Abstract
Background:
Recently, Acinetobacter has emerged as significant hospital pathogen, notoriously known to acquire antibiotic resistance to most of the commonly prescribed antimicrobials. Many risk factors are associated with Acinetobacter infections, especially in patients in intensive care unit (ICU). This study aims to isolate Acinetobacter from various clinical specimens and to determine its antimicrobial sensitivity pattern.
Materials and Methods:
Identification, speciation and antimicrobial sensitivity testing were performed using the standard microbiological techniques. Slime production was also tested by microtiter plate and tube method.
Results:
From the processed clinical specimens, 107 Acinetobacter strains (1.02%) were isolated of which 76 (0.74%) isolates were from general wards and 31 (11.96%) were from ICU. Significantly higher percentage of Acinetobacter strains was found in ICU compared with general wards (P < 0.05). Most common Acinetobacter infection was abscess. Infections were more common in males and were associated with major risk factors such as post-surgical, diabetes mellitus, catheterization, extended hospital stay and prolonged antibiotic usage. Acinetobacter baumanii was the most common species isolated to cause abscess, wound infection, etc. 62.61% and 28.97% isolates produced slime by microtiter plate and tube method. Imipenem was most sensitive drug followed by amikacin. Ceftazidime, cefotaxime, piperacillin were most resistant. 43.00% isolates were IPM resistant. A. baumanii was more resistant to commonly used antimicrobials.
Conclusion:
Acinetobacter nosocomial infections resistant to most antimicrobials have emerged, especially in ICU. Early identification and continued surveillance of prevalent organism will help prevent the spread of Acinetobacter in hospital environment.
Keywords: Acinetobacter, antimicrobial resistance, nosocomial pathogen
INTRODUCTION
Acinetobacter are Gram-negative Coccobacilli, strictly aerobic, non-motile, catalase positive, oxidase negative and lack pigmentation.[1] They are ubiquitous[2] free living saprophytes in soil and water.[3]
Up to 25% of healthy ambulatory adults exhibit cutaneous colonization by Acinetobacter and are the most common Gram-negative bacteria carried on the skin of hospital personnel.[4] They are usually opportunistic pathogens reported to cause a number of outbreaks of nosocomial infections such as septicemia, pneumonia, wound sepsis, endocarditis, meningitis, urinary tract infections and peritonitis,[5] but their predominant role is in ventilator associated pneumonia (VAP), in intensive care units (ICUs).[1]
Predisposing factors for Acinetobacter infections include the presence of prosthesis, endotracheal intubation, intravenous (I.V.) catheters and prior antibiotic therapy in a seriously ill-patient in hospital.[3] Such infections are often extremely difficult to treat because of widespread resistance to the major groups of antibiotics and long-term survival of bacteria in the hospital environment.[1]
Resistance to all known antibiotics has now emerged in Acinetobacter spp. with the majority of strains still being susceptible to carbapenems.[6] Multidrug-resistant (MDR) Acinetobacter infections are associated with increased time on mechanical ventilation, in the ICU and in the hospital. Treatment options are severely limited; carbapenems and colistin are the agents of choice. More research and greater emphasis on the prevention of health-care associated transmission of MDR Acinetobacter infection are essential.[7]
The aim of this study was to isolate Acinetobacter species from clinical specimens and to study the antimicrobial susceptibility pattern of Acinetobacter isolates.
MATERIALS AND METHODS
The study was carried out in the Department of Microbiology from August 2008 to September 2010. Relevant clinical specimens (sputum, blood, pus, urine, cerebrospinal fluid, peritoneal fluid etc.) were collected from inpatient and out-patient departments by standard collection procedures. No specific exclusion criteria envisaged. Specimens were processed by standard microbiological techniques.[3] Non-fermenters were initially separated and further identified as Acinetobacter spp. In Gram stain of direct smears Acinetobacter appeared as tiny, Gram-negative coccobacillary cells often appearing as diplococci.[5] All specimens were inoculated on 10% sheep blood agar and MacConkey agar and incubated at 37°C for 18-24 h.[3] Colonies on blood agar were 0.5-2 mm diameter, translucent to opaque (never pigmented), convex and entire. On MacConkey agar a faint pink tint was produced.[5] Gram stain, catalase, oxidase and motility tests were performed. Acinetobacter are Gram-negative Coccobacilli, non-motile, strictly aerobic, catalase positive and oxidase negative. Rapid utilization of 10% glucose was seen with O-F medium. Acinetobacter isolates were differentiated from other oxidase negative, non-motile organisms such as Centers for Disease Control and Prevention NO-1, Bordetella holmessii by nitrate reduction test and presence of brown soluble pigment.[5]
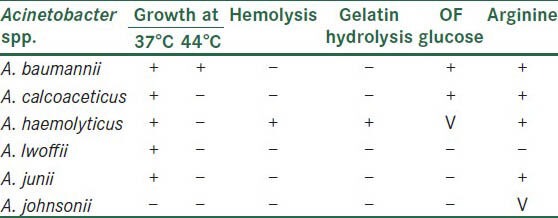
Acinetobacter isolates confirmed by the above standard microbiological tests were further speciated as per the following scheme of identification[3,5] [Table 1].
