Abstract
Background:
The scope of this study was to preserve whole detailed structure of dissected and decalcified bones, taken from used cadavers, by a new plastination technique.
Materials and Methods:
Specimens we used in this study were sheep femurs and human bones including pelvis, femur, tibia, and fibula. Bones, at first, fixed with 5% formalin and were decalcified with 5% nitric acid, and then were fixed again and washed under the tap water. The resulted flexible bones were dehydrated in −25°C acetone and degreased them in +25°C acetone. Then, the experimental and control specimen were placed in the vacuum chamber for forced impregnation with our new flexible unsaturated polyester resin (UP89 method) and silicon resin (S10 method), respectively. Finally, the strength and flexibility of plastinated decalcified specimens were investigated by tensometer, and the weight diversity was measured by digital balance.
Results:
Plastinated bones prepared by this technique were found to be dried, non-fragile, durable, odorless, non-greasy, and demonstrating all detailed structures of the bones. Tensile and weight tests results indicated that plastinated decalcified femurs have owned higher flexibility and strength but lesser weight than plastinated undecalcified femurs. The characteristics of both experimental and control groups of plastinated decalcified specimens were found to have no significant difference.
Conclusions:
Our synthesized resin found to be much more economical than conventional plastination method. In more details, properties of these new products were the same as, S10 method, from points of strength, flexibility and weight, but, since the money cost for producing them was about one fifth that of S10 method.
Keywords: Bones, decalcification, plastination, UP89 method
INTRODUCTION
Bone decalcification is an ongoing challenging aspect of pathology and histology research laboratories. Due to the difficulties in sectioning undecalcified bone, early investigators of a hundred years ago began examining thin ground sections of acid-soaked bone.[1] Over the years, strong mineral acids, weak organic acids, ion exchange resins, electrolytic devices, and chelators were used in decalcification methods. As originally published, many of these methods are still used today.[2,3] Albiin in 1991 described a new technique, decalcification by perfusion, for the softening of bony tissue. The perfusion technique considerably reduced the time needed to decalcify the tissue and preserved the morphology better than did the immersion procedure.[4] Our pilot study (Rabiei, 2012) proposed the new method of decalcification of bones before plastination for human cadavers because this method prepared bones of excellent quality for teaching.[5]
Paraffin impregnation was introduced by Hochstetter in 1925. Embedding of the organic tissue in plastic was introduced in the 1949.[6] Plastination was introduced by Von Hagense in 1978 who applied polymerizing resins for replacement instead of intermediary solvent.[7] Plastination is a method of conservation of anatomical specimens by curable polymers such as silicone, epoxy, and polyester resins. Subsequently, plastinated specimens are non-toxic, odorless, dry, and durable over the time. Plastination is carried out in many institutions worldwide and obtained great acceptance particularly because of the durability and the high teaching value of plastinated specimens. Plastination preserves tissues and organs that have been removed from the body of the deceased as well as the entire body itself. Plastination was performed in 5 steps of Fixation, Dehydration, Degreasing, Force Impregnation, and Curing.[8,9,10,11] The purpose of this study is to establish a new technique for decalcification and plastination of bone specimens, as the teaching anatomy tool and museum specimens, by our new flexible unsaturated polyester resin UP89.
MATERIALS AND METHODS
Materials
Specimens we used in this study were sheep femurs and some bones of a 65-years-old human who had donated his body for education and research affairs to the department of anatomy. The latter bones included: Pelvis, femur, tibia, and fibula. Our other material consists of: Silicon resin S10, S3 and S6 hardener (BIODUR), and flexible unsaturated polyester resin (UP89), synthesized in plastination laboratory in Anatomical Sciences and Molecular Biology Department of Isfahan University of Medical Sciences. The characteristics of synthesized resin are shown in Table 1. MEK Peroxide, Acetone, Deep freezer, acetonometer, heater, vacuum chamber, vacuum pump (3 m3/min for 30 L polymer mixture), tensometer (Universal test machine- DARTEC), and digital balance were used.
Table 1.
