Abstract
To create an effective well-ordered delivery platform still remains a challenge. Herein we fabricate vertically aligned alumina nanowire arrays via atomic layer deposition templated by carbon nanotubes. Using these arrays, a caspase-3/7 inhibitor was delivered into DC 2.4 cells and blocked apoptosis, as confirmed by fluorescence microscopy.
Currently, the most commonly used tools for interrogating living systems are on the micro-scale with typical dimensions down to 1 micron. These tools include micropipettes, which are generally used for patch clamp work within the field of electrophysiology. Nanotechnology has a lot to offer in regards to reducing the dimensions of probes and modifying their properties using a host of different materials other than the typical fused silica micropipette and capillary tubes. It was first demonstrated by McKnight et al. that carbon nanofibers with lengths of 6–10 μm and tip diameters from 20–50 nm could successfully interface with Chinese hamster ovary (CHO) cells3. Nano-scale materials had been introduced to living systems before this work, however, the materials were typically solution-borne and injected either in vitro or in vivo. Since then, many groups have reported various nano-scale material interfaces on-chip and witnessed large-scale cell viability. This is of utmost importance in order to maintain a high degree of selectivity in the perturbance of a particular cell line. Dramatically changing the environment of living tissue may skew results and lead to conclusions in vitro that are unrelated to conditions in vivo.
Materials used for interaction with living cells have a large range of variability in material composition and morphology including: silicon nanowires (NWs)1, carbon nanofiber-like structures2, gallium phosphide NWs12, and carbon-based nanosyringes templated by anodized aluminium oxide (AAO)6. From this small collection of works, it was Kim et al. and Hällstrom et al. that were first to demonstrate that anchored NWs could pass through the lipid bilayer of living cells without an external aide5,7. These two reports demonstrated the potential to perturb a living system without adversely affecting the viability of the cells and tissue functions with spontaneous spearing of the presented nanostructures through the lipid bilayer. And, importantly, the ability to anchor the materials used for interaction with living systems prevents the accumulation of the nanomaterials into potentially cytotoxic aggregates9. Within the available literature there exists a shortage in works where tethered nanostructures are introduced to living systems
Our previous discovery demonstrated that α-alumina is much more efficient at stimulating the immune system than the commercially utilized alum8. It was found that the autophagocytosis of the α-alumina nanoparticles (NPs) into B16-OVA cells is responsible for the improved cross-presentation of antigen. Therefore it follows that perhaps the endocytotic pathway is induced by the presence of the alumina NPs at the surface of the cell. We sought to explore the applicability of alumina for the presentation of a tetrapeptide directly into the cytosol to induce a phenotypic response from DC 2.4 cells. Instead of using NPs dispersed into solution, as in our previous work, arrays of alumina (NWs) were fabricated in order to precisely control their physical dimensions and composition with a large scale of uniformity over the extent of the fabricated arrays. CNTs produced by catalysed plasma-enhanced chemical vapor deposition (PECVD) were chosen as templates due to their ability to be precisely manipulated through fine control of process parameters. To ultimately create alumina nanowires, atomic layer deposition (ALD) was selected because of its ability to precisely control thickness through a layer-by-layer assembly technique. Combining these two fabrication processes, alumina NWs with large-scale uniformity were created for controlled delivery of the tetrapeptide in effort to elucidate a mechanism for cellular interaction with the alumina NWs. Other groups have reported the synthesis of alumina NW arrays using methods such as chemical etching of AAO membranes10. This particular route to alumina NW arrays is difficult to control and lacks the uniformity granted by ALD. The technique of coating CNTs with alumina via ALD has been previously reported in literature11, however, to our knowledge the application of alumina-coated CNT arrays for delivery of a biomolecule into living cells has not been reported.
