Abstract
Scoliosis in children poses serious problems including respiratory problems, trunk imbalance, and depression, as well as detracting from the child’s appearance. Scoliosis can also contribute to back pain later in life. Advanced surgical techniques allow for good correction and maintenance of progressive curves, and growth-sparing treatments are now available for patients with early-onset scoliosis (EOS). Posterior corrective surgeries using pedicle screw (PS) constructs, which allow curves to be corrected in three dimensions, has become the most popular surgical treatment for scoliosis. Several navigation systems and probes have been developed to aid in accurate PS placement. For thoracolumbar and lumbar curves, anterior surgery remains the method of choice. Growth-sparing techniques for treating EOS include growing rods, the Shilla method, anterior stapling, and vertical expandable prosthetic titanium rib, which was originally designed to treat thoracic insufficiency syndrome. However, these advanced surgical techniques do not always offer a perfect solution for pediatric scoliosis, and they are associated with complications such as infections and problems with instrumentation. Surgeons have developed several techniques in efforts to address these complications. We here review historic and recent advances in the surgical treatment of scoliosis in children, the problems associated with various techniques, and the challenges that remain to be overcome.
Scoliosis can affect a child’s appearance, hamper respiratory function and trunk balance, lead to depression, and cause back pain later in life. Moderate scoliotic curves are usually treated conservatively using a brace, but a curve that continues to progress despite treatment requires corrective surgery, either with or without fusion. Recent advances in surgical techniques have made it possible to obtain good correction and maintenance of progressive curves, and several growth-sparing techniques are now available for treating patients with early-onset scoliosis (EOS). However, these advanced surgical techniques do not provide a perfect solution for pediatric scoliosis, and some are associated with suboptimal clinical outcomes.
In this article, we review recent technical advances and problems associated with the surgical treatment of scoliosis in children, both from a historic perspective and with a view to the future.
The development of posterior-approach surgery
The surgical correction of scoliosis is particularly challenging, and surgeons have applied various methods in efforts to meet that challenge. In 1924, Hibbs [1] reported early results from 59 patients, most with paralytic scoliosis, who had undergone posterior fusion surgery. His fusion technique involved elevating the bone flaps from the laminae and turning the free end of the flaps upward or downward to bring them into contact with the adjacent decorticated laminae. He also used a traction jacket to correct the curve as much as possible before surgery. Based on his results, Hibbs recommended early surgical intervention, before gross, severe spinal deformity develops.
Modern instrumentation surgery for scoliosis was pioneered in the early 1950s by Harrington [2], who used a stainless steel rod-and-hook system to correct spinal deformities. Harrington’s system was first used in combination with bone-fusion techniques to treat poliomyelitis-induced scoliosis, and was thereafter applied, with various modifications, to idiopathic and other types of scoliosis. Although the advent of the Harrington system dramatically improved the prospects of scoliosis surgery, the system had several disadvantages including a requirement for long-term bed rest and the extended use of a plaster jacket after surgery, hook dislodgement, and other instrumentation failures, pseudarthrosis, and flat-back syndrome if physiological sagittal alignment was not restored. Moe et al. modified the Harrington rod with a square end, which allowed the rod to be contoured along the lumbar lordosis to prevent postoperative flat-back. Moe also reported a facet-fusion technique that substantially enhanced fusion rates, and this remains one of the most important surgical techniques for scoliosis surgery to date [3].
In 1973, Luque reported a segmental spinal-instrumentation method that used rods and sublaminar wiring [4]. Unlike Harrington’s system, which relied primarily on distractive force applied to the spine through hooks, Luque’s system used transverse forces applied segmentally though sublaminar wires. This system obviated the need for a postoperative cast. In 1982, Luque reported the outcomes of 65 consecutive patients who were treated for idiopathic or paralytic scoliosis using his system, and found a mean correction rate of 72 % with a correction loss of only 1.5°. Complications in this series included infection and pseudarthrosis, in two patients each. While Luque’s success was remarkable, the risk of neural injury during sublaminar wiring presented a major concern. Although there have been several reports of this complication occurring, sublaminar wiring can be done with reasonable safety when performed by experienced surgeons, and it has become a standard technique in spinal fixation. Several authors have reported combining Harrington’s rod system with Luque’s rod-and-sublaminar-wiring system (the Harri–Luque technique) for more stable instrumentation. The concept of segmental fixation and correction in modern instrumentation surgery derives from Luque’s work, although the Luque system itself is now rarely used.