Table 1.
Acinetobacter species identification
All Acinetobacter spp. were tested for slime production, an important virulence factor by two methods viz. microtiter plate method[8] and tube method.[9] In microtiter plate method, optical density (OD) of stained adherent bacteria was determined at 570 nm wavelength. If OD value is >0.240 then it was strong slime producer.[8] In tube method, biofilm formation was considered positive when a visible film lined the wall and bottom of the tube.[9]
Antimicrobial susceptibility testing[3] was performed by modified Kirby Bauer method[10] as per the Clinical and Laboratory Standards Institute guidelines.[11] Antibiotics tested were ceftazidime (CAZ), ciprofloxacin (CIP), imipenem (IPM), gentamicin, tobramycin (TOB), amikacin (AK), piperacillin-tazobactam (P/T), cefepime (CPM), cefotaxime (CTX), tetracycline, piperacillin (PIP), trimethoprim-sulfamethoxazole (COT), gatifloxacin (GAT).
Statistical analysis
P value was reported and a value of P < 0.05 was considered as a significant. The statistical analysis was performed using the Chi-square test.
RESULTS
In total, 107 Acinetobacter strains (1.02%) were isolated from the processed clinical specimens (10,453). Out of these 107 Acinetobacter isolates, 76 (0.74%) isolates were from general wards and 31 (11.96%) were from ICU. Significantly higher percentage of Acinetobacter strains were found in ICU compared with general wards (P < 0.05) [Table 2]. The most common Acinetobacter infection was abscess (28.03%), followed by pneumonia (23.86%), septicemia (17.75%), wound infection (16.84%) and urinary tract infection (12.14%) [Table 3]. Acinetobacter infections were more common in males (54.20%) as compared with females (45.80%). Major risk factor associated with Acinetobacter infection were post-surgical (37.50%), followed by diabetes mellitus (8.33%), I.V. catheterization (21.05%), extended hospital stay (10.52%) and mechanical ventilation (84.00%) [Table 3]. Most common Acinetobacter species isolated was Acinetobacter baumannii (79.43%) [Table 3]. A. baumannii was the most common species responsible for abscess (90.00%), wound infection (88.88%), septicemia (47.36%), urinary tract infection (61.54%) and pneumonia (92.00%). Out of 107 Acinetobacter isolates, slime production can be detected in 62.61% isolates by microtiter plate method, but in only 28.97% by tube method. Though laborious, microtiter plate method is the reliable and reproducible method for demonstration of slime production. The maximum sensitivity of Acinetobacter was seen to IPM (57.00%), AK (55.14%), followed by GAT (44.87%) and TOB (41.12%). Maximum resistance was observed to CAZ (100%), CTX (100%), PIP (100%) and P/T (86.92%). IPM resistance was seen in 46 (43.00%) Acinetobacter strains [Figure 1]. In general wards and in ICU, A. baumannii was more resistant to commonly used antimicrobials. Acinetobacter junii was more susceptible to the majority of the drugs used.
Table 2.
Distribution of specimens and Acinetobacter isolates
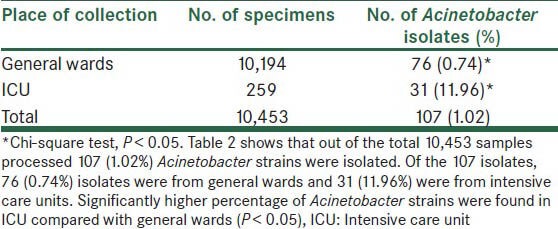
Table 3.
