes was decreased to almost 50% in mice deficient in ESAM on treatment with TNF-α and IL-1β (444). Thus, there appears to be a great deal of redundancy in the role of these endothelial adhesion molecules in mediating leukocyte transmigration. One of the key questions is whether they are activated sequentially and whether they have distinct roles in the transmigration response. Only with dissection of the specific elements of the transmigration response into discrete steps will it become clear whether the function of these adhesion molecules is, in fact, distinct.
D. High endothelial venules
The postcapillary vascular endothelium of all the secondary lymphoid organs except spleen is constitutively enriched in CAMs. These regions were classified as HEVs, enabling circulation of naive lymphocytes between the blood and lymph node. Approximately 25% of all the lymphocytes circulating in HEVs bind and emigrate into a single lymph node (via HEVs) every second (134). In addition to commonly expressed CAMs in normal venules, HEVs abundantly express highly glycosylated and sulfated forms of adhesion molecules, including glycosylation-dependent cell-adhesion molecule 1 (GLYCAM1), CD34, MAdCAM-1, and peripheral LN addressin (PNAd), which are commonly referred to as vascular addressins, enabling rapid homing of lymphocytes in lymphoid organs (296). Certain chronic inflammatory conditions such as rheumatoid arthritis, psoriasis, Hashimoto's thyroditis, Crohn's disease, ulcerative colitis, and multiple sclerosis are accompanied by development of HEVs in normal organs, facilitating a large influx of lymphocytes that contribute to chronic inflammation (8, 231). This de novo formation of organized lymphoid tissue in chronic inflammation diseases has been termed lymphoid neogenesis. Lymphotoxin-α, a member of TNF family, has been implicated in the development of secondary lymphoid organs in these diseases (179). Psoriasis is a common cutaneous inflammatory disorder in which the dermal microvasculature undergoes distinctive changes, including HEV formation, which facilitates trafficking of lymphocytes to the skin (253). There is an interesting correlation between Ang II-mediated activation of the immune system and hypertension (158, 450) with the increased risk of hypertension and cardiovascular mortality in psoriasis patients that is associated with elevated levels of Ang II (253, 285).
E. Regulation of CAMs by oxidative stress
There is considerable evidence indicating that extravasation of leukocytes in response to inflammatory stimuli is regulated by oxidative stress that is produced by leukocytes. The adhesion of neutrophils to the surface of endothelial cells has been demonstrated to elicit a biphasic response that is related to endogenous ROS production in endothelial cells (221). Oxidative stress can regulate the expression of endothelial CAMs by a direct activation of CAMs that presents on the surface and also by a transcription-dependent mechanism involving redox-sensitive transcription factors (i.e., NF-κB and AP-1). In this regard, Patel et al. (337) demonstrated that external application of H2O2 or t-butylhydroperoxide as early as 1 h in HUVECs was accompanied by transcriptional-independent surface translocation of P-selectin, leading to increased PMN adherence. Treatment of cells with an antibody against P-selectin or antioxidant treatment abolished neutrophil adhesion to endothelial cells. Antibodies directed against P-selectin abolished an O2•−mediated increase in rolling leukocytes and decreased the number of adherent leukocytes, suggesting an increase in surface expression of P-selectin by O2•−. Similar to this study, Gaboury et al. (124) demonstrated using the intra vital microscopy that the application of hypoxanthine/XO (O2•− generating system) in rat mesenteric artery caused a decrease in leukocyte-rolling velocity and increased the number of rolling and adherent leukocytes which could be blocked by antibodies against P-selectin. In addition to oxidative stress, inflammatory mediators such as thrombin, VEGF, IL-17, and TNF-α are known to enhance the surface expression of P-selectin (154, 254). Unlike P-selectin, expression of ICAM-1, VCAM-1, and E-selectin is regulated at transcription level in endothelial cells by oxidative stress, although there is also considerable evidence that a significant component of ICAM-1 up-regulation on the endothelial cell surface is due to phosphorylation of cell-surface-bound ICAM-1 at tyrosine residues (249).
The promoter region of ICAM-1 contains binding sites for inducible redox sensitive transcription factors such as NF-κB and AP-1 (239, 361). H2O2 increases the transcripts of ICAM-1 in HUVECs within 30 min, the response was sustained for approximately 2 h, and antioxidant treatment (catalase) or application of ICAM-1 antibody abrogated the H2O2-induced PMN adhesion (251). TNFα-induced NF-κB activation and ICAM-1 expression in endothelial cells is dependent on oxidants that are generated by the PMN NADPH oxidase complex (117). In p47phox−/− mice, TNFα-induced NF-κB activation and ICAM-1 expression were significantly attenuated as compared with the wild-type mice (117, 432). Moreover, PMNs from p47phox−/− mice showed markedly reduced adhesion to mouse lung vascular endothelial cells, indicating the essential role of oxidant signaling in NF-κB activation and ICAM-1 expression in endothelial cells (117). Oscillatory shear stress (OS) induces endothelial expression of ICAM-1, and monocyte adhesion is also dependent on ROS that is produced from NADPH oxidases (393). ECs obtained from p47phox−/− mice showed impaired adhesion to monocytes in response to OS and were restored when the cells were transfected with p47phox plasmid. Furthermore, OS increased mRNA levels of NOX2 and NOX1, and monocyte adhesion was shown to be blocked by siRNA against NOX1. Interestingly, Matheny et al. have shown that VCAM-1 ligand binding induced ROS generation via NADPH oxidases in endothelial cells and promoted lymphocyte migration by actin restructuring in endothelial cells (278). Inhibiting NADPH oxidase activity with DPI or Apocynin blocked lymphocytic migration across mouse endothelial cells to greater than 65% (278). Expression of VCAM-1 in HUVECs was up-regulated by cytokine IL-1β and enhanced ROS generation. Treatment with antioxidant pyrrolidinedithiocarbamate (PDTC) and N-acetyl cysteine (NAC) repressed greater than 90% of the IL-1β-induced increase in VCAM-1 expression (276). Increased expression of ICAM-1 and P-selectin was also observed in the mouse model of acute pancreatitis that was dependent on enhanced ROS production (412). In the mouse model of atherosclerosis, Ang II-induced expression of VCAM-1 was dependent on mitochondria-derived ROS (354). In addition, in patients suffering from obstructive sleep apnea, an increased expression of adhesion molecules CD15 (a counter-receptor for selectins on ECs), CD11c (counter-receptor for ICAM-1 on ECs) on monocytes was correlated to increased ROS generation (105, 381). These monocytes showed increased adherence in culture to human endothelial cells and generated more intracellular ROS compared with control subjects (105). Continuous positive airway pressure treatment of these patients decreased basal ROS production in monocytes and concomitantly decreased the expression of CD15 and CD11c on monocytes (105). The circulating levels of ICAM-1, VCAM-1, and E-Selectin are elevated in hyperlipidemic and hyperglycemic and noninsulin-dependent diabetic-patients in direct correlation with increased oxidative stress (58, 69, 86, 313, 352). Reducing oxidative stress by GSH administration or NAC or low-fat diet improved the circulating levels of adhesion molecules (5, 57, 85, 86). All of these reports indicate that increased expression of CAMs induced by oxidative stress can contribute to the pathogenesis of the inflammatory disorders.
IV. Structural Basis of Endothelial Integrity
The vascular endothelium lining the blood vessels forms a continuous, semi-permeable restrictive barrier enabling the passage of macromolecules, inflammatory cells, and fluid between the blood and interstitial space. There are two different routes of transport across the endothelial barrier, transcellular (across the cell) and paracellular (between the cells). The transcellular route is adopted by the larger solute macromolecules having molecular radius >3 nm (284). The plasma protein albumin is actively transported by transcellular route by binding to a docking protein gp60 present in caveoli that transport it across the endothelium (284). The solute molecules (such as urea, glucose, amino acids, and certain ions) having Mr <3 nm and inflammatory cells take a paracellular route of transport across endothelium. The paracellular route is tightly controlled by inter-endothelial junctions (IEJs) and TJs, which provides an unperturbed restrictive barrier to the endothelium (Fig. 8). The paracellular permeability is the major route of vascular leakage observed in a variety of inflammatory states. The paracellular permeability was first described by Majno and Palade in rat cremaster muscle microvessels treated with a subcutaneous injection of histamine. They found that treatment with histamine leads to the formation of 0.1–0.8-μm-wide gaps along venular endothelium (262, 263). Paracellular permeability enables the extravasation of protein-rich fluid from the luminal to abluminal side of the endothelium though gaps formed between endothelial cells. The resulting protein-rich fluid leads to edema formation, which is a characteristic feature of most of the inflammatory conditions. Many of the permeability enhancing agents such as VEGF, thrombin, and PAF exert their actions by forming gaps between endothelial cells. The composition of TJs and IEJs and their regulation by oxidative stress and permeability-enhancing agents is described next:
FIG. 8.
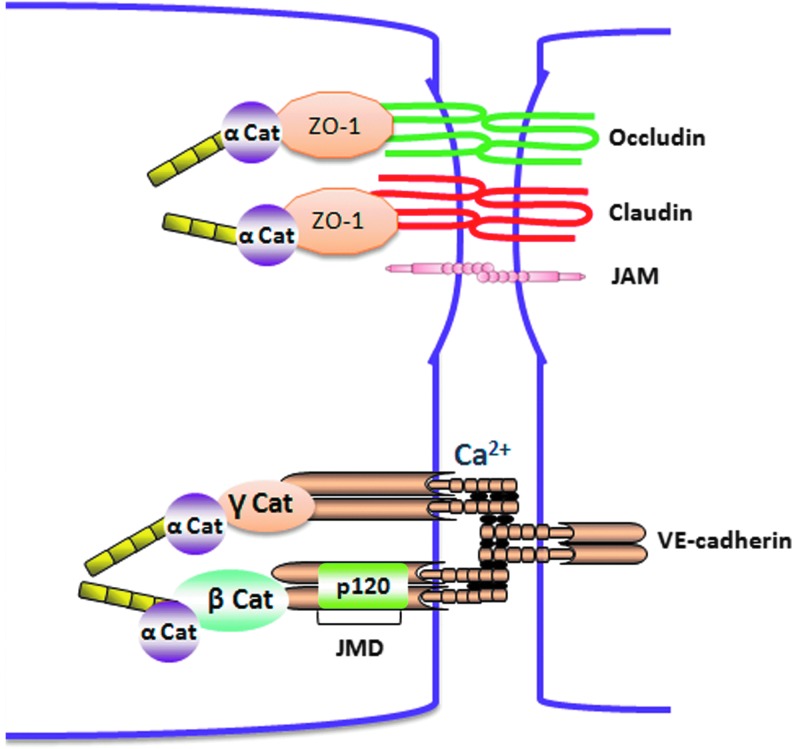
Structure of endothelial junctional proteins. Interaction between two adjacent endothelial cells is regulated by Adherens junction (AJ) and tight junction (TJ) proteins. AJs are formed by a homophilic interaction of transmembrane cadherin in a calcium-dependent manner that is linked to the actin cytoskeleton by supporting p120-, α-, β-, and γ-catenins. TJs consist of integral transmembrane proteins, claudin, occludin, and (JAM), form integral TJs between adjacent endothelial cells. Other accessory proteins, such as zonula occludens (ZO-1/2/3, etc.), are involved in structure support. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
A. Tight junctions
TJs are composed of transmembrane proteins occludins, claudins, and JAMs. The transmembrane proteins of TJs are connected to actin cytoskeleton by cytoplasmic adaptor proteins (zonula occludens [ZO]-1, -2, and -3, AF6, and cingulin). TJs in endothelial cells represent ∼20% of all the junctional complexes (454). The tightness of TJs depends on the protein composition and varies in different tissues ranging from almost complete tightening of the paracellular cleft for solutes (e.g., as in the blood brain barrier) to the formation of paracellular pores for specific ions. All transmembrane proteins of TJs, including occludins, claudins, and JAMs, possess a PDZ (postsynaptic protein disc large ZO-1)-binding motif in the C-terminal that binds to cytoplasmic adaptor proteins containing PDZ domain. Occludin consists of four transmembrane domains and two extracellular loops that enable a homotypic interaction with other occludin molecules and contributes to the TJ assembly. Arterial endothelial cells that are less permeable compared with vein endothelial cells have almost 18-fold greater expression of occludin (211). The C-terminal of occludin is linked to actin cytoskelton by cytoplasmic adaptor protein ZO-1. The role of occludin in maintaining TJ stability is not clear. In vitro studies showed that disruption of occludin function using antisense oligonucleotide or peptide antagonists against occludin molecules decreased transendothelial electrical resistance, whereas occludin−/− mice were still viable and showed no alternations in the structural and functional barrier properties of TJs (41, 211, 368). A compensatory up-regulation of other junctional proteins may account for the observed normal phenotype in these mice.
Claudins are transmembrane proteins of TJs that are similar to occludins and possess four transmembrane domains, two extracellular loops, and an N-terminal and C-terminal cytosolic domains. At least 24 claudins are identified in humans. Similar to occludin, the PDZ domain in the c-terminus of claudin interacts with ZO-1 and connects claudins with actin cytoskeleton. The composition of claudins varies in different endothelial barriers and, hence, determines whether the barrier is selectively permeable (e.g., the ion selective pores in the kidney) or impermeable (e.g., blood–brain barrier) (328). Pore-forming claudins such as claudin 2 (Cldn-2) form pores that are specific to monovalent cations and water and are found in Madin–Darby Canine Kidney (MDCK) cells. The claudins involved in the sealing of TJs include claudin 1, 3, 5, 11, and 19 (328). Of these claudins, claudin 5 is highly expressed in the blood–brain barrier, and claludin 5−/− null mice die within 10 h after birth (320). Although in these mice the development and morphology of blood vessels were not altered and showed no brain edema, however, it selectively increased the permeability to small molecules with MW <800 D (320).
JAMs belong to Ig superfamily of proteins consisting of a single-pass membrane protein with a long extracellular domain. At least three different isoforms of JAMs are known, including JAM-A, JAM-B, and JAM-C (18). The expression pattern of the JAMs varies significantly in different cell types. JAM-A is more widely distributed in epithelial cells, endothelial cells, monocytes, and neutrophils. The expression of JAM-C is exclusively restricted to endothelial cells, whereas JAM-B is more abundant in HEVs of lymphatic organs (16–18). The homophilic interaction between different JAMs contributes to the tightness of the TJs. Studies show that expression of JAM-A or JAM-C in CHO cells reduced the paracellular permeability to FITC dextran (17, 273). Moreover, antibodies directed against JAM-A significantly delayed the recovery of transepithelial electrical resistance during TJ reformation in epithelial cell monolayers (246). Orlova et al. (325) showed that JAM-C was mainly localized intracellularly in quiescent microvascular endothelial cells, and was recruited to junctions on stimulation with VEGF or histamine. Notably, disruption of JAM-C function decreased basal permeability and prevented the further increase in permeability induced by VEGF and histamine in human dermal microvascular endothelial cells in vitro and skin permeability in mice. JAMs are also known to interact with different integrins on adjacent cells. This heterophilic interaction regulated leukocyte–endothelial cell and leukocyte–platelet interactions (326, 329, 373).
ZOs belong to a membrane-associated guanylate kinase (GUK) homologue protein family. There are three ZO subtypes (ZO-1, -2, -3), and all of them possess three domains: PDZ, an Src homology 3 (SH3), and GUK (140, 305). ZO is an acronym for ZO. ZO-1 was the first TJ-specific protein identified (398). ZO1 acts as a scaffolding protein, as it interacts directly with claudins and also binds to the other Adherens junction (AJ) proteins such as α-catenin and to connexins in gap junctions (228). Through their PDZ, SH3, and GUK domains, ZOs are critical in recruiting signaling molecules to TJs, and thereby linking TJ proteins to the actin cytoskeleton (140).
B. Adherens junctions
AJs are composed of the cadherin family of transmembrane adhesion proteins that are anchored to the cytoskeletal network by intermediate cytoplasmic proteins from the catenin family, including α-, β-, γ-, and p120 catenins (31). There are two types of cadherins present in endothelial cells: VE-cadherin (vascular endothelial cadherin) and N-cadherin (N for neuronal) (315). Although both are abundantly expressed in endothelial cells, VE-cadherin mainly promotes the homotypic interaction between endothelial cells, and when present, it excludes N-cadherin from those sites (315). VE-cadherin is a homophilic binding protein that mediates the interaction of endothelial cells in a calcium-dependent manner. It has five cadherin-like repeats in its extracellular domain that oligomerize in a cis- and trans-manner with other VE-cadherin molecules between the same and adjacent cells (Fig. 8). The cytoplasmic tail of VE-cadherin contains two functional domains: juxtamembrane domain (JMD) and C-terminal domain (CTD). JMD of VE-cadherin binds p120-catenin, whereas CTD binds β-catenin and γ- catenin (also known as plakoglobin) (Fig. 8) (31). α-catenin by binding to β- and γ- catenin links the catenin-cadherin complex to the actin cytoskeleton. Both the N- and C-terminus of VE-cadherin are essential in the regulation of endothelial barrier function. Monoclonal antibodies targeted against N-terminus of VE-cadherin prevented the formation of IEJs and increased the microvascular permeability (70, 71). Interestingly, the C-terminal-truncated mutant of VE-cadherin in CHO cells did not impair the ability of cells to form aggregates; however, it greatly enhanced the intercellular junction permeability to high-molecular-weight molecules (56, 314). This finding suggests that the N-terminal region of VE-cadherin is required for cell–cell adhesion, whereas the C-terminal provides a tight restrictive barrier regulating paracellular permeability. VE-cadherin is essential for the development of endothelial barrier in embryos, and VE-cadherin knockout mice die in the embryonic stage due to abnormal vascular development (53). Moreover, expression of dominant negative VE-cadherin mutants also resulted in leaky junctions and an increase in microvascular permeability (430). Homotypic binding of VE-cadherin molecule between endothelial cells is stabilized by calcium, and chelation of calcium using EDTA destabilized the junctions and increased transendothelial permeability (125).
Catenins are equally important in regulating the stability of AJs by connecting them with actin cytoskelton. Deletion of β-catenin binding site on VE-cadherin caused lethality at 9.5 days of gestation (53). Similarly, conditional deletion of β-catenin in cultured endothelial cells reduced the ability of endothelial cells to maintain intercellular contacts and increased paracellular permeability (55). p120 catenin regulated the surface expression of VE-cadherin, and the depletion of p120 by siRNA decreased the VE-cadherin expression and increased the junctional permeability. In addition, p120 catenin acted as a scaffold protein by binding with two different tyrosine phosphatases, including Src homology 2 domain-containing tyrosine phosphatase-1 (SHP-1) and protein tyrosine phosphatases (PTPμ), which regulated VE-cadherin phosphorylation and stability of the junctions (210, 271, 477) (see next section).