Herein we report the use of CNT arrays as templates for the conformal growth of aluminium oxide via ALD for presentation to living cells. These arrays were then used as a means for direct access into DC 2.4 cells for efficiently delivering a caspase-3/7 inhibitor, an aldehyde-terminated tetrapeptide (Ac-DEVD-CHO). A phenotypic response to inhibit caspase-3/7, thereby preventing apoptosis induced by tumor necrosis factor-α (TNF-α), was achieved by culture of DC 2.4 cells on an amorphous alumina coated CNT array. Results were confirmed by fluorescence microscopy.
CNTs were grown by first depositing a 3.5 nm film of Ni on top of a Si wafer fragment with a 100 nm layer of SiO2 thermal oxide. The wafer was subsequently placed into a PECVD reactor maintained at 800 °C and 630 V for 30 min with feedstock of NH3 and C2H2 at flow rates of 200 and 50 sccm, respectively (shown in SI 2, ESI†). The wafer fragments were then used as-grown or placed into a flow-through hot-wall ALD reactor (Arradiance Gemstar) and maintained at 175 °C during 191 cycles of 500 ms pulses of trimethylaluminum (TMA) and H2O separated by 40 s nitrogen purges for an ultimate thickness of 25 nm (Fig. 2a and 2c). From here, the wafers were either used as-is or further processed by annealing at 950 °C for 2 h within an oxygen atmosphere (Fig. 2d and 2f). The wafers were then allowed to cool to room temperature before further experimentation (see scheme illustrated in Fig. 1). Annealing at 950 °C yielded hollow, polycrystalline alumina NW arrays that were found to be the γ-phase via electron beam diffraction (Fig. 2e) compared to the as-deposited amorphous alumina NWs (Fig. 2b). For electron diffraction measurements, bundles scraped from NW array substrates onto TEM grids containing greater than 50 NWs were selected using a selected area electron diffraction (SAED) aperture. The CNTs within the alumina coatings react with oxygen during the annealing process and degrade their covalent bonds by exceeding 400 °C within an oxygen atmosphere.
Fig. 2.
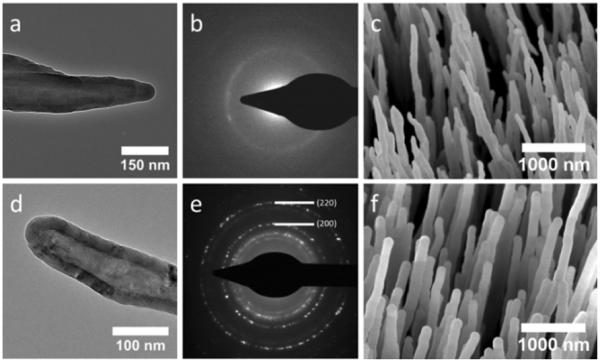
(a) TEM image of CNT coated with amorphous alumina via ALD, (b) electron diffraction image of a bundle of CNTs coated with amorphous alumina, (c) SEM image of amorphous alumina coated CNT array, (d) TEM image of hollow γ-alumina NW, (e) electron diffraction image of a bundle of hollow γ-alumina NWs, (f) SEM image of hollow γ-alumina NW array.
Fig. 1.
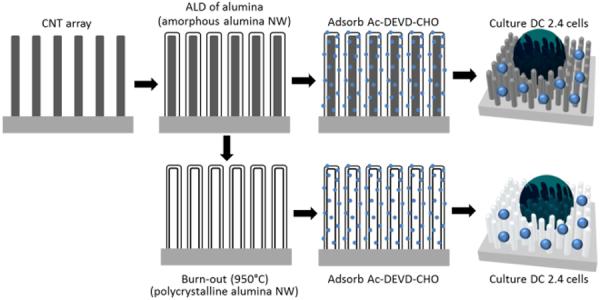
Schematic illustration of fabrication process and adsorption of the tetrapeptide Ac-DEVD-CHO. Amorphous alumina was deposited by atomic layer deposition onto as-grown CNT arrays and then either further processed by annealing at 950°C within an oxygen atmosphere or Ac-DEVD-CHO was directly absorbed onto the amorphous alumina NW array. After burn-out of the CNTs and production of polycrystalline alumina NWs, Ac-DEVD-CHO was similarly absorbed onto the polycrystalline NW array before cell culture.
