Abstract
The multidimensional construct of impulsivity is implicated in all phases of the addiction cycle. Substance dependent individuals (SDIs) demonstrate elevated impulsivity on both trait and laboratory tests of neurobehavioral impulsivity; however our understanding of the relationship between these different aspects of impulsivity in users of different classes of drugs remains rudimentary. The goal of this study was to assess for commonalities and differences in the relationships between trait and neurobehavioral impulsivity in heroin and amphetamine addicts. Participants included 58 amphetamine dependent (ADI) and 74 heroin dependent individuals (HDI) in protracted abstinence. We conducted principal components analyses (PCA) on two self-report trait and six neurobehavioral measures of impulsivity, which resulted in two trait impulsivity (action, planning) and four neurobehavioral impulsivity composites (discriminability, response inhibition efficiency, decision-making efficiency, quality of decision-making). Multiple regression analyses were used to determine whether neurobehavioral impulsivity is predicted by trait impulsivity and drug type. The analyses revealed a significant interaction between drug type and trait action impulsivity on response inhibition efficiency, which showed opposite relationships for ADIs and HDIs. Specifically, increased trait action impulsivity was associated with worse response inhibition efficiency in ADIs, but with better efficiency in HDIs. These results challenge the unitary account of drug addiction and contribute to a growing body of literature that reveals important behavioral, cognitive, and neurobiological differences between users of different classes of drugs.
Keywords: drug addiction, heroin, amphetamine, impulsivity
1. Introduction
Impulsivity, defined as a predisposition toward rapid unplanned reactions to internal and external stimuli without regard to the negative consequences of these reactions to self or others (Moeller et al., 2001) is one of the strongest predictors of the initiation and maintenance of drug addiction (de Wit, 2009; Moeller & Dougherty, 2002; Verdejo-Garcia, Perales, & Perez-Garcia, 2007) and is also reliably associated with increased risk for relapse and treatment failure (Moeller, Barratt, Dougherty, Schmitz, & Swann, 2001; Perry & Carroll, 2008). Impulsivity is a complex and multidimensional construct characterized by a variety of personality and neurocognitive manifestations (Cyders & Coskunpinar, 2011; Dick et al., 2010; Evenden, 1999). In general, measures of trait impulsivity assess self-reported and relatively stable personality characteristics, whereas measures of neurobehavioral impulsivity index performance on laboratory tests of behavior reflecting state-dependent neurocognitive processes.
Self-report trait impulsivity is itself multidimensional, with the number of proposed dimensions ranging from 2 to 15 depending on the specific measures used and samples tested (in Kirby & Finch, 2010). Although there is considerable disagreement in the literature over the number and nature of trait impulsivity dimensions, they typically include lack of planning (Kirby & Finch, 2010; Patton, Stanford, & Barratt, 1995; Whiteside & Lynam, 2001), lack of perseverence (Patton, Stanford, & Barratt, 1995; Whiteside & Lynam, 2001) and sensation seeking (Whiteside & Lynam, 2001; Zuckerman, 1994), among others. Although informative, self-report measures are limited by their reliance on subjective judgment (de Wit, 2009) and cannot be directly related to preclinical biological models of impulsivity (Evenden, 1999). Neurobehavioral measures of impulsivity overcome most of the problems associated with assessment of trait impulsivity (Rogers & Robbins, 2001). They are often based on preclinical models of impulsivity (Winstanley, Olausson, Taylor, & Jentsch, 2010) and their neural substrates are well delineated by neuroimaging studies with humans (Ersche et al., 2012; Moeller et al., 2005). Neurobehavioral impulsivity is typically measured with tests falling into one of two broad categories (Ersche et al., 2012; Winstanley et al., 2010): (a) Impulsive choice (“cognitive impulsivity”), assessed with decision-making tasks involving various risk, reward, and delay contingencies; and (b) Impulsive action (“motor impulsivity”), indexed by response inhibition tasks reflecting inability to inhibit motor responses. Recently, Weafer and colleagues (Weafer, Baggott, & de Wit, in press) demonstrated moderate to high test-retest reliability of “cognitive” (r range: .76–.89) and “motor” impulsivity dimensions (r range: .65–.73), indicating that they are reliable measures of impulsive behavior. Other “state-like” neurobehavioral measures of impulsivity assessing “impulsive choice” and “impulsive action” (e.g. Kirby, 2009; White, Lejuez, & de Wit, 2008) show comparable reliability to trait-like measures (Odum, 2011). These neurobehavioral dimensions of impulsivity are shown to be mediated by dissociable brain substrates and neurotransmitter systems (Kim & Lee, 2011; Sonuga-Barke, 2002) and to load separately in factor analyses (Broos et al., 2012; Lane, Cherek, Rhodes, Pietras, & Tcheremissine, 2003; Rogers et al., 1999; Sonuga-Barke, 2002).
A notable finding in the literature is that although many substance dependent individuals (SDIs) show impaired impulse control on neurobehavioral measures of impulsivity, certainly not all SDIs manifest such impairments, with some studies reporting that more than 1/3rd of SDIs demonstrate relatively spared impulse control even after many years of chronic drug use (Bechara & Damasio, 2002; Bechara & Martin, 2004). This raises the question of whether individual differences in some additional risk factors such as trait impulsivity may increase one’s vulnerability to neurobehavioral impairments in impulsivity. Most studies of this nature have focused on healthy individuals and reveal equivocal findings (in Cyders & Coskunpinar, 2011). Of the few studies that have included drug users, (Kjome et al., 2010) found that in a mixed group of controls and cocaine users, higher trait impulsivity was associated with impulsive action (response inhibition), but not with impulsive choice (decision-making). Another study using factor analysis found that results varied based on participant group, such that impulsive choice (delay discounting) loaded with self-reported trait impulsivity for controls, but with sensation seeking for drug users and individuals at risk for addiction (Meda et al., 2009). Clearly, more research is needed to understand the associations between trait and neurobehavioral impulsivity and how they relate to substance abuse factors (Dick et al., 2010; Meda et al., 2009; Winstanley et al., 2010).
