Abstract
Background:
Cardiovascular diseases (CVD) are the most serious cause of mortality in developed and developing countries. Epidemiological studies indicated that dyslipidemia is the major risk factor of CVD. Dyslipidemia can be modified either by proper lifestyle or medical intervention or by the combination of both. Conjugated linoleic acids (CLA) and ω3 fatty acids have beneficial effects on plasma lipids and lipoproteins. The aim of this study was to evaluate the effect of CLA and omega-3 fatty acids (ω-3 fatty acids) supplementation on lipid profile in atherosclerosis patient.
Materials and Methods:
This study was a 2-month clinical randomized trial. Ninety atherosclerotic patients with angiographically diagnosed coronary atherosclerosis who were referred to Emam Reza Heart Clinic of Shiraz University of Medical Sciences from February to march 2011 were selected if they fulfilled the inclusion criteria. The participants were randomly classified into 3 groups receiving 3 g/d CLA or 1 920 mg/d ω3 or placebo for 2 months. High-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), triglycerides (TG), and total cholesterol were measured before and after the intervention. This study was a two-month clinical randomized trial.
Results:
Data were analyzed using SPSS software (SPSS Inc, Chicago, version19). Although CLA did not appear to have a significant effect on TG, ω3 supplementation significantly reduced TG level. Consumption of CLA and ω3 supplementation did not significantly affect HDL cholesterol, LDL cholesterol, and total cholesterol.
Conclusions:
ω3 supplementation significantly reduced TG level but CLA and ω3 did not show significant changes in other indices of lipid profile in atherosclerotic patients.
Keywords: Atherosclerosis, conjugated linoleic acids, lipid profile, omega-3 fatty acids
INTRODUCTION
Cardiovascular diseases (CVD) are the most serious cause of mortality in developed and developing countries.[1] This disease is also the major cause of morbidity, mortality, and disability in Iranian people and accounts for nearly 50% of mortality each year.[2] Reduced high-density lipoprotein-cholesterol (HDL-C) concentrations and increased low-density lipoprotein-cholesterol (LDL-C) and triglycerides (TG) concentrations as well as increased total cholesterol-to-HDL-C ratio play an important role in the development of atherosclerosis.[3] Epidemiological studies indicated dyslipidemia as a major risk factor of CVD.[3] Dyslipidemia can be modified either by proper lifestyle or medical intervention or by the combination of both.[3] Nowadays, there is a widespread interest in health improvement properties of conjugated linoleic acids (CLA) and ω3 fatty acids.[4] Studies in animal model and human population revealed that CLA and ω3 fatty acids have beneficial effects on plasma lipids and lipoproteins.[5,6] CLA was found naturally in food from ruminant animals such as dairy and meat products.[7,8,9] For nearly a decade, the health benefits of CLA have been investigated in animal models.[10] Results have shown CLA to be anticarcinogenic, antiatherosclerosis, and antidiabetes. It has been observed that animals fed an atherogenic diet and supplemented with CLA had significantly less aortic lesions.[10,5,11,12] Kritchevsky has also found 30% regression of atherosclerotic lesions in supplemented rabbits with CLA.[12] An early study showed that CLA-fed rabbits had significantly reduced LDL cholesterol, but there was no significant change in the HDL levels.[12] There are a few studies conducted on the effect of CLA supplementation on atherosclerosis in human population. On the other hand, ω3 fatty acids are essential fatty acids that human body need for metabolic function.[13] There is considerable evidence from randomized controlled trials (RCTs) indicating that ω3 fatty acids from fish and fish oil are protective against atherosclerosis.[6,14,15] The results of these studies raised a lot of interest in the role of fish oil andomega-3 fatty acids in primary and secondary prevention of CVD. Omega-3 fatty acids play an important role to regulate genes that are critical for controlling lipid homeostasis.[16] Examples of foods high in ω3 fatty acids are certain fish, fish oils, canola oil, flaxseed, and certain vegetables.[17] To the best of our knowledge, this paper was the first human study which assessed the effect of CLA supplementation on atherosclerosis patients and due to high prevalence of atherosclerosis in Iranian population, this study was carried out to evaluate the effect of ω3 fatty acids and CLA on lipid profile in atherosclerotic patients.
