Abstract
Research in the past decade has revealed key components of the Hippo tumour-suppressor pathway and its critical role in organ size regulation and tumorigenesis. Recent progress has identified a wide range of upstream factors that control the Hippo pathway, which include cell-cell contact, various diffusible signals, and cognate receptors. Dysregulation of the Hippo pathway, caused by gene mutation or aberrant expression, promotes cell proliferation and tumorigenesis. Here, we discuss the current state of Hippo pathway research, primarily focusing on upstream regulators and protein-protein interactions as potential therapeutic targets. Consideration of pharmacological intervention of the Hippo pathway may provide novel avenues for future therapeutic treatment of human diseases, particularly in cancer.
Keywords: Hippo pathway, YAP, phosphorylation, GPCR, cancer
The Hippo Pathway: a new link from the outside to inside
Core Components of the Hippo pathway
The Hippo tumour suppressor pathway plays a key role in regulation of organ size, and dysregulation of the pathway results in tissue overgrowth and tumorigenesis [1–3]. Many components of the Hippo pathway were identified by Drosophila mosaic genetic screens to search for genes whose loss of function mutation leads to strong overgrowth phenotype [4]. In mammals, the core components of the Hippo pathway consist of serine/threonine kinases MST1/2 (homologues of Hippo/Hpo in Drosophila), Lats1/2 (homologues of Warts/Wts), and their adaptor proteins Sav1 (homologue of Salvador/Sav) and Mob (MOBKL1A and MOBKL1B; homologues of Mats), respectively [5–7]. The final effectors of Hippo tumour-suppressor pathway are the transcription co-activators YAP and its paralogue TAZ (homologues of Yorkie/Yki). The Hippo kinase cascade phosphorylates and inhibits YAP/TAZ/Yki by promoting its cytoplasmic retention and degradation, therefore inhibiting the growth promoting function of these transcription co-activators. The TEAD family transcription factors were identified as critical partners of YAP/TAZ, which drive the oncogenic potential of YAP/TAZ by inducing target genes involved in anti-apoptosis and proliferation such as CTFG, Cyr61 and FGF1 [8–10].
The Hippo pathway as mediator of extracellular diffusible signals
Although several properties of tissue architecture, such as apicobasal polarity, adhesion, and cell-cell contact inhibition, have been implicated in Hippo regulation, the extracellular diffusible signals mediating those biological features were unknown until the discovery that Hippo-YAP/TAZ pathway was linked to diverse G protein-coupled receptor (GPCR) ligands and receptor signaling [11–13]. GPCRs comprise the largest family of cell surface receptors (>800 members) involved in signal transduction, and represent the most prominent family of validated pharmacological targets [14–16]. Recent reports show that Gα12/13, Gαq/11, Gαi/o-coupled GPCRs activate YAP/TAZ and promote nuclear translocation [11–13], whereas Gαs-coupled GPCRs suppress YAP/TAZ activity [12, 17–19]. These findings open the possibility that GPCRs could be pharmacologically targeted to regulate the Hippo pathway. Upon stimulation of Gα12/13 coupled receptor agonists, the activated small GTPase Rho family inhibits Lats1/2 and facilitates YAP/TAZ nuclear activity. LPA, S1P and thrombin, which are ligands highly implicated in tumorigenesis, have been shown to activate YAP/TAZ [11, 12]. By contrast, dobutamine, a Gαs-coupled β-adrenergic agonist, inhibits YAP-dependent gene transcription [19]. Moreover, epinephrine and glucagon also inhibit YAP/TAZ activity by activation of Gαs-cAMP-PKA-Lats1/2 [18]. Aberrant expression and mutations in Gα proteins and GPCRs are implicated in cancer progression [15, 20]. Modulating the Hippo pathway by diverse agonists and antagonists of GPCRs could have clinical impact on human cancer in which YAP/TAZ are highly expressed and functionally relevant.
In addition to its interaction with GPCR ligands, the Hippo-YAP/TAZ pathway has been shown to mediate diverse growth factor signaling, which includes epidermal growth factor (EGF), insulin growth factor (IGF), transforming growth factor-beta (TGFβ) and Wnt pathways. YAP and TAZ have also been reported to interact with several effectors of those pathways such as β-catenin and Smad proteins [21]. However, not all reports are consistent regarding the mechanism and function of YAP/TAZ in these signaling processes. For example, it has been reported that the EGF-PI3K pathway does not affect YAP S127 phosphorylation and nuclear localization [12, 22], while another report indicates that it induced YAP activation [23]. In addition, PI3K inhibitor showed opposite effects on serum-induced YAP activation in different cell lines [12, 23]. Therefore, the effect of growth factors and PI3K on YAP/TAZ might be cell content dependent. Also, insulin/IGF signaling has been shown to activate Yki by PI3K-PDK1 in Drosophila [24], however, there is no solid evidence to date that has confirmed this mechanism in mammalian systems.
There are several reports demonstrating that the Hippo-YAP/TAZ pathway can suppress Wnt signaling by inhibiting the function of Dishevelled [25–27]. In contrast, a separate report showed that TAZ is an effector of Wnt signaling and mediates Wnt inducible gene expression [28]. Therefore, the functional relationship between YAP/TAZ and Wnt signaling is currently unclear and certainly requires further investigation. Since many of these growth factor signaling pathways have been previously implicated as therapeutic targets in cancer drug development, it will be important to clearly demonstrate how diverse signaling pathways can impinge on the Hippo pathway to properly regulate YAP/TAZ with drugs targeting different pathways.