Much of the research investigating impulsivity in SDIs has focused on the common effects of addiction to different types of drugs, based on findings that addictive drugs increase dopamine concentrations in the mesolimbic system, considered to be the neurobiological substrate of the rewarding effects of most drugs of abuse (Di Chiara & Imperato, 1988; Wise, 1978). More recently, researchers have emphasized the importance of investigating potential differences among commonly abused drugs such as heroin and amphetamines, given that they lead to increased dopamine transmission through different neural mechanisms (Badiani, Belin, Epstein, Calu, & Shaham, 2011; Wise, 1978) and have distinct effects on other neuromodulatory and neuropeptide systems (George & Koob, 2010). This line of research has begun to reveal different behavioral manifestations of impulsivity in heroin and amphetamine users (Fernandez-Serrano, Perez-Garcia, & Verdejo-Garcia, 2011; Verdejo-Garcia, Bechara, Recknor, & Perez-Garcia, 2007). For instance, stimulant users show greater deficits on tests of impulsive action compared with opiate users (Verdejo-Garcia et al., 2007), whereas tests of impulsive choice reveal more variable results (Bornovalova, Daughters, Hernandez, Richards, & Lejuez, 2005; Rogers et al., 1999; Verdejo-Garcia et al., 2007). Research in this field is significantly complicated by the high rates of polysubstance abuse and dependence among SDIs in North America and Western Europe, which makes it virtually impossible to investigate the unique effects of different types of drugs on neurocognitive functioning. Further, despite unequivocal evidence that impulsivity is not a unitary construct, very few studies to date have performed within-subjects comparisons of various trait and neurobehavioral dimensions of impulsivity (Broos et al., 2012) and to our knowledge, no study has investigated the relationships between trait and neurobehavioral impulsivity among stimulant and opiate users.
The current study used a within-subject multi-method design to assess for commonalities and differences in the relationships between different aspects of trait and neurobehavioral impulsivity among SDIs with a history of dependence on either amphetamines or heroin. The main goal of the study was to determine whether aspects of trait impulsivity would differentially predict neurobehavioral impulsivity in heroin and amphetamine addicts. In order to limit the confounding effects of polysubstance abuse and dependence, the study included SDIs who were largely mono-substance dependent on either amphetamines or heroin. Given evidence that amphetamine is associated with increased difficulties in impulsive action, we hypothesized that increased trait impulsivity would be associated with increased impulsive action in amphetamine, but not in heroin users. Further, in light of evidence that higher trait impulsivity is associated with impulsive action but not with impulsive choice, we expected no differential associations between trait impulsivity and impulsive choice among heroin and amphetamine users.
2. Materials and Methods
2.1 Participants
Participants included 58 individuals meeting DSM-IV diagnostic criteria for past dependence on amphetamines (ADI) and 74 individuals meeting diagnostic criteria for past dependence on heroin (HDI), evaluated at the Bulgarian Addictions Institute in Sofia, Bulgaria. Inclusion criteria included: 1) age between 18–50 years; 2) minimum of 8th grade education; 3) estimated IQ > 75; 4) no history of neurologic illness (including dementia secondary to substance abuse) or psychosis; 5) no history of penetrating head injury or closed head injury with a loss of consciousness > 30 minutes; 6) no history of psychotic or mood disorders, or current use of psychotropic medication; 7) HIV seronegative status, 8) no history of dependence on both amphetamines and heroin or current dependence on any substance; 9) negative breathalyzer test for alcohol and negative urine toxicology screen for opiates, cannabis, amphetamines, methamphetamines, benzodiazepines, barbiturates, cocaine, MDMA, and methadone.
2.2 Procedures
The study was approved by the Institutional Review Boards at University of Illinois at Chicago and the Medical University – Sofia on behalf of the Bulgarian Addictions Institute. Participants were recruited by distributing informational flyers in leading substance abuse treatment centers in Sofia or on popular internet addiction forums.
Initial screening was made by brief telephone interview to determine whether potential participants met inclusion criteria. Those who did were scheduled for their first testing session at the Bulgarian Addictions Institute. After signing an informed consent form, participants underwent rapid urine toxicology screens and a breathalyzer test for alcohol. Participants who were positive for either alcohol or drugs received no payment for the visit and were rescheduled for testing on another day. Participants who had not been tested for HIV in the last 6 months underwent a free rapid HIV test on site.
The study protocol was completed in two testing sessions of about 3.5 hours each. The first session included IQ estimation with the Raven’s Progressive Matrices (Raven & Raven, 1998) and assessment of substance use using the Substance Abuse Module of the Structured Clinical Interview for DSM-IV (SCID-SAM; First, Spitzer, Gibbon, & Williams, 1996) and a brief version of the Addiction Severity Index (ASI Lite; McLellan et al., 1985) estimating severity of drug and alcohol abuse, associated social problems, legal status, and medical and psychiatric issues. Participants also completed self-report measures assessing current levels of depression (BDI-II; Beck, Steer, Ball, & Ranieri, 1996) and anxiety (STAI; Speelberger, Gorsuch, & Lushene, 1971), as well as childhood symptoms of ADHD (WURS; Ward, Wender, & Reimherr, 1993).
The second visit was scheduled typically within a week from the first and included the trait and neurobehavioral impulsivity measures. Participants were paid 80 Bulgarian Leva (~$50) for their participation in the study.
All self-report and interview measures were translated into Bulgarian by the first author (JV) who is a native Bulgarian speaker and has lived in the country for 25 years. Some of the measures (e.g. SCID-SAM; BDI-II; STAI; WURS) were already translated and back-translated and used in our previous studies in Bulgaria (Vassileva et al., 2007; 2011). Back-translations into English were performed by current and former Bulgarian members of the research team that included both psychiatrists and psychologists. Back-translated items were reviewed and ones with inconsistencies were sent back for repeat back-translation until they were deemed effective. The final versions of the translated instruments were distributed and discussed within the wider research community in Sofia, Bulgaria, prior to being implemented in this and in our previous studies.