Distribution of Acinetobacter species, major risk factors and various infections (n = 107)
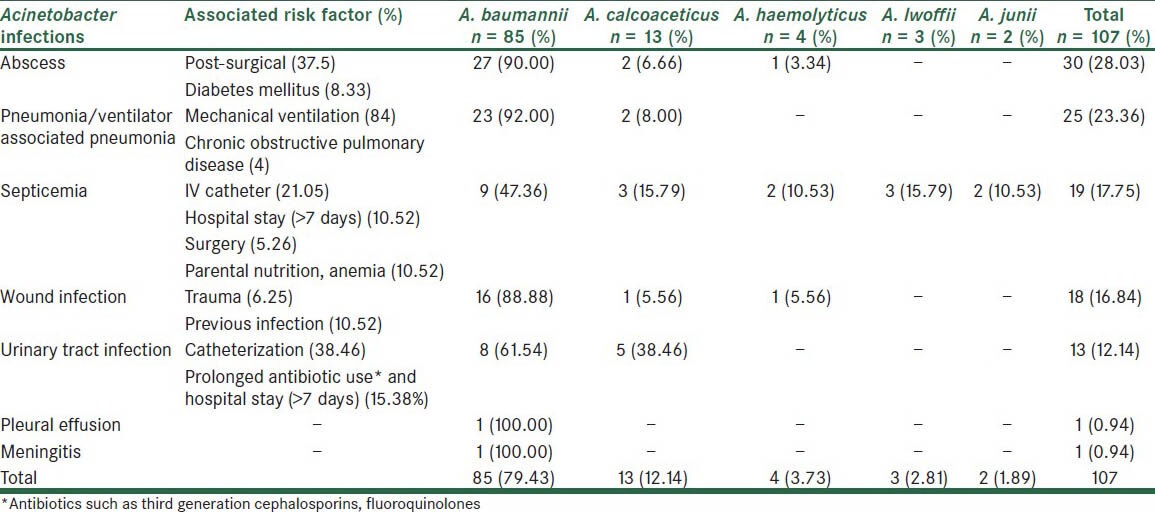
Figure 1.
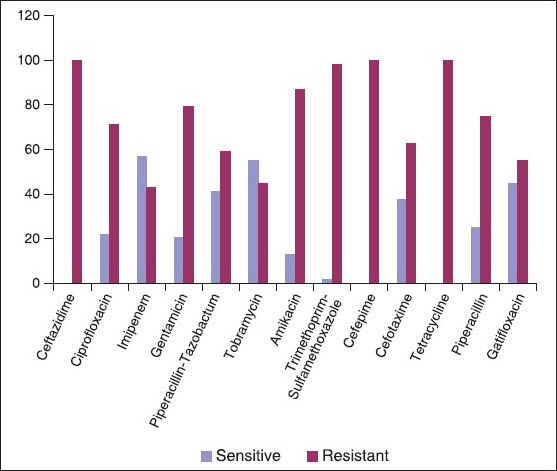
Antimicrobial sensitivity pattern of Acinetobacter isolates (n = 107)
DISCUSSION
Acinetobacter spp. is Gram-negative Coccobacilli that contribute profoundly to the burden of modern medicine. Acinetobacter spp. is the second most commonly isolated non-fermenter in human specimens (after Pseudomonas aeruginosa). They rank fourth (after P. aeruginosa, Stapylococcus aureus and Klebsiella pneumoniae) among the most frequent hospital acquired infectious agents.[12] Acinetobacter spp. have emerged as a cause of ICUs infection. Multiresistant Acinetobacter spp. have become established as “alert” pathogens, particularly in ICUs and are associated with outbreaks of infection.[13] Their ubiquitous nature in the ICU environment and inadequate infection control practice have continuously raised the incidence of Acinetobacter infections over the past two decades. The understanding and recognition of Acinetobacter infections in the ICU is critically needed.[14]
In our study, a total number of 107 (1.02%) Acinetobacter strains were isolated from processed clinical specimens. Houang et al.[15] reported a total of 1.32% Acinetobacter isolates from all clinical specimens, which was well comparable with our study. In our study, 31 (11.96%) Acinetobacter strains were isolated from clinical specimens from ICUs and Acinetobacter infections were more common in ICU as compared with general wards (P < 0.05) [Table 2]. Prashanth and Badrinath[16] reported 10.00% Acinetobacter infections in ICU. Patwardhan et al.[17] isolated 13.23% Acinetobacter isolates. Our findings are comparable with Patwardhan et al. Occurrence of Acinetobacter is contributed by several factors including immunosuppressed hosts, patients with severe underlying disease, previous use of antibiotics, duration of hospital stay and more frequent use of antibiotics in ICU. Patients in ICU are sicker and require more invasive monitoring and therapeutic procedures to survive. ICU environmental contamination appears to be another important source of Acinetobacter infection.[14] The development of ICU-acquired infections is strongly related to prolonged ICU stay and is associated with worse outcomes including increased morbidity and mortality.[18] In the present study, most common infection was abscess (28.03%), followed by pneumonia (23.86%), septicemia (17.75%), wound infection (16.84%) and urinary tract infection (12.14%) [Table 3]. Joshi et al.[19] reported that 27.50% of wound infection were caused by Acinetobacter. Acinetobacter ICU-acquired infections during the last decade represent a growing concern among clinicians and researchers. These infections most frequently involve the respiratory tract of intubated patients.[18] In our study, out of the 31 Acinetobacter isolates from ICU, 21 (67.74%) Acinetobacter were isolated from patients on mechanical ventilation causing VAP. Bennani et al.[20] reported 68.18% VAP ranging from 9% to 68% Acinetobacter infections. Our findings are comparable with Bennani et al.