The Hippo pathway as a mediator of contact inhibition and mechanotransduction
The Hippo-YAP/TAZ pathway has been implicated in diverse cellular and tissue properties, which includes apicobasal polarity, cell-cell adhesion, contact inhibition, planar cell polarity (PCP) and mechanotransduction [22, 29–32]. At high cell density, sensing neighboring cells through junctional structures is known to activate the Hippo pathway, leading to sequestration of YAP/TAZ at cell junctions, and proliferation arrest. Several components of the adherens junction (KIBRA, NF2, α-catenin, and E-cadherin) and tight junction (CRB, PATJ, PALS1, aPKC, PAR3, and AMOT) have been shown to interact with the core Hippo pathway to inhibit YAP/TAZ nuclear accumulation [31, 33–37]. Thus, overexpression of YAP or loss of junctional proteins leads to defects in cell polarity and contact inhibition. Furthermore, YAP/TAZ is activated and translocate into the nucleus on stiff matrix or upon cell attachment, which induce F-actin polymerization via activation of small Rho GTPases. It was demonstrated in vivo that the small Rho GTPase Cdc42 was essential for nuclear translocation of YAP during kidney development [38]. Tumors have altered mechanical properties and are surrounded by stiff matrix [39, 40], which may underlie the frequent upregulation of YAP/TAZ observed in human cancer. Recent study also demonstrated YAP activation in cancer-associated fibroblasts (CAF), which promote matrix stiffening and cancer progression as a feed-forward mechanism [41]. Regulation of the Hippo pathway by cytoskeleton and mechanical forces therefore adds another exciting aspect of how pharmacological regulators that inhibit actin polymerization, such as Rho kinases and ROCK inhibitors, could be used to suppress YAP/TAZ activity (Figure 1a) [29, 42].
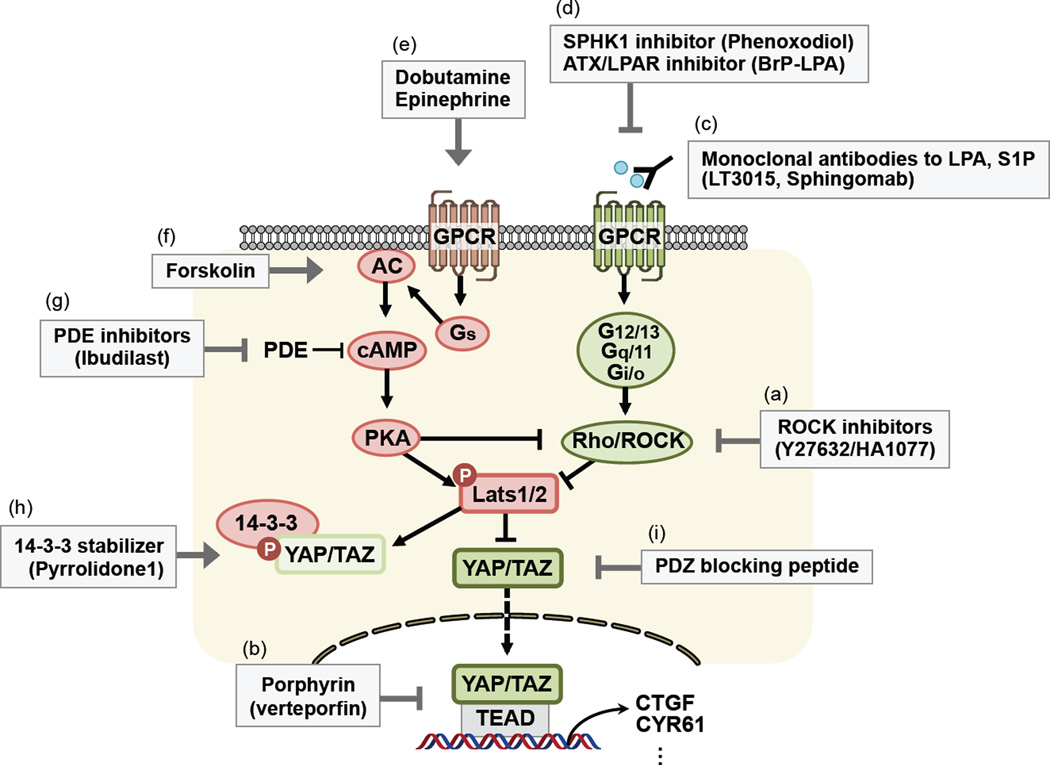
Figure 1. Regulation of Hippo pathway and putative targets for therapeutic intervention of YAP/TAZ inhibitors.
Activating components upstream of YAP/TAZ are in green, inhibitory components are in red, and putative YAP/TAZ inhibitors are in gray box. (a,c,d) Antagonists of Gα12/13-coupled GPCRs, LPA/S1P receptors, and downstream effectors Rho/ROCK activate Hippo pathway and inhibit YAP/YAZ activity. (e–g) Agonists of Gαs-coupled GPCR and increase in cAMP-PKA signaling activate Hippo pathway and inhibit YAP/TAZ activity. (b,h,i) Modulating physical interaction between YAP/TAZ and its binding partners abrogate YAP/TAZ nuclear translocation and target gene expression.
Mechanisms of YAP/TAZ inhibition by the Hippo pathway
Phosphorylation-dependent inhibitory mechanisms
Recent findings place the Hippo pathway as a central hub to functionally interact with and integrate diverse signaling networks. The most critical and best-characterized function of the Hippo pathway is to relay multiple signals and fine-tune the activity of YAP/TAZ transcription co-activators. Lats1/2 phosphorylates YAP on five [HxRxxS] consensus motifs [43]. Phosphorylation of YAP S127 promotes 14-3-3 binding which results in cytoplasmic sequestration, separating it from nuclear transcription factors and target gene promoters [8, 44, 45]. Therefore, YAP is inactive when it is phosphorylated on S127. Mutation of YAP S127 disrupts the 14-3-3 interaction and promotes nuclear translocation of YAP. Lats1/2 phosphorylation of corresponding sites in TAZ and Yki (by Wts in Drosophila) also generates 14-3-3 binding sites and inhibition by cytoplasmic retention [45, 46]. Phosphorylation by Lats1/2 can also suppress YAP activity by inducing proteasomal degradation. Phosphorylation of S381 in YAP, and of the corresponding site on TAZ, primes them for further phosphorylation by CK1δ/ε in the degradation motif (termed a phosphodegron), and subsequently, the β-TRCP E3 ubiquitin ligase binds to the phosphodegron, leading to polyubiquitination and degradation of YAP [43, 47].