C. Regulation of endothelial junctional proteins and associated cytoskeleton by oxidative stress
Oxidative stress produced by leukocytes at the site of inflammation plays a crucial role in initiating junctional disassembly. At the site of inflammation, AJs are disrupted by several mediators that are released by inflammatory cells, including ROS, cytokines, chemokines, thrombin, histamine, PAF, VEGF, and bradykinins. Most of these molecular mechanisms converge on mediating disruption of AJs and TJs, leading to gap formation between cells. These pathways in relation to oxidative stress are described next.
1. Regulation of TJs by oxidative stress
Among the TJ proteins, occludins have been well documented to be redox-sensitive proteins. Increased oxidative stress has been related to down-regulation of occludin expression, reduced membrane localization, and reduced tightness of the junctional barrier (229, 261). The C-terminal of occludin contains a coiled-coil (CC)-domain which carries cysteine residues that are essential for oligomerization of occludin by forming disfulfide bonds (280, 433, 434). Masking of free sulfhydryl groups or the presence of DTT prevented occludin dimerization, suggesting the formation of intermolecular disulfide bridges. The dimerization of full-length human occludin can also be prevented by the replacement of its cysteine 409 by alanine in the cytosolic C-terminal CC-domain (433). Oligomerization of occludin is modulated by the ratio of intracellular GSH/GSSG (433). Under physiological conditions, there is a 30- to 100-fold greater GSH than GSSG, thus maintaining a cytosolic reduced state (183, 184). Almost equal number of occludin monomers and oligomers are detected under physiological conditions (433). During inflammation, enhanced oxidative stress can drastically alter the GSH/GSSG ratio toward glutathione GSSG, which prevents oligomeric assembly of occludin. Along this line, inflammation or hypoxia/reoxygenation-induced oxidative stress resulted in reduced occludin oligomerization (279, 281). Mice and rat deficient in SOD1 also have decreased expression of occludin, claudin-5, and ZO-1 correlating with excessive ROS generation (260, 261, 472). These mice exhibited symptoms of amyotrophic lateral sclerosis-linked neurodegeneration (472). Similar to the in vivo studies, compounds such as 2,3-Dimethoxy-1,4-naphthoquinone (DMNQ), which enhance intracellular ROS generation or hypoxia/reoxygenation, were associated with decreased occludin expression and disruption of cadherin-β-catenin complex formation in cultured cerebral endothelial cells (229).
In addition, occludin is regulated by tyrosine phosphorylation in C-terminal region by enhanced oxidative stress. In the mouse model of atherosclerosis, Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) reduced the total occludin protein and increased occludin phosphorylation in aortic endothelial, which was associated with enhanced O2•− generation (89). Interestingly, SOD and catalase reversed the inhibitory effects of OxPAPC with catalase being more effective, suggesting that H2O2 is the predominant ROS species involved in mediating disassembly of TJ proteins (89). Kevil et al. (212) have demonstrated in HUVECs that treatment with H2O2 induced endothelial barrier disruption secondary to increased phosphorylation of occludin at serine residue. This phosphorylation was dependent on ERK 1/2 activation and was blocked by the ERK inhibitor PD-98059. In addition, Rao et al. (359) demonstrated that treatment of Caco-2 cells with xanthine+XO rapidly increased tyrosine phosphorylation of occludin, ZO-1, E-cadherin, and β-catenin, leading to endothelial barrier disruption, which was prevented by treatment with tyrosine kinase inhibitor genistein. There are two Tyr residues (Y398 and Y402) that are located in highly conserved CTD YETDYTT of human occludin, and are targets of c-Src-mediated phosphorylation. Deletion of the YETDYTT motif abolished c-Src-mediated phosphorylation of occludin and ZO-1 binding. Mutations of these residues to alanine in human occludin also abolished c-Src-mediated phosphorylation and regulation of ZO-1 binding (107). Gain-of-function mutations (Y398D/Y402D) resulted in a dramatic decrease of ZO-1 binding even in the absence of c-Src, whereas these mutants also failed to localize at the cell–cell contacts and sensitized MDCK cells for H2O2-induced barrier disruption (107). In addition to the role of tyrosine phosphorylation of occludin in junctional disassembly, it also appears to be essential in mediating recovery of the junctions. Meyer et al. (288) demonstrated in MDCK cells that during reassembly, tyrosine phosphorylation was localized near the lateral membrane. Inhibitors of tyrosine kinases genistein and PP-2 inhibited the recovery of transepithelial resistance and perturbed the re-localization of ZO-1 and occludin at the TJ.
2. Regulation of AJs by oxidative stress
The effects of oxidative stress and other inflammatory agonists such as thrombin and VEGF on AJ proteins are mediated by phosphorylation of key serine/tyrosine residues of VE-cadherin, β-catenin, and p120 catenin, which induce junctional disassembly. There are at least five known tyrosines (Y645, Y658, Y685, Y731, and Y733) and one serine residue (S665) in VE-cadherin that are targeted phosphorylation sites for permeability-enhancing agents. Tyrosine phosphorylation of VE-cadherin induces junctional disassembly by preventing interaction with its cytoplasmic binding partners and actin cytoskelton. Phosphorylation of these residues differs according to the inflammatory mediator used. The residues Y645 and Y658 located in the p120-catenin binding site and Y731 and Y733 located in the β-catenin binding site are the targeted phosphorylation sites during leukocyte trafficking (Fig. 10) (7, 418). Allingham et al (7) demonstrated that co-incubation of leukocytes and human endothelial cells enhanced tyrosine phosphorylation of VE-cadherin, specifically on residues Y658 and Y731, which was essential for trans-endothelial migration of leukocytes by inducing junctional opening (Fig. 9). Similar to this study, Turowski et al. (418) demonstrated that lymphocyte trafficking across the endothelial barrier is mediated by phosphorylation of Y645, Y731, and Y733 of VE-cadherin. Point mutations of these residues with phenylalanine suppressed the lymphocyte trafficking across endothelial monolayers by preventing junctional opening (7, 418). The agonist VEGF increased endothelial permeability by phosphorylating VE-cadherin at S665 by p21-activated kinase (PAK) and at Y658 and Y685 by c-Src kinase (130, 299). Phosphorylated S665 residue in VE-cadherin recruited β-arrestin to the site, resulting in clathrin-dependent endocytosis of VE-cadherin and junctional disassembly (130). Phosphorylation of Y685 prevented the binding of c-Src tyrosine kinase (CSK), which is an inhibitor of c-Src activity (30). VEGF-induced phosphorylation of VE-cadherin is dependent on enhanced ROS production and Rac activation in microvascular endothelial cells (299). Inhibitors of ROS generations such as DPI and NAC prevented phosphorylation of VE-cadherin at Y658 (299). In addition, double-point mutants of VE-cadherin Y658F, Y731F, suppressed VEGF-induced vascular permeability (299). In a similar study, van Wetering et al. (427) demonstrated that expression of a constitutively active form of Rac in HUVECs enhanced ROS production by activation of NADPH oxidases, resulting in tyrosine phosphorylation of VE-cadherin and α-catenin, leading to junctional disassembly. Moreover, endothelial junctional disassembly induced by blocking antibodies against extra-cellular domains of VE-cadherin also resulted in Rac activation, enhanced ROS generation, and tyrosine phosphorylation of β-catenin, which was dependent on Pyk2 activation (424). In another study, Tai et al. (407) demonstrated that enhanced ROS production by shear stress in endothelial cells activated PYK2, which can induce junctional disassembly. Similar to VEGF, thrombin is known to increase endothelial permeability by promoting tyrosine phosphorylation of VE-cadherin and associated proteins β-catenin, γ-catenin, and p120-catenin (419).
FIG. 10.
Signaling mechanisms of ROS-mediated increase in leukocyte migration and junctional permeability. During neutrophil migration, clustering of ICAM-1 by integrins leads to the activation of Rac, which induces intracellular ROS generation by NADPH oxidases. Increased ROS generation activates tyrosine kinase activity of c-Src and PYK2, which phosphorylates VE-cadherin at Y645, Y658, Y731, and Y733. The phosphorylation of these residues destabilizes the AJs by preventing interactions with the cytoplasmic proteins such as beta catenin and p120 catenin. Increased ROS enhances the expression of P-selectin on the endothelium by enabling secretion from Weibel–Palade bodies (WPB). In addition, ROS is known to activate NF-κB, which induces the expression of cell adhesion molecules such as ICAM-1, VCAM-1, and E-selectin; enhances neutrophil binding on the endothelium; and increases paracellular migration. Acute exposure of pro-inflammatory factors such as thrombin, lipopolysaccharide (LPS), and VEGF trigger NOX-mediated ROS formation and PLC-γ/cADPR activation, which resulted in an increase of [Ca2+]i by TRPC/TRPM. Increase in [Ca2+]i activates calmodulin and kinases, which, in turn, modified constituents of AJs and reorganized actin cytoskeleton and resulted in junction disassembly. Agonist-mediated ROS formation also induces transcription of pro-inflammatory genes by canonical NFκB pathway. ROS are also involved in activation of different kinases, including c-Src, PYK2, FAK, and PKC, and thus induce phosphorylation of AJ proteins and destabilize AJs. E-Sln, E-Selectin; P-Sln, P-Selectin; WPB, Weibel-Palade bodies; CaM, calmodulin; cADPR, cyclic ADP Ribose; DAG, diacylglycerol; FAK, focal adhesion kinase; GPCR, G protein coupled receptor; MLCK, myosin light-chain kinase; PLC, phospho lipase C; PKC, protein Kinase C; PYK2, protein tyrosine kinase; RTK, receptor tyrosine kinase; TRPC/M, transient receptor potential canonical/melastatin channel; TLR, toll-like receptor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 9.
Dynamic behavior of actin filament. Actin tread-milling is driven by ATP-hydrolysis. ATP-actin polymerizes at the plus end of filaments, while ADP-actin dissociates from the minus end. ADF and cofilin sever actin filaments at point end and thus promote the release of ADP-actin monomers. In the presence of Ca2+ gelsolin caps (+) end to block the filament growth. By inducing ATP/ADP exchange, profilin increases actin-ATP and promotes polymerization. Actin depolymerizing factor (ADF). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The low level of tyrosine phosphorylation of VE-cadherin under physiological conditions is regulated by the association with VE-protein tyrosine phosphatases (VE-PTP) that localized at cell–cell adhesion sites (316). Several PTPs, including PTP1B, SHP-2, and density-enhanced phosphatase-1 (DEP1)/CD148, are sensitive to inactivation by enhanced ROS production. The cysteine residue at the catalytic site of classical PTPs is sensitive to oxidation and is the target of specific inhibitors such as pervanadate (91, 287). All of these reports suggest that ROS-mediated activation of tyrosine kinases (c-Src and PYK2) is involved in phosphorylation of junctional proteins, resulting in junctional disassembly.
Independent of tyrosine kinases, oxidative stress during inflammation can also lead to generation of secondary by-products that enhance intracellular ROS generation and affect AJ assembly. One of such a by-product is 4-hydroxy-2-nonenal (4-HNE), which is generated by peroxidation of membrane lipids during inflammation and modifies AJ proteins by forming Michael adducts (421). The levels of 4HNE accumulate certain tissues ranging from 10 μM to 4 nM concentrations. Treatment of bovine lung microvascular endothelial cells to 4-HNE-induced ROS generation modified AJ proteins VE-cadherin and β-catenin by forming 4HNE adducts (Michael adducts) and decreased the transendothelial resistance (421).
3. Regulation of actin cytoskeleton by oxidative stress
The actin cytoskeleton of endothelial cells plays an indispensable role in maintaining endothelial cell morphology, junctional stability, and endothelial motility. According to Ingber et al (186), endothelial cells maintain a state of “tensegrity” in which the centrifugal tension generated by interconnected AJs, microtubules, and focal adhesion resist the centripetal tension generated by actomyosin cytoskeleton. The morphology of the cell, therefore, depends on the force that dominates. Thus, a decrease in the resistive force due to the loss of endothelial junctions results in rounded morphology of endothelial cells.
Oxidative stress and inflammatory agonists are well-known inducers of actin cytoskeleton reorganization in endothelial cells, leading to junctional opening and gap formation between endothelial cells. Actin is a 42-kDa protein that can be present either as a free monomeric form known as G-actin or as a part of a linear polymer microfilament called F-actin (Fig. 9). A direct effect of exogenous application of H2O2 on remodeling of actin cytoskeleton of endothelial cells has been documented in several reports. Endothelial monolayer treated with inflammatory agonists such as thrombin, histamine, and VEGF or directly with H2O2 correlated with decreased cortical actin and formation of polymerized F-actin (61, 182). Reorganization of actin ctyoskelton into F-actin increased centripetal tension and induced gap formation in endothelial cells (103, 349, 397).
Oxidative stress can affect actin cytoskeleton reorganization in two different ways: (i) a direct effect on actin cytoskeleton or actin regulatory proteins by oxidative modification and (ii) indirectly by influencing the signaling networks intrinsic to endothelial cells affecting polymerization of actin cytoskeleton. Both pathways are influenced by inflammatory agonists and oxidative stress. Oxidation of actin monomers (G-actin) by H2O2 alters its polymerization ability as well as its interaction with actin regulatory proteins filamin and α-actinin (77). H2O2 is known to induce actin polymerization at doses ranging from 0.2 to 5 mM with an effective dose (ED50) at 1 mM H2O2 (198, 324, 411). The cysteine and methionine residues in G-actin are particularly susceptible to oxidation. Exposure of G-actin to tert-butyl hydroperoxide (t-BH) in 1–20 mM concentration induced oxidation of Cys-374 of G-actin and altered the kinetics of actin polymerization (78), while treatment with H2O2 induced oxidation of Cys374 as well as Met44, Met47, Met176, Met190, Met269, and Met35 of actin monomers. Of all the residues studied, Cys-374 was the earliest target of H2O2-induced oxidation (291). The oxidative modification of these residues alters conformations of subdomain 1 (which regulates binding to actin-binding proteins) and subdomain 2 (regulates polymerization of actin filament) of actin (291). Interestingly, treatment with H2O2 did not increase actin polymerization at the barbed ends; however, it enhanced polymerization at the pointed end (Fig. 10) (297, 324). Inhibitors of ROS generation DPI or SOD mimetic (MnTmPyP) decreased the number of exposed barbed ends and reduced actin polymerization (297).
In addition, oxidative stress can influence cytoskeletal dynamics by direct modification of actin and actin-associated regulatory proteins through phosphorylation, nitration, carbonylation, disulfide formation, and glutathionylation (108, 109). s-glutathionylation of actin, in particular, has been detected in cells exposed to oxidative stress (59, 76, 123, 362). Moreover, actin was found to be the most abundant s-glutathionylated protein in PMNs and platelets in response to exposure to oxidants (59, 76). Actin-associated regulatory proteins affected by oxidative stress include cofilins, gelsolin, and filamin (Fig. 9). Cofilins or actin depolymerizing factor (AC/ADF) is a class of small (13–19 kDa) actin-binding proteins that regulates actin turnover in the cells through depolymerization of actin filaments. Cofilins exist in cells as both monomers and oligomers. The monomers exhibited the known severing activity, whereas the oligomers exhibited reduced severing activity but increased actin bundling activity (347). The transition from monomers to oligomers was regulated by reversible intermolecular disulfide bond formation between Cys39 and Cys147 of two adjacent cofilin units (347). Gelsolin regulated actin turnover in the cells by severing the barbed ends and capping actin filaments. Gelsolin is an unusual actin-binding protein that functions in two different redox environments. Plasma gelsolin functions in a Ca2+-rich, oxidizing environment and regulates the actin scavenger system of the blood. In contrast, cytoplasmic gelsolin works in Ca2+-poor, reducing environment of the cytoplasm (448). Under basal conditions, plasma gelsolin contains a disulfide bond between cysteines 215 and 228. This alters its sensitivity to calcium and the rate of severing F-actin, which includes binding to actin monomers and nucleation of actin polymerization (6, 448). Enhanced oxidative stress induced by Rac activation resulted in dissociation of gelsolin from actin filaments, exposing more barbed ends for actin polymerization (13, 167). Filamin is a 280 kDa actin-binding protein that plays a significant role in connecting F-actin with plasma membrane glycoproteins. In resting endothelial cells, filamin is constitutively phosphorylated by PKA, which protects it against calpain-mediated proteolysis. Treatment of HUVECs with 100 μM of H2O2 caused dephosphorylation of filamin by inhibiting PKA activity. This subsequently induced translocation of filamin from the membrane to the cytosol within 1 min and was accompanied by gap formation, which was attributed to rearrangement of the dense peripheral band of F-actin (168, 169). The translocation of filamin was inhibited by antioxidants such as TEMPO (nonspecific free radical scavenger) and Deferoxamine (iron chelator) (168). Altogether, enhanced intracellular oxidative stress is the major inducer of actin stress fiber formation, leading to junctional disassembly and gap formation between endothelial cells.
V. Signaling Mechanisms of Endothelial Barrier Disruption by Oxidative Stress
A. Altered intracellular Ca2+ regulation
Endothelial barrier integrity is formed by tight cell–cell and cell–matrix adhesions, and it is coupled to cytosolic Ca2+ levels (284). Increases in [Ca2+]i induced formation of inter-endothelial cell gaps and vascular hyperpermeability through different signaling pathways (Fig. 10) (256, 264, 371). Several reports suggest that an increase in [Ca2+]i leads to activation of Ca2+/calmodulin-dependent myosin light chain kinase (MLCK), which facilitates reorganization of actin cytoskeleton to induce changes in endothelial cell shape. Increasing cytosolic Ca2+ by thapsigargin (which depletes ER Ca2+ store) and calcium ionophore increased permeability in an HUVEC-derived cell line ECV304 (103, 127). Interestingly, thapsigargin induced activation of PKC-α, and phosphorylation of VE-cadherin-associated proteins was prevented by PKC inhibitor, calphostin C. These findings suggest that activation of PKC-α is critical in Ca2+-mediated increase in endothelial permeability (370, 371). An increase in [Ca2+]i has also been associated with activation of small GTPase RhoA. The inflammatory mediator such as thrombin is known to induce receptor-mediated Ca2+ entry followed by RhoA activation, thereby resulting in increased endothelial permeability (390).