MATERIALS AND METHODS
Patient: Triglyceride is an important variable in this study, so to determine the sample based on power = 80% and α = 0.05, the results of the Omrani et al.[18] study was used. Ninety atherosclerotic patients (40 males and 50 females) aged 30 to 60 years with angiographically diagnosed coronary atherosclerosis who were referred to Emam Reza Heart Clinic from February to march 2011were recruited for the study. They gave written informed consent. The study was approved by the Research Ethics Committee of Shiraz University of Medical Sciences. Volunteers had the following criteria: History of angina, myocardial infarction or bypass surgery, body mass index (BMI) between 18.5-24.9 kg/m2, no pregnancy, and no dietary supplements. Volunteers with acute heart failure, arrhythmia acute, or chronic inflammatory disease were excluded from the study. Most of the patients consumed lipid lowering drugs, so dosage and type of these drugs were kept consistent. Participants followed their regular diet and physical activity during the study. To determine the food intake and macro- and micronutrient consumptions of participants, FFQ questionnaire was fill out for each patient at the beginning of the study.
Study design: In this 2-month clinical randomized trial, the volunteers were randomly divided into 3 groups using BBR (Balance Block Randomization) protocol. According to previous population-based studies,[14,19] they were allocated to receive 3g/d CLA (3 × 1 g soft gel, a 50:50 isomer blend of cis9trance11 and trance10cis12), 1920 mg/d ω3 fatty acids (3 × 640 mg soft gel blend of 210 mg DHA and 310 mg EPA, Vitamin E, gelatine, and glycerin) and the placebo. CLA soft gel was obtained from Puritan's Pride (USA) and ω3 fatty acids soft gel was produced by seven seas Health Care (UK). Placebo was produced by Zahravi Pharmacy Company (Tehran-Iran). Participants asked not to take any vitamin or supplements during the trial. Each group was invited separately to take their supplements every two weeks and researcher supervised ingestion of supplements every week.
Blood sampling: At the beginning and the end of the 8-week supplementation trial, 5 cc fasting venous blood samples were collected and immediately centrifuged (3000 × g, 10 min, 4°C); then the plasma was spilled into a tube and stored at -70°C until analysis for lipid profiles.
Anthropometric assessment: Body weight was measured by Seca 713 scale, while the subjects were minimally clothed and their height was determined using measuring tape without shoes. Then, BMI (weight (kg)/hight2 (m)) was calculated.
Biochemical analysis: Plasma total cholesterol, triglyceride, LDL-cholesterol, and HDL-cholesterol concentrations were measured by using spectrophotometry methods. Total serum cholesterol, TG, and HDL-C was analyzed using a commercial kit supplied by Pars azmoon Co (Tehran, Iran). LDL-C is estimated using the Friedewald equation [Low-density lipoprotein cholesterol = Total cholesterol – [High-density lipoprotein cholesterol – Trigylcerides/5] (Friedewald et al., 1972).
Statistical analysis: Data were analyzed using SPSS software (SPSS Inc, Chicago, version19). Normality distributed data were expressed as mean ± standard deviation. Paired-sample t- test was used for within-group effects from baseline. Differences between groups from baseline to 8 weeks were assessed using ANOVA followed by a post hoc analysis. FFQ questionnaire was analyzed using Food Processor Nut4 software by incorporating the Iranian food table.