In the present study, Acinetobacter infections were more common in males (54.20%) as compared with females. This may be due to the fact that the males report more frequently to the hospitals compared with females. Prashanth and Badrinath[16] reported the infections to be more common in males (58.00%) compared with females (42.00%). Joshi et al.[19] reported 50.20% infection in males.
In the present study, out of 107 Acinetobacter cases major predisposing and associated risk factors were evident in many cases [Table 3]. Joshi et al.[19] reported existing debilitating chronic illness (20.20%), post-operative surgical (18.50%), trauma (3.30%), urinary catheterization (4.10%) as risk factors associated with Acinetobacter infections.
Currently at least 31 Acinetobacter genomospecies have been described. Acinetobacter johnsonii, Acinetobacter lwoffii and Acinetobacter radioresistant seem to be natural inhabitants of human skin and commensals in human oropharynx and vagina.[5] The digestive tract of patients within ICUs often serve as reservoirs for multiresistant A. baumannii strains involved in hospital outbreaks.[2] The most common site for A. baumannii infection is the respiratory tract and the most common manifestation is VAP and bloodstream infections. A. lwoffii has been more commonly associated with meningitis, A. junii rarely causes ocular infection and bacteremia.[5] In our study, out of the 107 Acinetobacter isolates, A. baumannii (79.43%) was the most common species to cause Acinetobacter infection [Table 3]. From 140 Acinetobacter isolates, Joshi et al.[19] isolated 70.00% A. baumannii, 1.40% Acinetobacter calcoaceticus, 6.40% Acinetobacter haemolyticus, 8.60% A. junii and 1.40% A. johnsonii. Prashanth and Badrinath[16] isolated 71.42% A. baumannii, 10.02% A. lwoffii, 4.08% A. haemolyticus and 2.04% strains of A. junii.
The ability of Acinetobacter strains to adhere to surfaces is an important mechanism in the pathogenicity. It frequently causes infections associated with medical devices, e.g., vascular catheters, cerebrospinal fluid shunts or Foley catheters. Biofilm formation is a well-known pathogenic mechanism in such infections.[21] Biofilms have clinical and therapeutic implications, because biofilms preserve bacteria from the action of hosts defensive mechanisms and antimicrobial activity against bacteria in biofilms might be substantially diminished.[21] In the present study, out of total 107 Acinetobacter isolates, 67 (62.61%) Acinetobacter isolates produced slime by microtiter plate method, but only 31 (28.97%) isolates by Tube method. Rodríguez-Baño et al.[21] reported 63.00% biofilm production in Acinetobacter isolates. Our findings are comparable with Rodríguez-Baño et al.