In addition, evidence indicates that ROS activates Ca2+ signaling and influences different cellular events which trigger inflammation. Chelation of extracellular Ca2+ by BAPTA suppressed H2O2 formation (312). An increase in intracellular Ca2+ was associated with enhanced H2O2 generation (101). The converse is also true. H2O2 caused a dose-dependent rise in [Ca2+]i of endothelial cells (172). ROS-mediated regulation of intracellular free Ca2+ concentration is a major mechanism of increased vascular permeability, the hallmark of inflammation. However, to date, little is known about the mechanism of ROS-induced Ca2+ entry in endothelial cells and how this relationship promotes the loss of endothelial barrier integrity.
The principal Ca2+ entry pathway in endothelial cells is via store-operated calcium (SOC) channels and receptor-operated calcium (ROC) channels. Since transient receptor potential (TRP) channels are believed to underlie the molecular makeup of many ROC channels (TRPC3, 6 and 7) and at least some SOC channels (TRPC1 and 4) (333), it is logical to presume that TRPC proteins are sensitive to regulation by ROS. TRPC7 (also known as transient receptor potential melastatin 2 (TRPM2), TRPC6, and TRPC3/TRPC4 have been shown to be regulated by ROS in the endothelium (156, 408). In one study, Balzer et al. (22) provided strong evidence for a central role of TRP channels in oxidant-induced vascular pathophysiology. They showed that expression of a dominant negative fragment of TRPC3 suppressed oxidant-induced membrane currents in endothelial cells. To further analyze the role of TRPC3 as a redox sensor, Poteser et al. (353) stably transfected HEK293 cells with TRPC3, and these cell types displayed a large increase in membrane conductance in response to cholesterol oxidase. Generation of redox cation conductance by over-expression of TRPC3 suggests that TRPC3 is an important redox-sensitive cation channel. Using immunoprecipitation and Forster Resonance Energy Transfer (FRET), it was shown that TRPC4 interacts with TRPC3 to form heteromers, representing a potential redox-sensitive channel complex. The requirement of TRPC3/C4 heteromerization to serve as a sensor for cellular redox state was further evident by the finding that redox-activated TRPC3 cation conductance was inhibited by expression of dominant negative mutants of TRPC4 (353).
Recent studies have demonstrated that TRPC6 also represents a target of ROS in different disease models (98, 443, 446). Using lung ischemia–reperfusion edema (LIRE) model, Weissmann et al. (446) elegantly showed that NOX2-mediated O2•− activates TRPC6 and thereby induces Ca2+ influx in endothelial cells. They also found that NOX2−/− and TRPC6−/− mice were equally protected against ischemia-induced lung injury. Thus, TRPC6 also represents a target for ROS in different pathophysiological conditions.
TRPM2, of the melastatin subfamily, originally named TRPC7 (307), is no longer considered a member of the TRPC subfamily. The TRPM subfamily only exhibits limited homology to the other TRP family ion channels, and functional channels comprising members of this subfamily are believed to be homotetramers (277). CTD of TRPM2 share 39% sequence identity to the human nucleoside diphosphate-linked moiety X-type motif 9 (NUDT9) ADPR pyrophosphatase (173, 343, 372, 386) Despite significant homology with NUDT9 ADP pyrophosphate, TRPM2 has low levels of ADP-ribose (ADPR) pyrophosphatase activity (343, 372). Intracellular application of ADPR, the substrate of the NUDT9 enzyme, specifically induced TRPM2 gating (343). A further mode of TRPM2 activation is through oxidative stress (164, 392, 445). Different mechanisms have been proposed for H2O2-mediated TRPM2 gating. Perradud et al. (344) showed that oxidative stress activates production of ADP-ribose in mitochondria, which are then released to the cytosol and function as a second messenger to stimulate TRPM2 gating. In contrast, Hara et al. (164) demonstrated that TRPM2 is a Ca2+-permeable cation channel and can be activated by H2O2 via action of NAD+. Direct oxidation of TRPM2 by H2O2 has also been reported as a mechanism of activation of the channel (445).
Hecquet et al. (171) showed that H2O2 induced TRPM2-like cation current that was increased by over-expression of TRPM2 and by 3-deaza-cADP ribose but inhibited by a specific TRPM2 small interfering RNA and by PARP inhibitors in human pulmonary artery endothelial cells (Fig. 11). These data clearly favor an indirect mechanism of activation of TRPM2 channels by H2O2 via the formation of ADPR. Using Ca2+ add-back protocol designed to rule out indirect effects of H2O2 on Ca2+ entry via Ca2+ store depletion, Hecquet et al. also showed that H2O2 increased intracellular Ca2+ by stimulating Ca2+ entry without provoking Ca2+ store depletion. More importantly, this study showed the importance of TRPM2 in mediating the H2O2-induced increase in endothelial permeability, which could be prevented partly by application of TRPM2 small interfering RNA and TRPM2-specific antibody (171). The short variant of TRPM2, which lacks the pore domain and acts as a dominant-negative form by inhibiting the formation of functional homotetrameric channels, also significantly reduced H2O2-mediated microvascular hyper-permeability (171). In another article, Di et al. (95) addressed the role of TRPM2 in phagocytic cells and showed a protective role for TRPM2 in LPS-induced lung inflammation. This involved the influx of cations via TRPM2, which prevented membrane depolarization induced by transfer of electrons from NADPH to NADPH oxidase. Therefore, the net effect of TRPM2 activation was to reduce the production of ROS, which, in turn, modulated the lung inflammatory response to E. coli.
FIG. 11.
H2O2-mediated ionic current and increase in endothelial barrier permeability depends on TRPM2-L expression. (a) Current–voltage relationship for membrane current induced by H2O2 in untransfected, TRPM2-L–overexpressing and TRPM2 siRNA–transfected cells. (b) Bar graph quantifying peak currents (in pA) of (a). (c, d) human arterial endothelial cells were plated to confluence on gold electrodes without or with control/TRPM2 siRNA and then treated with 300 μM H2O2. H2O2-mediated decrease in trans-endothelial electrical resistance (TER) was significantly attenuated in cells transfected by TRPM2-specific siRNAs compared with untreated control or control group transfected with scrambled siRNA. The abscissa indicates time in hours; the ordinate, normalized resistance (relative to basal value). Modified from Hecquet et al. (171). *p<0.05. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
B. Myosin light chain kinase
Endothelial cells contain contractile machinery, which includes actin and myosin. Phosphorylation of regulatory myosin light chain (MLC) leads to activation of the endothelial contractile elements and disrupts endothelial barrier function. (127). Permeability increasing mediators such as thrombin, histamine, and H2O2 enhance MLC phosphorylation, which precedes the onset of endothelial contraction (Fig. 10) (303, 387, 470). The myosin phosphorylation in the endothelial cells is regulated by two enzymes: nonmuscle MLCK and myosin-associated protein phosphatase. Activated MLCK directly phosphorylates MLC at Thr-18 and Ser-19 site, which induced isometric tension development and actin polymerization in endothelial cells (136). Zhao et al. (470) reported that H2O2 also induced MLC phosphorylation in endothelial cells. Though the inhibition of MLCK blocked H2O2-induced MLC phosphorylation, yet it did not affect H2O2-induced actin and myosin assembly. Therefore, MLC phosphorylation does not appear to regulate H2O2-induced actin and myosin assembly but may play a role in induced actin rearrangement. The role of MLCK was also studied in hyperoxia-induced lung injury model (422). This study demonstrated that NADPH oxidase-mediated ROS generation during hyperoxia was inhibited by silencing nmMLCK in lung endothelial cells. In addition, nmMLCK−/− mice show decreased ROS production, phosphorylation of MLC, and pulmonary vascular leak. These results provide strong evidence for the involvement of nonmuscle MLCK in the assembly and activation of NADPH oxidase and ROS formation during hyperoxia.
Depletion of MLCK by using siRNA was also shown to reduce LPS-induced phosphorylation of mono and diphospho-MLC and restored barrier function (43). Inflammatory mediators are known to inactivate myosin-associated phosphatases, which further promoted MLC phosphorylation (111, 112). Phosphorylation of MLC phosphatase subunit (MYPT1) was increased by ROS followed by cell contraction (199). Taken together, ROS-mediated activation of MLC kinase contributes to actin reorganization in endothelial cells.
C. The Ras superfamily of GTPases
The Ras family of small GTP-binding proteins are activated by a variety of agonists, growth factors, TNF-α, G-proteins, and ROS and regulate a wide range of biological processes, including reorganization of the actin cytoskeleton, co-ordinated migration, proliferation, tumorigenesis, and cell barrier functions (2, 32, 147). This small GTPases superfamily is subdivided into five subfamilies: Rho, Ras, Rab, Ran, and Arf (394). These proteins act as switches by cycling between active (GTP-bound) and inactive (GDP-bound) conformations. The activation state of these small molecules is regulated by: GEFs, which promote exchange of GTP for GDP; GTPase-activating proteins (GAPs), which inactivate G proteins by enhancing the GTP-hydrolysis; and guanine-nucleotide-dissociation inhibitors, which bind to GDP-bound small proteins and regulate translocation between membranes and the cytosol (394). The active form of these small molecules interacts with their downstream targets and mediates their biological functions.
The mammalian Rho GTPase family consists of more than 20 distinct members, while the Ras family has 36 members. In this section, we focus on the regulation of endothelial barrier functions by the best-characterized members of RhoGTPase family: RhoA, Rac1, and CDC42 and Ras family GTPase Rap1 (449). RhoA activation downstream of several endothelial permeability-increasing mediators causes loss of endothelial junctions by enhancing actomyosin contractility and increasing isometric tension at the cell margins (426). However, Rac1 and Cdc42 are required for the assembly and maturation of endothelial junctions, and their activity increases during junction formation (144, 226, 234, 449). In contrast, other studies showed that activation of Rac1 downstream of VEGF and other growth factors increased endothelial permeability (226, 234). This points to the notion that an optimal level of Rac activity is required to maintain the stability of endothelial junctions and either inhibition or hyper-activation can disrupt the AJs.
Sundaresan et al. (400) showed that activated Rac1 increased the ROS generation in fibroblasts. This was further confirmed by multiple reports in response to different stimuli in endothelial cells (92, 463). Over-expression of active Rac1 induced tyrosine phosphorylation of VE-cadherin and increased endothelial permeability in an ROS-dependent manner (427). Contrary to this, active RhoA induced pronounced stress fiber formation but did not induce loss of cell–cell adhesion, indicating that Rac-mediated ROS generation was specifically involved in loss of cell–cell adhesion (427).
The role of RhoA family GTPases has also been investigated in endothelial cells under hypoxic condition. Rac1 and RhoA oppose each other during changes in oxygen tension. In addition, expression of dominant negative Rac1 (N17Rac1) strongly inhibited RhoA and NADPH oxidase activity, suggesting that Rac1 acted upstream of RhoA during hypoxia/reoxygenation stress (427, 455). Interestingly, there are also other mechanisms through which ROS can directly affect RhoA activity and RhoA-mediated cytoskeletal rearrangement. Campbell et al. (176) found that the treatment of purified recombinant GTPase with superoxide in vitro resulted in guanine nucleotide dissociation. Furthermore, using cysteine to alanine mutants, Aghajanian et al. (2) demonstrated that ROS-mediated activation of RhoA was dependent on cysteines 16 and 20.
It has been known for several years that Cdc42 activation is required to maintain endothelial barrier function. Barrier-stabilizing effect of oxidized phospholipids was blocked by siRNA targeting Cdc42 (38), and expression of constitutively active Cdc42 abolished LPS-induced lung vascular permeability in vivo (357). These studies suggest an obligatory role of Cdc42 for barrier maintenance. Although there is little information regarding the direct effects of ROS on Cdc42 function, studies showed the involvement of Cdc42 in ethanol-induced NOX activation and ROS generation and subsequently, reorganization of actin filaments in endothelial cells (437). Further studies are needed to fully understand the mechanisms by which this important modulator of the cell cytoskeleton regulates endothelial barrier function under oxidant stress conditions.
D. PKCs
The PKC isoforms are divided into three subclasses according to their structure, activation, and substrate requirements. The calcium-dependent conventional or classical PKC isoforms (α, β1/2, and γ) are regulated by diacylglycerol (DAG) or phosphatidylserine (PS), whereas the novel PKCs are Ca2+ independent (δ, ε, η, θ, and μ) and activated by DAG or PS. The atypical PKCs (ζ, λ) are regulated by PS and are independent of DAG and Ca2+ (250, 334). The PKC activity is regulated by its translocation to the membrane and phosphorylation and generally denotes enzyme activation (334). PKC is the target for multiple tyrosine phosphorylations by various tyrosine kinases, including Src family kinases (304, 396). These modifications further increase kinase activity of PKC (243). Several PKC inhibitors such as staurosporine, H7, calphostin C, bisindolylmaleimide, and chelerytherine inhibited endothelial hyper-permeability in response to different pro-inflammatory agents (133, 200, 341, 358, 460). A number of studies have demonstrated that membrane translocation and activation of the atypical isoforms of PKCζ is required in thrombin-mediated increase in endothelial permeability (244, 294). Depletion of PKCζ by siRNA or overexpression of dominant negative mutants of PKCζ abolished thrombin-induced hyper-permeability by inhibiting phosphorylation of MLC and activation of RhoA (294). In agreement with these findings, PKC-α was shown to induce phosphorylation of p120-catenin in response to thrombin or LPS, which reduced p120-catenin binding affinity for VE-cadherin and subsequently disrupted endothelial junction integrity (429). TNF-α-mediated endothelial barrier dysfunction was shown to be dependent on an increase in PKC-α activity. Furthermore, the antisense of PKC-α reduced the TNF-α-induced increase in endothelial permeability (120). Other studies have reported that VEGF can increase vascular permeability through activation of PKC-β in vivo and, importantly, oral administration of PKC β-isoform-selective inhibitors partially inhibited VEGF-induced vascular permeability (4). Moreover, Titchenell et al. (414) have shown that pseudo-substrate peptide inhibitors of atypical PKC isoform such as aPKC-I-PD and aPKC-I-diCl can inhibit VEGF-induced endothelial permeability. These studies support a prominent role of different PKC isoforms in microvascular leakage associated with different pro-inflammatory mediators. Increased PKC activity has also been associated with various vascular disorders such as atherosclerosis, hypoxia, and ischemia reperfusion (138, 174, 286, 447).
In the endothelium, H2O2 is also known to activate multiple PKC isoforms (224, 225). There are different mechanisms through which ROS can activate PKC. All PKC isoforms contain redox-sensitive cysteine residues in both regulatory and catalytic domains, which are required for autoinhibition and catalytic activity, respectively. Direct oxidative modification of the regulatory domain results in increased PKC activity (143). ROS can also regulate PKC activation by generating different mediators and cofactors, including PLC and PLD (267, 438). Oxidant-mediated activation of PKC also occurred through phosphorylation of distinct residues of different PKC isoforms (224). Several lines of evidence suggest that PKC is likely to play a permissive role in H2O2-mediated endothelial permeability and lung edema (200, 389, 470). Johnson et al. (200) showed that H2O2-induced lung edema and stress fiber formation in ECs were inhibited by protein kinase blocker H-7. Another study demonstrated that the selective inhibition of PKC by calphostin C abolished H2O2-mediated myosin light-chain phosphorylation in ECs (470). Taher et al. (406) demonstrated H2O2-inducing translocation of PKC from cytosol to membrane and increased membrane-associated PKC activity. Thus, PKCs act as a key mediator of inflammation and microvascular hyper-permeability under stimulated conditions.
E. Toll-like receptors
TLRs are a subset of pathogen-associated pattern recognition receptors family that are classified on the basis of homology to the cytoplasmic domain of the interleukin-1 receptor (IL-1R) family, also known as the Toll/IL-1R (TIR) domain (391). A total of 10 and 14 TLRs have been identified in human and mouse, respectively (63, 405). TLRs are expressed in a wide range of immune cells, including DCs, macrophages, PMNs, and certain nonimmune cells such as endothelial and epithelial cells (28, 220). Among all members, TLR4 is the most widely characterized and well-established receptor for LPS signaling. LPS-binding protein recognizes the lipid-A moiety of LPS and brings LPS to the cell surface to form a ternary complex with CD14, which facilitates the transfer of LPS to TLR4 and MD-2 (306). This complex then initiates intracellular signals through two adaptor proteins, TRIF and myD88, which recruit multiple cytoplasmic signaling molecules TRAF6, TIRAP and the kinases RICK and IRAK, and activates downstream signaling components involving JNK, p38, and NF-κB (84). A number of studies showed that LPS-TLR4 axis is involved in endothelial barrier disruption by different pathways. LPS-mediated TLR4 activation induced tyrosine phosphorylation of junctional proteins, including VE-cadherin, p120-catenin, and γ-catenin through activation of Src family kinases to open the endothelial paracellular pathway (139). Liu et al. (248) showed that LPS/TLR4 signaling activated Src family kinases by enhancing the interaction of TRAF6 with cSrc and Fyn and promoting transendothelial albumin flux. In another study, Wang et al. (442) reported a direct role of p120-catenin in the regulation of LPS-mediated increase in endothelial permeability. They showed that the inhibition of p120-catenin expression in endothelial cells increased MyD88 and TLR4 association, ICAM-1 expression and enhanced the severity of LPS-induced lung inflammation.
Increasing evidence suggests that enhanced ROS generation is central in the activation of TLR-mediated signaling pathways. Ryan et al. (365) showed that ROS are involved in LPS/TLR4-mediated IL-8 signaling. Antioxidant treatment decreased LPS-induced nuclear translocation of NF-κB as well as IL-8 production in human monocytes. Park et al. (336) have proposed a direct mechanism through which TLR4 regulates ROS formation. They demonstrated that TLR4 interacted with the COOH-terminal region of NOX4 in HEK293 cells, and knockdown of NOX4 inhibited LPS-mediated NF-κB activation (335). NOX4 was also shown to be required for LPS-induced ROS generation and NF-κB activation in endothelial cells (335). Knockdown of NOX4 significantly inhibited LPS-induced MCP1 production, ICAM-1 expression, and adhesion of monocytes to endothelial cells (335). TLR4 also induced TLR2 expression in human endothelial cells in an NF-κB-dependent manner (119). A unique mechanism of cross-talk between TLR4/TLR2 has been proposed; that is, the role of PMN NADPH oxidase complex in mediating the cross-talk between TLR4 and TLR2 (116). Impairment of PMN NADPH oxidase markedly decreased the LPS-induced NF-κB activation and TLR2 up-regulation in endothelial cells (116). In addition, LPS did not up-regulate the TLR2 expression in TLR4 knockout mice, indicating that PMN NADPH oxidase mediates TLR2 up-regulation in endothelial cells and is an important mechanism that is responsible for amplifying PMN transmigration to the site of infection (118).