RESULTS
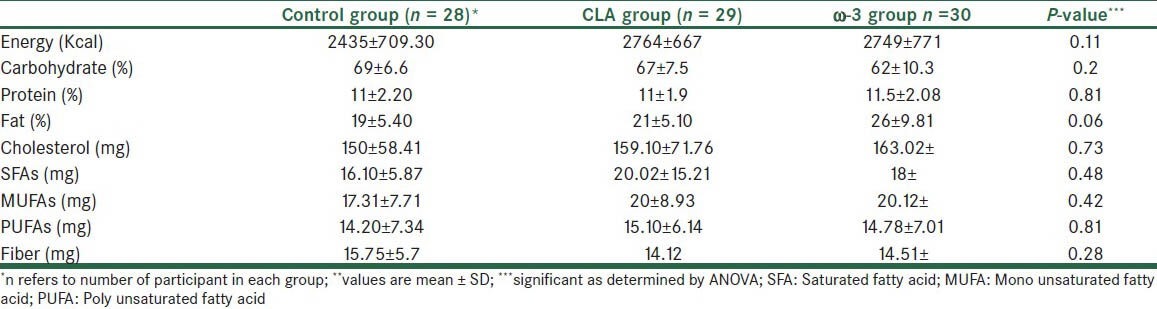
Three patients were excluded during the study and finally data from 87 patients (39 men and 48 women) were collected and analyzed and with on average over 95% of supplements being apparently consumed by trial participants. Three groups were well matched in different variables before intervention. Analysis of sex distribution by ANOVA test shows no significant differences between groups [Table 1]. Also, there was no significant difference for the dosage and type of lipid lowering drugs between the groups. As shown in Table 1, the age, weight, height, BMI, disease duration, and biochemical markers did not differ significantly between groups before the study. Concerning differences in food intake between patients, analysis of food frequency questionnaire showed no differences for the mean intake of energy, carbohydrate, protein, fat, fiber, and intake between the patients [Table 2]. More results will be presented in a separate article.
Table 1.
Baseline characteristics of the study population
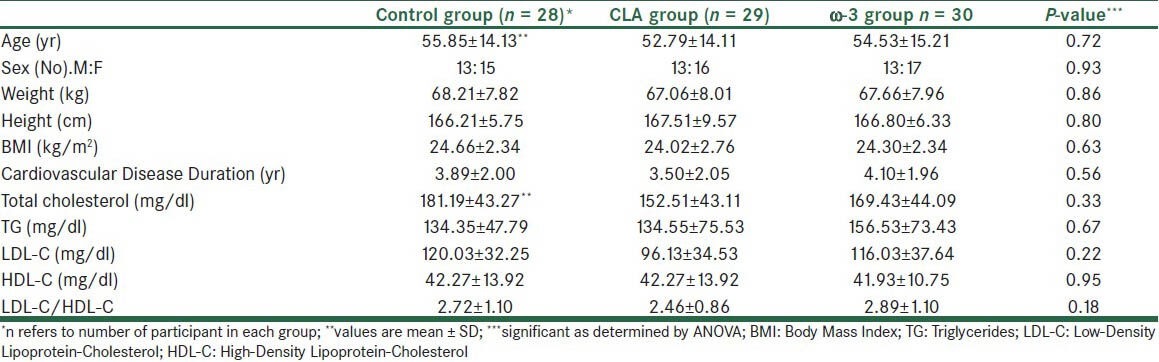
Table 2.
Food intake measurements from food frequency questionnaire of the study population
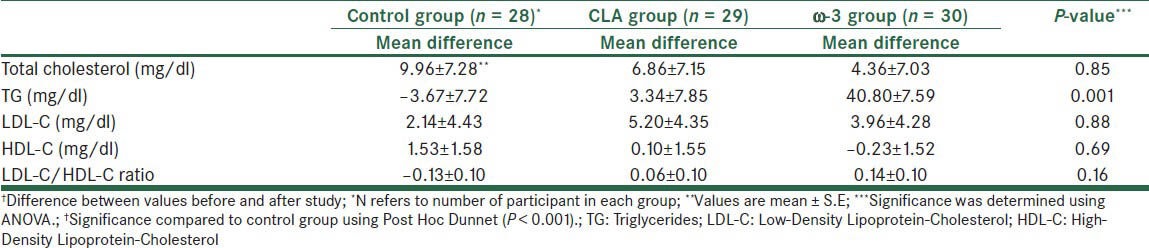
At the end of the study, comparison to the baseline, the level of TG differed significantly in the ω3 group but it did not differ significantly in the CLA group. As shown in Table 3, there were no significant changes in mean differences of LDL-C, HDL-C, and LDL-C/HDL-C ratio. However, mean differences of TG change significantly in the ω3 group relative to control group (P<0.001).
Table 3.
Mean differences† Changes in biochemical indices at the end of the study
DISCUSSION
Functional foods such as CLA may aid in controlling the increasing prevalence of diseases. The amount of CLA in the diet has been estimated to be ~152 mg/d in women and 212 mg/d in men.