2.3 Measures
2.3.1 Trait Impulsivity Measures
Barratt Impulsiveness Scale (BIS-11)
The Barratt Impulsiveness Scale – 11th revision (BIS-11) (Patton, Stanford, & Barratt, 1995) is a 30 item self-report instrument assessing three separate dimensions of trait impulsivity: motor, attentional, and non-planning.
UPPS Impulsive Behavior Scale
The UPPS Impulsive Behavior Scale (Whiteside & Lynam, 2001) was designed based on the Five Factor Model of personality (Costa & McCrae, 1992). It includes four subscales: negative urgency, lack of planning, lack of perseverance, and sensation seeking.
2.3.2 Neurobehavioral Impulsivity Measures
Tests of Impulsive Choice
Iowa Gambling Task (IGT)
The IGT (Bechara, Tranel, & Damasio, 2000) is a computerized decision-making task where participants are asked to choose between four decks of cards with each deck associated with a certain amount of reward money. Decks associated with higher monetary rewards (i.e., A & B) occasionally also involve a large penalty, whereas decks associated with smaller monetary rewards (i.e., C & D) occasionally also involve a small penalty (i.e. loss of money). Participants were told that the goal of the task is to win as much money as possible. As participants were unable to predict when the penalties would occur, it was beneficial to choose cards from the decks associated with lower penalties (i.e., “good” decks: C & D) instead of those with high penalties (i.e., “bad” decks: A & B). Healthy participants typically learn to select cards from the “good” decks instead of the “bad” decks as the task progresses, thereby achieving a higher cumulative reward value. The total net score used in analyses was calculated by subtracting the number of “bad” deck selections from the number of “good” deck selections.
Cambridge Gambling Task (CGT)
The CGT is a computerized task developed to assess decision-making and risk-taking where the decision-making contingencies are apparent on every trial and do not require learning. In this task, participants were required to guess whether a yellow token is hidden under a red or a blue box presented in variable ratios of blue to red boxes on each trial (i.e., 9:1, 8:2, 7:3, 6:4), which unambiguously provide the outcome probabilities of winning or losing, thereby allowing the assessment of decision-making under risk rather than ambiguity. After guessing, participants were asked to bet a proportion of their points on the confidence of their choice. The primary outcome scores, Deliberation Time and Quality of Decision Making, were selected based on previous studies with SDIs (Rogers et al., 1999). Deliberation time represents the mean amount of time taken between time of presentation of the colored box display and choice of colored box. Quality of Decision Making represents the proportion of trials on which the participant chooses the most likely outcome (e.g. choosing red when display shows 8 red: 2 blue boxes).
Delayed Reward Discounting Task (DRDT)
Delayed reward discounting was measured with the Monetary Choice Questionnaire (Kirby, Petry, & Bickel, 1999), consisting of 27 items wherein the participant chooses between smaller immediate rewards available today or larger rewards available at delays ranging from 7 to 186 days. The discount rate parameter (k) was estimated using procedures described in detail in (Kirby et al., 1999) and in our previous study (Vassileva, Georgiev, Martin, Gonzalez, & Segala, 2011). Higher values of k represent increased tendency to discount future rewards and are regarded as a measure of impulsivity. One participant demonstrating more than 2 inconsistent choices on one of the 3 magnitude conditions was excluded from analyses. The overall DRDT scores were not found to be normally distributed and were log transformed.
Tests of Impulsive Action
Immediate Memory Task (IMT)
The IMT is a modified continuous performance task with more complex demands on inhibitory control, working memory, and sustained attention (Dougherty, Marsh D.M., Mathias, & Steinberg, 2002). Participants were instructed to press a response button when two consecutive 5-digit numbers were identical and to avoid responding on catch trials that differed from the target number. The primary dependent variables were discriminability (d′), an index of the ability to discriminate between correct targets and nontarget catch trials, and response time latency for target trials, an index of rapid-response impulsivity. The measure d′ is derived from signal detection theory and is a measurement of the distance between the mean distribution for noise and the mean distribution for signal combined with noise (Dougherty et al., 2002).
Stop Signal Task
The GoStop Impulsivity paradigm (Dougherty, Mathias, Marsh, & Jagar, 2005) is a stop signal task requiring participants to attend to a consecutive series of five digit numbers and to respond when the number presented is identical to the previous number. Participants are instructed to respond to target trials and withhold responding on “stop” trials when the color of the target stimuli changes from black to red. The primary variable of interest was the average response inhibition ratio, which is calculated by dividing the failures to inhibit a response (“stop” trial responses) by the correct detections (“go” trial responses) averaged across four different stop signal delays (50ms, 150ms, 250ms, 350ms). Increased proportion of responses to “stop” trials relative to “go” trials has been proposed to be related to impulsivity characterized by impaired response inhibition.
Go/No-Go Task
The Go/No-Go task (Lane, Moeller, Steinberg, Buzby, & Kosten, 2007) is a response inhibition task, comprised of a series of two–element visual stimuli presented side by side near the center of a computer screen. Participants were instructed to respond on “go” trials when the two elements were identical, but to withhold responding on “no-go” trials when the elements were different. The primary dependent variable was d′, an index of discriminability, measuring the ability to discriminate between correct targets and “no go” trials.
3. Results
3.1 Group Characteristics
We conducted an initial series of between-group (HDI vs. ADI) comparisons on demographic and substance use variables. As shown in Table 1, the HDI group was significantly older, whereas a larger proportion of ADIs demonstrated past dependence on cannabis. Kruskal-Wallis tests of significance were used for between-group comparisons of ASI scores, duration of drug use, and time since last use because data were not normally distributed. ASI alcohol use scores were significantly higher for ADIs compared with HDIs. HDIs showed longer duration of use of alcohol and of drug of dependence (amphetamine for ADI; heroin for HDI), although there were no group differences in length of abstinence, indexed as time since participants last met dependence criteria. There were no group differences in sex, IQ, years of education, ASI drug scores, or proportion of participants meeting criteria for past dependence on alcohol.
Table 1.