VI. Oxidative Stress and Tissue Injury
Accumulation of activated macrophages at the site of injury is the characteristic feature of chronic inflammatory diseases (236). Macrophages are evolutionary “designed” to eliminate pathogens by producing excessive oxidative stress, which, if excessive and unchecked, can lead to tissue damage. However, the current literature has shown that macrophages after undergoing a phenotypic shift also help in tissue repair. According to current classification, there are two distinct populations of macrophages: M1 or classically activated macrophages, which contribute to tissue injury by releasing large quantities of highly reactive cytotoxic oxidants to destroy pathogens and M2 or alternatively activated macrophages, which suppress inflammation and help in wound resolution by phagocytizing dead neutrophils and synthesizing molecules that are responsible for tissue remodeling such as TGFβ, VEGF, and EGF (236, 302). Both classes of macrophages have a distinct cytokine profile that distinguish their behavior; M1 macrophages produce excessive oxidative stress and secrete pro-inflammatory cytokines TNFα, IL-1, and IL-6, whereas M2 macrophages secrete anti-inflammatory cytokines IL-4, IL-10, and IL-13 (236). The classically activated macrophages induce tissue injury by releasing chemokines and reactive oxidants that induce cell death by activation of cell death receptors, culminating in caspase activation via either an extrinsic pathway (i.e., mitochondria independent) or an intrinsic pathway (i.e., mitochondrial dependent) (64). In addition, oxidative stress produced by macrophages can induce cell death through creating an imbalance in antioxidant GSH equilibrium (64). Activation of caspases appears to be central to most of the pathways of cell death.
A. Extrinsic pathways of cell death and regulation by ROS
The extrinsic pathway of cell death is mediated by cell death receptors, which after binding with their ligands initiate protein–protein interactions, resulting in activation of the initiator caspases. There are four major cell death receptors, including TNF receptor 1 (TNFR1), TNF-related apoptosis-inducing ligand receptor 1 (TRAIL-R1), TRAIL receptor 2 (TRAIL-R2), and Fas receptor. The ligands that bind to these receptors belong to TNF superfamily of cytokines TNFα, Fas ligand, and TRAIL (64). TNF-α-induced cell death has been attributed to excessive ROS generation-sustained JNK activation and caspase activation (Fig. 12) (90, 369, 457). TNF-α is a principal plieotropic cytokine that is secreted by activated macrophges which possesses a variety of biological properties such as production of inflammatory cytokines, cell proliferation, and cell death. It was first defined by William Coley in his findings that cancer patients have necrotic tumors when they developed bacterial infections and was formally referred to as Coley's toxins (452). The active component inducing cell death in tumors was later identified as a cytokine secreted by macrophages and hence, termed tumor necrosis factor (68). TNFR1 signaling under resting conditions is inhibited by association with a 60-kD cytoplasmic protein known as silencer of death domains (SODD). Within minutes of TNFR1 activation by TNF-α, this auto-inhibition is released, the adaptor proteins TNFR1-associated death domain (TRADD) and Fas-associated death domain (FADD) are recruited, and the collective death-inducing signaling complex (DISC) is internalized. Release of endosomal DISC to the cytosol further recruits initiator procaspase-8, resulting in its activation (64). Similar to TNFR1, the apoptosis induced by FasR has been attributed to enhanced ROS generation via NADPH oxidases and caspase activation. The apoptosis induced by FasR likewise depends on the recruitment of FADD and pro-caspase-8 activation. A key feature of FasR-mediated apoptosis is regulation by FLICE inhibitory protein (FLIP), which inhibits apoptosis by preventing the binding of procaspase-8 to FADD (189). The enhanced ROS generation down-regulates FLIP protein and executes FasR-dependent apoptosis (436). The extent of caspase-8 activation is an essential determinant of cells undergoing mitochondrial independent or dependent apoptosis (377). Extensive caspase-8 activation directly activated effector caspases such as caspase-3 and induced cell death, whereas low caspase-8 activation activated an amplification loop that was dependent on the mitochondria (described in the next section).
FIG. 12.
Schematic representation of ROS-mediated cell death and cell survival signaling pathways. The intricate balance between cell death and cell survival is largely modulated by intracellular ROS generation. In general, high intracellular ROS generation causes cell death by activation of cell death pathways (mitochondrial dependent and independent), whereas low levels of ROS acts as a signaling molecules that help in cell survival. The major cell death receptors such as TNF receptor–1 (TNFR1) and FasR cause cell death by enhancing high intracellular ROS production. The binding of TNF alpha to TNFR1 recruits proteins such as TNFR1-associated death domain (TRADD) and TNFα-receptor-associated factor 2 (TRAF2) to activate the TNFR1 signaling. The associated TRADD further recruits Fas-associated death domain (FADD) and pro-caspase-8, and this whole signaling complex known as death-inducing signaling complex (DISC) is endocytosed, resulting in caspase-8 activation. Similar to TNFR1, FasR recruits FADD and activates caspase-8. The extent of caspase-8 activation determines whether a cell will follow a mitochondrial-dependent pathway or an independent pathway. Low caspase-8 activation follows a mitochondrial-dependent amplification loop that is accompanied by caspase-8-mediated cleavage of pro-apoptotic bid (truncated bid [t-bid]), which causes Cyt-c release via oligomerization of Bax/Bak proteins in mitochondria. The released cytochrome c then subsequently binds apoptosis activation factor-1 (Apaf-1) and caspase-9 and activates effector caspases such as caspase-3, which causes cell death. In the event of significant caspase-8 activation, it directly activates casapse-3 independent of mitochondria. High intracellular ROS induces sustained JNK activation and causes mitochondrial cytochrome c release-dependent cell death. High intracellular ROS also causes cardiolipin (CL) oxidation, which is translocated to the outer membrane and provides the docking site for t-bid, facilitating the cytochrome c-release. In contrast, low levels of intracellular ROS mediate a transient JNK activation that helps in cell survival by activating AP-1 transcription factor and anti-apoptotic genes. TNFR1 signaling also activates NF-κB transcription factor by recruiting TRAF-2 and receptor-interacting kinase 1 (RIP1) proteins. NF-κB activation is involved in the transcription of manganese SOD (MnSOD) and anti-apoptotic signaling proteins such as cIAP-2 and Bcl-xL. MnSOD puts a negative feedback loop on enhanced ROS generation and switches off the apoptotic pathway. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
However, TNF-α does not always induce apoptosis in all cells, because it can alternatively activate at least two cell survival pathways (Fig. 12). TNF-α activates NF-κB by recruiting TNFα-receptor-associated factor 2 (TRAF2) and receptor-interacting kinase 1 (RIP1), which has pro-survival effects by induction of anti-apoptotic proteins (e.g., anti-apoptotic proteins of Bcl2 family and caspase inhibitors) and suppressed TNF-α induced ROS generation by enhancing expression of MnSOD (205, 369). Further, TNF-α mediated an early and transient activation of JNK/Jun, which contributed to cell survival in conjunction with NF-κB by activation of the AP-1 transcription factor (232). In the absence of NF-κB, a massive accumulation of H2O2 leads to prolonged activation of JNK kinase, which induced cell death (90, 205, 369).
Although caspase activation and enhanced ROS generation are central to the mechanism of apoptosis, there are reports suggesting no significant role of ROS in the progression of apoptosis and showing that reduced ROS generation is, in fact, the cause of apoptosis (15, 122). This is surprising, as the efficacy of pharmacological antioxidants such as GSH, NAC, deferoxamine (283, 420, 425), and antioxidant enzymes such as catalase or GPx (149, 283) in preventing apoptosis have been shown by several groups. Other studies, however, were unable to demonstrate any role of antioxidants in preventing apoptosis (15, 66, 122). These differences may be ascribed to the use of different cell types, different methods of measuring ROS, and the particular antioxidants tested. In addition, plasma membrane permeability of different cell types to antioxidant enzymes can greatly influence the analysis.
B. Intrinsic pathways of cell death and regulation by ROS
The intrinsic pathways of cell death are dependent on increased mitochondrial outer membrane permeability (MOMP), which causes mitochondria-to-cytosol release of apoptogenic proteins such as Cytochrome-c (Cyt-c), Smac/Diablo, apoptosis-inducing factor (AIF), and endonuclease G, which trigger cell death in either a caspase-dependent or -independent manner (Fig. 12) (151, 230). This phenomenon of increased MOMP can be induced by excessive calcium entry, high oxidative stress, and compounds, which result in mitochondrial membrane depolarization (417). The released Cyt-c then binds to apoptosis activation factor-1 (Apaf-1) and recruits initiator pro-caspase 9, which undergoes auto-activation. This whole complex is collectively known as “apoptosome” (151) (Fig. 12). Caspase-9 then activated the effector caspases such as caspase-3. Smac/Diablo also facilitates the activation of effector caspases by removing the blockage by inhibitor of apoptosis proteins (IAPs) (417). AIF, on activation, induced DNA condensation and cleavage (50). EndoG-mediated DNA fragmentation is the final event in the apoptosis. The mitochondrial release of Cyt-c is considered the major determinant of cell death.
The pro-apoptotic member of Bcl-2 family such as Bid (BH3 interacting-domain death agonist) can enhance MOMP by cleavage with caspase-8. The truncated bid after caspases cleavage then induces oligomerization of pro-apoptotic proteins Bax/Bak, which then integrated in the outer mitochondrial membrane and triggered Cyt-c release (342). However, recombinant oligomeric Bax protein alone contributed only 18% of Cyt-c release from brain mitochondria (342). When combined together, recombinant Bax and complex I inhibitors (which is known to induce oxidative stress) induced approximately 65% of the mitochondrial Cyt-c release (342), indicating that oxidative stress is the major trigger in Cyt-c release from mitochondria. The release of Cyt-c further enhanced ROS generation from mitochondria because of uncoupling of ETC. An anionic phospholipid specific to mitochondria known as cardiolipin (CL) helps in anchoring of Cyt-c to the inner mitochondrial membrane. Enhanced oxidative stress leads to oxidation of CL, which decreases the affinity binding with Cyt-c (218, 388). A growing number of reports have strongly implicated the role of oxidized CL in Cyt-c release (64, 203, 309, 327). The content of oxidized CL was increased in mitochondria isolated from ischemic myocardium, which potentially contributed to the ischemia/reperfusion injury (345). However, the mechanism of oxidized CL-mediated Cyt-c release is not yet clear. According to Gonzalvez et al. (141, 142), oxidized CL is translocated to the outer mitochondrial membrane provided a docking site for tBid, enabling mitochondrial outer membrane permeabilization and Cyt-c movement. However, contradictory reports indicate that oxidized CL does not play a role in Bax-mediated pore formation (194, 195). Interestingly, Kagan et al. showed that a pool of CL-bound mitochondrial Cyt-c functions as a peroxidase that catalyzes peroxidation of CL (204). The peroxidase activity of Cyt-c can be stimulated at a lower H2O2 concentration when bound to CL-containing membranes compared with unbound (35), suggesting that increased oxidative stress can enhance the peroxidase activity of Cyt-c, enabling CL peroxidation and execution of cell death.
Independent of apoptosis by Bcl-2 family members, the mitochondrial permeability transition (MPT) induced by the opening of mitochondrial permeability transition pore (mPTP) can also lead to cell death. Unlike MOMP, mPTP enhances permeability of inner mitochondrial membrane and is independent of Cyt-c release. The opening of the mPTP causes mitochondrial swelling due to massive influx of solute and water, resulting in mitochondrial depolarization and cessation of ATP synthesis (215). mPTP is the underlying event in primary necrosis. However, apoptotic cells can undergo secondary necrosis if their removal is delayed. In contrast to primary necrosis, mitochondrial depolarization in secondary necrosis can be coincident or followed by Cyt-c release. mPTP is a megapore spanning the inner and outer mitochondrial membranes, which is hypothesized to consist of at least three components (72): outer membrane channel known as VDAC, inner membrane channel known as adenine-nucleotide translocase (ANT), and cyclophilin-D (CypD) present in mitochondrial matrix. CypD, although present in the mitochondrial matrix, becomes associated with the inner mitochondrial membrane during the MPT (72, 160). Although MOMP and mPTP appear to be distinct pathways, a cross-talk has been reported between them. Mice deficient in Bax/Bak render cells resistant to mPTP opening and necrosis (451). Superoxide-induced mitochondrial Cyt-c release in HepG2 cells depends on VDAC, and blockers of VDAC inhibited the release (259). ANT and cyclophilin D are also targets of oxidative stress, and their oxidative modification has been implicated in an increase in mitochondrial permeability and Cyt-c release (20, 135). Mice deficient in CypD were protected from ischemia/reperfusion-induced cell death in vivo and primary hepatocytes and fibroblasts isolated from CypD null mice were protected from Ca2+ overload and oxidative stress-induced cell death (20). Therefore, it seems that there is an intricate relationship between these two pathways which is likely modulated by oxidative stress during inflammation.
VII. Conclusion and Future Remarks
Intensive research into the mechanisms of inflammation in the last decade has described the outlines of the intricate relationship between oxidative stress and inflammation. Clearance of pathogens from the body is the principle objective of inflammation for which ROS produced by phagocytic cells plays a fundamental role. The ambient levels of ROS are important for homeostasis of cells; whereas excessive ROS are important in killing pathogens. If ROS are not controlled by an array of sophisticated antioxidant mechanisms, it leads to inflammatory tissue injury. The antioxidant machinery of the cell, thus, plays an essential role in setting up this intricate balance. Despite an understanding of the role of oxidants in the inflammatory progression, treatments utilizing a variety of antioxidant approaches have not yet been successful. Failure of antioxidant clinical trials (207) indicates gaps in our general understanding whether oxidative stress is salutatory or detrimental in inflammation. Since oxidants have homeostatic and well-calibrated signaling functions in cells, untargeted antioxidant therapy may not prove to be efficacious or may be efficacious under unique circumstances. It is also possible that the distribution of antioxidants at specific sites in specific cells will be effective as opposed to a generalized alteration in the redox balance. A related issue is the compartmentalized nature of ROS production that is not easily accessible to antioxidants (146, 213). While it is clear that ROS are important to the pathogenesis of inflammation and tissue injury, much remains to be learned about how ROS function physiologically and how they contribute to the mechanism of inflammation and tissue injury.
Sources of Funding
This work was supported by the AHA Midwest affiliate Postdoctoral fellowship (13POST16640000) to M.M., U.S. National Institutes of Health grants P01 HL77806, P01 HL 060678, R01 HL 045638, and R01 HL 090152 to A.B.M. and HL66109 and ES11863 to S.P.R.