To meet therapeutic need levels (3-6g/d), use of CLA supplements and CLA-enriched foods is necessary.[20] In Nicolosi and Nestel's study, CLA supplementation reduces TG in rats.[21,22] CLA isomers function as PPARs ligands, thereby increasing PPAR activity. PPAR is an important transcription factor in hepatic lipid metabolism. So, the TG-lowering effect of CLA may be related to its effect on PPAR.[23] In a report by Mougios et al., 0.7 g/d CLA for 4 weeks decreased TG levels. However in this study, CLA supplementation did not have any significant effect on serum TG.[24] Some studies have demonstrated no effect of CLA on plasma TG.[25,26] In most of these studies and present study, patients had normal serum TG; therefore, the non-significant effect of CLA on TG may be partly attributed to this fact.
On the other hand, in some animal studies, CLA supplementation reduces cholesterol in rats[21,22] but in most human studies including the present one, CLA did not have any significant effect on total cholesterol and LDL-C.[25,26] As reported by Tholstrup and Riserus, CLA supplementation decreases HDL-C concentration. However in some reports, mixture of CLA isomer did not affect HDL-C concentration.[27,28] We also observed no effect of c9t11 and t10c12 CLA isomers on HDL-C levels.
In a recent study by Nazara et al.,[27] enrichment of yogurt with CLA did not have any significant effect on serum lipids. In most of human studies, CLA supplementation did not affect blood lipids, which are in contrast with those of most animal studies.[19] The variety of species can account for inconsistent results. Although in the animal model study, animals were fed atrogenic diet and were hyperlipidemic, in most of human studies the participants were normolipidemic.
Several studies show that ω3 fatty acids have hypolipidemic properties.[17] ω3 fatty acids decrease production of TG in the liver by a direct effect on DGAT (Diacyl Glycerol Acyl Transferase) and consequently decrease VLDL production.[29] In addition, these fatty acids increase β-oxidation of fatty acids and ultimately decrease the availability of fatty acids to biosynthesis of TG in the liver.[29] Another possible mechanism is that ω3 fatty acids increase LPL activity and TG clearance.[29] So ω3 fatty acids reduce fasting plasma TG and VLDL in patients with high or normal TG.[29] In the present study, TG concentration decreased significantly in ω3 group relative to control group. The effect of ω3 fatty acids on LDL-C and total cholesterol has been controversial.[30] In this study, ω3 fatty acids did not have any significant effect on serum LDL-C and total cholesterol level. Despite the reduced TG and VLDL levels with ω3 fatty acids supplementation, the rise in LDL as observed with fish oil supplementation can be due to the increased conversion of VLDL particles into LDL.[31] In addition, there is evidence that fish oil down-regulates the receptor in hepatic cells.
Increased HDL-C in response to ω3 fatty acid supplementation was observed in most, but not all, studies. In the present study, HDL-C concentration increased although it was not significant.[32] Major decreases were observed only when high doses of fish oil were given. The major effect of fish oil on HDL-C is due to reduction in activity of cholesterol ester transfer protein which transfers cholesterol esters from HDLs to VLDLs and LDLs. In the present study, HDL-C concentration increased although it was not significant.
The strengths of the current study included high follow-up rates, high statistical power, and narrow confidence intervals of the outcomes. Finally, we employed both male and female volunteers.
However to determine the food intake of participants, FFQ questionnaire was fill out for each patient at the beginning of the study; the limitation of this study was that we did not assess dietary intake and physical activity during the study although the randomized design should have clearly lowered the risk of such bias, and all subjects were instructed to maintain their regular diet and physical activity. On the other hand, in the present study the measured biochemical indices were normal in the beginning of the study and the patients had normal BMI. So, these might have affected our results.
In conclusion, this study demonstrates helpful effects of ω3 fatty acids on atherosclerosis risk factors, although results were not significant for most indices. Since a few human studies evaluated CLA effect on atherosclerosis risk factors, more research with large sample size and high dose of supplements is required for CLA supplementation to bring up definite comments in this regard.