In 1988, Cotrel and Doubusset [5] described a multi-segmental system, called CD instrumentation, in which multiple laminal and pedicle hooks—and later, pedicle screws—were placed on the concave and convex sides of the curve. This allowed segmental fixation with multiple hooks, translation of the scoliotic curve, and the creation of kyphosis by rod rotation. Cotrel and Doubusset claimed that CD instrumentation allowed for a shorter fusion area, derotation of the spine, and the creation of kyphosis and lordosis in the thoracic spine and lumbar spine, respectively. However, some researchers questioned the derotation effect obtained through CD instrumentation, and this system had the disadvantages of high technical demands, bulky implants, and frequent postoperative decompensation. To prevent postoperative decompensation, Lenke et al. [6] created strict guidelines for selective thoracic fusion. Although the original CD instrumentation has been abandoned, it laid the foundation for the spinal instrumentation used today.
In 1994, Suk et al. [7] reported the first in a case series using pedicle screws (PSs) for fixation of the thoracic curve, and compared the results of three different surgical constructs: hooks only, screws only, or a combination of screws and hooks. They found that PS constructs provided better correction of frontal, sagittal, and rotational deformity with less loss of correction, a shorter fusion area, and less risk of neurological complications. In Japan in 1992, Abe et al. [8] also reported using PS constructs to correct thoracic curves in three patients, with a 78 % correction rate. Several researchers have since compared surgical results between patients treated with PS-only constructs and those with hybrid constructs of hooks, sublaminar wires, and pedicle screws. Kim et al. [9] compared the outcomes for patients treated with PS or hybrid constructs (29 each), and found that pedicle screw constructs offered better correction of the major curve and more improved pulmonary function than hybrid constructs, while the junctional change, lowest instrumented vertebra, time in surgery, and postoperative SRS-24 outcome scores were similar in both groups. Other authors echoed Kim’s findings in retrospective comparative studies and systemic reviews, reporting that PS constructs provided better, or at least similar, correction and maintenance of the main curve, and required fewer revisions due to their biomechanical stability [10] (Fig. 1). PS constructs have an important advantage in that vertebrae can be derotated directly with the PSs, thus reducing vertebral rotation by 42–60 % and reducing thoracic and lumbar humps [10, 11] (Fig. 2). PS constructs also facilitate osteotomies, including Ponte and pedicle-subtraction osteotomies and posterior vertebral column resection, so that even rigid and severe curves can be corrected efficiently without anterior procedures [12] (Fig. 3). PS constructs also obviate the need for an autologous iliac bone graft; in most patients with adolescent idiopathic scoliosis (AIS), this is replaced by a graft of local bone, with or without bone extenders. Thus, PS fixation is presently the most popular posterior surgical method for treating AIS, while, as in conventional scoliosis surgery, the meticulous release of the facet joints, ligaments, and muscles remains paramount.
Fig. 1.
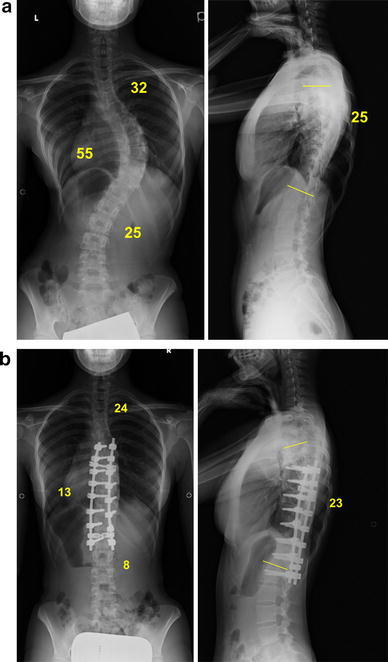
A 16-year-old girl with AIS (Lenke type 1AN) treated using PS constructs. The main thoracic curve of 55° was corrected to 13° with a fusion area ranging from T6 (one level below the upper end vertebra) to L1 (one level above the stable vertebra). a Radiograph before surgery. b Radiograph after surgery
Fig. 2.
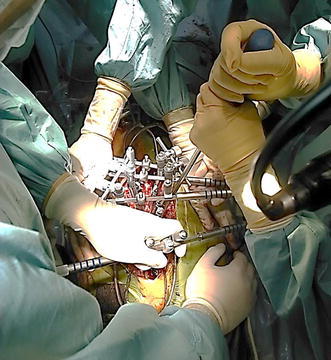
Direct vertebral derotation maneuver via pedicle screws
Fig. 3.