Abbreviations Used
- 4-HNE
4-hydroxy-2-nonenal
- ADMA
asymmetrical dimethyl arginines
- AIF
apoptosis-inducing factor
- AJ
Adherens junction
- Ang II
angiotensin II
- ANT
adenine-nucleotide translocase
- Apaf-1
apoptosis activation factor-1
- ARDS
acute respiratory distress syndrome
- ARE
antioxidant response element
- ASC
apoptosis-associated speck-like protein
- BH4
tetrahydrobiopterin
- CAMs
cell adhesion molecules
- CC
coiled-coil
- CD99L2
CD99 antigen like 2
- CL
cardiolipin
- CTD
C-terminal domain
- Cyt-c
Cytochrome-c
- DAG
diacylglycerol
- DAMP
danger-associated molecular pattern
- DCs
dendritic cells
- DISC
death-inducing signaling complex
- eNOS
endothelial NOS
- ESAM
endothelial cell-selective adhesion molecule
- ESL-1
E-selectin ligand-1
- ETC
electron transport chain
- F-actin
filamentous actin
- FAD
flavin-adenine dinucleotide
- FADD
Fas-associated death domain
- GEF
guanine nucleotide exchange factor
- GMP-140
granule membrane protein-140
- GPx
glutathione peroxidase
- GSH
glutathione
- GUK
guanylate kinase
- H2O2
hydrogen peroxide
- HEVs
high endothelial venules
- HOCl
hypochlorous acid
- ICAM-1
intercellular adhesion molecule-1
- IEJs
inter-endothelial junctions
- IL-1
interleukin-1
- iNOS
inducible NOS
- JAMs
junctional adhesion molecules
- JMD
juxtamembrane domain
- Keap1
Kelch-like ECH-associated protein 1
- LFA-1
leukocyte function–associated antigen 1
- LPS
lipopolysaccharide
- MDCK
Madin Darby Canine Kidney
- MLCK
myosin light chain kinase
- MnSOD
manganese SOD
- MOMP
mitochondrial outer membrane permeability
- MPT
mitochondrial permeability transition
- mPTP
mitochondrial permeability transition pore
- MtROS
mitochondrial derived ROS
- NAC
N-acetyl cysteine
- NLR
nod like receptors
- nNOS
neuronal NOS
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nrf2
NF-E2-related factor 2
- OH•
hydroxyl radical
- PAF
platelet activating factor
- PAMP
pathogen-associated molecular pattern
- PMNs
polymorphonuclear neutrophils
- PRRs
pattern-recognition receptors
- PS
phosphatidylserine
- PSGL-1
P-selectin glycoprotein ligand-1
- RIP1
receptor-interacting kinase 1
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBP-2
thioredoxin-binding protein-2
- TEMs
tetraspanin-enriched microdomains
- TIR
Toll/IL-1R
- TJs
tight junctions
- TLRs
Toll-like receptors
- TNFR1
TNF-α receptor–1
- TRAF2
TNFα-receptor-associated factor 2
- TRAIL-R1
TNF-related apoptosis-inducing ligand receptor 1
- TRAPS
tumor necrosis factor receptor-associated periodic syndrome
- Trx
thioredoxin
- VCAM-1
vascular cell adhesion molecule-1
- VDAC
voltage-dependent anion channels
- VDUP1
vitamin D3 up-regulated protein 1
- VLA4
very late antigen 4
- XDH
xanthine dehydrogenase
- XO
xanthine oxidase
References
- 1.Abid MR, Schoots IG, Spokes KC, Wu SQ, Mawhinney C, and Aird WC. Vascular endothelial growth factor-mediated induction of manganese superoxide dismutase occurs through redox-dependent regulation of forkhead and IkappaB/NF-kappaB. J Biol Chem 279: 44030–44038, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian A, Wittchen ES, Campbell SL, and Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One 4: e8045, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ago T, Kuroda J, Pain J, Fu C, Li H, and Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, and King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes 46: 1473–1480, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Aikawa M, Sugiyama S, Hill CC, Voglic SJ, Rabkin E, Fukumoto Y, Schoen FJ, Witztum JL, and Libby P. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation 106: 1390–1396, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Allen PG. Functional consequences of disulfide bond formation in gelsolin. FEBS Lett 401: 89–94, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Allingham MJ, van Buul JD, and Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 179: 4053–4064, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Aloisi F. and Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 6: 205–217, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, and Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol 128: 1243–1253, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alp NJ. and Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Amara N, Goven D, Prost F, Muloway R, Crestani B, and Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 65: 733–738, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, and Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Arcaro A. The small GTP-binding protein Rac promotes the dissociation of gelsolin from actin filaments in neutrophils. J Biol Chem 273: 805–813, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Arita Y, Harkness SH, Kazzaz JA, Koo HC, Joseph A, Melendez JA, Davis JM, Chander A, and Li Y. Mitochondrial localization of catalase provides optimal protection from H2O2-induced cell death in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 290: L978–L986, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Aronis A, Melendez JA, Golan O, Shilo S, Dicter N, and Tirosh O. Potentiation of Fas-mediated apoptosis by attenuated production of mitochondria-derived reactive oxygen species. Cell Death Differ 10: 335–344, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Arrate MP, Rodriguez JM, Tran TM, Brock TA, and Cunningham SA. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J Biol Chem 276: 45826–45832, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Aurrand-Lions M, Duncan L, Ballestrem C, and Imhof BA. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J Biol Chem 276: 2733–2741, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Aurrand-Lions M, Johnson-Leger C, Wong C, Du PL, and Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood 98: 3699–3707, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Babior BM. NADPH oxidase: an update. Blood 93: 1464–1476, 1999 [PubMed] [Google Scholar]
- 20.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, and Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, and Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation 106: 544–549, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Balzer M, Lintschinger B, and Groschner K. Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovasc Res 42: 543–549, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Banfi B, Clark RA, Steger K, and Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278: 3510–3513, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, and Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 157: 1233–1245, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barreiro O, Zamai M, Yanez-Mo M, Tejera E, Lopez-Romero P, Monk PN, Gratton E, Caiolfa VR, and Sanchez-Madrid F. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol 183: 527–542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, and Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest 108: 1513–1522, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barthel SR, Gavino JD, Descheny L, and Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets 11: 1473–1491, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basu S. and Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol 286: L887–L892, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Basuroy S, Bhattacharya S, Leffler CW, and Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 296: C422–C432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, and Vestweber D. Association of Csk to VE-cadherin and inhibition of cell proliferation. EMBO J 24: 1686–1695, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazzoni G. and Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 84: 869–901, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Beckers CM, van Hinsbergh VW, and van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost 103: 40–55, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol 9: 836–844, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Belaiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, and Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med 42: 446–459, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, and Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry 45: 4998–5009, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bendall JK, Cave AC, Heymes C, Gall N, and Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105: 293–296, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, and Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282: 1183–1192, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, and Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Bixel MG, Li H, Petri B, Khandoga AG, Khandoga A, Zarbock A, Wolburg-Buchholz K, Wolburg H, Sorokin L, Zeuschner D, Maerz S, Butz S, Krombach F, and Vestweber D. CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood 116: 1172–1184, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Bixel MG, Petri B, Khandoga AG, Khandoga A, Wolburg-Buchholz K, Wolburg H, Marz S, Krombach F, and Vestweber D. A CD99-related antigen on endothelial cells mediates neutrophil but not lymphocyte extravasation in vivo. Blood 109: 5327–5336, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Blaschuk OW, Oshima T, Gour BJ, Symonds JM, Park JH, Kevil CG, Trocha SD, Michaud S, Okayama N, Elrod JW, Alexander JS, and Sasaki M. Identification of an occludin cell adhesion recognition sequence. Inflammation 26: 193–198, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Block K, Gorin Y, and Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogatcheva NV, Zemskova MA, Poirier C, Mirzapoiazova T, Kolosova I, Bresnick AR, and Verin AD. The suppression of myosin light chain (MLC) phosphorylation during the response to lipopolysaccharide (LPS): beneficial or detrimental to endothelial barrier? J Cell Physiol 226: 3132–3146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, and Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res 87: 99–105, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Borregaard N. Neutrophils, from marrow to microbes. Immunity 33: 657–670, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Bowler RP, Nicks M, Tran K, Tanner G, Chang LY, Young SK, and Worthen GS. Extracellular superoxide dismutase attenuates lipopolysaccharide-induced neutrophilic inflammation. Am J Respir Cell Mol Biol 31: 432–439, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Brady TC, Chang LY, Day BJ, and Crapo JD. Extracellular superoxide dismutase is upregulated with inducible nitric oxide synthase after NF-kappa B activation. Am J Physiol 273: L1002–L1006, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, and Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208: 519–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bunting M, Harris ES, McIntyre TM, Prescott SM, and Zimmerman GA. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr Opin Hematol 9: 30–35, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Cande C, Cecconi F, Dessen P, and Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci 115: 4727–4734, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Carlsson LM, Jonsson J, Edlund T, and Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci U S A 92: 6264–6268, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carman CV. and Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol 167: 377–388, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de GA, Poelmann R, Lupu F, Herbert JM, Collen D, and Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98: 147–157, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, and Barazzone AC. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 180: 972–981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, and Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol 162: 1111–1122, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caveda L, Martin-Padura I, Navarro P, Breviario F, Corada M, Gulino D, Lampugnani MG, and Dejana E. Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin). J Clin Invest 98: 886–893, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ceriello A, Falleti E, Motz E, Taboga C, Tonutti L, Ezsol Z, Gonano F, and Bartoli E. Hyperglycemia-induced circulating ICAM-1 increase in diabetes mellitus: the possible role of oxidative stress. Horm Metab Res 30: 146–149, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Ceriello A, Novials A, Ortega E, La SL, Pujadas G, Testa R, Bonfigli AR, Esposito K, and Giugliano D. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes 61: 2993–2997, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chai YC, Ashraf SS, Rokutan K, Johnston RB, Jr., and Thomas JA. S-thiolation of individual human neutrophil proteins including actin by stimulation of the respiratory burst: evidence against a role for glutathione disulfide. Arch Biochem Biophys 310: 273–281, 1994 [DOI] [PubMed] [Google Scholar]
- 60.Chandel NS, Trzyna WC, McClintock DS, and Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol 165: 1013–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Chen MZ, Zhu X, Sun HQ, Mao YS, Wei Y, Yamamoto M, and Yin HL. Oxidative stress decreases phosphatidylinositol 4,5-bisphosphate levels by deactivating phosphatidylinositol-4-phosphate 5-kinase beta in a Syk-dependent manner. J Biol Chem 284: 23743–23753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Q, Powell DW, Rane MJ, Singh S, Butt W, Klein JB, and McLeish KR. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol 170: 5302–5308, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Chuang TH. and Ulevitch RJ. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw 11: 372–378, 2000 [PubMed] [Google Scholar]
- 64.Circu ML. and Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48: 749–762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clark R. and Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol 125: 629–637, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Clement MV. and Stamenkovic I. Superoxide anion is a natural inhibitor of FAS-mediated cell death. EMBO J 15: 216–225, 1996 [PMC free article] [PubMed] [Google Scholar]
- 67.Clerch LB, Wright A, Chung DJ, and Massaro D. Early divergent lung antioxidant enzyme expression in response to lipopolysaccharide. Am J Physiol 271: L949–L954, 1996 [DOI] [PubMed] [Google Scholar]
- 68.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res 3–11, 1991 [PubMed] [Google Scholar]
- 69.Cominacini L, Fratta PA, Garbin U, Campagnola M, Davoli A, Rigoni A, Zenti MG, Pastorino AM, and Lo C V. E-selectin plasma concentration is influenced by glycaemic control in NIDDM patients: possible role of oxidative stress. Diabetologia 40: 584–589, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, and Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood 97: 1679–1684, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, and Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A 96: 9815–9820, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crompton M, Virji S, Doyle V, Johnson N, and Ward JM. The mitochondrial permeability transition pore. Biochem Soc Symp 66: 167–179, 1999 [DOI] [PubMed] [Google Scholar]
- 73.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 1797: 897–906, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Dal SD, Moreira AP, Freitas A, Silva JS, Rossi MA, Ferreira SH, and Cunha FQ. Nitric oxide inhibits neutrophil migration by a mechanism dependent on ICAM-1: role of soluble guanylate cyclase. Nitric Oxide 15: 77–86, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Dal SD, Paron JA, de Oliveira SH, Ferreira SH, Silva JS, and Cunha FQ. Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide 9: 153–164, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Dalle-Donne I, Giustarini D, Colombo R, Milzani A, and Rossi R. S-glutathionylation in human platelets by a thiol-disulfide exchange-independent mechanism. Free Radic Biol Med 38: 1501–1510, 2005 [DOI] [PubMed] [Google Scholar]
- 77.DalleDonne I, Milzani A, and Colombo R. H2O2-treated actin: assembly and polymer interactions with cross-linking proteins. Biophys J 69: 2710–2719, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DalleDonne I, Milzani A, and Colombo R. The tert-butyl hydroperoxide-induced oxidation of actin Cys-374 is coupled with structural changes in distant regions of the protein. Biochemistry 38: 12471–12480, 1999 [DOI] [PubMed] [Google Scholar]
- 79.Dang PM, Cross AR, and Babior BM. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558. Proc Natl Acad Sci U S A 98: 3001–3005, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dang PM, Cross AR, Quinn MT, and Babior BM. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558 II. Proc Natl Acad Sci U S A 99: 4262–4265, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dang PM, Dewas C, Gaudry M, Fay M, Pedruzzi E, Gougerot-Pocidalo MA, and El BJ. Priming of human neutrophil respiratory burst by granulocyte/macrophage colony-stimulating factor (GM-CSF) involves partial phosphorylation of p47(phox). J Biol Chem 274: 20704–20708, 1999 [DOI] [PubMed] [Google Scholar]
- 82.Dang PM, Fontayne A, Hakim J, El BJ, and Perianin A. Protein kinase C zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol 166: 1206–1213, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, and El-Benna J. A specific p47phox-serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest 116: 2033–2043, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dauphinee SM. and Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest 86: 9–22, 2006 [DOI] [PubMed] [Google Scholar]
- 85.De MG, Bravi MC, Laurenti O, Cassone-Faldetta M, Armiento A, Ferri C, and Balsano F. Influence of reduced glutathione infusion on glucose metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism 47: 993–997, 1998 [DOI] [PubMed] [Google Scholar]
- 86.De MG, Bravi MC, Laurenti O, Cassone-Faldetta M, Proietti A, De LO, Armiento A, and Ferri C. Reduction of oxidative stress by oral N-acetyl-L-cysteine treatment decreases plasma soluble vascular cell adhesion molecule-1 concentrations in non-obese, non-dyslipidaemic, normotensive, patients with non-insulin-dependent diabetes. Diabetologia 41: 1392–1396, 1998 [DOI] [PubMed] [Google Scholar]
- 87.Dekker LV, Leitges M, Altschuler G, Mistry N, McDermott A, Roes J, and Segal AW. Protein kinase C-beta contributes to NADPH oxidase activation in neutrophils. Biochem J 347 Pt 1: 285–289, 2000 [PMC free article] [PubMed] [Google Scholar]
- 88.DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, and Nauseef WM. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest 101: 455–463, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeMaio L, Rouhanizadeh M, Reddy S, Sevanian A, Hwang J, and Hsiai TK. Oxidized phospholipids mediate occludin expression and phosphorylation in vascular endothelial cells. Am J Physiol Heart Circ Physiol 290: H674–H683, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Deng Y, Ren X, Yang L, Lin Y, and Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell 115: 61–70, 2003 [DOI] [PubMed] [Google Scholar]
- 91.Denu JM. and Dixon JE. Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Curr Opin Chem Biol 2: 633–641, 1998 [DOI] [PubMed] [Google Scholar]
- 92.Deshpande SS, Angkeow P, Huang J, Ozaki M, and Irani K. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J 14: 1705–1714, 2000 [DOI] [PubMed] [Google Scholar]
- 93.Dewas C, Dang PM, Gougerot-Pocidalo MA, and El-Benna J. TNF-alpha induces phosphorylation of p47(phox) in human neutrophils: partial phosphorylation of p47phox is a common event of priming of human neutrophils by TNF-alpha and granulocyte-macrophage colony-stimulating factor. J Immunol 171: 4392–4398, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Dewas C, Fay M, Gougerot-Pocidalo MA, and El-Benna J. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. J Immunol 165: 5238–5244, 2000 [DOI] [PubMed] [Google Scholar]
- 95.Di A, Gao XP, Qian F, Kawamura T, Han J, Hecquet C, Ye RD, Vogel SM, and Malik AB. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol 13: 29–34, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DiMauro S. and Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med 348: 2656–2668, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Ding Y, Winters A, Ding M, Graham S, Akopova I, Muallem S, Wang Y, Hong JH, Gryczynski Z, Yang SH, Birnbaumer L, and Ma R. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J Biol Chem 286: 31799–31809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dostert C, Petrilli V, Van BR, Steele C, Mossman BT, and Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doughan AK, Harrison DG, and Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Dreher D. and Junod AF. Differential effects of superoxide, hydrogen peroxide, and hydroxyl radical on intracellular calcium in human endothelial cells. J Cell Physiol 162: 147–153, 1995 [DOI] [PubMed] [Google Scholar]
- 102.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 103.Dudek SM. and Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001 [DOI] [PubMed] [Google Scholar]
- 104.Dupont GP, Huecksteadt TP, Marshall BC, Ryan US, Michael JR, and Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. J Clin Invest 89: 197–202, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dyugovskaya L, Lavie P, and Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 165: 934–939, 2002 [DOI] [PubMed] [Google Scholar]
- 106.El BJ, Faust RP, Johnson JL, and Babior BM. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J Biol Chem 271: 6374–6378, 1996 [DOI] [PubMed] [Google Scholar]
- 107.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, and Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem 284: 1559–1569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.England K. and Cotter TG. Direct oxidative modifications of signalling proteins in mammalian cells and their effects on apoptosis. Redox Rep 10: 237–245, 2005 [DOI] [PubMed] [Google Scholar]
- 109.England K, O'Driscoll C, and Cotter TG. Carbonylation of glycolytic proteins is a key response to drug-induced oxidative stress and apoptosis. Cell Death Differ 11: 252–260, 2004 [DOI] [PubMed] [Google Scholar]
- 110.Erzurum SC, Danel C, Gillissen A, Chu CS, Trapnell BC, and Crystal RG. In vivo antioxidant gene expression in human airway epithelium of normal individuals exposed to 100% O2. J Appl Physiol 75: 1256–1262, 1993 [DOI] [PubMed] [Google Scholar]
- 111.Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, and Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem 273: 21867–21874, 1998 [DOI] [PubMed] [Google Scholar]
- 112.Essler M, Staddon JM, Weber PC, and Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol 164: 6543–6549, 2000 [DOI] [PubMed] [Google Scholar]
- 113.Etzioni A, Frydman M, Pollack S, Avidor I, Phillips ML, Paulson JC, and Gershoni-Baruch R. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med 327: 1789–1792, 1992 [DOI] [PubMed] [Google Scholar]
- 114.Evans R, Patzak I, Svensson L, De FK, Jones K, McDowall A, and Hogg N. Integrins in immunity. J Cell Sci 122: 215–225, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Everitt EA, Malik AB, and Hendey B. Fibronectin enhances the migration rate of human neutrophils in vitro. J Leukoc Biol 60: 199–206, 1996 [DOI] [PubMed] [Google Scholar]
- 116.Fan J, Frey RS, and Malik AB. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest 112: 1234–1243, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fan J, Frey RS, Rahman A, and Malik AB. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem 277: 3404–3411, 2002 [DOI] [PubMed] [Google Scholar]
- 118.Fan J. and Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med 9: 315–321, 2003 [DOI] [PubMed] [Google Scholar]
- 119.Faure E, Thomas L, Xu H, Medvedev A, Equils O, and Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol 166: 2018–2024, 2001 [DOI] [PubMed] [Google Scholar]
- 120.Ferro T, Neumann P, Gertzberg N, Clements R, and Johnson A. Protein kinase C-alpha mediates endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol 278: L1107–L1117, 2000 [DOI] [PubMed] [Google Scholar]
- 121.Forstermann U. and Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 122.Franco R, Panayiotidis MI, and Cidlowski JA. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J Biol Chem 282: 30452–30465, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, Bachi A, Vandekerckhove J, Gianazza E, and Ghezzi P. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci U S A 99: 3505–3510, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gaboury JP, Anderson DC, and Kubes P. Molecular mechanisms involved in superoxide-induced leukocyte-endothelial cell interactions in vivo. Am J Physiol 266: H637–H642, 1994 [DOI] [PubMed] [Google Scholar]
- 125.Gao X, Kouklis P, Xu N, Minshall RD, Sandoval R, Vogel SM, and Malik AB. Reversibility of increased microvessel permeability in response to VE-cadherin disassembly. Am J Physiol Lung Cell Mol Physiol 279: L1218–L1225, 2000 [DOI] [PubMed] [Google Scholar]
- 126.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, and Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J Immunol 168: 3974–3982, 2002 [DOI] [PubMed] [Google Scholar]
- 127.Garcia JG, Davis HW, and Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 163: 510–522, 1995 [DOI] [PubMed] [Google Scholar]
- 128.This reference has been deleted.