Notably, many known activators of the core Hippo pathway are components of cell adhesion, such as the basolateral surface, adherens, and tight junctions, implying that the cell adhesion and polarity complex activates Hippo kinase activity and promotes YAP/TAZ phosphorylation by Lats1/2 [2, 31]. In addition, PKA has recently been shown to inhibit YAP by activating Lats1/2 and subsequent phosphorylation of YAP on S127 and S381 [17].
Physical interaction-mediated inhibitory mechanisms
Protein-protein interactions mediated by specific modular domains are crucial in governing the dynamic signaling network of the Hippo-YAP/TAZ pathway. YAP and TAZ have WW domains that recognize PPxY motifs in proteins. YAP/TAZ also have C-terminal motifs that can be recognized by PDZ domains. Of note, the high prevalence of WW domains and PPxY (PY) motifs, as well as PDZ, provides means to predict possible interaction of Hippo pathway components [48, 49]. Furthermore, many components of the Hippo pathway contain SARAH (Salvador/RASSF/Hippo) domains that can interact with each other. DCHS1/2 (Drosophila homologue of Dachsous), Fat1-4 (homologue of Fat), CRB1-3 (homologue of Crumbs), MST1/2, and Lats1/2 contain one or multiple PY motifs. In addition, angiomotin (AMOT) [33, 50, 51] and PTPN14 [52, 53], which have emerged as negative regulators of YAP/TAZ, also contain PY motifs. The PY motif was identified as the first ligand of the YAP WW domain [54]. ITCH, Sav1 and KIBRA in the Hippo pathway, as well as the effectors YAP and TAZ, harbor the WW domain. Among the abundant WW-PY interaction within the Hippo network, the PY motifs on Lats1/2, AMOT, and PTPN14 have been shown to directly interact with WW domains on YAP/TAZ and thereby convey inhibitory signals by sequestering YAP/TAZ in the cytosol or junctional compartments [2, 48].
PDZ domains in junctional proteins (PATJ, ZO-2) interact directly with the PDZ binding motif (PDZbm) of YAP/TAZ, or indirectly via PDZbm of KIBRA or AMOT [31, 55–57]. The SARAH domain mediates RASSF-MST-Sav1 interaction [58, 59]; however, whether SARAH domain interaction confers an inhibitory effect on YAP/TAZ has not been documented. A detailed summary of nomenclatures and domain/motif information is shown in Table 1. Thus, categorizing the modularity in the Hippo pathway will help selecting protein targets as well as predicting the outcome of compounds that influence protein-protein interaction on YAP/TAZ activity.
Table 1.
Function and modular domains of mammalian Hippo pathway components
| Human | Drosophila | Domains and Motifs | YAP/TAZ activity | ||
|---|---|---|---|---|---|
| WW | PY | Others | |||
| The Core Hippo Pathway | |||||
| Mst1/2 | Hippo | SARAH | ↓ | ||
| Sav1/WW45 | Salvador | 2-WW | SARAH | ↓ | |
| Lats1/2 | Warts | 2-PY, 1-PY | ↓ | ||
| MOBKL1A/B | Mats | ↓ | |||
| YAP1/2 | Yorkie | 1-WW, 2-WW | PDZbm | ||
| TAZ | Yorkie | 1-WW | PDZbm | ||
| TEAD1-4 | Scalloped | ||||
| Apicobasal Cell Polarity | |||||
| KIBRA | Kibra | 2-WW | PDZbm | ↓ | |
| FRMD6 | Expanded | FERM | ↓ | ||
| NF2/Merlin | Merlin | FERM | ↓ | ||
| CRB1-3 | Crumbs | 1-PY | PDZbm | ↓ | |
| aPKC | aPKC | ↓ | |||
| PALS1 | Sdt | PDZ | ↓ | ||
| PATJ | PatJ | PDZ | ↓ | ||
| AMOT | 2-PY | PDZbm | ↓ | ||
| AMOTL1/2 | 2-PY | PDZbm | ↓ | ||
| PAR3/6 | Baz/Par6 | PDZ | ↓ | ||
| E-cadherin | E-cadherin | ↓ | |||
| α-Catenin | α-Catenin | ↓ | |||
| SCRIB | Scribble | PDZ | ↓ | ||
| LGL1/2 | Lgl | ↓ | |||
| DLG | Dlg | PDZ | ↓ | ||
| Other Modulators | |||||
| FT1/2 | Fat | 2-PY, 4-PY | ↓ | ||
| DCHS1/2 | Dachsous | ↓ | |||
| RASSF1-6 | dRASSF | SARAH | ↑↓ | ||
| PTPN14 | Pez | 2-PY | ↓ | ||
| PKA | PKA | ↓ | |||
| WBP2 | Wbp2 | 3-PY | ↑ | ||
| HIPK2 | Hipk | ↑ | |||
| ITCH | SuDx | 4-WW | ↑ | ||
| ZO-2 | ZO-2 | PDZ | ↑↓ | ||
| PP1 | PP1 | ↑ | |||
| PP2A | STRIPAK | ↑ | |||
| ASPP1/2 | dASPP | 1-PY | ↑ | ||
| RhoA | RhoA | ↑ | |||
| Gα12/13 | cta | ↑ | |||
| 14-3-3 | 14-3-3 | ↓ | |||
Domains and motifs are based on human sequences.