Characteristics of synthesized flexible unsaturated polyester resin

Methods
To evaluate this method, human bones including pelvis, femur, tibia, and fibula and 12 sheep femur bones, all approximately the same size, were used. All of human bones and half of the sheep femur bones (number = 6) were decalcified and plastinated with UP89 and S10 resins, and the other half (number = 6) were plastinated without decalcification. At the end, we compared decalcified and undecalcified plastinated specimens with UP89 and S10 resins by tensile test, compression test, and weight test. Subsequently, we compared efficiency of plastinated decalcified specimens versus plastinated undecalcified specimens and also efficiency of our new synthesized resin versus silicon resin. Our protocol was as the following of eight steps:
Fixation: In order to avoid decomposition of the soft tissue of bones over time, before performing other steps, the samples were fixed with 5% formalin at 5°C.[12]
Decalcification: For decalcification, 5% nitric acid was used at 42°C.[12] The specimens had to be removed from the decalcifying solution as soon as the decalcification process was completed, otherwise the histologic and cytologic detail might be harmed. Endpoint of decalcification was measured by needle pinning and ease of cutting. (The needle had to pass through the bone without any force).[13] Decalcification was investigated by X-ray radiology for each bone after decalcification as a standard method.[14,15,16] In this step, all of the human bones and half of the sheep femur bones were decalcified.
Neutralizing: When the tissue was completely decalcified with mineral acid techniques, the tissue should be neutralized before washing by treatment with alkali. For neutralizing the remained acid, the specimens were put in 5% sodium sulfate solution for 24 hours.[12,13,14]
Washing: Bones were put in tap water to remove all traces of alkali.[12]
Re-fixation: Extended washings can create swelling of the tissue, so the specimens were re-fixed in 5% formalin at 5°C to obtain appropriate density before plastination.[3]
Dehydration: Decalcified and non-decalcified specimens were transferred to 95% acetone bath at −25°C, and acetone purity was measured using acetonometer every 2 days. After a week, the acetone inside the stainless steel container was replaced with pure acetone, and this process was repeated until complete dehydration was achieved, i.e., when the acetone percentage remained constant.[7,8,9,10,11]
Degreasing: The specimens were transferred to acetone bath at 25°C. During degreasing, the acetone was changed whenever it became visibly yellowish. This step was stopped when the acetone no longer became discolored.[17]
Forced impregnation: In this step, half of the specimens (3 decalcified sheep femurs, 3 undecalcified sheep femurs, and 1 decalcified human hip, femur, tibia, and fibula) were put into a container of S10 resin and the other half were placed in a container of UP89 resin and 2% peroxide (MEKP) was added to resin. Then, the containers were placed in the vacuum chamber up to the pressure of 5 mm Hg.[18,19,20] After 14 days and replacing the acetone by resin, the samples were taken out of the vacuum chamber. Impregnation was checked by watching the bubble formation on the surface of the resin and by means of a vacuum gauge. Bubbling will be stopped usually within 2 weeks, which is the sign of a full impregnation.[17]
Curing: When forced impregnation was completed, specimens were removed from the vacuum chamber and excess polymer drained back into the chamber. For curing of impregnated specimens by S10 resin, we used conventional gas curing method by S3 hardener. UP89 impregnated specimens were carried out in the UV and heat cabinet to complete curing.[17,18,19,20]
Mechanical tests and weight test
To compare our new plastination method (UP89) with that of the conventional one (S10) for undecalcified and decalcified bones, we used tensile test (to show strength of bones), compression test (to show flexibility of bones), and weight test (to show difference between decalcified and undecalcified plastinated bones weight) for 4 groups (n = 3) of plastinated specimens consist of group 1: Decalcified bones plastinated with UP89 (D- UP89), group 2: Undecalcified bones plastinated with UP89 (UD- UP89), group 3: Decalcified bones plastinated with S10 (D- S10), and group 4: Undecalcified bones plastinated with S10 (UD- S10). For weight test, 12 femur (n = 3 for each group) were weighed before and after decalcification and after plastination. For mechanical tests, 12 femur cutting in the same block in size 5 × 1 × 1 cm were used (n = 3 for each group).[3,15] In tensile test, for comparison of samples strength, we determined maximum force to break the specimen in constant speed (V = 5 mm/min) by tensometer. In compression test, for comparison of samples flexibility, we determined maximum deflection (mm) of the specimen in constant force of 0.8 kN by tensometer.[11,12]
Statistical analysis
To compare the result of tensile test, compression test, and weight test among the 4 groups (D- UP89), (UD- UP89), (D- S10), (UD- S10) of plastinated specimens, Mann-Whitney test was used. Differences with P < 0.05 were interpreted as significant.[11,15]
RESULTS
We found that the bones needed 20 days to be well-fixed. Decalcification time by this method ranged from 29 to 40 hours for different human bones [Table 2]. After decalcification, bones were X-rayed to show all the calcium was removed. Specimens become rigid after 2 days (re-fixation). For dehydration, at least, 3 bathes of −25°C acetone for 2-3 weeks are essential to remove the water and ensure good dehydration. The specimens were impregnated with UP89 and S10 under the vacuum for 2 weeks. Curing was complete after 4 months.