- 129.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH, and Imhof BA. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS One 6: e14665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gavard J. and Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8: 1223–1234, 2006 [DOI] [PubMed] [Google Scholar]
- 131.Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, and Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension 50: 189–196, 2007 [DOI] [PubMed] [Google Scholar]
- 132.Geiszt M, Kopp JB, Varnai P, and Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ginsberg J. and Murray PG. Phorbol ester and phospholipase C-mediated differentiated thyroid function in vitro: the effects of protein kinase C inhibition and downregulation. Thyroid 1: 195–200, 1991 [DOI] [PubMed] [Google Scholar]
- 134.Girard JP. and Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today 16: 449–457, 1995 [DOI] [PubMed] [Google Scholar]
- 135.Giron-Calle J, Zwizinski CW, and Schmid HH. Peroxidative damage to cardiac mitochondria. II. Immunological analysis of modified adenine nucleotide translocase. Arch Biochem Biophys 315: 1–7, 1994 [DOI] [PubMed] [Google Scholar]
- 136.Goeckeler ZM. and Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol 130: 613–627, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goetz DJ, el-Sabban ME, Pauli BU, and Hammer DA. Dynamics of neutrophil rolling over stimulated endothelium in vitro. Biophys J 66: 2202–2209, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goldberg M, Zhang HL, and Steinberg SF. Hypoxia alters the subcellular distribution of protein kinase C isoforms in neonatal rat ventricular myocytes. J Clin Invest 99: 55–61, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, and Goldblum SE. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem 283: 13437–13449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gonzalez-Mariscal L, Quiros M, and Diaz-Coranguez M. ZO proteins and redox-dependent processes. Antioxid Redox Signal 15: 1235–1253, 2011 [DOI] [PubMed] [Google Scholar]
- 141.Gonzalvez F, Bessoule JJ, Rocchiccioli F, Manon S, and Petit PX. Role of cardiolipin on tBid and tBid/Bax synergistic effects on yeast mitochondria. Cell Death Differ 12: 659–667, 2005 [DOI] [PubMed] [Google Scholar]
- 142.Gonzalvez F, Pariselli F, Dupaigne P, Budihardjo I, Lutter M, Antonsson B, Diolez P, Manon S, Martinou JC, Goubern M, Wang X, Bernard S, and Petit PX. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ 12: 614–626, 2005 [DOI] [PubMed] [Google Scholar]
- 143.Gopalakrishna R. and Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A 86: 6758–6762, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gopalakrishnan S, Dunn KW, and Marrs JA. Rac1, but not RhoA, signaling protects epithelial adherens junction assembly during ATP depletion. Am J Physiol Cell Physiol 283: C261–C272, 2002 [DOI] [PubMed] [Google Scholar]
- 145.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, and Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 146.Gorlach A, Klappa P, and Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal 8: 1391–1418, 2006 [DOI] [PubMed] [Google Scholar]
- 147.Gorovoy M, Neamu R, Niu J, Vogel S, Predescu D, Miyoshi J, Takai Y, Kini V, Mehta D, Malik AB, and Voyno-Yasenetskaya T. RhoGDI-1 modulation of the activity of monomeric RhoGTPase RhoA regulates endothelial barrier function in mouse lungs. Circ Res 101: 50–58, 2007 [DOI] [PubMed] [Google Scholar]
- 148.Goto H, Nishikawa T, Sonoda K, Kondo T, Kukidome D, Fujisawa K, Yamashiro T, Motoshima H, Matsumura T, Tsuruzoe K, and Araki E. Endothelial MnSOD overexpression prevents retinal VEGF expression in diabetic mice. Biochem Biophys Res Commun 366: 814–820, 2008 [DOI] [PubMed] [Google Scholar]
- 149.Gouaze V, Andrieu-Abadie N, Cuvillier O, Malagarie-Cazenave S, Frisach MF, Mirault ME, and Levade T. Glutathione peroxidase-1 protects from CD95-induced apoptosis. J Biol Chem 277: 42867–42874, 2002 [DOI] [PubMed] [Google Scholar]
- 150.Graf E, Mahoney JR, Bryant RG, and Eaton JW. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem 259: 3620–3624, 1984 [PubMed] [Google Scholar]
- 151.Green DR. and Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004 [DOI] [PubMed] [Google Scholar]
- 152.Green K, Brand MD, and Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53Suppl 1: S110–S118, 2004 [DOI] [PubMed] [Google Scholar]
- 153.Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, and Shah AM. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol 47: 817–826, 2006 [DOI] [PubMed] [Google Scholar]
- 154.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, and Lichtman AH. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 188: 6287–6299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, and Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal 11: 2505–2516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Groschner K, Hingel S, Lintschinger B, Balzer M, Romanin C, Zhu X, and Schreibmayer W. Trp proteins form store-operated cation channels in human vascular endothelial cells. FEBS Lett 437: 101–106, 1998 [DOI] [PubMed] [Google Scholar]
- 157.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, and Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, and Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hafezi-Moghadam A, Thomas KL, Prorock AJ, Huo Y, and Ley K. L-selectin shedding regulates leukocyte recruitment. J Exp Med 193: 863–872, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Halestrap AP, Clarke SJ, and Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res 61: 372–385, 2004 [DOI] [PubMed] [Google Scholar]
- 161.Hampton MB, Kettle AJ, and Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92: 3007–3017, 1998 [PubMed] [Google Scholar]
- 162.Han D, Antunes F, Canali R, Rettori D, and Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278: 5557–5563, 2003 [DOI] [PubMed] [Google Scholar]
- 163.Handy DE. and Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal 16: 1323–1367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, and Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9: 163–173, 2002 [DOI] [PubMed] [Google Scholar]
- 165.Hara-Chikuma M, Chikuma S, Sugiyama Y, Kabashima K, Verkman AS, Inoue S, and Miyachi Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J Exp Med 209: 1743–1752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Haraldsen G, Kvale D, Lien B, Farstad IN, and Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol 156: 2558–2565, 1996 [PubMed] [Google Scholar]
- 167.Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, and Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell 82: 643–653, 1995 [DOI] [PubMed] [Google Scholar]
- 168.Hastie LE, Patton WF, Hechtman HB, and Shepro D. Filamin redistribution in an endothelial cell reoxygenation injury model. Free Radic Biol Med 22: 955–966, 1997 [DOI] [PubMed] [Google Scholar]
- 169.Hastie LE, Patton WF, Hechtman HB, and Shepro D. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. J Cell Physiol 172: 373–381, 1997 [DOI] [PubMed] [Google Scholar]
- 170.He C. and Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43: 67–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hecquet CM, Ahmmed GU, Vogel SM, and Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102: 347–355, 2008 [DOI] [PubMed] [Google Scholar]
- 172.Hecquet CM. and Malik AB. Role of H(2)O(2)-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb Haemost 101: 619–625, 2009 [PMC free article] [PubMed] [Google Scholar]
- 173.Heiner I, Eisfeld J, Warnstedt M, Radukina N, Jungling E, and Luckhoff A. Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J 398: 225–232, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hempel A, Lindschau C, Maasch C, Mahn M, Bychkov R, Noll T, Luft FC, and Haller H. Calcium antagonists ameliorate ischemia-induced endothelial cell permeability by inhibiting protein kinase C. Circulation 99: 2523–2529, 1999 [DOI] [PubMed] [Google Scholar]
- 175.Henderson RB, Lim LH, Tessier PA, Gavins FN, Mathies M, Perretti M, and Hogg N. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J Exp Med 194: 219–226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Heo J. and Campbell SL. Mechanism of redox-mediated guanine nucleotide exchange on redox-active Rho GTPases. J Biol Chem 280: 31003–31010, 2005 [DOI] [PubMed] [Google Scholar]
- 177.Heyworth PG, Cross AR, and Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol 15: 578–584, 2003 [DOI] [PubMed] [Google Scholar]
- 178.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, and Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: E14–E22, 2001 [DOI] [PubMed] [Google Scholar]
- 179.Hjelmstrom P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J Leukoc Biol 69: 331–339, 2001 [PubMed] [Google Scholar]
- 180.Ho YS. Transgenic and knockout models for studying the role of lung antioxidant enzymes in defense against hyperoxia. Am J Respir Crit Care Med 166: S51–S56, 2002 [DOI] [PubMed] [Google Scholar]
- 181.Hogg N, Patzak I, and Willenbrock F. The insider's guide to leukocyte integrin signalling and function. Nat Rev Immunol 11: 416–426, 2011 [DOI] [PubMed] [Google Scholar]
- 182.Huot J, Houle F, Marceau F, and Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res 80: 383–392, 1997 [DOI] [PubMed] [Google Scholar]
- 183.Hwang C, Lodish HF, and Sinskey AJ. Measurement of glutathione redox state in cytosol and secretory pathway of cultured cells. Methods Enzymol 251: 212–221, 1995 [DOI] [PubMed] [Google Scholar]
- 184.Hwang C, Sinskey AJ, and Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257: 1496–1502, 1992 [DOI] [PubMed] [Google Scholar]
- 185.Ichimura H, Parthasarathi K, Quadri S, Issekutz AC, and Bhattacharya J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J Clin Invest 111: 691–699, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci 116: 1157–1173, 2003 [DOI] [PubMed] [Google Scholar]
- 187.Inoue K, Takano H, Koike E, Warabi E, Yanagawa T, Yanagisawa R, and Ishii T. Peroxiredoxin I is a negative regulator of Th2-dominant allergic asthma. Int Immunopharmacol 9: 1281–1288, 2009 [DOI] [PubMed] [Google Scholar]
- 188.Inoue N, Ramasamy S, Fukai T, Nerem RM, and Harrison DG. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res 79: 32–37, 1996 [DOI] [PubMed] [Google Scholar]
- 189.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, and Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature 388: 190–195, 1997 [DOI] [PubMed] [Google Scholar]
- 190.Ishii T, Warabi E, and Yanagawa T. Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr 50: 91–105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, and Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, and Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, and Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8: 379–391, 2003 [DOI] [PubMed] [Google Scholar]
- 194.Iverson SL, Enoksson M, Gogvadze V, Ott M, and Orrenius S. Cardiolipin is not required for Bax-mediated cytochrome c release from yeast mitochondria. J Biol Chem 279: 1100–1107, 2004 [DOI] [PubMed] [Google Scholar]
- 195.Iverson SL. and Orrenius S. The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys 423: 37–46, 2004 [DOI] [PubMed] [Google Scholar]
- 196.Jankov RP, Kantores C, Pan J, and Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol 294: L233–L245, 2008 [DOI] [PubMed] [Google Scholar]
- 197.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, and Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med 45: 329–335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jin BY, Lin AJ, Golan DE, and Michel T. MARCKS protein mediates hydrogen peroxide regulation of endothelial permeability. Proc Natl Acad Sci U S A 109: 14864–14869, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jin L, Linder AE, Mills TM, and Webb RC. Inhibition of the tonic contraction in the treatment of erectile dysfunction. Expert Opin Ther Targets 7: 265–276, 2003 [DOI] [PubMed] [Google Scholar]
- 200.Johnson A, Phillips P, Hocking D, Tsan MF, and Ferro T. Protein kinase inhibitor prevents pulmonary edema in response to H2O2. Am J Physiol 256: H1012–H1022, 1989 [DOI] [PubMed] [Google Scholar]
- 201.Jones PL, Ping D, and Boss JM. Tumor necrosis factor alpha and interleukin-1beta regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-beta and NF-kappaB. Mol Cell Biol 17: 6970–6981, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jornot L. and Junod AF. Response of human endothelial cell antioxidant enzymes to hyperoxia. Am J Respir Cell Mol Biol 6: 107–115, 1992 [DOI] [PubMed] [Google Scholar]
- 203.Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, and Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med 37: 1963–1985, 2004 [DOI] [PubMed] [Google Scholar]
- 204.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, and Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1: 223–232, 2005 [DOI] [PubMed] [Google Scholar]
- 205.Kamata H, Honda S, Maeda S, Chang L, Hirata H, and Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120: 649–661, 2005 [DOI] [PubMed] [Google Scholar]
- 206.Kamp DW, Shacter E, and Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park) 25: 400–410; 413, 2011 [PubMed] [Google Scholar]
- 207.Katsiki N. and Manes C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin Nutr 28: 3–9, 2009 [DOI] [PubMed] [Google Scholar]
- 208.Kawahara T, Kohjima M, Kuwano Y, Mino H, Teshima-Kondo S, Takeya R, Tsunawaki S, Wada A, Sumimoto H, and Rokutan K. Helicobacter pylori lipopolysaccharide activates Rac1 and transcription of NADPH oxidase Nox1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am J Physiol Cell Physiol 288: C450–C457, 2005 [DOI] [PubMed] [Google Scholar]
- 209.Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149: 43–50, 2000 [DOI] [PubMed] [Google Scholar]
- 210.Keilhack H, Hellman U, van HJ, van RF, Godovac-Zimmermann J, and Bohmer FD. The protein-tyrosine phosphatase SHP-1 binds to and dephosphorylates p120 catenin. J Biol Chem 275: 26376–26384, 2000 [DOI] [PubMed] [Google Scholar]
- 211.Kevil CG, Okayama N, Trocha SD, Kalogeris TJ, Coe LL, Specian RD, Davis CP, and Alexander JS. Expression of zonula occludens and adherens junctional proteins in human venous and arterial endothelial cells: role of occludin in endothelial solute barriers. Microcirculation 5: 197–210, 1998 [PubMed] [Google Scholar]
- 212.Kevil CG, Oshima T, Alexander B, Coe LL, and Alexander JS. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol 279: C21–C30, 2000 [DOI] [PubMed] [Google Scholar]
- 213.Kietzmann T. Intracellular redox compartments: mechanisms and significances. Antioxid Redox Signal 13: 395–398, 2010 [DOI] [PubMed] [Google Scholar]
- 214.Kikuchi N, Ishii Y, Morishima Y, Yageta Y, Haraguchi N, Yamadori T, Masuko H, Sakamoto T, Yanagawa T, Warabi E, Ishii T, and Hizawa N. Aggravation of bleomycin-induced pulmonary inflammation and fibrosis in mice lacking peroxiredoxin I. Am J Respir Cell Mol Biol 45: 600–609, 2011 [DOI] [PubMed] [Google Scholar]
- 215.Kim JS, He L, and Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304: 463–470, 2003 [DOI] [PubMed] [Google Scholar]
- 216.Kim KS, Takeda K, Sethi R, Pracyk JB, Tanaka K, Zhou YF, Yu ZX, Ferrans VJ, Bruder JT, Kovesdi I, Irani K, Goldschmidt-Clermont P, and Finkel T. Protection from reoxygenation injury by inhibition of rac1. J Clin Invest 101: 1821–1826, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kinnula VL. and Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 167: 1600–1619, 2003 [DOI] [PubMed] [Google Scholar]
- 218.Kirkland RA, Adibhatla RM, Hatcher JF, and Franklin JL. Loss of cardiolipin and mitochondria during programmed neuronal death: evidence of a role for lipid peroxidation and autophagy. Neuroscience 115: 587–602, 2002 [DOI] [PubMed] [Google Scholar]
- 219.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, and Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8: pii:, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kluwe J, Mencin A, and Schwabe RF. Toll-like receptors, wound healing, and carcinogenesis. J Mol Med (Berl) 87: 125–138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kokura S, Wolf RE, Yoshikawa T, Granger DN, and Aw TY. Molecular mechanisms of neutrophil-endothelial cell adhesion induced by redox imbalance. Circ Res 84: 516–524, 1999 [DOI] [PubMed] [Google Scholar]
- 222.Kolaczkowska E. and Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159–175, 2013 [DOI] [PubMed] [Google Scholar]
- 223.Kong XJ, Lee SL, Lanzillo JJ, and Fanburg BL. Cu,Zn superoxide dismutase in vascular cells: changes during cell cycling and exposure to hyperoxia. Am J Physiol 264: L365–L375, 1993 [DOI] [PubMed] [Google Scholar]
- 224.Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, and Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A 94: 11233–11237, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, Ohmae K, Kikkawa U, and Nishizuka Y. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc Natl Acad Sci U S A 98: 6587–6592, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kouklis P, Konstantoulaki M, Vogel S, Broman M, and Malik AB. Cdc42 regulates the restoration of endothelial barrier function. Circ Res 94: 159–166, 2004 [DOI] [PubMed] [Google Scholar]
- 227.Krause KH. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol 42: 256–262, 2007 [DOI] [PubMed] [Google Scholar]
- 228.Kremerskothen J, Stolting M, Wiesner C, Korb-Pap A, van Vliet V, Linder S, Huber TB, Rottiers P, Reuzeau E, Genot E, and Pavenstadt H. Zona occludens proteins modulate podosome formation and function. FASEB J 25: 505–514, 2011 [DOI] [PubMed] [Google Scholar]
- 229.Krizbai IA, Bauer H, Bresgen N, Eckl PM, Farkas A, Szatmari E, Traweger A, Wejksza K, and Bauer HC. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol Neurobiol 25: 129–139, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kroemer G. and Reed JC. Mitochondrial control of cell death. Nat Med 6: 513–519, 2000 [DOI] [PubMed] [Google Scholar]
- 231.Krueger G. and Ellis CN. Psoriasis—recent advances in understanding its pathogenesis and treatment. J Am Acad Dermatol 53: S94–S100, 2005 [DOI] [PubMed] [Google Scholar]
- 232.Lamb JA, Ventura JJ, Hess P, Flavell RA, and Davis RJ. JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell 11: 1479–1489, 2003 [DOI] [PubMed] [Google Scholar]
- 233.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 234.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, and Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell 13: 1175–1189, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, and Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Laskin DL, Sunil VR, Gardner CR, and Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol 51: 267–288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, and Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1288, 2001 [DOI] [PubMed] [Google Scholar]
- 238.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr., Dionne L, Lu N, Huang S, and Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A 93: 9782–9787, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ledebur HC. and Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem 270: 933–943, 1995 [DOI] [PubMed] [Google Scholar]
- 240.Lee CF, Qiao M, Schroder K, Zhao Q, and Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res 106: 1489–1497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, and Shah AM. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res 90: 143–150, 2002 [DOI] [PubMed] [Google Scholar]
- 242.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, and Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal 10: 1687–1698, 2008 [DOI] [PubMed] [Google Scholar]
- 243.Li W, Mischak H, Yu JC, Wang LM, Mushinski JF, Heidaran MA, and Pierce JH. Tyrosine phosphorylation of protein kinase C-delta in response to its activation. J Biol Chem 269: 2349–2352, 1994 [PubMed] [Google Scholar]
- 244.Li X, Hahn CN, Parsons M, Drew J, Vadas MA, and Gamble JR. Role of protein kinase Czeta in thrombin-induced endothelial permeability changes: inhibition by angiopoietin-1. Blood 104: 1716–1724, 2004 [DOI] [PubMed] [Google Scholar]
- 245.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, and Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11: 376–381, 1995 [DOI] [PubMed] [Google Scholar]
- 246.Liang TW, DeMarco RA, Mrsny RJ, Gurney A, Gray A, Hooley J, Aaron HL, Huang A, Klassen T, Tumas DB, and Fong S. Characterization of huJAM: evidence for involvement in cell-cell contact and tight junction regulation. Am J Physiol Cell Physiol 279: C1733–C1743, 2000 [DOI] [PubMed] [Google Scholar]
- 247.Liby KT, Yore MM, and Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 7: 357–369, 2007 [DOI] [PubMed] [Google Scholar]
- 248.Liu A, Gong P, Hyun SW, Wang KZ, Cates EA, Perkins D, Bannerman DD, Puche AC, Toshchakov VY, Fang S, Auron PE, Vogel SN, and Goldblum SE. TRAF6 protein couples Toll-like receptor 4 signaling to Src family kinase activation and opening of paracellular pathway in human lung microvascular endothelia. J Biol Chem 287: 16132–16145, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liu G, Vogel SM, Gao X, Javaid K, Hu G, Danilov SM, Malik AB, and Minshall RD. Src phosphorylation of endothelial cell surface intercellular adhesion molecule-1 mediates neutrophil adhesion and contributes to the mechanism of lung inflammation. Arterioscler Thromb Vasc Biol 31: 1342–1350, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Liu Q, Ning W, Dantzer R, Freund GG, and Kelley KW. Activation of protein kinase C-zeta and phosphatidylinositol 3′-kinase and promotion of macrophage differentiation by insulin-like growth factor-I. J Immunol 160: 1393–1401, 1998 [PubMed] [Google Scholar]