As noted by the Infectious Disease Society of America, Acinetobacter is “a prime example of mismatch between unmet medical need and the current antimicrobial research and development pipeline.” Acinetobacter spp. are notorious for their ability to acquire antibiotic resistance.[22] Antimicrobial resistance among Acinetobacter spp. has increased substantially in the past decade and has created a major public health dilemma. The most potent antibiotic drug class currently available are the carbapenems, but resistant strains have emerged.[7] We have studied the antimicrobial resistance pattern among Acinetobacter isolates by Kirby-Bauer disc diffusion method. In our study, Acinetobacter isolates showed resistance to most of the antibiotics available. Maximum sensitivity was observed to IPM (57.00%), AK (55.14%), followed by GAT (44.87%) and TOB (41.12%). Maximum resistance was observed to CAZ (100%), CTX (100%), PIP (100%), CPM (98.13%) and P/T (86.92%). IPM resistance was seen in 46 (43.00%) Acinetobacter strains [Figure 1]. Sinha et al.[23] reported maximum sensitivity to meropenem (86.00%), CIP (36.00%), AK (33.00%), CPM (26.00%), CAZ (26.00%) and maximum resistance was reported to PIP (90.00%) and CTX (87.00%). Acinetobacter spp. is universally resistant to penicillin, ampicillin and cephalothin. Various susceptibility to second and third generation cephalosporins have been reported.[5] Acinetobacter species possess a wide array of β-lactamases that hydrolyze and confer resistance to penicillins, cephalosporins and carbapenems. AmpC cephalosporinases are chromosomally encoded and confer resistance to broad-spectrum cephalosporins. Class D oxacillin-hydrolyzing-type enzymes, Class B metallo β-lactamases (MBLs), hydrolyze a broad array of antimicrobial agents, including carbapenems. Increasing antimicrobial resistance leaves few therapeutic options for MDR Acinetobacter infection. The Meropenem Yearly Susceptibility Test Information Collection surveillance program has documented discordance that favors IPM as the more potent agent, compared with meropenem, for treatment of MDR Acinetobacter infection.[7] In the present study, 43.00% of Acinetobacter were IPM resistant. Out of these, 60.71% were imipenem resistant A. baumannii (IRAB) compared with 16.66% A. calcoaceticus in general wards and 34.48% IRAB in ICU. Sinha et al.[23] reported 35.00% IPM resistant Acinetobacter. Lee et al.[24] reported 21.18% IRAB. Corbella et al.[25] reported 36.00% carbapenem resistant A. baumannii from the patients admitted to ICU.
CONCLUSIONS
Acinetobacter are the “superbugs” of the modern hospital environment causing significant proportion of infections in specific patient populations, especially in critically-ill patients in the ICU. As ubiquitous organisms (fortunately of low virulence), with few requirements for growth and survival, Acinetobacter spp. are prone to persist indefinitely in the hospital environment and to cause infections periodically when iatrogenic factors are present, i.e., overuse of broad spectrum antibiotics and high-risk patients. This situation, together with the fact that Acinetobacter isolates have inherent and/or easily acquired mechanisms of resistance against many of the available antimicrobial agents, makes this pathogen one of the most significant microbial challenges of the current era. Antibiotic resistance is attributed to production of extended spectrum beta-lactamase, MBL, loss of outer membrane proteins, efflux pumps and biofilm formation. Are there ways to control or limit the spread of these multiresistant strains? Is it still possible to treat Acinetobacter infections? First, it is necessary to improve microbiological techniques for early and more accurate identification and laboratory vigilance to prevent inappropriate empirical treatment. Second, newer strategies for antibiotic use should be employed to reduce selection pressure, including more frequent rotation of antibiotic groups or sequential use of antibiotic classes. The development of totally new antibiotics with novel bacterial molecular target sites may constitute therapeutic alternatives within the next few years. Nevertheless, continued surveillance of prevalent organisms in ICUs, combined with preventive measures (e.g., isolation precautions, hand disinfection, efficient sterilization of instruments) remains absolutely essential in efforts to prevent or limit the spread of Acinetobacter infection. Continued awareness to maintain good housekeeping, control of the environment including equipment decontamination, strict attention to hand washing, isolation procedures and control of antibiotic usage, especially in high-risk areas, appear most likely measures to control the spread of Acinetobacter spp. in hospitals.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bergogne-Bérézin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: Microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–65. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riley W. Acinetobacter and Moraxella. In: Borriello SP, Murray PR, Funke G, editors. Topley and Wilson's Microbiology and Microbial Infections: Bacteriology. 10th ed. Vol. 2. London: Hodder Arnold Publication; 2005. pp. 1301–11. [Google Scholar]