ACKNOWLEDGEMENT
The present study was funded by the grant number 89-5407 and IRCT201103146067N1 from Shiraz University of Medical Sciences. The authors thank Dr. Ahmadinia, the manager of ARMAN SETAD Company, for supplying ω3 fatty acids supplements. The authors gratefully acknowledge all patients who attended this study. The authors hereby declare that the investigation undertaken and described in this article has been extracted from the thesis of our Msc student of nutrition sciences, Ms Fereshte Aliasghari, the second author of this article. The authors also thank Dr. Nasrin Shokrpour at Center for Development of Clinical Research of Namazee Hospital for editorial assistance.
Footnotes
Source of Support: The present study was funded by the grant number 89-5407 and IRCT201103146067N1 from Shiraz University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Nissinen A, Berrios X, Puska P. Community-based noncommunicable disease interventions: Lessons from developed countries for developing ones. Bull World Health Organ. 2001;79:963–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Hatmi ZN, Tahvildari S, Gafarzadeh Motlag A, Sabouri Kashani A. Prevalence of coronary artery disease risk factors in Iran: A population based survey. BMC Cardiovasc Disord. 2007;7:32. doi: 10.1186/1471-2261-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jawalekar S, Kulkarni U, Surve V, Deshmukh Y. Status of lipid profile, MDA and protein carbonyl in patients with cardiovascular diseases. Arch Appl Sci Res. 2010;2:8–14. [Google Scholar]
- 4.Sneddon AA, Tsofliou F, Fyfe CL, Matheson I, Jackson DM, Horgan G, et al. Effect of a conjugated linoleic acid and ù-3 fatty acid mixture on body composition and adiponectin. Obesity. 2008;16:1019–24. doi: 10.1038/oby.2008.41. [DOI] [PubMed] [Google Scholar]
- 5.McLeod RS, LeBlanc AM, Langille MA, Mitchell PL, Currie DL. Conjugated linoleic acids, atherosclerosis, and hepatic very-low-density lipoprotein metabolism. Am J Clin Nutr. 2004;79(6 Suppl):1169–74. doi: 10.1093/ajcn/79.6.1169S. [DOI] [PubMed] [Google Scholar]
- 6.Holub BJ. Docosahexaenoicacid(DHA) and cardiovascular disease risk factors. Prostaglandins Leukot Essent Fatty Acids. 2009;81:199–204. doi: 10.1016/j.plefa.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Whigham LD, Cook ME, Atkinson RL. Conjugated linoleic acid: Implications for human health. Pharmacol Res. 2000;42:503–10. doi: 10.1006/phrs.2000.0735. [DOI] [PubMed] [Google Scholar]
- 8.Kritchevskya D, Teppera SA, Wrighta S, Czarneckib SK, Wilson TA, Nicolosi RJ. Conjugated linoleic acid isomer effects in atherosclerosis: Growth and regression of lesions. Lipids. 2004;39:611–6. doi: 10.1007/s11745-004-1273-8. [DOI] [PubMed] [Google Scholar]
- 9.Banni S, Angioni E, Contini MS, Carta G, Casu V, Lengo GA, et al. Conjugated linoleic acid and oxidative stress. J Am Oil Chem Soc. 1998;75:261–7. [Google Scholar]
- 10.Benjamin S, Spener F. Conjugated linoleic acids as functional food: An insight into their health benefits. Nutr Metab. 2009;6:36. doi: 10.1186/1743-7075-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagao K, Yanagita T. Conjugated fatty acids in food and their healthbenefits. J Biosci Bioeng. 2005;100:152–7. doi: 10.1263/jbb.100.152. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura YK, Flintoff-Dye N, Omaye ST. Conjugated linoleic acid modulation of risk factors associated with atherosclerosis. Nutr Metab. 2008;5:20–40. doi: 10.1186/1743-7075-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manjula B, Shivalinge Gowda KP, Ahamed SM, Nagarjan T, Gaurav G. Role of omega fatty acid in human body. Asian J Res Chem. 2009;2:93–9. [Google Scholar]
- 14.Kris-Etherton PM, Harris WS, Appel LJ Nutrition Committee. Fish consumption, fish oil, Omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:e31. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 15.Connor SL, Connor WE. Are fish oils beneficial in the prevention and treatment of coronary artery disease. Am J Clin Nutr. 1997;6(4 Suppl):1020–31S. doi: 10.1093/ajcn/66.4.1020S. [DOI] [PubMed] [Google Scholar]