Demographic, Psychiatric, and Substance Use Characteristics of Participants
| ADI | HDI | Test Statistic | p | |
|---|---|---|---|---|
| n | 58 | 74 | ||
| Age | 22.59 (3.70) | 29.24 (4.61) | F = 80.34 | <.01 |
| Education (years) | 12.40 (1.89) | 13.01 (2.37) | F = 2.63 | .11 |
| Raven Estimated IQ | 106.28 (13.40) | 104.05 (12.65) | F = 0.95 | .33 |
| Sex (% male) | 76 | 80 | χ2 = 0.28 | .59 |
| Hepatitis C Seropositive | 0 | 44 | ||
| Addiction Severity Index | ||||
| Alcohol | .11 (.12) | .06 (.11) | χ2 = 11.12 | <.01 |
| Drug | .01 (.02) | .03 (.06) | χ2 = 0.12 | .73 |
| Duration of Substance Use in Years | ||||
| Alcohol | 8.0 (3.5) | 11.2 (5.4) | U = 1014.5 | <.01 |
| Amphetamine | 3.3 (2.3) | 0.1 (0.8) | U = 55.5 | <.01 |
| Heroin | 0 | 7.5 (3.4) | ||
| Length of Abstinence (time since last met dependence criteria) | ||||
| Days (median, IQR) | 913 (1073) | 730 (1390) | χ2 = .03 | .87 |
| Past Substance Dependence (%) | ||||
| Alcohol | 7 | 8 | χ2 = 0.05 | .82 |
| Cannabis | 39 | 19 | χ2 = 6.26 | .01 |
| Sedatives | 0 | 0 | ||
| Cocaine | 0 | 0 | ||
| Hallucinogens | 0 | 0 | ||
| Other Drugs | 0 | 0 | ||
| Polysubstance | 0 | 0 | ||
| Trait Impulsivity (z-scores) | ||||
| Planning | −0.01 (0.92) | 0.01 (0.77) | t = 0.2 | .87 |
| Action | 0.12 (0.74) | −0.09 (0.69) | t = −1.7 | .09 |
| Neurobehavioral Impulsivity (z-scores) | ||||
| Discriminability | −0.10 (0.79) | 0.08 (0.84) | t = −1.2 | .22 |
| Response Inhibition | −0.10 (0.56) | 0.08 (0.58 | t = −1.7 | .09 |
| Decision-Making | −0.04 (0.60) | 0.03 (0.71) | t = −0.5 | .61 |
| Quality Decision-Making | −0.10 (0.80) | 0.08 (0.73) | t = −1.3 | .20 |
| Psychiatric Comorbidities | ||||
| BDI-II | 7.8 (6.1) | 9.0 (6.5) | t = 1.1 | .29 |
| STAI-State | 34.0 (7.3) | 36.3 (8.9) | t = 1.6 | .11 |
| STAI-Trait | 39.9 (10.2) | 42.1 (10.1) | t = 1.2 | .22 |
| SRPS | 29.4 (10.9) | 31.6 (9.9) | t = 1.2 | .24 |
| WURS | 27.9 (15.1) | 31.4 (15.6) | t = 1.3 | .19 |
Note. Unless otherwise noted, entries represent means and standard deviations; BDI-II = Beck Depression Inventory-II; STAI = State-Trait Anxiety Inventory; SRPS = Self-Report Psychopathy Scale; WURS = Wender Utah Rating Scale
3.2 Principal Components Analysis
For data reduction purposes, principal components analyses (PCA) (Abdi & Williams, 2010) with varimax rotation including all participants were performed separately for the trait and neurobehavioral scores.
Measures of trait impulsivity
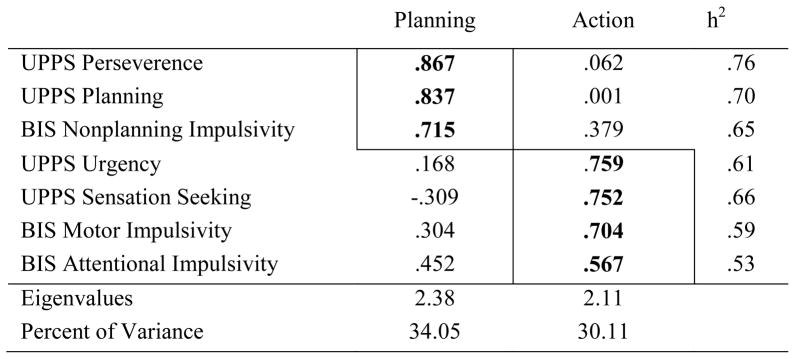
The PCA of trait impulsivity revealed two factors (in Table 2A): (1) Planning impulsivity (BIS Nonplanning, UPPS Planning, UPPS Perseverence) and (2) Action impulsivity (BIS Motor Impulsivity, BIS Attentional Impulsivity, UPPS Negative Urgency, UPPS Sensation Seeking). Based on the results of the PCA, composite scores were computed by z-transforming raw scores and deriving the sum to form a composite score.
Table 2A.
Principal Components Analysis factor loadings and communalities (h2) for the trait impulsivity scores
|
Measures of neurobehavioral impulsivity
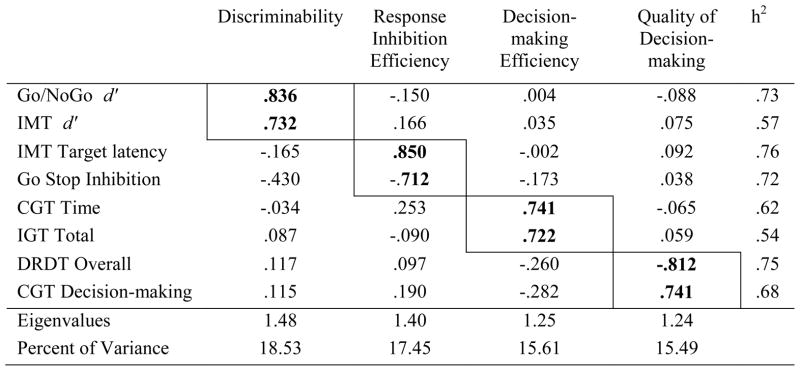
As shown in Table 2B, the PCA for neurobehavioral impulsivity revealed four factors: (1) Discriminability (Go/No-go d′, IMT d′), (2) Response inhibition efficiency (Go Stop Inhibition Ratio, IMT Target latency), (3) Decision-making efficiency (CGT Deliberation Time, IGT Total), and (4) Quality of decision-making (DRDT Overall, CGT Quality of Decision Making). Scores were transformed such that high scores reflected better performance for all measures. Based on the results of the PCAs, composite scores were computed by z-transforming raw scores and deriving the sum.