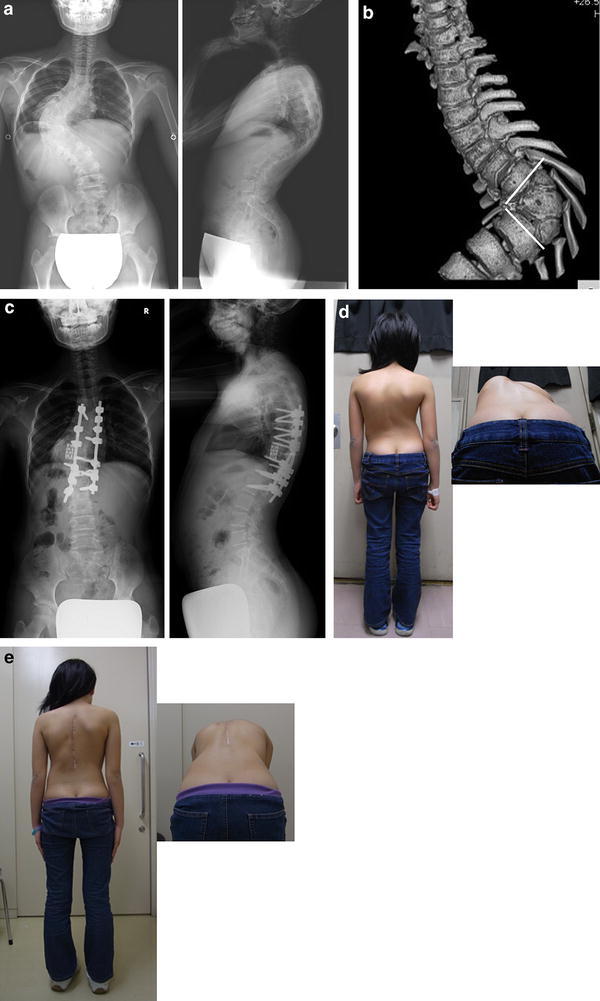
A 12-year-old girl with severe congenital scoliosis treated by posterior vertebral column resection. a Radiographs before surgery. b 3-dimensional CT showing three consecutive anomalous vertebrae which were resected. c Radiographs after surgery. d Appearance before surgery. e Appearance after surgery
Accurate PS placement is essential, and this requires precise anatomical information. Watanabe et al. [13] found that while 91 % of the pedicles examined had sufficient cancellous channels for screw placement, 9 % had insufficient cancellous channels, making screw placement extremely difficult, if not impossible. Insufficient cancellous channel was observed mostly in the concave area of the curve in the upper and middle thoracic spine. The reported accuracy of PS placement for the thoracic spine ranges widely, from 1.5 to 58 %. Suk et al. [7, 11, 12] reported using Kirschner wires as pedicle markers. Lenke et al. [9] reported a free-hand technique using a specially designed pedicle probe. Several surgeons have developed navigation systems to improve placement accuracy. A meta-analysis by Tian et al. [14] found that PS placement was significantly more accurate with the aid of a navigation method rather than with conventional free-hand placement, and that PSs were placed more accurately using a three-dimensional fluoroscopy-based or CT-based navigation system than with a two-dimensional fluoroscopy-based system. While these navigation systems improve the accuracy of PS placement, they are time-consuming and expensive, and they do not entirely eliminate the risk of malpositioned screws. Recently, Watanabe et al. [15] reported a ball-tip probe technique that uses a blunt, flexible probe to prepare the hole for the PS, with a placement accuracy of 95 %, a significant improvement in accuracy compared to the 65 % accuracy obtained with conventional free-hand placement.
Despite these advantages, PS constructs have several drawbacks. These include a steep learning curve, the potential for neurovascular injuries due to screw malposition, postoperative shoulder imbalance, difficulties in maintaining physiologic thoracic kyphosis, and higher instrumentation costs. Several surgeons have attempted to overcome these drawbacks, producing some effective techniques. For example, to prevent postoperative shoulder imbalance, Matsumoto et al. [16] reported using a short fusion strategy to simultaneously achieve both shoulder balance and acceptable correction of the main thoracic curve. To restore physiological thoracic kyphosis, Ito et al. [17] used simultaneous double-rod rotation to correct AIS curves.
The development of anterior-approach surgery
Dwyer developed an anterior instrumentation system using a titanium cable and screws to correct scoliosis. After thorough discectomy and a morselized rib graft, the cable was threaded through the screw heads, and a tensioning device was applied to approximate the adjacent vertebral bodies. Fusion was achieved in 91 % of 51 patients treated with this device; however, there was a loss of correction in 19 patients [18], and others experienced loss of lumbar lordosis and instrumentation failure. Zielke et al. [19] developed ventrale derotations-spondylodesis (VDS), an anterior instrumentation system that was claimed to allow derotation and restoration of lordosis of the thoracolumbar spine, and to yield better correction than either the Harrington or Dwyer systems. After following 53 patients for at least 10 years after undergoing treatment with Dwyer or Zielke instrumentation [20], Otani reported a 62 % correction rate, a 6 % rate of instrumentation failure, and patient satisfaction in most cases. However, other researchers have reported implant failure, loss of correction, progressive kyphosis, and pseudarthrosis in association with the VDS system. Kaneda et al. [21] treated 25 patients with thoracolumbar or lumbar curves using an anterior dual-rod system, and obtained a correction rate of 83 % for scoliosis and 86 % for rotation with restoration of lumbar lordosis. This two-rod system is biomechanically robust enough to prevent loss of correction after surgery. Sudo et al. [22] recently published results after following 30 patients treated with this dual-rod system for a mean of 17.2 years. The mean correction rate of the thoracolumbar/lumbar curve and the loss of correction at follow-up were 79.8 % and 3.4°, respectively, and a scoliosis-specific questionnaire revealed that the patients maintained a good quality of life. Thoracoscopic anterior correction surgery, a minimally invasive alternative for treating a single thoracic curve, yields correction rates comparable to conventional anterior or posterior approaches [23]. However, thoracoscopic anterior correction has been associated with high rates of pseudarthrosis, implant failure, and pulmonary complications, and it has fallen out of popularity.