Hippo signaling in human cancers
Uncontrolled tissue overgrowth induced by inactivation mutants of the Hippo pathway was seen originally in Drosophila but has recently been recapitulated in mammals; overexpression of YAP/TAZ or inactivation mutants of Hippo components induce transformation in mammalian cells [29, 46, 60] and promote tumorigenesis in transgenic mice models [61–64]. In humans, hereditary or sporadic inactivating mutations in neurofibromatosis tumour suppressor NF2 (Merlin), which is a Hippo component upstream of MST1/2, promote brain tumor development [65]. Inactivation of NF2 in mice liver results in hepatocellular carcinoma (HCC) and bile duct tumours [61, 62] that show a similar phenotype to mice harboring mutations in MST1/2 and Sav1 [63, 64]. Importantly, heterozygous deletion of YAP is able to prevent the liver pathology, which provides strong evidence that NF2 functions upstream of the Hippo pathway to restrict YAP activity [61]. It is noteworthy that primary cells isolated from patients with the NF2 mutation demonstrated F-actin abnormalities and morphological changes [65]. Since the YAP activation status is sensitive to the actin cytoskeleton, altered actin organization by NF2 mutations may be a factor leading to YAP activation.
On the other hand, TEAD1 Y421H mutation is associated with human genetic disease, Sveinsson’s chorioretinal atrophy (SCRA), which is defined by degeneration and atrophy of the choroid [66]. Recent structural studies on YAP-TEAD interaction clearly show that Y421 in TEAD1 and its equivalent in TEAD4 form a hydrogen bond with a conserved serine residue in YAP, which is disrupted by the Y to H mutation, revealing the structural basis of YAP-TEAD interaction and emphasizing their critical function in tissue growth [10, 67, 68]. Although Hippo pathway genes are infrequently mutated in human cancers, numerous studies report the altered expression and activity of the Hippo pathway, and YAP/TAZ in wide range of human cancers [69–71]. The overexpression of YAP/TAZ oncogenes, as well as epigenetic silencing of Hippo components are commonly reported in human cancer [1, 72, 73], suggesting that non-mutational mechanisms may also contribute to tumorigenesis. These studies firmly suggest that manipulation of Hippo pathway and relevant upstream regulators by small molecules may provide means to control cell growth by restricting the oncogenic potential of YAP/TAZ-TEAD transcription factor complex.
Targeting Hippo-YAP/TAZ pathway for drug development
Accumulating evidence suggests that the Hippo tumour-suppresser pathway, and its effectors, the YAP/TAZ-TEAD transcription complex, may be potential targets for anticancer therapy. Most importantly, the recent discovery of GPCRs as regulators of the Hippo-YAP/TAZ pathway has broadened the scope of upstream targets to include wide variety of extracellular ligands and receptors. Another intriguing aspect of Hippo pathway is the structural basis of each component interacting through well-characterized modules such as WW domain and PY motifs. These properties of the Hippo network impart significant potential and advantages to be an attractive target for drug development.
Targeting YAP/TAZ-TEAD complex for drug development
The key downstream effectors of Hippo pathway are the YAP/TAZ transcription coactivators that bind to TEAD transcription factors to induce target gene expression. In this regard, recent expansion of the Hippo pathway components, and upstream regulatory mechanisms are all in the context of controlling the nuclear activity of YAP/TAZ-TEAD complex. Hence, designing compounds that directly dissociate the interaction between YAP/TAZ and TEAD will be the most direct and effective approach to suppress YAP/TAZ-induced oncogenic events [3, 49].
A screening of >3300 drugs by Pan and colleagues led to the identification of the Porphyrin family, such as verteporfin (VP), hematoporphyrin (HP), and protoporphyrin IX (PPIX), as YAP inhibitors [74] (Figure 1b). Porphyrin abrogates the interaction between YAP and TEAD. Most importantly, VP abolishes liver overgrowth induced by YAP overexpression or by inactivation of Nf2 in vivo [74], which clearly demonstrates the therapeutic potential of disrupting YAP/TAZ-TEAD interaction. Direct inhibition of YAP-TEAD may reduce possible side effects that might be caused by targeting upstream components that likely affect multiple intracellular signaling besides Hippo-YAP.
GPCR-Hippo signaling pathway as druggable targets
GPCRs function as key transducers of extracellular signaling molecules (lipids, small molecules, and peptides/proteins) to the inside of the cell by utilizing heterotrimeric G proteins and a number of secondary messengers. GPCRs are favorable targets of drugs as more than 25% of therapeutic drugs are targeting GPCRs either directly or indirectly [15, 20]. Recent deep sequencing of the cancer genome revealed nearly 20% of human cancers harbor mutations in GPCR signaling, which includes high frequency of mutations in GPCRs (TSHR, SMO, GRMs) and G proteins in many prevalent malignancies [15].
Among the Gα proteins, Gα12/13 and Gαq/11 (and Gαi/o to a lesser extent) have been shown to be potent activators of YAP and TAZ. In hyperplastic mammary gland obtained from LPA receptor 1 or 2 overexpressing transgenic mice, YAP/TAZ were dephosphorylated and accumulated in the nucleus [12]. Of note, LPA and autotaxin (ATX) have been suggested as biomarkers for human cancers, such as ovarian cancer, HCC, and lymphoma [75, 76]. In addition to LPA, S1P and thrombin also stimulate YAP/TAZ by binding to their cognate receptors, which results in activation of Gα12/13-Rho GTPase and inhibition of Lats1/2 [11, 12]. Therefore, antagonizing LPA, S1P, and thrombin-mediated receptor signaling will be attractive strategies to suppress YAP/TAZ activity in cancer [77]. Phosphatase-resistant LPA analogues have been developed as receptor antagonist, such as α-fluoromethylene and α-hydroxymethylene phosphonates [78]. Monoclonal antibodies with high specificity to LPA and S1P have been developed [79, 80] (Figure 1c). For example, the S1P-blocking antibody Sphingomab has been shown to reduce lung tumor metastasis [81]. In addition, ATX, which generates LPA from lysophosphatidylcholine, plays a role in breast cancer invasion and metastasis [82]. Sphingosine kinase 1 (SPHK1), which generates S1P, is also increased in cancer and shows oncogenic effects [83]. Phenoxodiol, an isoflavone-derivative SPHK1 inhibitor, is under clinical trial in patients with advanced cancer [84]. Therefore, simultaneous inhibition of both receptor-ligand interaction and ligand production may provide an effective approach to blocking LPA and S1P actions, thereby inhibiting tumors with elevated signaling of these pathways. One example is the palmitoyl α-bromomethylenephosphonate-1 (BrP-LPA), which can antagonize LPA receptor 1–4 as well as inhibit ATX activity [85] (Figure 1d).