Table 2B.
Principal Components Analysis factor loadings and communalities (h2) for the neurobehavioral impulsivity scores
|
3.3 Determination of Covariates
We identified covariates to be included in analyses by determining whether each of the variables found to differ between participant groups significantly predicted either the trait or the neurobehavioral composite scores. Based on the results of these regression analyses, age and ASI alcohol scores were included as covariates in the regression analyses. Self-report psychiatric symptoms such as depression, anxiety, ADHD, and psychopathy did not correlate with neurobehavioral composite measures (see Table 4) and were not utilized as potential covariates.
Table 4.
Correlation Matrix of Impulsivity Measures and Demographic, Substance Use, and Psychiatric Characteritics
| Dis | RIE | DME | QDM | Plan | Action | Age | Sex | IQ | Edu | HCV | Last Alc Use | Last Drug Use | Yrs Alc Use | Yrs Her Use | Yrs Amp Use | ASI-Alc | ASI-Drug | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dis | -- | .31* | .02 | −.02 | .00 | −.05 | .22* | −.02 | .36* | .23* | −.01 | −.03 | .08 | .21* | .06 | −.05 | .03 | −.09 |
| RIE | .31* | -- | −.05 | −.04 | −.04 | −.04 | .06 | −.04 | .21* | −.01 | .01 | .04 | .04 | .05 | .15 | −.17 | −.04 | −.12 |
| DME | .02 | −.05 | -- | .01 | −.01 | −.01 | .00 | .07 | .10 | −.10 | .10 | .19* | .04 | −.06 | .07 | −.16 | −.02 | .03 |
| QDM | −.02 | −.04 | .01 | -- | .06 | −.02 | .03 | .03 | .03 | .16 | .04 | .16 | .14 | .03 | .04 | −.06 | −.21* | −.11 |
| Action | .00 | −.04 | −.01 | .06 | -- | .36* | .05 | −.01 | −.06 | −.02 | .04 | −.06 | −.12 | .17 | .06 | −.06 | .16 | .13 |
| Plan | −.05 | −.05 | .06 | −.02 | .36* | -- | −.01 | −.04 | .04 | .00 | .23* | −.11 | −.22* | .01 | −.07 | .02 | .06 | −.03 |
| Age | .22* | .06 | .00 | .03 | .05 | −.01 | -- | −.21* | −.09 | .25* | −.31* | .10 | .34* | .68* | .66* | −.32* | .02 | −.01 |
| Sex | −.02 | −.04 | .07 | .03 | −.01 | −.04 | −.21* | -- | .24* | .07 | .15 | −.08 | .12 | −.19* | −.03 | −.09 | −.02 | −.07 |
| IQ | .36* | .21* | .10 | .03 | −.06 | .04 | −.09 | .24* | -- | .30* | .01 | −.01 | .08 | .10 | −.07 | −.07 | −.03 | −.09 |
| Edu | .23* | −.01 | −.10 | .16 | −.02 | .00 | .25* | .07 | .30* | -- | −.05 | .13 | .21* | .28* | .12 | −.10 | −.04 | −.11 |
| HCV | −.01 | .01 | .10 | .04 | .04 | .23* | −.31* | .15 | .01 | −.05 | -- | −.10 | −.12 | −.17 | −.35* | .16 | .02 | −.03 |
| Last Alc Use | −.03 | .04 | .19* | .16 | −.06 | −.11 | .10 | −.08 | −.01 | .13 | −.10 | -- | .28* | .05 | .21* | −.17 | −.24* | .12 |
| Last Drug Use | .08 | .04 | .04 | .14 | −.12 | −.22* | .34* | .12 | .08 | .21* | −.12 | .28* | -- | .17 | .35* | −.28* | −.11 | −.13 |
| Yrs Alc Use | .21* | .05 | −.06 | .03 | .17 | .01 | .68* | −.19* | .01 | .28* | −.17 | .05 | .17 | -- | .42* | −.10 | .10 | −.05 |
| Yrs Her Use | .06 | .15 | .07 | .04 | .06 | −.07 | .66* | −.03 | −.07 | .12 | −.35* | 21* | .35* | .42* | -- | −.59* | −.05 | .16 |
| Yrs Amp Use | −.05 | −.17 | −.16 | −.06 | −.06 | .02 | −.32* | −.09 | −.07 | −.10 | .16 | −.17 | −.28* | −.10 | −.59* | -- | .29* | −.12 |
| ASI-Alc | .03 | −.04 | −.02 | −.21* | .16 | .06 | .02 | −.02 | −.03 | −.04 | .02 | −.24* | −.11 | .10 | −.05 | .29* | -- | −.13 |
| ASI-Drug | −.09 | −.12 | .03 | −.11 | .13 | −.03 | −.01 | −.07 | −.09 | −.11 | −.03 | .12 | −.13 | −.05 | .16 | −.12 | −.13 | -- |
| BDI-II | −.06 | .03 | −.12 | −.05 | .34* | .30* | .16 | .05 | −.19* | −.03 | .08 | .01 | −.10 | .12 | .19* | −.19* | −.03 | .10 |
| STAI-S | −.11 | −.02 | .04 | −.08 | .43* | .16 | .10 | .05 | −.07 | .11 | −.08 | .02 | .04 | .05 | .16 | −.19* | .05 | .13 |
| STAI-T | −.08 | .04 | −.06 | .05 | .40* | .28* | .09 | .13 | −.08 | .05 | .07 | .02 | −.04 | .11 | .16 | −.20* | −.01 | .13 |
| SRPS | −.14 | −.02 | −.08 | .02 | .38* | .45* | .16 | −.28* | −.36* | −.05 | −.02 | −.01 | −.14 | .20* | .11 | −.01 | −.05 | .10 |
| WURS | .03 | .03 | .02 | .06 | .10 | .25* | .10 | −.13 | −.09 | −.08 | .04 | .12 | .07 | .03 | .12 | −.05 | −.18* | −.07 |
Note.