DC 2.4 cells were cultured on both the amorphous alumina and γ-alumina arrays with and without adsorbed coatings of Ac-DEVD-CHO. Next, the cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE), a membrane-permeable dye used for establishing the location of the cells, and propidium iodide (PI) which is not membrane-permeable and will indiscriminately stain the DNA of a cell only when the membrane has been compromised.
As previously mentioned, alumina NW arrays created via ALD onto CNT templates were chosen due to their large-scale uniformity and the ability to precisely control their physical parameters such as diameter and length. Another motivation for alumina coating was to improve the rigidity of the high-aspect ratio structures and provide a biocompatible surface for presentation to living cells. Without the coating, CNTs will form bundles due to capillary forces after exposure to liquids4 (Fig. 3a and 3c). Once stuck together, the effect is generally irreversible and creates larger effective tip diameters and therefore could hamper the biocompatibility of the array. Additionally, the effective surface area of the array decreases with the increased bundle diameter therefore limiting the amount of biomolecules exposed to cells. Other researchers have used coatings such as polypyrrole (PPy) to maintain separation and augmented biocompatibility through use of a collagen ECM coating when interacting with PC12 cells, a neuron-like cell line4. Although cell proliferation had been improved with the applied ECM coating, the growth rate still did not approach that of the as-grown CNT arrays. In our experiments, cultured DC 2.4 cells on unmodified CNTs exhibited whole-cell staining of PI suggesting the stained DNA had leaked into the nucleus and indicating cytotoxic interactions with the bare CNT arrays (shown in SI 1, ESI†). Cultured DC 2.4 cells on alumina NW arrays (Fig. 3b) had a clear difference in cell morphology than with the bare CNT arrays (Fig. 3a). It is interesting to note that the presence of extended filopodia is much more prevalent for the cells cultured on the bare CNT array than with the alumina NW array. Also, the NWs were engulfed by the filopodia of the cell rather than the filopodia and central cell body as with the CNT arrays (Fig. 3d).
Fig. 3.
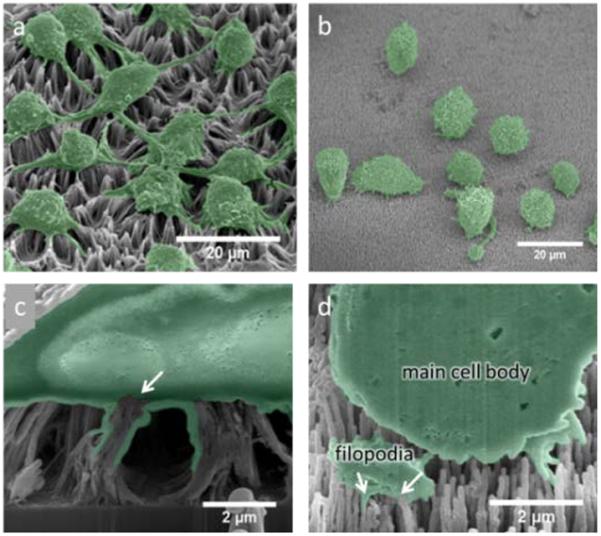
(a) Representative SEM image of DC 2.4 cells on a bare CNT array and (b) on an amorphous alumina-coated CNT array. (c) FIB cross-section of a DC 2.4 cell cultured on a CNT array, the arrow indicates point of CNT engulfing resulting in a broken membrane, (d) FIB cross-section of a DC 2.4 cell on an alumina-coated CNT array, arrows indicate engulfing of individual alumina NWs through a sectioned portion of filopodia.