In contrast to other Gα proteins, Gαs-coupled GPCR agonists increase Lats1/2 activity, leading to suppression of YAP and TAZ activity. Dobutamine and epinephrine, which are β-adrenergic receptor agonists, as well as forskolin and phosphodiesterase (PDE) inhibitors, which elevate cAMP level, activate Lats1/2 and promote YAP/TAZ phosphorylation and inhibition [17–19]. Recently, cAMP-PKA has been identified as potent activator of Hippo pathway [12, 17, 18]. PKA activates Lats1/2 either by directly phosphorylating Lats1/2 [17] or by suppressing RhoA activity [18]. When PKA is inhibited, YAP activity is increased and thus capable to suppress anoikis and promote anchorage-independent growth, suggesting that Gαs-cAMP-PKA activating molecules may have therapeutic benefit in inhibiting YAP/TAZ-induced tumorigenesis. Elevated intracellular cAMP concentration can arrest growth, induce apoptosis, and suppress cancer cell migration in various cancers such as breast cancer and colon cancer [86]. Forskolin activates adenylyl cyclase and increases intracellular cAMP concentration. Topical forskolin pretreatment shows protective effect against UV-induced carcinogenesis in mice [87]. In addition, several forskolin derivatives, such as FSK88 and coleusin factor (CF), have been shown to inhibit proliferation and induce cell cycle arrest and apoptosis in human gastric cancer cells both in vitro and in vivo [88, 89].
Overexpression of PDE isoforms has been reported in various cancers [86]. Phosphodiesterase (PDE) inhibitors also increase cAMP levels. For example, Rolipram, a PDE4 inhibitor, suppresses tumour growth and augments anticancer effect of chemotherapy in brain tumour cells [90]. In addition, combination of low dose of forskolin with rolipram induces growth arrest in chemoresistant colon cancer cells [91]. Dobutamine/epinephrine (β-adrenergic receptor agonists), forskolin, IBMX/theophylline (nonselective PDE inhibitors), and rolipram/ibudilast (PDE4 selective inhibitors) are all shown to inhibit YAP/TAZ by increasing cAMP level and activating PKA-Lats1/2 pathway [18] (Figure 1 e–g). In this regard, the regulation of Hippo-YAP/TAZ pathway may be a useful readout for predicting the potential therapeutic effects of drugs that modulate cAMP-PKA activity.
However, it is noteworthy that the Gαs-coupled GPCR signaling pathway also displays a pro-tumorigenic role in some tumour types [15]. Therefore, the effects of cAMP and PKA on cell growth are likely to be cell-context dependent. Activating mutations in Gαs proteins or Gαs-coupled GPCRs are found in a number of cancers, especially in growth-hormone-secreting pituitary tumours and thyroid adenomas, in which cAMP has been shown to stimulate proliferation and hormone secretion [92]. Also, in some cases, β-adrenergic signaling has been documented to contribute to cancer progression and epithelial-mesenchymal transition (EMT) [93]. It will be important to test if certain mutations in Gαs, or other β-adrenergic pathways beside cAMP-PKA, such as Src-STAT3, could activate YAP/TAZ by circumventing the inhibitory effect of PKA-Hippo pathway. For example, β-adrenergic antagonists (“β-blockers”) have been implicated as possible anticancer drugs [93]. However, it is strongly advised that the activation status of YAP/TAZ by these β-blockers should be monitored in preclinical studies. In addition, combination therapy of β-blockers together with direct inhibitors of YAP/TAZ activity such as verteporfin (VP) should be considered, because this may allow for inhibition of β-adrenergic signaling without activation of YAP/TAZ.
Modular protein domains in Hippo pathway; the WW domain-PY motif
One of the most unique features of Hippo pathway is the unusual prevalence of modular structures; WW domains and its interaction with PPxY (PY)-containing cognate ligands [48]. To date, the WW domains of YAP/TAZ have been shown to interact with several components harboring the PY motif, which can be categorized into two groups: the first group consists of Lats1/2, AMOT, AMOTL1/2, and PTPN14, which negatively regulate YAP/TAZ activity by WW-PY interaction; the second group consists of WBP2 and ASPP2, which promote YAP/TAZ activation through WW-PY interaction. Similar to 14-3-3 binding to YAP/TAZ, the first group of partners, Lats1/2 PTPN14, and AMOT proteins, sequester YAP/TAZ in the cytosolic compartments, therefore suppressing its oncogenic properties [2, 33, 44, 49, 52]. In this regards, it is conceivable to speculate that small molecules or adaptors, which can stabilize those WW-PY complexes, may harbor anti-proliferative effects. By contrast, the second group, WBP2 and ASPP2, promote YAP/TAZ dephosphorylation and tissue growth through WW-PY interaction [94, 95]. In this case, molecules disrupting such interaction would inhibit YAP/TAZ activity. Effort has been made to build a structural model of the WW domain of YAP bound to digitoxin, which is a cardiac glycoside that has been predicted to have affinity for WW domains by in silico analysis [49]. These studies show potential opportunities for chemical modification of digitoxin to compete specifically with PY ligands that bind to YAP WW domains. In addition, the phosphorylation status adjacent to PY motif of Smad1/5 conferred selectivity toward WW domain of YAP [96]. Phosphorylation of Smad PY motif by CDK8/9 increased the binding affinity of YAP WW domain, whereas phosphorylation by GSK3 diminished their interaction. Therefore, phosphomimetic peptides containing PY motif could be used as blockers of the PY motif-mediated interaction with YAP. However, inhibitors of WW-PY interaction should be carefully designed since WW domains of YAP/TAZ convey both positive, as well as inhibitory signals. For example, it will be important to analyze the relative expression level of different binding partners depending on cell and tissue context. Moreover, the large numbers of WW domain containing proteins in the cell may complicate specificity issues of such compounds.