The development of a classification system for AIS
The recent development of an AIS classification system has done much to advance the field of surgical scoliosis correction. In 1983, King et al. [24] divided AIS thoracic curves into Types I–V and recommended selective thoracic fusion for a Type II (major thoracic) curve of less than 80°, using the neutral and stable vertebra as the lower instrumented vertebra. King’s classification system was used for many years, although it described only the thoracic curve, and the interobserver reliability was not high. More recently, Lenke et al. [25] reported a comprehensive, validated system that divides AIS curves into six basic types, subdivided by lumbar spine modifiers that describe the relationship between the apical vertebra of the lumbar curve and the central sacral vertical line (A, B, C), and by the sagittal profile (hypo, normal, hyper). Based on the classification, Lenke recommended surgical fusion of all structural curves, which were defined as curves of 25° or more on bending films, or a local kyphosis greater than 20°. This classification system has made the determination of surgical strategies for AIS simpler and more reliable.
Surgeries for EOS
Growing-rod technique
One of the more remarkable recent advances in the treatment of scoliosis is for EOS, a condition that presents extreme treatment challenges. EOS should be treated surgically if conservative treatment fails or the patient will have severe deformity, restrictive pulmonary dysfunction, cor pulmonale, and early mortality. The surgical treatment should not restrict the growth of the trunk and thoracic cavity. Therefore, many growth-sparing techniques have been developed in which the curved spine is either not fused, or is fused only at the level of anchor placements instead of being fused throughout the curve. Distraction-based surgery using a single subcutaneous distraction rod, described first by Harrington, has been used with various modifications [26]. Although this method effectively corrected and prevented the progression of curves to a degree, it was also associated with implant failure, anchor dislodgement, and spontaneous fusion. Akbarnia et al. [27] popularized a dual growing rod technique, which enhanced the stability of the construct and reduced the risk of implant failure. This surgical technique uses craw hooks or PSs for the cephalad and caudal foundations, and connects two rods, placed on either side of the spine, with connectors (Fig. 4). The rods are lengthened every 6 months through the connectors until the skeletal structure matures, at which time the growing rods are removed and the patient undergoes final fusion surgery. After following 13 patients with dual growing rods through final fusion surgery, Akbarnia et al. [27] reported that the Cobb angle improved from an initial 81.0° to 27.7° after the final fusion and that patients averaged 5.7 cm of spinal growth during the treatment period, with the greater growth and correction occurring in patients with more frequent rod lengthening. Bess et al. [28] evaluated 140 patients who had undergone a total of 897 growing rod procedures, and reported that 81 (58 %) had experienced at least one complication and that the complication rate increased by 24 % for each additional surgical procedure. In a multi-center study conducted in Japan, Watanabe et al. [29] reported that complications occurred in 50 of 88 patients (57 %) treated with growing rods, including implant-related failures (72 %), infections (16 %), neurological impairments (3 %), and 11 others, and that a larger upper-thoracic scoliotic curve, thoracic kyphosis, and a larger number of rod-lengthening procedures increased the risk of complications. Thus, dual growing rod techniques are effective in controlling EOS curves, although increasing the number of rod-lengthening procedures increases the rate of complications. Magnetically controlled growing rods, developed to overcome this challenge, are now in clinical use, and early results are promising [30].
Fig. 4.