- 16.Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H. Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur Heart J. 2012;33:436–43. doi: 10.1093/eurheartj/ehr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harper CR, Jacobson TA. The role of omega-3 fatty acid in the prevention of coronary heart disease. Arch Intern Med. 2001;161:2185–92. doi: 10.1001/archinte.161.18.2185. [DOI] [PubMed] [Google Scholar]
- 18.Omrani GH, Mazloom Z, Savid M, Rashidi AA. Effect of omega-3 fatty acids on glycemic control and lipid profile in patients with type 2 diabetes. J Diabetes Metab Disord. 2003;2:34–9. [Google Scholar]
- 19.Rainer L, Heiss CJ. Conjugated linoleic acid: Health implications and effects on body composition. J Am Diet Assoc. 2004;104:963–8. doi: 10.1016/j.jada.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Joseph SV, Jacques H, Plourde M, Mitchell PL, McLeod RS, Jones PJ. Conjugated linoleic acid supplementation for 8 weeks does not affect body composition, lipid profile, or safety biomarkers in overweight, hyperlipidemic men. J Nutr. 2011;141:1286–91. doi: 10.3945/jn.110.135087. [DOI] [PubMed] [Google Scholar]
- 21.Baddini Feitoza A, Fernandes Pereira A, Ferreira da Costa N, Gonçalves Ribeiro B. Conjugated linoleic acid (CLA): Effect modulation of body composition and lipid profile. Nutr Hosp. 2009;24:422–8. [PubMed] [Google Scholar]
- 22.Nestel P, Fujii A, Allen T. The cis-9,trans-11 isomer of conjugated linoleic acid (CLA) lowers plasma triglyceride and raises HDL cholesterol concentrations but does not suppress aortic atherosclerosis in diabetic apoE-deficient mice. Athrosclerosis. 2006;189:282–7. doi: 10.1016/j.atherosclerosis.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Noone EJ, Roche HM, Nugent AP, Gibney MJ. The effect of dietary supplementation using isomeric blend of conjugated linoleic acid on lipid metabolism in healthy human subjects. Br J Nutr. 2002;88:243–51. doi: 10.1079/BJN2002615. [DOI] [PubMed] [Google Scholar]
- 24.Mougiosa V, Matsakasa A, Petridoua A, Ring S, Sagredosc A, Melissopoulou A, et al. Effect of supplementation with conjugated linoleic acid on human serum lipids and body fat. J Nutr Biochem. 2001;12:585–94. doi: 10.1016/s0955-2863(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyaa A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem. 2006;17:789–810. doi: 10.1016/j.jnutbio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Smedman A, Vessby B. Conjugated linoleic acid supplementation in humans-Metabolic. Effects Lipids. 2001;36:773–81. doi: 10.1007/s11745-001-0784-7. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds C, Roche H. Conjugated linoleic acid and inflammatory cell signaling. Prostaglandins Leukot Essent Fatty Acids. 2010;82:199–204. doi: 10.1016/j.plefa.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Riserus U, Arner P, Brismar K, Vessby B. Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care. 2002;25:1516–21. doi: 10.2337/diacare.25.9.1516. [DOI] [PubMed] [Google Scholar]
- 29.Harris WS, Connor WE, Inkeles SB, Illingworth DR. Dietary omega-3 fatty acids prevent carbohydrate-induced hypertriglyceridemia. Metabolism. 1984;33:1016–9. doi: 10.1016/0026-0495(84)90230-0. [DOI] [PubMed] [Google Scholar]
- 30.Montrol VM, Farmer A, Wollan PC, Dinneen SF. Fish oil supplementation in type 2 diabetes. Diabetes Care. 2000;23:1407–15. doi: 10.2337/diacare.23.9.1407. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson TA. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am J Clin Nutr. 2008;87:1981–90S. doi: 10.1093/ajcn/87.6.1981S. [DOI] [PubMed] [Google Scholar]
- 32.von Schacky C. n-3 Fatty acids and the prevention of coronary atherosclerosis. Am J Clin Nutr. 2000;71(1 Suppl):224–7. doi: 10.1093/ajcn/71.1.224s. [DOI] [PubMed] [Google Scholar]