Table 2.
Decalcification time for human bones by 5% nitric acid at 42°C

The plastinated bones prepared with both UP89 and S10 methods were dry, clean, non-fragile, light, and odorless, and all of them maintained their original shape and natural look.
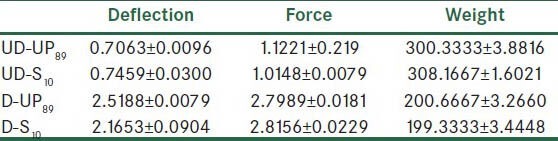
The results (mean ± SD) of the compression test, tensile test, and weight test for 4 groups of plastinated specimens are shown in Table 3. Also, the result of Mann-Whitney test for comparison of 4 groups of plastinated specimens is shown in Table 4.
Tables 3.
The results (mean ± SD) of compression test (F = 0.8 kN), tensile test (V = 5 mm/min), and weight test for 4 groups (n = 3) of plastinated specimens
Tables 4.
The result of Mann-Whitney test (P- value) for comparison of 4 groups of plastinated specimens one by one for compression test, tensile test, and weight test

The comparison of the results of Mann-Whitney test [Tables 4] for deflection, force, and weight indicated that there was no significant difference between undecalcified bones plastinated by S10 and UP89 (group 1). Also indicated that there was no significant difference between decalcified bones plastinated by S10 and UP89 (group 2). A comparison of results of Mann-Whitney test [Tables 4] for deflection, force, and weight indicated that there was significant difference between decalcified and undecalcified bones plastinated by S10 (group 3). Also indicated that there were significant differences between decalcified and undecalcified bones plastinated by UP89 (group 4).
DISCUSSION
According to Page et al. (1996), formic acid mixtures are decalcifying agents used most often; it decalcifies slowly enough for controlled decalcification and does not need to be watched as carefully as other acid decalcifying agents. Hydrochloric and nitric acid mixtures are usually suggested for rapid decalcification. They work very quickly and have to be watched carefully.[14] Callis and Sterchi (1998) emphasized that larger specimens would show some damage to the tissue morphology from certain acid decalcification. Unfortunately, this cannot be avoided with the larger specimens. Tissues should never remain in acid decalcifying solution over a 48 to 72 hours weekend without daily chemical or X-ray testing. They should be removed from the decalcifying solution, rinsed, and placed into 10% formalin.[3] We checked the endpoint of decalcification as measured by needle pinning and ease of cutting, and the specimens were removed from the decalcifying solution as soon as the decalcification process was completed. As Kiviranta et al. (1980) mentioned, the time needed for decalcification of osseous structures depends upon 2 factors: First, the minimum diffusing distance of the specimen and second, the density of the bone.[21] In the present study, it was shown that a period of 29 to 40 hours is needed to obtain a sufficient decalcification of human bones when immersed in the 5% nitric acid. According to Witter et al. (2000), decalcification is one of the most time-consuming steps in the preparation of tissue samples for histological purposes. There are various techniques to accelerate this procedure, such as putting specimens in a microwave oven, which shows the accretion is an effect of high temperature.[15] In an attempt to obtain decalcified specimens in shorter periods of time, we used the high temperature up to 42°C.