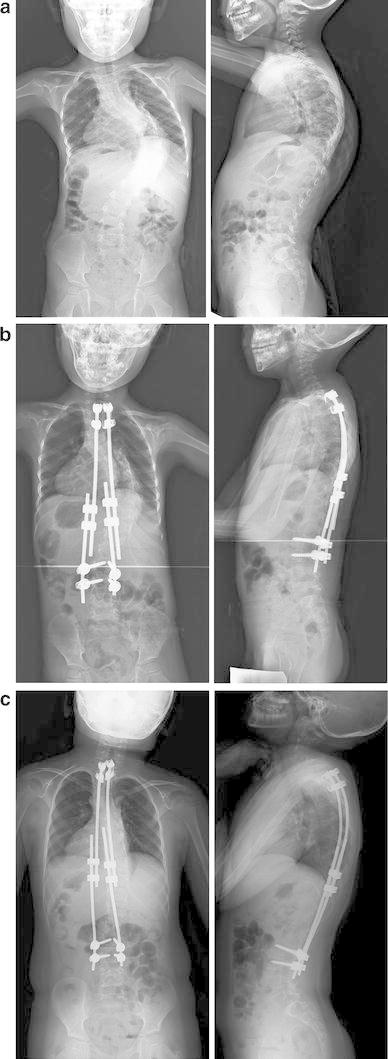
A 4-year-old boy with scoliosis associated with congenital myopathy treated using dual growing rods. a Radiograph before surgery. b Radiograph immediately after growing rod placement. c Radiograph at the latest follow-up (5 years after the rod placement)
Vertical expandable prosthetic titanium rib (VEPTR)
Campbell established the concept of thoracic insufficiency syndrome (TIS) as instability of the thorax to support normal respiration or lung growth [31, 32]. TIS is caused by three types of congenital and acquired pathologies: Type I, rib absence and scoliosis; Type II, fused ribs and scoliosis; and Type III, hypoplastic thorax, as occurs in the Jarcho–Levin and Jeune syndromes. Campbell developed VEPTR to maximize the thoracic volume and correct deformities of the thorax and spine in patients with TIS [32]. With expansion thoracotomy and, if necessary, osteotomy of the fused ribs and constricting fibrous tissues on the concave side of the curve, one VEPTR rod is placed between cradles set on the rib and a hook set on the lumbar spine or pelvis, and a secondary rod is placed between cradles set on the ribs (Fig. 5). These rods are extended every 4–6 months. VEPTR has recently been applied to other types of EOS in place of growing rods, because VEPTR does not require wide exposure of the spine. Campbell and colleagues obtained promising results with VEPTR in lengthening the spine, correcting deformity, and increasing the space available for the lungs. However, this was obtained at the cost of frequent complications, including construct dislodgement or migration, cardiopulmonary morbidities, and infections. VEPTR has been available in Japan since July 2009, owing to the efforts of Dr. N. Kawakami at Meijo Hospital [33].
Fig. 5.
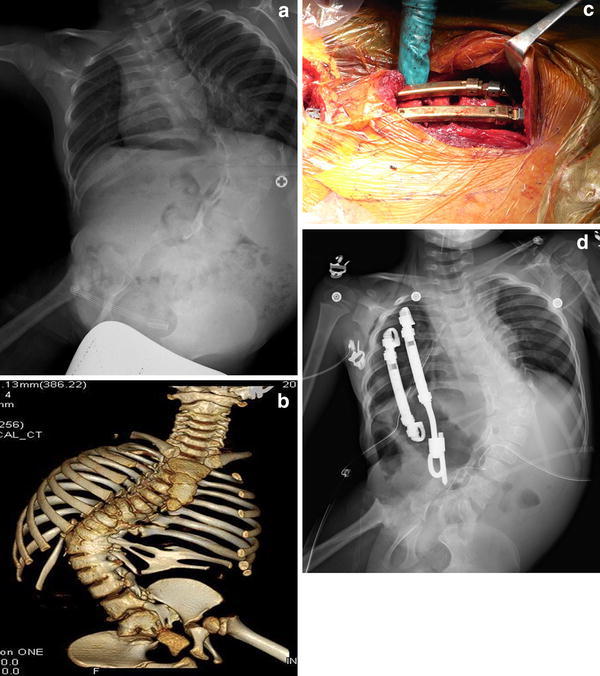
Congenital scoliosis with fused ribs treated using VEPTR. Expansion thoracotomy was conducted by dividing the fused ribs. a Radiograph before surgery. b 3-dimensional CT showing congenital fused ribs and vertebrae. c Intraoperative photo showing expansion thoracotomy and placement of VEPTR. d Radiograph after surgery
Other surgical methods for EOS
Growth-guidance techniques allow the unfused spine to grow along rods, thereby controlling scoliosis and maintaining the longitudnal growth of the spine. Luque attempted the first growth-guidance technique in the late 1970s, using Luque rods. This technique, called the Luque trolley method, was almost abandoned because other surgeons could not replicate Luque’s results. McCarthy developed the Shilla technique, in which the apex of the scoliosis is fixed with PSs and rods are attached at the upper and lower ends of the curve on gliding PSs, allowing the rods to glide through the unlocked screw heads as the spine grows along the constructs [34]. Although long-term follow-up results are not yet available, this technique is a promising surgical option for EOS.