In order to ascertain whether the TNF-α applied to the solution would signal apoptosis, the cultured cells were first incubated for 48 h and then 2 ng/mL of TNF-α was introduced to the culture. All NW arrays behaved as expected with PI staining present (Fig. 4c and 4e). In a separate experiment, the arrays were first incubated with 100 mM of Ac-DEVD-CHO caspase-3/7 inhibitor for 1 h at 37 °C. Subsequently, the arrays were incubated with cells for 48 h and, again, exposed to 2 ng/mL of TNF-α.
Fig. 4.
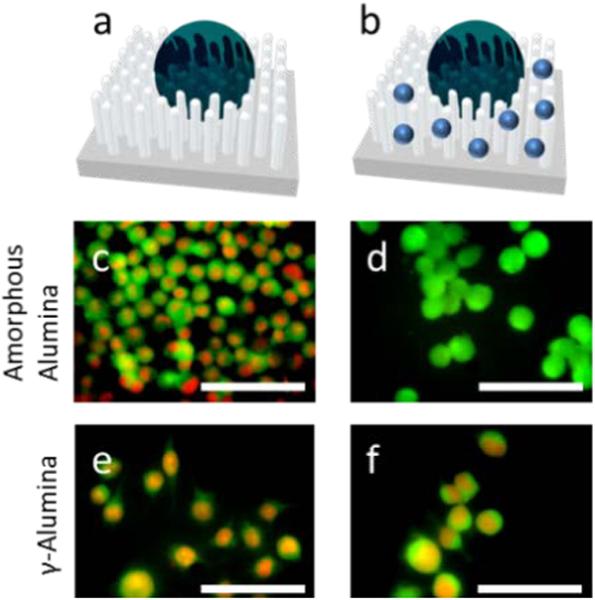
(a) Representation of a cell on the nanostructure array without inhibitor and (b) with the inhibitor (Ac-DEVD-CHO represented by blue dots), (c) and (e) DC 2.4 cells incubated onto amorphous alumina and γ-alumina arrays, respectively, with introduction of TNF-α, (d) and (f) DC 2.4 cells incubated with the same arrays as in (c) and (e) except coated with Ac-DEVD-CHO via adsorption. All scale bars represent 60 μm. In all images, propidium iodide (PI) staining is represented by red and the CFDA membrane dye is represented by green.
Only the amorphous alumina substrate exhibited living cells after the 48 h incubation period. Similar to a recent study, there was concern about the inhibitor being released from the substrate during incubation and causing inhibition through solution and not from delivery via the amorphous NW array1. Ac-DEVD-CHO was therefore coincubated with the DC 2.4 cells on the same type of amorphous alumina NW array. The concentrations for coincubation were determined by measuring the concentration of released Ac-DEVD-CHO during the 48 h incubation period through a caspase-3 fluorimetry assay kit. Coincubation revealed that the concentration of released inhibitor peptide was not sufficient to prevent apoptosis (shown in SI 3, ESI†). Therefore, the results indicate that the amorphous alumina substrate is required for delivery of the inhibitor peptide. However, a concentration gradient is present at the NW array interface where the concentration is much higher close to the NW array surface before the inhibitory peptide is released into the bulk culture media. A continued study concerning the time-lapse interactions of dendritic cells with NW arrays is ongoing.
Nanostructures that approach perpendicular to a cell membrane surface have been found to be the method of endocytosis that requires the minimum amount of energy12. This provides a natural physicomechanical means to explain why cells are able to uptake 1D materials of great length because the cell first recognizes the tip as a particle thereby initiating a natural endocytotic pathway that is unable to detect the length of the entering 1D material. This may be the reason for 1D material cytotoxicity, however, it demonstrates that living cells will recognize the tip of vertically aligned NWs in an array as tethered particles which induce a phenotypic endocytosis of the presented NWs. Therefore vertically aligned arrays of 1D materials can be utilized as a controlled means of assessing biological effector uptake through mimicking of comparable NP adjuvants or other related vectors within the field of nanomedicine. Alumina coatings provided by ALD onto CNTs are able to maintain their orientation during presentation into the cell within a liquid media and can take advantage of natural particle recognition pathways of the cells.