Plastic embedding for preservation of tissues and organs introduced in 1949 (Bennet et al., 1976). As Wolfe state (1956), many of these polymers were introduced to be used in electron microscopy or for enzymatic histochemistry at the light microscopic level.[22] Von Hagense in 1978 applied polymerizing resins (such as silicon resin) for replacement instead of intermediary solvent. In this study, we applied UP89 technique for plastination to examine the new synthesized resin instead of the silicon resin in the conventional (S10) technique. The specimens obtained by UP89 technique were found to show acceptable appearance for teaching anatomy and museum specimens. The specimens were odorless, dry, and flexible compared to the specimens prepared by the S10 technique. Since the cost was observed to be lower than the prepared plastinated specimens by the S10 technique, the synthesized resin was found to be much more appropriate and economical for plastination of bones. The use of plastinated prosections for teaching anatomy to medical students and residents is well-known (Holladay and Hudson, 1989).[23] Baptista et al. (1989) argue that the relationship between anatomical structures can be examined, as well as the identification of different tissue layers, nerves, and vessels.[24] Our plastinated bones were dry to touch, clean, non-fragile, non-toxic, odorless and all of them maintained their original shapes, and all parts of the bones (groove, tubercle, cartilage, and ligament) were preserved. They were easy to handle and had a very light weight. Seibold R. et al. (1991) applied sectioning technique, histological stains, and plastination for both undecalcified and decalcified bones for the preparation of transparent decalcified bone sections.[25] In this study, we used decalcification and bulk plastination for preparation of plastinated bone prosections for teaching anatomy. Cannas and Fuda (1991) utilized long-term fixed specimens for plastination for purpose of both teaching and observation.[26] Bones from old human cadavers by period of time may result in deterioration occurring in the different parts of the bones. So, we propose this new method of decalcification of bones and plastination for long-term fixed human cadavers.
ACKNOWLEDGEMENTS
This study was financially supported, Department of Anatomical Sciences and Molecular Biology, School of Medicine, Isfahan University of Medical Science, Isfahan, Iran.
Footnotes
Source of Support: Department of Anatomical Sciences and Molecular Biology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Brain EB. The Preparation of Decalcified Sections. In: Thomas CC, editor. Springfield; 1966. pp. 3–4. [Google Scholar]
- 2.Lillie RD, Fuller HM. 4th ed. New York: McCraw-Hill Book Company; 1960. Histopathologictechnic and practical histochemistry; pp. 787–807. [Google Scholar]
- 3.Callis G, Sterchi D. Decalcification of bone: Literature review and practical study of various decalcifying agents. Methodsand their effects on bone histology. J Histotechnol. 1998;21:49–58. [Google Scholar]
- 4.Nilsson M, Hellstrom S, Albiin N. Decalcification by perfusion. A new method for rapid softening of temporal bones. HistolHistopath. 1991;6:415–20. [PubMed] [Google Scholar]
- 5.Rabiei AA, Esfandiary E, SetayeshMehr M, Shamosi A, Mardani M, Dashti GR. Decalcified Bone Plastination by the new UP89 resin. J Int Soc Plastination. 2012;24:51. [Google Scholar]
- 6.Bennet HS, Wyrick AD, Lee SW, McNeil JH. Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technol. 1976;51:71–97. doi: 10.3109/10520297609116677. [DOI] [PubMed] [Google Scholar]
- 7.Weiglein AH. Preservation and Plastination. Clin Anat. 2002;15:445. doi: 10.1002/ca.10038. [DOI] [PubMed] [Google Scholar]