Future perspectives
In treating scoliosis, modern surgical techniques and instrumentation make it possible to obtain good curve correction and osseous fusion. However, the optimal goal of scoliosis surgery is eventually to obtain a mobile, pain-free spine with good balance and physiologic alignment. Bets et al. treated 28 AIS patients with anterior vertebral body stapling to preserve mobility, and after following these patients for at least 2 years [35], reported a success rate of 87 % for all lumber curves, 79 % for thoracic curves smaller than 35°, and a failure to correct thoracic curves larger than 35°. Thus, it would be ideal that a patient with a mild AIS curve that is likely to progress later can be treated with fusionless or minimally invasive surgery. To make this determination, however, requires a highly accurate method of predicting curve progression. Lonstein and Carlson proposed predictive factors for curve progression, including Risser signs, initial curve magnitude, and age [36]. However, because the accuracy and predictive value of their formula may not be optimal, some investigators developed a genome-based prognostic test, which is now available in the United States [37]. Notably, however, the testing kit has only been validated with Caucasian patients, and our genomic study in Japanese AIS patients failed to replicate the test results [38, 39]. Therefore, genes related to the onset and progression of AIS should be identified independently for different ethnic populations. Genome-wide association studies by Takahashi et al. [40] and Koh et al. [41] identified LBX1 and GPR 126, respectively, as candidate AIS genes, and Miyake et al. [42] found a single-nucleotide polymorphism that was significantly associated with the severity of the curve.
These genomic approaches, together with AIS patients’ clinical data, will help us to predict accurately the onset and progression of scoliotic curves in AIS, with the goal of intervening early and treating patients with less invasive techniques.
Conclusions
Advances in the surgical treatment of AIS have made it possible to obtain good correction of scoliotic curves, but these treatments are still associated with several disadvantages. It is important to find reliable methods for predicting curve progression, and to develop less invasive methods for the surgical correction of pediatric scoliosis.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Hibbs A., II A report of fifty-nine cases of scoliosis treated by the fusion operation. J Bone Joint Surg Am. 1924;6:3–37. [PubMed] [Google Scholar]
- 2.Harrington PR. Treatment of scoliosis: correction and internal fixation by spine instrumentation. J Bone Joint Surg Am. 2002;84:591–634. [PubMed] [Google Scholar]
- 3.Moe JH. Critical analysis of methods of fusion for scoliosis. An evaluation in two hundred and sixty-six patients. J Bone Joint Surg Am. 1958;40:529–554. [PubMed] [Google Scholar]
- 4.Luque ER. Segmental spinal instrumentation for correction of scoliosis. Clin Orthop Relat Res. 1982;163:192–198. [PubMed] [Google Scholar]
- 5.Cotrel Y, Dubousset J, Guillaumat M. New universal instrumentation in spinal surgery. Clin Orthop Relat Res. 1988;227:10–23. [PubMed] [Google Scholar]
- 6.Lenke LG, Bridwell KH, Baldus C, Blanke K. Preventing decompensation in King type II curves treated with Cotrel-Dubousset instrumentation. Strict guidelines for selective thoracic fusion. Spine. 1992;17(8 Suppl):S274–81. [DOI] [PubMed]
- 7.Suk SI, Lee CK, Min HJ, Cho KH, Oh JH. Comparison of Cotrel–Dubousset pedicle screws and hooks in the treatment of idiopathic scoliosis. Int Orthop. 1994;18:341–346. doi: 10.1007/BF00187077. [DOI] [PubMed] [Google Scholar]
- 8.Abe E, Shimada Y, Okuyama K. Experiences in scoliosis surgery using pedicular screwing. Tohoku Seikeisaigaigeka Kiyou. 1992;36:327–331 (in Japanese).
- 9.Kim YJ, Lenke LG, Kim J, Bridwell KH, Cho SK, Cheh G, Sides B. Comparative analysis of pedicle screw versus hybrid instrumentation in posterior spinal fusion of adolescent idiopathic scoliosis. Spine. 2006;31:291–298. doi: 10.1097/01.brs.0000197865.20803.d4. [DOI] [PubMed] [Google Scholar]
- 10.Ledonio CG, Polly DW, Jr, Vitale MG, Wang Q, Richards BS. Pediatric pedicle screws: comparative effectiveness and safety: a systematic literature review from the Scoliosis Research Society and the Pediatric Orthopaedic Society of North America task force. J Bone Joint Surg Am. 2011;93:1227–1234. doi: 10.2106/JBJS.J.00678. [DOI] [PubMed] [Google Scholar]
- 11.Lee SM, Suk SI, Chung ER. Direct vertebral rotation: a new technique of three-dimensional deformity correction with segmental pedicle screw fixation in adolescent idiopathic scoliosis. Spine. 2004;29:343–349. doi: 10.1097/01.BRS.0000109991.88149.19. [DOI] [PubMed] [Google Scholar]