p < .05; Dis = Neurobehavioral Discriminability; RIE = Neurobehavioral Response Inhibition Efficiency; DME = Neurobehavioral Decision-Making Efficiency; QDM = Neurobehavioral Quality of Decision-Making; Plan = Trait Planning Impulsivity; Action = Trait Action Impulsivity; Edu = Education; HCV = Hepatitis C Virus; Alc = Alcohol; ASI = Addiction Severity Index; Her = Heroin; Amp = Amphetamine; BDI = Beck Depression Inventory-II; STAI-S/T = State-Trait Anxiety Inventory-State/Trait; SRPS = Self-Reporty Psychopathy Scale
3.4 Regression models
To determine whether self-report trait impulsivity would predict neurobehavioral impulsivity composite scores, we used hierarchical regressions (one for each trait composite score). In Step 1, we entered covariates. In Step 2, we entered participant group and the trait composite score. As shown in Table 3, we found that trait impulsivity did not significantly predict neurobehavioral impulsivity for any combination of composite scores.
Table 3.
Multiple Regression Results for trait composite scores predicting neurobehavioral composite scores
| Neurobehavioral Composite Scores
|
||||
|---|---|---|---|---|
| Discrimination | Response Inhibition Efficiency | Decision Making Efficiency | Quality of Decision Making | |
|
| ||||
| Step 1: | ||||
| β: Age | .21* | .08 | .01 | −.07 |
| β: ASI Alcohol | .02 | −.04 | −.02 | −.21* |
| R2 | .04 | .01 | .00 | .05 |
| Step 2: | ||||
| Planning Impulsivity | ||||
| β: Group | .04 | −.14 | −.06 | −.12 |
| β: Planning Imp | .04 | −.06 | −.02 | .11 |
| R2 change | .00 | .01 | .00 | .02 |
| Action Impulsivity | ||||
| β: Group | −.04 | −.13 | −.07 | −.11 |
| β: Action Imp | −.04 | −.02 | .06 | −.02 |
| R2 change | .002 | .01 | .01 | .01 |
| Step 3: | ||||
| Planning Impulsivity | ||||
| β: Group X Planning Imp. | −.09 | −.16 | .05 | .16 |
| R2 change | .01 | .03 | .00 | .03 |
| Action Impulsivity | ||||
| β: Group X Action Imp. | .00 | −.34** | .08 | −.07 |
| R2 change | .00 | .10 | .01 | .00 |
p < .05,
p < .01
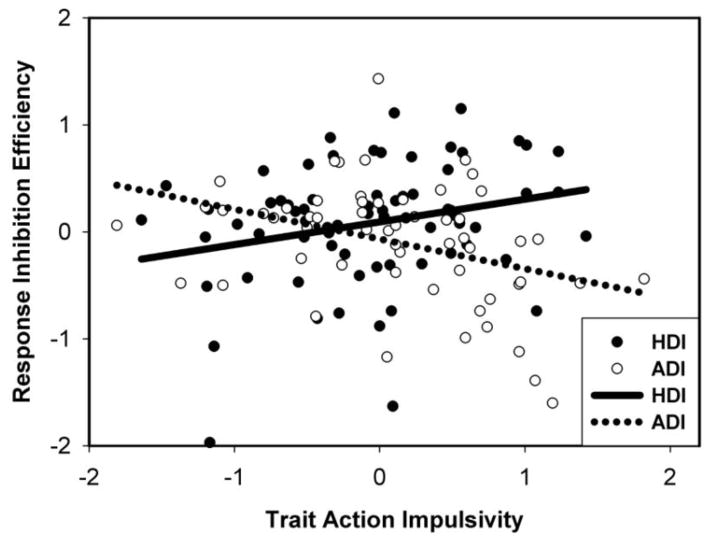
Next, to investigate whether trait impulsivity predicted variance in neurobehavioral impulsivity differently for ADIs and HDIs, we entered the interaction between participant group and trait impulsivity in Step 3. When these interactions significantly predicted the neurobehavioral score, we completed separate regression analyses for each drug group with trait composite scores in the second step. As shown in Table 3, after applying a Bonferroni correction for multiple comparisons, we found that the neurobehavioral response inhibition efficiency composites were predicted by an interaction between participant group and trait action impulsivity composite (Figure 1). When this relationship was explored separately for participant groups, ADIs showed a significant negative relationship between trait action impulsivity and neurobehavioral response inhibition efficiency (β = −.43, p = .001) while HDIs showed a significant positive association (β = .24, p = .05).
Figure 1.
Neurobehavioral Response Inhibition Efficiency composite scores as a function of Trait Action Impulsivity composite scores for heroin dependent (HDI) and amphetamine dependent (ADI) individuals
4. Discussion
Current results reveal that greater trait action impulsivity is associated with lower response inhibition efficiency in ADIs, whereas the opposite pattern is observed in HDIs. This indicates that the effects of trait impulsivity on neurobehavioral impulsivity in drug users are dependent on the particular drug class and on the specific type of impulsivity. The interaction between trait impulsivity and drug class suggests that it could be a more important determinant of behavior than either factor alone. These results contribute to the growing literature on the differential effects of specific drug classes (Badiani et al., 2011) and distinct aspects of impulsivity affected in SDIs (Winstanley et al., 2010).
An obvious question arises about the reasons for the different relationships between trait and neurobehavioral impulsivity in ADIs and HDIs. These group differences could be due to documented neurobiological differences between heroin and amphetamines (Badiani et al., 2011) such as the activation of μ-opioid receptors by heroin (George & Koob, 2010) and differences in the effects of these drugs on dopamine in the prefrontal cortex (Di Chiara, 1999). Of note, recent computational modeling analyses of the IGT in participants from the same sample as in the current study reveal additional dissociations in aspects of decision-making, such that ADIs are characterized by increased sensitivity to reward, whereas HDIs demonstrate reduced sensitivity to loss (Ahn et al., 2012).