14-3-3 and PDZ binding motif in YAP and TAZ as druggable targets
Among the inhibitory mechanisms of YAP/TAZ by the Hippo pathway identified to date, the cytosolic sequestration induced by 14-3-3 interaction is the best-characterized and appears the most important mechanism in YAP/TAZ inhibition [22, 45, 46, 97, 98]. Lats1/2 phosphorylate YAP on five HxRxxS consensus motifs, including S127 and S381, which are the most crucial sites to repress its oncogenic activity. The 14-3-3 protein family has seven isoforms in humans and directly binds to phosphorylated serine or threonine residues in specific sequence motifs. 14-3-3 proteins play pivotal role in physiological processes such as gene expression, signal transduction, enzyme activation, and apoptosis, which lead to broad spectrum of human diseases when dysregulated. Accumulating evidence has established a strong connection between dysregulation of 14-3-3 and many types of cancer such as lung, breast, head and neck cancers [99, 100]. Recently, the structure of the 14-3-3 binding domain of YAP (YAP pS127 peptide) in complex with 14-3-3σ isoform has been resolved at 1.15 Å resolution, which shows similar binding mechanism to other known target proteins [101]. It is interesting to note that 14-3-3σ is the only known 14-3-3 isoform that harbors tumor suppressor function in epithelial cells [99]. Several molecules have been developed to either disrupt or stabilize 14-3-3 interaction with its binding partners, such as FOBISIN101 and Pyrrolidone 1 [99] (Figure 1h). In the case of YAP/TAZ and 14-3-3σ interaction, it will be most interesting to test the effect of 14-3-3/client protein stabilizers in combination with Hippo pathway activators such as PDE inhibitors, which will simultaneously activate Lats1/2 and stabilize 14-3-3 binding to YAP/TAZ.
YAP and TAZ contain highly conserved PDZ binding motifs (-FLTWL) at their C-termini, which are required for nuclear translocation and target gene expression as well as binding to the plasma membrane [98]. Recently, ZO-2, which is a tight junction protein in epithelial cells, has been shown to interact with YAP through the PDZ domain and promote YAP nuclear translocation [55] (Figure 1i). In addition, most of the cell polarity complex proteins, such as Crumbs (CRB3/PALS1/PATJ), PAR (PAR3/PAR6), and the SCRIB complex (Scribble/DLG), also harbor multiple PDZ domains, which are suggested to interact with Hippo pathway and negatively regulate YAP/TAZ [31, 36]. For example, loss of apicobasal polarity during the EMT process changes the subcellular localization of Scribbled, which inhibits the Hippo pathway, resulting in TAZ activation [102]. Thus, PDZ interaction with YAP/TAZ may either inhibit or stimulate YAP/TAZ activity. A thorough functional characterization would be needed before one may consider interfering the PDZ and YAP/TAZ interaction for therapeutic purpose.
Serine/Threonine Protein Phosphatase Inhibitors
The activation of YAP/TAZ is induced by dephosphorylation of consensus Lats1/2 phosphorylation sites followed by nuclear accumulation and target gene expression [43]. The dephosphorylation of YAP/TAZ is rapid upon stimulation with GPCR agonists, such as LPA and thrombin, which suggests the dynamic engagement of protein phosphatases in this process [11, 12]. Recently, it has been shown that PP1A and PP2A phosphatase interact with YAP/TAZ and induce dephosphorylation, thereby disrupting the binding between YAP/TAZ and 14-3-3 [35, 95, 103].
Selective serine/threonine phosphatases have been targeted for anticancer drug development, such as cantharidin, fostriecin, and their analogs, which are inhibitors of PP1 and PP2A [104]. Phosphatases play crucial roles in the progression through cell cycle, and these inhibitors promote G2/M phase growth arrest, aberrant mitotic spindles, and apoptosis in wide range of tumor cells both in vitro and in vivo. Notably, Lats1/2 activation also results in G2/M cell cycle arrest through CDK1 inhibition [105], which resembles the effect of PP1 and PP2A inhibitors. Although, the role of YAP/TAZ in mitotic exit and cytokinesis has not yet been documented, these results suggest that stabilizing Lats1/2 phosphorylation on YAP/TAZ with phosphatase inhibitors may synergistically induce growth arrest and apoptosis.