- 12.Suk SI, Kim JH, Kim WJ, Lee SM, Chung ER, Nah KH. Posterior vertebral column resection for severe spinal deformities. Spine. 2002;27:2374–2382. doi: 10.1097/00007632-200211010-00012. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe K, Lenke LG, Matsumoto M, Harimaya K, Kim YJ, Hensley M, Stobbs G, Toyama Y, Chiba K. A novel pedicle channel classification describing osseous anatomy: how many thoracic scoliotic pedicles have cancellous channels? Spine. 2010;35:1836–1842. doi: 10.1097/BRS.0b013e3181d3cfde. [DOI] [PubMed] [Google Scholar]
- 14.Tian NF, Huang QS, Zhou P, Zhou Y, Wu RK, Lou Y, Xu HZ. Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J. 2011;20:846–859. doi: 10.1007/s00586-010-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe K, Matsumoto M, Tsuji T, Ishii K, Takaishi H, Nakamura M, Toyama Y, Chiba K. Ball tip technique for thoracic pedicle screw placement in patients with adolescent idiopathic scoliosis. J Neurosurg Spine. 2010;13:246–252. doi: 10.3171/2010.3.SPINE09497. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M, Watanabe K, Ogura Y, Okada E, Hosogane N, Chiba K, Toyama Y. Short fusion strategy for Lenke type 1 thoracic curve using pedicle screw fixation. J Spinal Disord Tech. 2013;26:93–97. doi: 10.1097/BSD.0b013e31823ac2e8. [DOI] [PubMed] [Google Scholar]
- 17.Ito M, Abumi K, Kotani Y, Takahata M, Sudo H, Hojo Y, Minami A. Simultaneous double-rod rotation technique in posterior instrumentation surgery for correction of adolescent idiopathic scoliosis. J Neurosurg Spine. 2010;12:293–300. doi: 10.3171/2009.9.SPINE09377. [DOI] [PubMed] [Google Scholar]
- 18.Dwyer AF, Schafer MF. Anterior approach to scoliosis. Results of treatment in fifty-one cases. J Bone Joint Surg Br. 1974;56:218–224. [PubMed] [Google Scholar]
- 19.Zielke K, Stunkat R, Beaujean F. Ventrale derotations-spondylodesis. Arch Orthop Unfallchir. 1976;85:257–277. doi: 10.1007/BF00415189. [DOI] [PubMed] [Google Scholar]
- 20.Otani K, Saito M, Sibasaki K. Anterior instrumentation in idiopathic scoliosis: a minimum follow-up of 10 years. Int Orthop. 1997;21:4–8. doi: 10.1007/s002640050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneda K, Shono Y, Satoh S, Abumi K. New anterior instrumentation for the management of thoracolumbar and lumbar scoliosis. Application of the Kaneda two-rod system. Spine. 1996;21:1250–1262. doi: 10.1097/00007632-199605150-00021. [DOI] [PubMed] [Google Scholar]
- 22.Sudo H, Ito M, Kaneda K, Shono Y, Abumi K. Long-term outcomes of anterior dual-rod instrumentation for thoracolumbar and lumbar curves in adolescent idiopathic scoliosis: a twelve to twenty-three-year follow-up study. J Bone Joint Surg Am. 2013;95:e49. doi: 10.2106/JBJS.L.00781. [DOI] [PubMed] [Google Scholar]
- 23.Newton PO, Upasani VV, Lhamby J, Ugrinow VL, Pawelek JB, Bastrom TP. Surgical treatment of main thoracic scoliosis with thoracoscopic anterior instrumentation. Surgical technique. J Bone Joint Surg Am. 2009;91(Suppl 2):233–248. doi: 10.2106/JBJS.I.00368. [DOI] [PubMed] [Google Scholar]
- 24.King HA, Moe JH, Bradford DS, Winter RB. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am. 1983;65:1302–1313. [PubMed] [Google Scholar]
- 25.Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169–1181. [PubMed] [Google Scholar]
- 26.Moe JH, Kharrat K, Winter RB, Cummine JL. Harrington instrumentation without fusion plus external orthotic support for the treatment of difficult curvature problems in young children. Clin Orthop Relat Res. 1984;185:35–45. [PubMed] [Google Scholar]
- 27.Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine. 2005;30:S46–S57. doi: 10.1097/01.brs.0000175190.08134.73. [DOI] [PubMed] [Google Scholar]
- 28.Bess S, Akbarnia BA, Thompson GH, Sponseller PD, Shah SA, El Sebaie H, Boachie-Adjei O, Karlin LI, Canale S, Poe-Kochert C, Skaggs DL. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am. 2010;92:2533–2543. doi: 10.2106/JBJS.I.01471. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe K, Uno K, Suzuki T, Kawakami N, Tsuji T, Yanagida H, Ito M, Hirano T, Yamazaki K, Minami S, Kotani T, Taneichi H, Imagama S, Takeshita K, Yamamoto T, Matsumoto M. Risk factors for complications associated with growing-rod surgery for early-onset scoliosis. Spine. 2013;38:E464–8. [DOI] [PubMed]
- 30.Cheung KM, Cheung JP, Samartzis D, Mak KC, Wong YW, Cheung WY, Akbarnia BA, Luk K. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. Lancet. 2012;379(9830):1967–1974. doi: 10.1016/S0140-6736(12)60112-3. [DOI] [PubMed] [Google Scholar]
- 31.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85:399–408. doi: 10.1302/0301-620X.85B3.13429. [DOI] [PubMed] [Google Scholar]
- 32.Campbell RM., Jr VEPTR: past experience and the future of VEPTR principles. Eur Spine J. 2013;22(Suppl 2):S106–S117. doi: 10.1007/s00586-013-2671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawakami N, Tsuji T, Saito T, Nohara A, Obara T, Miyasaka K, Sato M, Ito K, Uno K, Matsumoto M, Yanagida H, Watanabe K, Committee of VEPTR, Japanese Scoliosis Society. Operative indications and surgical technique/complications of VEPTR. J Spine Res. 2011;2:43–51 (in Japanese).