- 3.Collee JG, Fraser AG, Marmion BP, Simmons A. 14th ed. New York: Churchill-Livingstone; 1999. Mackie and McCartney Practical Medical Microbiology. [Google Scholar]
- 4.Allen DM, Hartman BJ. Acinetobacter species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 5th ed. Vol. 2. Philadelphia: Churchill Livingstone; 2000. pp. 2239–44. [Google Scholar]
- 5.Koneman EW, Allen SD, Jande WM, Schreckenberger PC, Winn WC., Jr . 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. Koneman's Colour Atlas and Textbook of Diagnostic Microbiology. [Google Scholar]
- 6.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maragakis LL, Perl TM. Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–63. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 8.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, et al. Adherence of coagulase-negative Staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–26. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 11.Wayne, PA, USA: CLSI; 2008. Clinical and Laboratory Standard Institute. Performance Standard for Antimicrobial Susceptibility Testing; Eighteenth Informational Supplement; M100-S18. [Google Scholar]
- 12.Shete VB, Ghadage DP, Muley VA, Bhore AV. Acinetobacter septicemia in neonates admitted to intensive care units. J Lab Physicians. 2009;1:73–6. doi: 10.4103/0974-2727.59704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agodi A, Zarrilli R, Barchitta M, Anzaldi A, Di Popolo A, Mattaliano A, et al. Alert surveillance of intensive care unit-acquired Acinetobacter infections in a Sicilian Hospital. Clin Microbiol Infect. 2006;12:241–7. doi: 10.1111/j.1469-0691.2005.01339.x. [DOI] [PubMed] [Google Scholar]
- 14.Rungruanghiranya S, Somboonwit C, Kanchanapoom T. Acinetobacter infection in the intensive care unit. J Infect Dis Antimicrob Agents. 2005;22:77–92. [Google Scholar]
- 15.Houang ET, Chu YW, Leung CM, Chu KY, Berlau J, Ng KC, et al. Epidemiology and infection control implications of Acinetobacter spp. in Hong Kong. J Clin Microbiol. 2001;39:228–34. doi: 10.1128/JCM.39.1.228-234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prashanth K, Badrinath S. Nosocomial infections due to Acinetobacter species: Clinical findings, risk and prognostic factors. Indian J Med Microbiol. 2006;24:39–44. doi: 10.4103/0255-0857.19893. [DOI] [PubMed] [Google Scholar]
- 17.Patwardhan RB, Dhakephalkar PK, Niphadkar KB, Chopade BA. A study on nosocomial pathogens in ICU with special reference to multiresistant Acinetobacter baumannii harbouring multiple plasmids. Indian J Med Res. 2008;128:178–87. [PubMed] [Google Scholar]
- 18.Falagas ME, Karveli EA, Siempos II, Vardakas KZ. Acinetobacter infections: A growing threat for critically ill patients. Epidemiol Infect. 2008;136:1009–19. doi: 10.1017/S0950268807009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi SG, Litake GM, Satpute MG, Telang NV, Ghole VS, Niphadkar KB. Clinical and demographic features of infection caused by Acinetobacter species. Indian J Med Sci. 2006;60:351–60. [PubMed] [Google Scholar]
- 20.Bennani B, Selmani R, Mahmoud M, Nejjari C, Kanjaa N. Nosocomial pneumonia in mechanically ventilated patients: Prospective study in intensive care unit of Fez University Hospital. Saudi J Anaesth. 2008;2:46–51. [Google Scholar]
- 21.Rodríguez-Baño J, Martí S, Soto S, Fernández-Cuenca F, Cisneros JM, Pachón J, et al. Biofilm formation in Acinetobacter baumannii: Associated features and clinical implications. Clin Microbiol Infect. 2008;14:276–8. doi: 10.1111/j.1469-0691.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 22.Coelho JM, Turton JF, Kaufmann ME, Glover J, Woodford N, Warner M, et al. Occurrence of carbapenem-resistant Acinetobacter baumannii clones at multiple hospitals in London and Southeast England. J Clin Microbiol. 2006;44:3623–7. doi: 10.1128/JCM.00699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha M, Srinivasa H, Macaden R. Antibiotic resistance profile & extended spectrum beta-lactamase (ESBL) production in Acinetobacter species. Indian J Med Res. 2007;126:63–7. [PubMed] [Google Scholar]
- 24.Lee SO, Kim NJ, Choi SH, Hyong Kim T, Chung JW, Woo JH, et al. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: A case-control study. Antimicrob Agents Chemother. 2004;48:224–8. doi: 10.1128/AAC.48.1.224-228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbella X, Montero A, Pujol M, Domínguez MA, Ayats J, Argerich MJ, et al. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol. 2000;38:4086–95. doi: 10.1128/jcm.38.11.4086-4095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