Concluding remarks
The Hippo pathway has gained significant attention in the last few years, owing to its diverse biological functions such as cell contact inhibition, apicobasal cell polarity, mechanotransduction, and cell survival. Uncontrolled Hippo pathway activity is directly relevant to various aspects in cancer biology, which includes cell proliferation, EMT, and metastasis. Several factors have contributed to the rapid expansion of the field; i) its fundamental importance in tissue and organ growth, ii) the highly conserved functional network that synergized research from Drosophila to mammals, iii) the unusual prevalence of modular domains mediating the protein-protein interaction, and iv) wide range of upstream signals encompassing GPCRs, cell contact, and mechanotransduction. Rapid progress in the field has prompted researchers to recognize the Hippo-YAP/TAZ signaling pathway as a therapeutic target for anticancer drug development. As suggested in the current review, there are numerous potential drug targets that directly or indirectly modulate the Hippo pathway. However, further research is necessary to address several issues relevant to these therapeutic opportunities. Many of the upstream regulators, such as GPCRs, although they are highly druggable targets, have broad physiological functions, and hence general interference of GPCR signaling may cause side effects. In this regard, targeting the core components of the Hippo pathway would be an attractive approach. However, a key caveat for this approach is that majority of kinases such as MST1/2 and Lats1/2 are tumor suppressors, which requires activators to inhibit YAP/TAZ. Recently, mir-135b, an intronic microRNA, was shown to target multiple components in the Hippo pathway, including Lats2, which promoted lung tumor growth and metastasis in mice [106]. Inhibition of such negative regulators of Hippo pathway could provide alternative means to activate Hippo pathway. The abundant protein-protein interactions in the Hippo pathway offer multiple targets for intervention; however, developing drugs to enhance or interfere protein-protein interaction is a rather challenging task. Notably, YAP/TAZ have also been implicated in stem cell biology [107, 108], which suggests inhibitors of the Hippo kinase cascade may have value of treating wound healing and tissue regeneration. Further in-depth characterization of regulatory mechanisms will provide valuable information for drug development of targeting the Hippo-YAP/TAZ pathway.
Highlights.
We outline identified upstream regulators & modular structures of the Hippo pathway
We propose anticancer drug targets in the Hippo pathway.
Acknowledgements
We thank all the members of the laboratory for helpful discussions. Research in the laboratory of K.L.G. is supported by grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey KF, et al. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 4.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 5.Wu S, et al. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 6.Callus BA, et al. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 7.Praskova M, et al. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao B, et al. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamar JM, et al. The Hippo pathway target YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo JS, et al. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller E, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 15.O'Hayre M, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smrcka AV. Molecular targeting of Galpha and Gbetagamma subunits: a potential approach for cancer therapeutics. Trends Pharmacol Sci. 2013;34:290–298. doi: 10.1016/j.tips.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M, et al. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32:1543–1555. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu FX, et al. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao Y, et al. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem. 2011;150:199–208. doi: 10.1093/jb/mvr063. [DOI] [PubMed] [Google Scholar]
- 20.Kan Z, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 21.Varelas X, Wrana JL. Coordinating developmental signaling: novel roles for the Hippo pathway. Trends Cell Biol. 2012;22:88–96. doi: 10.1016/j.tcb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan R, et al. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A. 2013;110:2569–2574. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strassburger K, et al. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367:187–196. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Imajo M, et al. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varelas X, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Barry ER, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azzolin L, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Zhao B, et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halder G, et al. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 31.Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem J. 2011;436:213–224. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- 32.Wada K, et al. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 33.Zhao B, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varelas X, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Schlegelmilch K, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol. 2012;23:803–811. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Kim NG, et al. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reginensi A, et al. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9:e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butcher DT, et al. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 43.Zhao B, et al. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao Y, et al. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 45.Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lei QY, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu CY, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sudol M, Harvey KF. Modularity in the Hippo signaling pathway. Trends Biochem Sci. 2010;35:627–633. doi: 10.1016/j.tibs.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Sudol M, et al. Structures of YAP protein domains reveal promising targets for development of new cancer drugs. Semin Cell Dev Biol. 2012;23:827–833. doi: 10.1016/j.semcdb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, et al. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan SW, et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, et al. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26:1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, et al. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32:1266–1273. doi: 10.1038/onc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen HI, Sudol M. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci U S A. 1995;92:7819–7823. doi: 10.1073/pnas.92.17.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oka T, et al. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J. 2010;432:461–472. doi: 10.1042/BJ20100870. [DOI] [PubMed] [Google Scholar]
- 56.Duning K, et al. KIBRA modulates directional migration of podocytes. J Am Soc Nephrol. 2008;19:1891–1903. doi: 10.1681/ASN.2007080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duning K, et al. Polycystin-2 activity is controlled by transcriptional coactivator with PDZ binding motif and PALS1-associated tight junction protein. J Biol Chem. 2010;285:33584–33588. doi: 10.1074/jbc.C110.146381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang E, et al. Structural insight into dimeric interaction of the SARAH domains from Mst1 and RASSF family proteins in the apoptosis pathway. Proc Natl Acad Sci U S A. 2007;104:9236–9241. doi: 10.1073/pnas.0610716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheel H, Hofmann K. A novel interaction motif, SARAH, connects three classes of tumor suppressor. Curr Biol. 2003;13:R899–R900. doi: 10.1016/j.cub.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Overholtzer M, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benhamouche S, et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee KP, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gutmann DH, et al. Functional analysis of neurofibromatosis 2 (NF2) missense mutations. Hum Mol Genet. 2001;10:1519–1529. doi: 10.1093/hmg/10.14.1519. [DOI] [PubMed] [Google Scholar]