Our finding that increased trait action impulsivity was associated with worse neurobehavioral response inhibition efficiency in ADIs is consistent with previous studies in healthy control populations (Cyders & Coskunpinar, 2011; Swann, Bjork, Moeller, & Dougherty, 2002) and in cocaine users (Kjome et al., 2010). These results are also consistent with animal studies providing evidence that impulsivity negatively affects behavior in stimulant users (Dalley et al., 2007). In contrast, our finding that greater trait impulsivity is associated with better response inhibition efficiency in HDIs was not expected and underscores our still rudimentary understanding of the complex role of impulsivity in heroin addiction. The animal literature reveals that trait impulsivity is not related to increased heroin use (McNamara, Dalley, Robbins, Everitt, & Belin, 2010) or to reinstatement of heroin use after abstinence in rats (Schippers, Binnekade, Schoffelmeer, Pattij, & De Vries, 2012), as commonly observed in studies of stimulant use (Dalley et al., 2007). Further, there are some indications from human studies that longstanding and pre-existing factors such as trait impulsivity or personality disorders such as psychopathy may lead to improved instead of impaired impulse control in HDIs. For example, polysubstance (primarily heroin and crack cocaine) users with antisocial personality traits demonstrate better decision making compared with non-antisocial polysubstance users (Vassileva, Gonzalez, Bechara, & Martin, 2007). Further, although relatively “pure” psychopathic HDIs show poorer decision-making compared with non-psychopathic HDIs (Vassileva et al., 2007), they show better attentional performance on tasks of response inhibition (Vassileva et al., 2011). Therefore, the effects of personality traits or disorders on neurobehavioral performance may depend on whether SDIs are monosubstance or polysubstance dependent and on the specific drug of dependence.
Aside from the group differences in the relationship between trait action impulsivity and neurobehavioral response inhibition efficiency, we did not find differences in relationships between other aspects of impulsivity. Even though SDIs in acute withdrawal (Kirby et al., 1999; Rogers et al., 1999), or SDIs abstinent for less than a year (Fernandez-Serrano, Perez-Garcia, Schmidt Rio-Valle, & Verdejo-Garcia, 2010) have consistently demonstrated impairments on tests of decision-making compared with controls, in our study decision-making ability was not significantly affected by trait action impulsivity. It is possible that response inhibition abilities are more vulnerable than decision-making abilities to between-subject variation in self-report trait impulsivity, as suggested by findings of studies with control participants (Cyders & Coskunpinar, 2011; Swann et al., 2002).
It is also important to consider our results in the context of the protracted abstinence stage of the addiction cycle, which characterized most of our participants. Animal studies reveal that impulsive behavior typically decreases after discontinuation of drug use (Winstanley et al., 2010). Studies with humans have demonstrated that withdrawal symptoms such as dysphoria, anhedonia, irritability, and sleep disturbances (Koob & Le Moal, 2008) decrease with increased length of abstinence in SDIs (Coffey, Dansky, Carrigan, & Brady, 2000). Yet, SDIs in protracted abstinence remain vulnerable to the negatively reinforcing effects of relapse due to prefrontal dysregulation characterized by aversive psychological states (Koob & Le Moal, 2008). Studies have also demonstrated that compared to controls, abstinent HDIs continue to show greater trait impulsivity (Nielsen et al., 2012). In light of our limited knowledge about the effects of heroin and amphetamine dependence in the protracted abstinence stage of the addiction cycle, our results increase our understanding of this relatively less well-studied stage and of the brain’s recovery of function after discontinuation of drug use.
When considering the chronic relapsing nature of addiction, our findings raise questions about whether different mechanisms underlie ADIs and HDIs susceptibility to relapse and suggest that treatment interventions should be tailored to the specific type of drug of dependence. Given that response inhibition deficits have been associated with poorer treatment outcomes in stimulant users (Brewer, Worhunsky, Carroll, Rounsaville, & Potenza, 2008), it is possible that ADIs who report increased trait action impulsivity and concurrent deficits in response inhibition may be at greater risk of relapse. If trait action impulsivity assesses a more transient and state-dependent aspect of impulsivity (de Wit, 2009), then our results would suggest that ADIs are at greater risk for relapse when self-reporting increased trait impulsivity and interventions could therefore focus on increasing impulse control in ADIs during these times. Conversely, if trait action impulsivity measures a longstanding characteristic that does not fluctuate, then interventions could target response inhibition in ADIs with higher trait action impulsivity scores. Future studies should address how the relationship between different aspects of impulsivity corresponds to relapse risk in order to determine the best targets for intervention.
Several potential caveats warrant discussion. First, we caution against over-interpreting these findings until future replications, given the small sample sizes of our subgroups, which raise questions about the stability of the observed differences. In addition, we did not utilize trait-like psychiatric indices of impulsivity such as symptoms of antisocial personality disorder or psychopathy, which may have obscured the role of these important clinical constructs. Further, there was higher self-reported prevalence of hepatitis C (HCV) among HDIs relative to ADIs in the current sample, which may have confounded the results. However, we believe that this is unlikely, given that in a previous study (Martin et al., 2004) HCV had no effect on neurocognitive impulsivity and that in the current study HCV was not related to any of the trait or state impulsivity variables.
In conclusion, we found that ADIs demonstrate a negative relationship between trait action impulsivity and response inhibition efficiency whereas HDIs demonstrate a positive relationship. These results contribute to the growing literature on neurobiological differences between different classes of drugs and suggest that different aspects of impulsivity are manifested in unique interdependent ways that are specific to the particular drug of dependence.
Highlights.
Assess relationships between trait and neurobehavioral impulsivity
Compare heroin and amphetamine addicts
Discovered opposite relationships in heroin vs. amphetamine addicts
Trait impulsivity is associated with worse response inhibition in amphetamine addicts
Trait impulsivity is associated with better response inhibition in heroin addicts
Acknowledgments
The authors thank Rada Naslednikova and Ivaylo Raynov for their assistance with data collection. Research reported in this publication was supported by the Fogarty International Center and the National Institute on Drug Abuse (NIDA) under award number R01DA021421 (JV).