- 251.Lo SK, Janakidevi K, Lai L, and Malik AB. Hydrogen peroxide-induced increase in endothelial adhesiveness is dependent on ICAM-1 activation. Am J Physiol 264: L406–L412, 1993 [DOI] [PubMed] [Google Scholar]
- 252.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, and Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51: 319–325, 2008 [DOI] [PubMed] [Google Scholar]
- 253.Lowe PM, Lee ML, Jackson CJ, To SS, Cooper AJ, and Schrieber L. The endothelium in psoriasis. Br J Dermatol 132: 497–505, 1995 [DOI] [PubMed] [Google Scholar]
- 254.Lowenstein CJ, Morrell CN, and Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med 15: 302–308, 2005 [DOI] [PubMed] [Google Scholar]
- 255.Luhn K, Wild MK, Eckhardt M, Gerardy-Schahn R, and Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet 28: 69–72, 2001 [DOI] [PubMed] [Google Scholar]
- 256.Lum H, Del Vecchio PJ, Schneider AS, Goligorsky MS, and Malik AB. Calcium dependence of the thrombin-induced increase in endothelial albumin permeability. J Appl Physiol 66: 1471–1476, 1989 [DOI] [PubMed] [Google Scholar]
- 257.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Madamanchi NR. and Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 100: 460–473, 2007 [DOI] [PubMed] [Google Scholar]
- 259.Madesh M. and Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol 155: 1003–1015, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Maier CM. and Chan PH. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 8: 323–334, 2002 [DOI] [PubMed] [Google Scholar]
- 261.Maier CM, Hsieh L, Crandall T, Narasimhan P, and Chan PH. A new approach for the investigation of reperfusion-related brain injury. Biochem Soc Trans 34: 1366–1369, 2006 [DOI] [PubMed] [Google Scholar]
- 262.Majno G. and Palade GE. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol 11: 571–605, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Majno G, Palade GE, and Schoefl GI. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol 11: 607–626, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Malik AB. and Fenton JW. Thrombin-mediated increase in vascular endothelial permeability. Semin Thromb Hemost 18: 193–199, 1992 [DOI] [PubMed] [Google Scholar]
- 265.Mambole A, Bigot S, Baruch D, Lesavre P, and Halbwachs-Mecarelli L. Human neutrophil integrin alpha9beta1: up-regulation by cell activation and synergy with beta2 integrins during adhesion to endothelium under flow. J Leukoc Biol 88: 321–327, 2010 [DOI] [PubMed] [Google Scholar]
- 266.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, and Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature 421: 748–753, 2003 [DOI] [PubMed] [Google Scholar]
- 267.Mangat R, Singal T, Dhalla NS, and Tappia PS. Inhibition of phospholipase C-gamma 1 augments the decrease in cardiomyocyte viability by H2O2. Am J Physiol Heart Circ Physiol 291: H854–H860, 2006 [DOI] [PubMed] [Google Scholar]
- 268.Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, and Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res 6: 11, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, and Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 1: 393–399, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Margis R, Dunand C, Teixeira FK, and Margis-Pinheiro M. Glutathione peroxidase family—an evolutionary overview. FEBS J 275: 3959–3970, 2008 [DOI] [PubMed] [Google Scholar]
- 271.Mariner DJ, Anastasiadis P, Keilhack H, Bohmer FD, Wang J, and Reynolds AB. Identification of Src phosphorylation sites in the catenin p120ctn. J Biol Chem 276: 28006–28013, 2001 [DOI] [PubMed] [Google Scholar]
- 272.Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A 79: 7634–7638, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, and Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142: 117–127, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Martinon F, Mayor A, and Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol 27: 229–265, 2009 [DOI] [PubMed] [Google Scholar]
- 275.Martyn KD, Frederick LM, von LK, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 276.Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, and Medford RM. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest 92: 1866–1874, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Maruyama Y, Ogura T, Mio K, Kiyonaka S, Kato K, Mori Y, and Sato C. Three-dimensional reconstruction using transmission electron microscopy reveals a swollen, bell-shaped structure of transient receptor potential melastatin type 2 cation channel. J Biol Chem 282: 36961–36970, 2007 [DOI] [PubMed] [Google Scholar]
- 278.Matheny HE, Deem TL, and Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol 164: 6550–6559, 2000 [DOI] [PubMed] [Google Scholar]
- 279.McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, Brooks TA, and Davis TP. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem 106: 2395–2409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, and Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem 103: 2540–2555, 2007 [DOI] [PubMed] [Google Scholar]
- 281.McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, Lochhead JJ, and Davis TP. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem 110: 58–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.McKelvey TG, Hollwarth ME, Granger DN, Engerson TD, Landler U, and Jones HP. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol 254: G753–G760, 1988 [DOI] [PubMed] [Google Scholar]
- 283.Medan D, Wang L, Toledo D, Lu B, Stehlik C, Jiang BH, Shi X, and Rojanasakul Y. Regulation of Fas (CD95)-induced apoptotic and necrotic cell death by reactive oxygen species in macrophages. J Cell Physiol 203: 78–84, 2005 [DOI] [PubMed] [Google Scholar]
- 284.Mehta D. and Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 285.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, and Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 31: 1000–1006, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Meier M. and King GL. Protein kinase C activation and its pharmacological inhibition in vascular disease. Vasc Med 5: 173–185, 2000 [DOI] [PubMed] [Google Scholar]
- 287.Meng TC, Fukada T, and Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9: 387–399, 2002 [DOI] [PubMed] [Google Scholar]
- 288.Meyer TN, Schwesinger C, Ye J, Denker BM, and Nigam SK. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J Biol Chem 276: 22048–22055, 2001 [DOI] [PubMed] [Google Scholar]
- 289.Miesel R. and Zuber M. Elevated levels of xanthine oxidase in serum of patients with inflammatory and autoimmune rheumatic diseases. Inflammation 17: 551–561, 1993 [DOI] [PubMed] [Google Scholar]
- 290.Miller EW, Dickinson BC, and Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A 107: 15681–15686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Milzani A, Rossi R, Di SP, Giustarini D, Colombo R, and DalleDonne I. The oxidation produced by hydrogen peroxide on Ca-ATP-G-actin. Protein Sci 9: 1774–1782, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Minami T, Horiuchi K, Miura M, Abid MR, Takabe W, Noguchi N, Kohro T, Ge X, Aburatani H, Hamakubo T, Kodama T, and Aird WC. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J Biol Chem 279: 50537–50554, 2004 [DOI] [PubMed] [Google Scholar]
- 293.Ming XF, Rajapakse AG, Yepuri G, Xiong Y, Carvas JM, Ruffieux J, Scerri I, Wu Z, Popp K, Li J, Sartori C, Scherrer U, Kwak BR, Montani JP, and Yang Z. Arginase II Promotes Macrophage Inflammatory Responses Through Mitochondrial Reactive Oxygen Species, Contributing to Insulin Resistance and Atherogenesis. J Am Heart Assoc 1: e000992, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Minshall RD, Vandenbroucke EE, Holinstat M, Place AT, Tiruppathi C, Vogel SM, van Nieuw Amerongen GP, Mehta D, and Malik AB. Role of protein kinase Czeta in thrombin-induced RhoA activation and inter-endothelial gap formation of human dermal microvessel endothelial cell monolayers. Microvasc Res 80: 240–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, and Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 296.Miyasaka M. and Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 4: 360–370, 2004 [DOI] [PubMed] [Google Scholar]
- 297.Moldovan L, Moldovan NI, Sohn RH, Parikh SA, and Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res 86: 549–557, 2000 [DOI] [PubMed] [Google Scholar]
- 298.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, and Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E58–E65, 2002 [DOI] [PubMed] [Google Scholar]
- 299.Monaghan-Benson E. and Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem 284: 25602–25611, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, and Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res 106: 1363–1373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Moore KL, Eaton SF, Lyons DE, Lichenstein HS, Cummings RD, and McEver RP. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J Biol Chem 269: 23318–23327, 1994 [PubMed] [Google Scholar]
- 302.Mosser DM. and Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Moy AB, Van EJ, Bodmer J, Kamath J, Keese C, Giaever I, Shasby S, and Shasby DM. Histamine and thrombin modulate endothelial focal adhesion through centripetal and centrifugal forces. J Clin Invest 97: 1020–1027, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Moyers JS, Bouton AH, and Parsons SJ. The sites of phosphorylation by protein kinase C and an intact SH2 domain are required for the enhanced response to beta-adrenergic agonists in cells overexpressing c-src. Mol Cell Biol 13: 2391–2400, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Muller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, and Krause G. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J Biol Chem 280: 3747–3756, 2005 [DOI] [PubMed] [Google Scholar]
- 306.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, and Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 3: 667–672, 2002 [DOI] [PubMed] [Google Scholar]
- 307.Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, and Shimizu N. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 54: 124–131, 1998 [DOI] [PubMed] [Google Scholar]
- 308.Naik E. and Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 208: 417–420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Nakagawa Y. Initiation of apoptotic signal by the peroxidation of cardiolipin of mitochondria. Ann N Y Acad Sci 1011: 177–184, 2004 [DOI] [PubMed] [Google Scholar]
- 310.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, and Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12: 222–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Nakamura T, Nakamura H, Hoshino T, Ueda S, Wada H, and Yodoi J. Redox regulation of lung inflammation by thioredoxin. Antioxid Redox Signal 7: 60–71, 2005 [DOI] [PubMed] [Google Scholar]
- 312.Nakamura T, Suchard SJ, Abe A, Shayman JA, and Boxer LA. Role of diradylglycerol formation in H2O2 and lactoferrin release in adherent human polymorphonuclear leukocytes. J Leukoc Biol 56: 105–109, 1994 [DOI] [PubMed] [Google Scholar]
- 313.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, and Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol 39: 1145–1150, 2002 [DOI] [PubMed] [Google Scholar]
- 314.Navarro P, Caveda L, Breviario F, Mandoteanu I, Lampugnani MG, and Dejana E. Catenin-dependent and -independent functions of vascular endothelial cadherin. J Biol Chem 270: 30965–30972, 1995 [DOI] [PubMed] [Google Scholar]
- 315.Navarro P, Ruco L, and Dejana E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol 140: 1475–1484, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, and Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J 21: 4885–4895, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nishikawa T. and Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 9: 343–353, 2007 [DOI] [PubMed] [Google Scholar]
- 318.Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, Matsumura T, and Araki E. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res Clin Pract 77Suppl 1: S161–S164, 2007 [DOI] [PubMed] [Google Scholar]
- 319.Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, Matsumura T, and Araki E. Impact of mitochondrial ROS production on diabetic vascular complications. Diabetes Res Clin Pract 77Suppl 1: S41–S45, 2007 [DOI] [PubMed] [Google Scholar]
- 320.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, and Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161: 653–660, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Notcovich C, Diez F, Tubio MR, Baldi A, Kazanietz MG, Davio C, and Shayo C. Histamine acting on H1 receptor promotes inhibition of proliferation via PLC, RAC, and JNK-dependent pathways. Exp Cell Res 316: 401–411, 2010 [DOI] [PubMed] [Google Scholar]
- 322.O'Dwyer MJ, Dempsey F, Crowley V, Kelleher DP, McManus R, and Ryan T. Septic shock is correlated with asymmetrical dimethyl arginine levels, which may be influenced by a polymorphism in the dimethylarginine dimethylaminohydrolase II gene: a prospective observational study. Crit Care 10: R139, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.O'Malley Y, Fink BD, Ross NC, Prisinzano TE, and Sivitz WI. Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J Biol Chem 281: 39766–39775, 2006 [DOI] [PubMed] [Google Scholar]
- 324.Omann GM, Harter JM, Burger JM, and Hinshaw DB. H2O2-induced increases in cellular F-actin occur without increases in actin nucleation activity. Arch Biochem Biophys 308: 407–412, 1994 [DOI] [PubMed] [Google Scholar]
- 325.Orlova VV, Economopoulou M, Lupu F, Santoso S, and Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J Exp Med 203: 2703–2714, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ostermann G, Weber KS, Zernecke A, Schroder A, and Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol 3: 151–158, 2002 [DOI] [PubMed] [Google Scholar]
- 327.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, and Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A 99: 1259–1263, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Overgaard CE, Daugherty BL, Mitchell LA, and Koval M. Claudins: control of barrier function and regulation in response to oxidant stress. Antioxid Redox Signal 15: 1179–1193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, and Kita T. Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol 163: 553–557, 1999 [PubMed] [Google Scholar]
- 330.Ozaki M, Kawashima S, Yamashita T, Hirase T, Namiki M, Inoue N, Hirata K, Yasui H, Sakurai H, Yoshida Y, Masada M, and Yokoyama M. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest 110: 331–340, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Pache JC, Carnesecchi S, Deffert C, Donati Y, Herrmann FR, Barazzone-Argiroffo C, and Krause KH. NOX-4 is expressed in thickened pulmonary arteries in idiopathic pulmonary fibrosis. Nat Med 17: 31–32, 2011 [DOI] [PubMed] [Google Scholar]
- 332.Pacher P, Nivorozhkin A, and Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58: 87–114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Parekh AB. and Putney JW, Jr., Store-operated calcium channels. Physiol Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 334.Parekh DB, Ziegler W, and Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J 19: 496–503, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Park HS, Chun JN, Jung HY, Choi C, and Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 72: 447–455, 2006 [DOI] [PubMed] [Google Scholar]
- 336.Park HS, Jung HY, Park EY, Kim J, Lee WJ, and Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173: 3589–3593, 2004 [DOI] [PubMed] [Google Scholar]
- 337.Patel KD, Zimmerman GA, Prescott SM, McEver RP, and McIntyre TM. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol 112: 749–759, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, and Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal 11: 747–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Pendyala S. and Natarajan V. Redox regulation of Nox proteins. Respir Physiol Neurobiol 174: 265–271, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, and Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11: 841–860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Peng J, He F, Zhang C, Deng X, and Yin F. Protein kinase C-alpha signals P115RhoGEF phosphorylation and RhoA activation in TNF-alpha-induced mouse brain microvascular endothelial cell barrier dysfunction. J Neuroinflammation 8: 28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M, Przedborski S, and Vila M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci U S A 102: 19126–19131, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, and Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411: 595–599, 2001 [DOI] [PubMed] [Google Scholar]
- 344.Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, and Scharenberg AM. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem 280: 6138–6148, 2005 [DOI] [PubMed] [Google Scholar]
- 345.Petrosillo G, Di VN, Ruggiero FM, Pistolese M, D'Agostino D, Tiravanti E, Fiore T, and Paradies G. Mitochondrial dysfunction associated with cardiac ischemia/reperfusion can be attenuated by oxygen tension control. Role of oxygen-free radicals and cardiolipin. Biochim Biophys Acta 1710: 78–86, 2005 [DOI] [PubMed] [Google Scholar]
- 346.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, and Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484, 2006 [DOI] [PubMed] [Google Scholar]
- 347.Pfannstiel J, Cyrklaff M, Habermann A, Stoeva S, Griffiths G, Shoeman R, and Faulstich H. Human cofilin forms oligomers exhibiting actin bundling activity. J Biol Chem 276: 49476–49484, 2001 [DOI] [PubMed] [Google Scholar]
- 348.Phillips ML, Schwartz BR, Etzioni A, Bayer R, Ochs HD, Paulson JC, and Harlan JM. Neutrophil adhesion in leukocyte adhesion deficiency syndrome type 2. J Clin Invest 96: 2898–2906, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Phillips PG, Lum H, Malik AB, and Tsan MF. Phallacidin prevents thrombin-induced increases in endothelial permeability to albumin. Am J Physiol 257: C562–C567, 1989 [DOI] [PubMed] [Google Scholar]
- 350.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, and Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med 203: 2569–2575, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Phillipson M, Kaur J, Colarusso P, Ballantyne CM, and Kubes P. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS One 3: e1649, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Piconi L, Quagliaro L, Da RR, Assaloni R, Giugliano D, Esposito K, Szabo C, and Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly(ADP-ribose) polymerase. J Thromb Haemost 2: 1453–1459, 2004 [DOI] [PubMed] [Google Scholar]
- 353.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, and Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3–TRPC4 heteromeric channels in endothelial cells. J Biol Chem 281: 13588–13595, 2006 [DOI] [PubMed] [Google Scholar]
- 354.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, and Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol 20: 645–651, 2000 [DOI] [PubMed] [Google Scholar]
- 355.Rahman I, Biswas SK, and Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 533: 222–239, 2006 [DOI] [PubMed] [Google Scholar]
- 356.Rahman I. and MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med 28: 1405–1420, 2000 [DOI] [PubMed] [Google Scholar]
- 357.Ramchandran R, Mehta D, Vogel SM, Mirza MK, Kouklis P, and Malik AB. Critical role of Cdc42 in mediating endothelial barrier protection in vivo. Am J Physiol Lung Cell Mol Physiol 295: L363–L369, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ramirez MM, Kim DD, and Duran WN. Protein kinase C modulates microvascular permeability through nitric oxide synthase. Am J Physiol 271: H1702–H1705, 1996 [DOI] [PubMed] [Google Scholar]
- 359.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, and Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368: 471–481, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, and Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol 182: 7264–7271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Roebuck KA. and Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol 66: 876–888, 1999 [DOI] [PubMed] [Google Scholar]
- 362.Rokutan K, Thomas JA, and Johnston RB, Jr., Phagocytosis and stimulation of the respiratory burst by phorbol diester initiate S-thiolation of specific proteins in macrophages. J Immunol 147: 260–264, 1991 [PubMed] [Google Scholar]
- 363.Romagnoli M, Gomez-Cabrera MC, Perrelli MG, Biasi F, Pallardo FV, Sastre J, Poli G, and Vina J. Xanthine oxidase-induced oxidative stress causes activation of NF-kappaB and inflammation in the liver of type I diabetic rats. Free Radic Biol Med 49: 171–177, 2010 [DOI] [PubMed] [Google Scholar]