Despite the clear physicomechanical relationship for inducing endocytosis, there is also surface chemistry dependence. Amorphous alumina coatings possess a different surface than the polycrystalline γ-alumina. Although both contain the same materials, the amorphous coated CNT arrays were the only surface preferred for delivery of Ac-DEVD-CHO. Further study is required to understand the relationship between surface chemistry of alumina and particle recognition of living cells.
The ability to induce phenotypic responses from the DC 2.4 cells with unmodified amorphous alumina NW arrays allows for a potential platform for inducing several other responses by delivering other peptides, proteins, DNA, and other biological effectors. Experiments have demonstrated that the as-produced amorphous alumina-coated CNT arrays are necessary for delivering Ac-DEVD-CHO for successful inhibition of apoptosis induced by the introduction of TNF-α. Straightforward fabrication routes such as PECVD of CNTs and ALD of amorphous alumina allow the fine control of physical parameters to create vertically aligned 1D nanostructures with large-scale uniformity. This platform has the ability to be tailored with precise control of process parameters for widespread application in high-throughput drug screening and the assessment of cellular uptake performance.
Supplementary Material
Acknowledgments
This work is supported in part by the Maseeh College of Engineering at Portland State University, the National Science Foundation REU (No. 1263339), and the National Institute of Health (J.D.S.) (No. R01GM082940).
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/c000000x/
Notes and references
- 1.Shalek A, Robinson J, Karp E, Lee J, Ahn D, Yoon M, Sutton A, Jorgolli M, Gertner R, Gujral T, MacBeath G, Yang E, Park H. Proc. Natl. Acad. Sci. 2009;107:1870–1875. doi: 10.1073/pnas.0909350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melechko A, Desikan R, McKnight T, Klein K, Rack P. J. Phys. D: Appl. Phys. 2009;42:1–28. [Google Scholar]
- 3.McKnight T, Melechko A, Griffin G, Guillorn M, Merkulov V, Serna F, Hensley D, Doktycz M, Lowndes D, Simpson M. Nanotechnology. 2003;14:551–556. [Google Scholar]
- 4.Nguyen-Vu T, Chen H, Cassell A, Andrews R, Meyyappan M, Li Jun. Small. 2006;1:89–94. doi: 10.1002/smll.200500175. [DOI] [PubMed] [Google Scholar]
- 5.Kim W, Ng J, Kunitake M, Conklin B, Yang P. JACS Comm. 2007;129:7228–7229. doi: 10.1021/ja071456k. [DOI] [PubMed] [Google Scholar]
- 6.Park S, Kim Y, Kim W, Jon S. Nano Letters. 2009;9:1325–1329. doi: 10.1021/nl802962t. [DOI] [PubMed] [Google Scholar]
- 7.Hällström W, Mårtensson T, Prinz C, Gustavsson P, Montelius L, Samuelson L, Kanje M. Nano Letters. 2007;7:2960–2965. doi: 10.1021/nl070728e. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Li Y, Jiao J, Hu H. Nature Nanotechnology. 2011;6:645–650. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacerda L, Bianco A, Prato M, Kostarelos K. Adv. Drug Delivery Rev. 2006;58:1460–1470. doi: 10.1016/j.addr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Min B, Cho K, Kim S, Park J, Lee Y, Kim N, Lee M, Park S, Moon J. Journal of Crystal Growth. 2003;254:443–448. [Google Scholar]
- 11.Tian Y, Meng G, Gao T, Sun S, Xie T, Peng X, Ye C, Zhang L. Nanotechnology. 2004;15:189–191. [Google Scholar]
- 12.Shi X, Bussche A, Hurt R, Kane A, Gao H. Nature Nanotechnology. 2011;6:714–719. doi: 10.1038/nnano.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