Role of Funding Source
This research was funded by the National Institute on Drug Abuse (NIDA) and the Fogarty International Center (FIC) R01DA021421 to Jasmin Vassileva. NIDA and FIC had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We gratefully acknowledge the assistance of Rada Naslednikova and Ivaylo Raynov in testing study participants.
Footnotes
Contributors
JV designed the study and wrote the manuscript. JP conducted the statistical analyses and wrote sections of the manuscript. MW conducted some of the statistical analyses and provided feedback on the manuscript. GV and KB implemented the study in Bulgaria and wrote sections of the manuscript. FGM, EM, and RG provided feedback and edits to the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors declare no conflict of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdi H, Williams LJ. Principal component analysis. Wiley Interdisciplinary Reviews: Computational Statistics. 2010;2:433–459. [Google Scholar]
- Ahn W, Vasilev G, Lee SH, Busemeyer J, Krurschke J, Bechara A, Vassileva J. Double dissociation of stimulant and opiate addiction on reward sensitivity and loss aversion. 42nd Annual Meeting of the Society for Neuroscience; New Orleans, LA. 2012. [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nature Reviews Neuroscience. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Experimental and Clinical Psychopharmacology. 2005;13:311–318. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, et al. The relationship between impulsive choice and impulsive action: a cross-species translational study. PLoS One. 2012;7:e36781. doi: 10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Dansky BS, Carrigan MH, Brady KT. Acute and protracted cocaine abstinence in an outpatient population: a prospective study of mood, sleep and withdrawal symptoms. Drug Alcohol Dependence. 2000;59:277–286. doi: 10.1016/s0376-8716(99)00126-x. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Cyders MA, Coskunpinar A. The relationship between self-report and lab task conceptualizations of impulsivity. Journal of Research in Personality. 2011;46:121–124. [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Euopean Journal of Pharmacology. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, et al. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW, Steinberg JL. Immediate and Delayed Memory Tasks: A computerized behavioral measure of memory, attention and impulsivity. Behavior Research Methods, Instruments, and Computers. 2002;34:391–398. doi: 10.3758/bf03195467. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behavior Research Methods. 2005;37:82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. Journal of Psychopharmacology. 2010;24:1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Verdejo-Garcia A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neuroscience and Biobehavioral Reviews. 2011;35:377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis I disorders. Biometric Research Department; New York: 1996. [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee D. Prefrontal cortex and impulsive decision making. Biological Psychiatry. 2011;69:1140–1146. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Finch JC. The hierarchical structure of self-reported impulsivity. Personality and Individual Differences. 2010;48:704–713. doi: 10.1016/j.paid.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN. One-year temporal stability of delay-discount rates. Psychonomic Bulletin & Review. 2009;16:457–462. doi: 10.3758/PBR.16.3.457. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology General. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kjome KL, Lane SD, Schmitz JM, Green C, Ma L, Prasla I, et al. Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Research. 2010;178:299–304. doi: 10.1016/j.psychres.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Rhodes HM, Pietras CJ, Tcheremissine OV. Relationships among laboratory and psychometric measures of impulsivity: Implications in substance abuse and dependence. Addictive Disorders and Their Treatment. 2003;2:33–40. [Google Scholar]
- Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. American Journal of Drug and Alcohol Abuse. 2007;33:717–726. doi: 10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index. Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D. Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology. 2010;212:453–464. doi: 10.1007/s00213-010-1974-9. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, et al. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behavioral Pharmacology. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. American Journal of Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM. Impulsivity and substance abuse: What is the connection? Addictive Disorders and Their Treatment. 2002;1:3–10. [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller G, Barratt E, Dougherty D, Schmitz J, Swann A. Psychiatric aspects of impulsivity. American Journal of Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Ho A, Bahl A, Varma P, Kellogg S, Borg L, et al. Former heroin addicts with or without a history of cocaine dependence are more impulsive than controls. Drug and Alcohol Dependence. 2012;124:113–120. doi: 10.1016/j.drugalcdep.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: Trait variable? Behavioural Processes. 2011;87:1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Oxford, U.K: Oxford Psychologists Press; 1998. [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, deWit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences. 2006;40:305–315. [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Schippers MC, Binnekade R, Schoffelmeer AN, Pattij T, De Vries TJ. Unidirectional relationship between heroin self-administration and impulsive decision-making in rats. Psychopharmacology. 2012;219:443–452. doi: 10.1007/s00213-011-2444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biological Psychiatry. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Vassileva J, Georgiev S, Martin E, Gonzalez R, Segala L. Psychopathic heroin addicts are not uniformly impaired across neurocognitive domains of impulsivity. Drug and Alcohol Dependence. 2011;114:194–200. doi: 10.1016/j.drugalcdep.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva J, Gonzalez R, Bechara A, Martin EM. Are all drug addicts impulsive? Effects of antisociality and extent of multidrug use on cognitive and motor impulsivity. Addictive Behaviors. 2007;32:3071–3076. doi: 10.1016/j.addbeh.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva J, Petkova P, Georgiev S, Martin EM, Tersiyski R, Raycheva M, et al. Impaired decision-making in psychopathic heroin addicts. Drug and Alcohol Dependence. 2007;86:287–289. doi: 10.1016/j.drugalcdep.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug and Alcohol Dependence. 2007;91:213–219. doi: 10.1016/j.drugalcdep.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addicive Behaviors. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. American Journal of Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Weafer J, Baggott MJ, de Wit H. Test-retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Experimental and Clinical Psychopharmacology. doi: 10.1037/a0033659. in press. [DOI] [PMC free article] [PubMed]
- White TL, Lejuez CW, de Wit H. Test-retest characteristics of the Balloon Analogue Risk Task. Experimental and Clinical Psychopharmacology. 2008;16:565–570. doi: 10.1037/a0014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcoholism: Clinical and Experimental Research. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Catecholamine theories of reward: a critical review. Brain Research. 1978;152:215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. New York, NY: Cambridge University Press; 1994. [Google Scholar]