- 364.Rowlands DJ, Islam MN, Das SR, Huertas A, Quadri SK, Horiuchi K, Inamdar N, Emin MT, Lindert J, Ten VS, Bhattacharya S, and Bhattacharya J. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J Clin Invest 121: 1986–1999, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ryan KA, Smith MF, Jr., Sanders MK, and Ernst PB. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect Immun 72: 2123–2130, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Sage PT. and Carman CV. Settings and mechanisms for trans-cellular diapedesis. Front Biosci 14: 5066–5083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, and Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456: 264–268, 2008 [DOI] [PubMed] [Google Scholar]
- 368.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, and Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11: 4131–4142, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, and Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 22: 3898–3909, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, and Tiruppathi C. Ca(2+) signalling and PKCalpha activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol 533: 433–445, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Sandoval R, Malik AB, Naqvi T, Mehta D, and Tiruppathi C. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 280: L239–L247, 2001 [DOI] [PubMed] [Google Scholar]
- 372.Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, and Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293: 1327–1330, 2001 [DOI] [PubMed] [Google Scholar]
- 373.Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, and Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med 196: 679–691, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Saran M. and Bors W. Oxygen radicals acting as chemical messengers: a hypothesis. Free Radic Res Commun 7: 213–220, 1989 [DOI] [PubMed] [Google Scholar]
- 375.Sasawatari S, Yoshizaki M, Taya C, Tazawa A, Furuyama-Tanaka K, Yonekawa H, Dohi T, Makrigiannis AP, Sasazuki T, Inaba K, and Toyama-Sorimachi N. The Ly49Q receptor plays a crucial role in neutrophil polarization and migration by regulating raft trafficking. Immunity 32: 200–213, 2010 [DOI] [PubMed] [Google Scholar]
- 376.Sbarra AJ. and Karnovsky ML. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem 234: 1355–1362, 1959 [PubMed] [Google Scholar]
- 377.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, and Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17: 1675–1687, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Schrauwen P. and Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 53: 1412–1417, 2004 [DOI] [PubMed] [Google Scholar]
- 379.Schroder K. and Tschopp J. The inflammasomes. Cell 140: 821–832, 2010 [DOI] [PubMed] [Google Scholar]
- 380.Schulz E, Wenzel P, Munzel T, and Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid Redox Signal 20: 308–324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, and Grimminger F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 162: 566–570, 2000 [DOI] [PubMed] [Google Scholar]
- 382.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, and Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 383.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, and Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, and Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 385.Sharma S, Smith A, Kumar S, Aggarwal S, Rehmani I, Snead C, Harmon C, Fineman J, Fulton D, Catravas JD, and Black SM. Mechanisms of nitric oxide synthase uncoupling in endotoxin-induced acute lung injury: role of asymmetric dimethylarginine. Vascul Pharmacol 52: 182–190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Shen BW, Perraud AL, Scharenberg A, and Stoddard BL. The crystal structure, and mutational analysis of human NUDT9. J Mol Biol 332: 385–398, 2003 [DOI] [PubMed] [Google Scholar]
- 387.Shi S, Verin AD, Schaphorst KL, Gilbert-McClain LI, Patterson CE, Irwin RP, Natarajan V, and Garcia JG. Role of tyrosine phosphorylation in thrombin-induced endothelial cell contraction and barrier function. Endothelium 6: 153–171, 1998 [DOI] [PubMed] [Google Scholar]
- 388.Shidoji Y, Hayashi K, Komura S, Ohishi N, and Yagi K. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochem Biophys Res Commun 264: 343–347, 1999 [DOI] [PubMed] [Google Scholar]
- 389.Siflinger-Birnboim A, Goligorsky MS, Del Vecchio PJ, and Malik AB. Activation of protein kinase C pathway contributes to hydrogen peroxide-induced increase in endothelial permeability. Lab Invest 67: 24–30, 1992 [PubMed] [Google Scholar]
- 390.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, and Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem 282: 7833–7843, 2007 [DOI] [PubMed] [Google Scholar]
- 391.Slack JL, Schooley K, Bonnert TP, Mitcham JL, Qwarnstrom EE, Sims JE, and Dower SK. Identification of two major sites in the type I interleukin-1 receptor cytoplasmic region responsible for coupling to pro-inflammatory signaling pathways. J Biol Chem 275: 4670–4678, 2000 [DOI] [PubMed] [Google Scholar]
- 392.Smith MA, Herson PS, Lee K, Pinnock RD, and Ashford ML. Hydrogen-peroxide-induced toxicity of rat striatal neurones involves activation of a non-selective cation channel. J Physiol 547: 417–425, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, and Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 95: 773–779, 2004 [DOI] [PubMed] [Google Scholar]
- 394.Spindler V, Schlegel N, and Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res 87: 243–253, 2010 [DOI] [PubMed] [Google Scholar]
- 395.Steegmaier M, Levinovitz A, Isenmann S, Borges E, Lenter M, Kocher HP, Kleuser B, and Vestweber D. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature 373: 615–620, 1995 [DOI] [PubMed] [Google Scholar]
- 396.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev 88: 1341–1378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Stelzner TJ, Weil JV, and O'Brien RF. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J Cell Physiol 139: 157–166, 1989 [DOI] [PubMed] [Google Scholar]
- 398.Stevenson BR, Siliciano JD, Mooseker MS, and Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103: 755–766, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Stralin P. and Marklund SL. Multiple cytokines regulate the expression of extracellular superoxide dismutase in human vascular smooth muscle cells. Atherosclerosis 151: 433–441, 2000 [DOI] [PubMed] [Google Scholar]
- 400.Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, and Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J 318 (Pt 2): 379–382, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Sunil VR, Shen J, Patel-Vayas K, Gow AJ, Laskin JD, and Laskin DL. Role of reactive nitrogen species generated via inducible nitric oxide synthase in vesicant-induced lung injury, inflammation and altered lung functioning. Toxicol Appl Pharmacol 261: 22–30, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, and Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci U S A 106: 250–255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, and Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24: 317–327, 2006 [DOI] [PubMed] [Google Scholar]
- 404.Sydow K. and Munzel T. ADMA and oxidative stress. Atheroscler Suppl 4: 41–51, 2003 [DOI] [PubMed] [Google Scholar]
- 405.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, and Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A 101: 3516–3521, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Taher MM, Garcia JG, and Natarajan V. Hydroperoxide-induced diacylglycerol formation and protein kinase C activation in vascular endothelial cells. Arch Biochem Biophys 303: 260–266, 1993 [DOI] [PubMed] [Google Scholar]
- 407.Tai LK, Okuda M, Abe J, Yan C, and Berk BC. Fluid shear stress activates proline-rich tyrosine kinase via reactive oxygen species-dependent pathway. Arterioscler Thromb Vasc Biol 22: 1790–1796, 2002 [DOI] [PubMed] [Google Scholar]
- 408.Takahashi N, Kozai D, Kobayashi R, Ebert M, and Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium 50: 279–287, 2011 [DOI] [PubMed] [Google Scholar]
- 409.Takeuchi O. and Akira S. Pattern recognition receptors and inflammation. Cell 140: 805–820, 2010 [DOI] [PubMed] [Google Scholar]
- 410.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, and Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem 278: 25234–25246, 2003 [DOI] [PubMed] [Google Scholar]
- 411.Taulet N, Delorme-Walker VD, and DerMardirossian C. Reactive oxygen species regulate protrusion efficiency by controlling actin dynamics. PLoS One 7: e41342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Telek G, Scoazec JY, Chariot J, Ducroc R, Feldmann G, and Roz C. Cerium-based histochemical demonstration of oxidative stress in taurocholate-induced acute pancreatitis in rats. A confocal laser scanning microscopic study. J Histochem Cytochem 47: 1201–1212, 1999 [DOI] [PubMed] [Google Scholar]
- 413.Thannickal VJ. and Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 414.Titchenell PM, Lin CM, Keil JM, Sundstrom JM, Smith CD, and Antonetti DA. Novel atypical PKC inhibitors prevent vascular endothelial growth factor-induced blood-retinal barrier dysfunction. Biochem J 446: 455–467, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Touyz RM. and Montezano AC. Vascular Nox4: a multifarious NADPH oxidase. Circ Res 110: 1159–1161, 2012 [DOI] [PubMed] [Google Scholar]
- 416.Tretter L, Sipos I, and Adam-Vizi V. Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson's disease. Neurochem Res 29: 569–577, 2004 [DOI] [PubMed] [Google Scholar]
- 417.Tsujimoto Y. and Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12: 835–840, 2007 [DOI] [PubMed] [Google Scholar]
- 418.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, and Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci 121: 29–37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Ukropec JA, Hollinger MK, Salva SM, and Woolkalis MJ. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J Biol Chem 275: 5983–5986, 2000 [DOI] [PubMed] [Google Scholar]
- 420.Um HD, Orenstein JM, and Wahl SM. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol 156: 3469–3477, 1996 [PubMed] [Google Scholar]
- 421.Usatyuk PV, Parinandi NL, and Natarajan V. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem 281: 35554–35566, 2006 [DOI] [PubMed] [Google Scholar]
- 422.Usatyuk PV, Singleton PA, Pendyala S, Kalari SK, He D, Gorshkova IA, Camp SM, Moitra J, Dudek SM, Garcia JG, and Natarajan V. Novel role for non-muscle myosin light chain kinase (MLCK) in hyperoxia-induced recruitment of cytoskeletal proteins, NADPH oxidase activation, and reactive oxygen species generation in lung endothelium. J Biol Chem 287: 9360–9375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, and Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol 178: 1279–1293, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.van Buul JD, Anthony EC, Fernandez-Borja M, Burridge K, and Hordijk PL. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating beta-catenin tyrosine phosphorylation. J Biol Chem 280: 21129–21136, 2005 [DOI] [PubMed] [Google Scholar]
- 425.van den Dobbelsteen DJ, Nobel CS, Schlegel J, Cotgreave IA, Orrenius S, and Slater AF. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J Biol Chem 271: 15420–15427, 1996 [DOI] [PubMed] [Google Scholar]
- 426.van Hinsbergh VW. and van Nieuw Amerongen GP. Intracellular signalling involved in modulating human endothelial barrier function. J Anat 200: 549–560, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, and Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci 115: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 428.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, and Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol 285: C343–C352, 2003 [DOI] [PubMed] [Google Scholar]
- 429.Vandenbroucke St AE, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, and Malik AB. PKCalpha activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ Res 111: 739–749, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Venkiteswaran K, Xiao K, Summers S, Calkins CC, Vincent PA, Pumiglia K, and Kowalczyk AP. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta-catenin. Am J Physiol Cell Physiol 283: C811–C821, 2002 [DOI] [PubMed] [Google Scholar]
- 431.Visner GA, Dougall WC, Wilson JM, Burr IA, and Nick HS. Regulation of manganese superoxide dismutase by lipopolysaccharide, interleukin-1, and tumor necrosis factor. Role in the acute inflammatory response. J Biol Chem 265: 2856–2864, 1990 [PubMed] [Google Scholar]
- 432.Vogel SM, Orrington-Myers J, Broman M, and Malik AB. De novo ICAM-1 synthesis in the mouse lung: model of assessment of protein expression in lungs. Am J Physiol Lung Cell Mol Physiol 291: L496–L501, 2006 [DOI] [PubMed] [Google Scholar]
- 433.Walter JK, Castro V, Voss M, Gast K, Rueckert C, Piontek J, and Blasig IE. Redox-sensitivity of the dimerization of occludin. Cell Mol Life Sci 66: 3655–3662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Walter JK, Rueckert C, Voss M, Mueller SL, Piontek J, Gast K, and Blasig IE. The oligomerization of the coiled coil-domain of occludin is redox sensitive. Ann N Y Acad Sci 1165: 19–27, 2009 [DOI] [PubMed] [Google Scholar]
- 435.Wang D, Malo D, and Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/− mouse mutants. J Immunol 184: 582–590, 2010 [DOI] [PubMed] [Google Scholar]
- 436.Wang L, Azad N, Kongkaneramit L, Chen F, Lu Y, Jiang BH, and Rojanasakul Y. The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J Immunol 180: 3072–3080, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Wang X, Ke Z, Chen G, Xu M, Bower KA, Frank JA, Zhang Z, Shi X, and Luo J. Cdc42-dependent activation of NADPH oxidase is involved in ethanol-induced neuronal oxidative stress. PLoS One 7: e38075, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Wang XT, McCullough KD, Wang XJ, Carpenter G, and Holbrook NJ. Oxidative stress-induced phospholipase C-gamma 1 activation enhances cell survival. J Biol Chem 276: 28364–28371, 2001 [DOI] [PubMed] [Google Scholar]
- 439.Wang Y, Feinstein SI, Manevich Y, Ho YS, and Fisher AB. Lung injury and mortality with hyperoxia are increased in peroxiredoxin 6 gene-targeted mice. Free Radic Biol Med 37: 1736–1743, 2004 [DOI] [PubMed] [Google Scholar]
- 440.Wang Y, Herrera AH, Li Y, Belani KK, and Walcheck B. Regulation of mature ADAM17 by redox agents for L-selectin shedding. J Immunol 182: 2449–2457, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Wang Y, Phelan SA, Manevich Y, Feinstein SI, and Fisher AB. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am J Respir Cell Mol Biol 34: 481–486, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Wang YL, Malik AB, Sun Y, Hu S, Reynolds AB, Minshall RD, and Hu G. Innate immune function of the adherens junction protein p120-catenin in endothelial response to endotoxin. J Immunol 186: 3180–3187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Wang Z, Wei X, Zhang Y, Ma X, Li B, Zhang S, Du P, Zhang X, and Yi F. NADPH oxidase-derived ROS contributes to upregulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cell Physiol Biochem 24: 619–626, 2009 [DOI] [PubMed] [Google Scholar]
- 444.Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, Li H, Nasdala I, Brandau O, Fassler R, Butz S, Krombach F, and Vestweber D. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med 203: 1671–1677, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, and Luckhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 277: 23150–23156, 2002 [DOI] [PubMed] [Google Scholar]
- 446.Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Schnitzler M, Brandes RP, Grimminger F, Meissner M, Freichel M, Offermanns S, Veit F, Pak O, Krause KH, Schermuly RT, Brewer AC, Schmidt HH, Seeger W, Shah AM, Gudermann T, Ghofrani HA, and Dietrich A. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun 3: 649, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Weissmann N, Voswinckel R, Hardebusch T, Rosseau S, Ghofrani HA, Schermuly R, Seeger W, and Grimminger F. Evidence for a role of protein kinase C in hypoxic pulmonary vasoconstriction. Am J Physiol 276: L90–L95, 1999 [DOI] [PubMed] [Google Scholar]
- 448.Wen D, Corina K, Chow EP, Miller S, Janmey PA, and Pepinsky RB. The plasma and cytoplasmic forms of human gelsolin differ in disulfide structure. Biochemistry 35: 9700–9709, 1996 [DOI] [PubMed] [Google Scholar]
- 449.Wennerberg K, Rossman KL, and Der CJ. The Ras superfamily at a glance. J Cell Sci 118: 843–846, 2005 [DOI] [PubMed] [Google Scholar]
- 450.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, and Munzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124: 1370–1381, 2011 [DOI] [PubMed] [Google Scholar]
- 451.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW, O'Rourke B, and Kitsis RN. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A 109: 6566–6571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Wiemann B. and Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 64: 529–564, 1994 [DOI] [PubMed] [Google Scholar]
- 453.Wojciak-Stothard B, Entwistle A, Garg R, and Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol 176: 150–165, 1998 [DOI] [PubMed] [Google Scholar]
- 454.Wojciak-Stothard B, Potempa S, Eichholtz T, and Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 114: 1343–1355, 2001 [DOI] [PubMed] [Google Scholar]
- 455.Wojciak-Stothard B, Tsang LY, and Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L749–L760, 2005 [DOI] [PubMed] [Google Scholar]
- 456.Wolff B, Burns AR, Middleton J, and Rot A. Endothelial cell “memory” of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med 188: 1757–1762, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Woo CH, Eom YW, Yoo MH, You HJ, Han HJ, Song WK, Yoo YJ, Chun JS, and Kim JH. Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem 275: 32357–32362, 2000 [DOI] [PubMed] [Google Scholar]
- 458.Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, and Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood 113: 6246–6257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Woodfin A, Voisin MB, and Nourshargh S. Recent developments and complexities in neutrophil transmigration. Curr Opin Hematol 17: 9–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Wu HL, Albrightson C, and Nambi P. Selective inhibition of rat mesangial cell proliferation by a synthetic peptide derived from the sequence of the C2 region of PKCbeta. Peptides 20: 675–678, 1999 [DOI] [PubMed] [Google Scholar]
- 461.Xu J, Wang F, Van KA, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, and Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114: 201–214, 2003 [DOI] [PubMed] [Google Scholar]
- 462.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, and Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood 106: 584–592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Yeh LH, Park YJ, Hansalia RJ, Ahmed IS, Deshpande SS, Goldschmidt-Clermont PJ, Irani K, and Alevriadou BR. Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am J Physiol 276: C838–C847, 1999 [DOI] [PubMed] [Google Scholar]
- 464.Yore MM, Kettenbach AN, Sporn MB, Gerber SA, and Liby KT. Proteomic analysis shows synthetic oleanane triterpenoid binds to mTOR. PLoS One 6: e22862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Zarbock A, Deem TL, Burcin TL, and Ley K. Galphai2 is required for chemokine-induced neutrophil arrest. Blood 110: 3773–3779, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Zarbock A, Ley K, McEver RP, and Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118: 6743–6751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Zhang K. and Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Zhang WJ, Wei H, and Frei B. Genetic deficiency of NADPH oxidase does not diminish, but rather enhances, LPS-induced acute inflammatory responses in vivo. Free Radic Biol Med 46: 791–798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Zhang WJ, Wei H, Tien YT, and Frei B. Genetic ablation of phagocytic NADPH oxidase in mice limits TNFalpha-induced inflammation in the lungs but not other tissues. Free Radic Biol Med 50: 1517–1525, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Zhao Y. and Davis HW. Hydrogen peroxide-induced cytoskeletal rearrangement in cultured pulmonary endothelial cells. J Cell Physiol 174: 370–379, 1998 [DOI] [PubMed] [Google Scholar]
- 471.Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM, McDermott BJ, and Grieve DJ. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res 70: 9287–9297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O'Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, and Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci 11: 420–422, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Zhou R, Tardivel A, Thorens B, Choi I, and Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11: 136–140, 2010 [DOI] [PubMed] [Google Scholar]
- 474.Zhou R, Yazdi AS, Menu P, and Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]
- 475.Zhu JH. and Lei XG. Lipopolysaccharide-induced hepatic oxidative injury is not potentiated by knockout of GPX1 and SOD1 in mice. Biochem Biophys Res Commun 404: 559–563, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Zoja C, Corna D, Nava V, Locatelli M, Abbate M, Gaspari F, Carrara F, Sangalli F, Remuzzi G, and Benigni A. Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am J Physiol Renal Physiol 304: F808–F819, 2013 [DOI] [PubMed] [Google Scholar]
- 477.Zondag GC, Reynolds AB, and Moolenaar WH. Receptor protein-tyrosine phosphatase RPTPmu binds to and dephosphorylates the catenin p120(ctn). J Biol Chem 275: 11264–11269, 2000 [DOI] [PubMed] [Google Scholar]