- 66.Fossdal R, et al. A novel TEAD1 mutation is the causative allele in Sveinsson's chorioretinal atrophy (helicoid peripapillary chorioretinal degeneration) Hum Mol Genet. 2004;13:975–981. doi: 10.1093/hmg/ddh106. [DOI] [PubMed] [Google Scholar]
- 67.Chen L, et al. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24:290–300. doi: 10.1101/gad.1865310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Z, et al. Structural insights into the YAP and TEAD complex. Genes Dev. 2010;24:235–240. doi: 10.1101/gad.1865810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 70.Song M, et al. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012;32:3827–3834. [PubMed] [Google Scholar]
- 71.Steinhardt AA, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill VK, et al. Frequent epigenetic inactivation of KIBRA, an upstream member of the Salvador/Warts/Hippo (SWH) tumor suppressor network, is associated with specific genetic event in B-cell acute lymphocytic leukemia. Epigenetics. 2011;6:326–332. doi: 10.4161/epi.6.3.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu MZ, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Houben AJ, Moolenaar WH. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 2011;30:557–565. doi: 10.1007/s10555-011-9319-7. [DOI] [PubMed] [Google Scholar]
- 76.Xu Y, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 77.Murph M, Mills GB. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000476. [DOI] [PubMed] [Google Scholar]
- 78.Prestwich GD, et al. Phosphatase-resistant analogues of lysophosphatidic acid: agonists promote healing, antagonists and autotaxin inhibitors treat cancer. Biochim Biophys Acta. 2008;1781:588–594. doi: 10.1016/j.bbalip.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fleming JK, et al. Biochemical and structural characterization of lysophosphatidic Acid binding by a humanized monoclonal antibody. J Mol Biol. 2011;408:462–476. doi: 10.1016/j.jmb.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wojciak JM, et al. The crystal structure of sphingosine-1-phosphate in complex with a Fab fragment reveals metal bridging of an antibody and its antigen. Proc Natl Acad Sci U S A. 2009;106:17717–17722. doi: 10.1073/pnas.0906153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ponnusamy S, et al. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol Med. 2012;4:761–775. doi: 10.1002/emmm.201200244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu S, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15:539–550. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cuvillier O, et al. Activation of sphingosine kinase-1 in cancer: implications for therapeutic targeting. Curr Mol Pharmacol. 2010;3:53–65. doi: 10.2174/1874467211003020053. [DOI] [PubMed] [Google Scholar]
- 84.Kelly MG, et al. Phase II evaluation of phenoxodiol in combination with cisplatin or paclitaxel in women with platinum/taxane-refractory/resistant epithelial ovarian, fallopian tube, or primary peritoneal cancers. Int J Gynecol Cancer. 2011;21:633–639. doi: 10.1097/IGC.0b013e3182126f05. [DOI] [PubMed] [Google Scholar]
- 85.Jiang G, et al. Alpha-substituted phosphonate analogues of lysophosphatidic acid (LPA) selectively inhibit production and action of LPA. Chem Med Chem. 2007;2:679–690. doi: 10.1002/cmdc.200600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Savai R, et al. Targeting cancer with phosphodiesterase inhibitors. Expert Opin Investig Drugs. 2010;19:117–131. doi: 10.1517/13543780903485642. [DOI] [PubMed] [Google Scholar]
- 87.D'Orazio JA, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- 88.Li Z, Wang J. A forskolin derivative, FSK88, induces apoptosis in human gastric cancer BGC823 cells through caspase activation involving regulation of Bcl-2 family gene expression, dissipation of mitochondrial membrane potential and cytochrome c release. Cell Biol Int. 2006;30:940–946. doi: 10.1016/j.cellbi.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 89.Sun B, et al. Coleusin factor exerts cytotoxic activity by inducing G0/G1 cell cycle arrest and apoptosis in human gastric cancer BGC-823 cells. Cancer Lett. 2011;301:95–105. doi: 10.1016/j.canlet.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 90.Goldhoff P, et al. Targeted inhibition of cyclic AMP phosphodiesterase-4 promotes brain tumor regression. Clin Cancer Res. 2008;14:7717–7725. doi: 10.1158/1078-0432.CCR-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McEwan DG, et al. Chemoresistant KM12C colon cancer cells are addicted to low cyclic AMP levels in a phosphodiesterase 4-regulated compartment via effects on phosphoinositide 3-kinase. Cancer Res. 2007;67:5248–5257. doi: 10.1158/0008-5472.CAN-07-0097. [DOI] [PubMed] [Google Scholar]
- 92.Bourne HR, et al. Hydrolysis of GTP by the alpha-chain of Gs and other GTP binding proteins. Proteins. 1989;6:222–230. doi: 10.1002/prot.340060304. [DOI] [PubMed] [Google Scholar]
- 93.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chan SW, et al. WW domain-mediated interaction with Wbp2 is important for the oncogenic property of TAZ. Oncogene. 2011;30:600–610. doi: 10.1038/onc.2010.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu CY, et al. PP1 cooperates with ASPP2 to dephosphorylate and activate TAZ. J Biol Chem. 2011;286:5558–5566. doi: 10.1074/jbc.M110.194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aragon E, et al. A Smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev. 2011;25:1275–1288. doi: 10.1101/gad.2060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao B, et al. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanai F, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao J, et al. 14-3-3 proteins as potential therapeutic targets. Semin Cell Dev Biol. 2011;22:705–712. doi: 10.1016/j.semcdb.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hermeking H. The 14-3-3 cancer connection. Nat Rev Cancer. 2003;3:931–943. doi: 10.1038/nrc1230. [DOI] [PubMed] [Google Scholar]
- 101.Schumacher B, et al. Structure of a 14-3-3sigma-YAP phosphopeptide complex at 1.15 A resolution. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:978–984. doi: 10.1107/S1744309110025479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 103.Wang P, et al. PP1A-mediated dephosphorylation positively regulates YAP2 activity. PLoS One. 2011;6:e24288. doi: 10.1371/journal.pone.0024288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swingle MR, et al. Structure-activity relationship studies of fostriecin, cytostatin, and key analogs, with PP1, PP2A, PP5, and(beta12-beta13)-chimeras (PP1/PP2A and PP5/PP2A), provide further insight into the inhibitory actions of fostriecin family inhibitors. J Pharmacol Exp Ther. 2009;331:45–53. doi: 10.1124/jpet.109.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hergovich A, Hemmings BA. Hippo signalling in the G2/M cell cycle phase: lessons learned from the yeast MEN and SIN pathways. Semin Cell Dev Biol. 2012;23:794–802. doi: 10.1016/j.semcdb.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin CW, et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat Commun. 2013;4:1877. doi: 10.1038/ncomms2876. [DOI] [PubMed] [Google Scholar]
- 107.Lian I, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barry ER, Camargo FD. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr Opin Cell Biol. 2013;25:247–253. doi: 10.1016/j.ceb.2012.12.006. [DOI] [PubMed] [Google Scholar]