- 34.Tis JE, Karlin LI, Akbarnia BA, Blakemore LC, Thompson GH, McCarthy RE, Tello CA, Mendelow MJ, Southern EP, Growing Spine Committee of the Scoliosis Research Society Early onset scoliosis: modern treatment and results. J Pediatr Orthop. 2012;32:647–657. doi: 10.1097/BPO.0b013e3182694f18. [DOI] [PubMed] [Google Scholar]
- 35.Betz RR, Ranade A, Samdani AF, Chafetz R, D’Andrea LP, Gaughan JP, Asghar J, Grewal H, Mulcahey MJ. Vertebral body stapling: a fusionless treatment option for a growing child with moderate idiopathic scoliosis. Spine. 2010;35:169–176. doi: 10.1097/BRS.0b013e3181c6dff5. [DOI] [PubMed] [Google Scholar]
- 36.Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984;66:1061–1071. [PubMed] [Google Scholar]
- 37.Ward K, Ogilvie JW, Singleton MV, Chettier R, Engler G, Nelson LM. Validation of DNA-based prognostic testing to predict spinal curve progression in adolescent idiopathic scoliosis. Spine. 2010;35:E1455–E1464. doi: 10.1097/BRS.0b013e3181ed2de1. [DOI] [PubMed] [Google Scholar]
- 38.Ogura Y, Takahashi Y, Kou I, Nakajima M, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Yonezawa I, Tsuji T, Suzuki T, Sudo H, Kotani T, Watanabe K, Chiba K, Toyama Y, Matsumoto M, Ikegawa S. A replication study for association of 53 single nucleotide polymorphisms in a scoliosis prognostic test with progression of adolescent idiopathic scoliosis in Japanese. Spine. 2013;38:1375–1379. doi: 10.1097/BRS.0b013e3182947d21. [DOI] [PubMed] [Google Scholar]
- 39.Ogura Y, Takahashi Y, Kou I, Nakajima M, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Yonezawa I, Tsuji T, Suzuki T, Sudo H, Kotani T, Watanabe K, Chiba K, Toyama Y, Matsumoto M, Ikegawa S. A replication study for association of 5 single nucleotide polymorphisms with curve progression of adolescent idiopathic scoliosis in Japanese patients. Spine. 2013;38:571–575. doi: 10.1097/BRS.0b013e3182761535. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi Y, Kou I, Takahashi A, Johnson TA, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Tsuji T, Suzuki T, Sudo H, Kotani T, Watanabe K, Chiba K, Hosono N, Kamatani N, Tsunoda T, Toyama Y, Kubo M, Matsumoto M, Ikegawa S. A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nat Genet. 2011;43:1237–1240. doi: 10.1038/ng.974. [DOI] [PubMed] [Google Scholar]
- 41.Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, Qiu X, Sharma S, Takimoto A, Ogura Y, Jiang H, Yan H, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Hosono N, Tsuji T, Suzuki T, Sudo H, Kotani T, Yonezawa I, Londono D, Gordon D, Herring JA, Watanabe K, Chiba K, Kamatani N, Jiang Q, Hiraki Y, Kubo M, Toyama Y, Tsunoda T, Wise CA, Qiu Y, Shukunami C, Matsumoto M, Ikegawa S. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45:676–679. doi: 10.1038/ng.2639. [DOI] [PubMed] [Google Scholar]
- 42.Miyake A, Kou I, Takahashi Y, Johnson TA, Ogura Y, Dai J, Qiu X, Takahashi A, Jiang H, Yan H, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Hosono N, Tsuji T, Suzuki T, Sudo H, Kotani T, Yonezawa I, Kubo M, Tsunoda T, Watanabe K, Chiba K, Toyama Y, Qiu Y, Matsumoto M, Ikegawa S. Identification of a susceptibility locus for severe adolescent idiopathic scoliosis on chromosome 17q24.3. PLOS ONE. 2013. e72802. [DOI] [PMC free article] [PubMed]