- 8.Von Hagens G, Tiedemann K, Kriz W. The current potential of plastination. Anat Embryol (Berl) 1987;175:411–21. doi: 10.1007/BF00309677. [DOI] [PubMed] [Google Scholar]
- 9.Weiglein AH. Plastination in the Neurosciences. Acata Anat. 1997;158:6–9. doi: 10.1159/000147902. [DOI] [PubMed] [Google Scholar]
- 10.Raoof A. Using a Room-temperature plastination technique in assessing prenatal changes in the human spinal cord. J Int Soc Plastination. 2001;16:5–8. [Google Scholar]
- 11.Pashaei S. A Brief Review on the History, Methods and applications of plastination. Int J Morphol. 2010;28:1075–9. [Google Scholar]
- 12.Sheehan DC, Hrapchak BB. 2nd Ed. St. Louis: The C. V Mosby Company; 1980. Theory and Practice of Histotechnology; pp. 89–107. [Google Scholar]
- 13.Trudel G, Seki M, Uhthoff H. Optimization of perfusion decalcification for bones and joints in rats. Clin Anat. 2000;260:222–7. doi: 10.1002/1097-0185(20001101)260:3<222::AID-AR20>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Page KM, Stevens A, Lowe J, Bancroft JD, Bone . Theory and Practice of Histologica1 Technique. In: Bancroft JD, Stevens A, editors. NY: Churchill Livingstone; 1996. pp. 309–39. [Google Scholar]
- 15.Witter K, Matulova P, Misek I. The effects of two different decalcification procedures on size and structure of embryonic epithelial tissue in objects prepared for light microscopy. Anat Histol Embryol. 2000;29:351–5. doi: 10.1046/j.1439-0264.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- 16.Machado-Silveiro LF, González-López S, González-Rodríguez MP. Decalcification of root canal dentine by citric acid, EDTA and sodium citrate. Int Endod J. 2004;37:365–9. doi: 10.1111/j.1365-2591.2004.00813.x. [DOI] [PubMed] [Google Scholar]
- 17.Riepertinger A, Heuckendorf E. E 20 color injection and plastination of the brain. J Int Soc Plastination. 1993;7:8–12. [Google Scholar]
- 18.Setayesh MM, Esfandiari E, Rabiei AA, Hanaei SM, Rashidi B. Comparing two methods of plastination and glycerin preservation to study skeletal system after Alizarin red-Alcian blue double staining. Adv Biomed Res. 2013;2:1–4. doi: 10.4103/2277-9175.108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabiei AA. PHD Thesis. Isfahan: Isfahan University of Medical Science; 2003. Fabrication of polymer production of the whole human body with mass plastination method. [Google Scholar]
- 20.Rabiei AA, Asadi MH, Esfandiari E, Taghipour M, Bahadoran H, Setayesh M. Preparation of flexible plastinated sheets of human brain by P87 polyester. J Isfahan Med Sch. 2011;28:1961–6. [Google Scholar]
- 21.Kiviranta I, Tammi M, Lappalainen R, Kuusela T, Helminen HJ. The rate of calcium extraction during EDTA decalcification from thin bone slices as assessed with atomic absorption spectrophotometry. Histochemistry. 1980;68:119–27. doi: 10.1007/BF00489507. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe K. Plastic-embedded hearts- Cleared and corroded specimens. AMA Arch Pathol. 1956;61:153–8. [PubMed] [Google Scholar]
- 23.Holladay SD, Hudson L. Use of plastinated brains in teaching neuroanatomy at the North Carolina State University, College of Veterinary Medicine. J Int Soc Plastination. 1989;3:15–7. [Google Scholar]
- 24.Baptista CAC, Skie M, Yeasting RA, Ebraheim N, Jackson WT. Plastination of the wrist: Potential uses in education and clinical medicine. J Int Soc Plastination. 1989;3:18–21. [Google Scholar]
- 25.Seibold R, Eitel F, Waldner H, Brunner U, von Hagens G. A new application of plastination in bone histology. Unfallchirurg. 1991;94:624–33. [PubMed] [Google Scholar]
- 26.Cannas M, Fuda P. Plastination of old formalin- fixed specimens. J Int Soc Plastination. 1991;5:11–5. [Google Scholar]


