Abstract
Purpose
To develop and evaluate variable-density (VD) spiral first-pass perfusion pulse sequences for improved efficiency and off-resonance performance and to demonstrate the utility of an apodizing density compensation function (DCF) to improve SNR and reduce dark-rim artifact caused by cardiac motion and Gibbs Ringing.
Methods
Three variable density spiral trajectories were designed, simulated, and evaluated in 18 normal subjects, and in 8 patients with cardiac pathology on a 1.5T scanner.
Results
By utilizing a density compensation function (DCF) which intentionally apodizes the k-space data, the side-lobe amplitude of the theoretical PSF is reduced by 68%, with only a 13% increase in the FWHM of the main-lobe as compared to the same data corrected with a conventional VD DCF, and has an 8% higher resolution than a uniform density spiral with the same number of interleaves and readout duration. Furthermore, this strategy results in a greater than 60% increase in measured SNR as compared to the same VD spiral data corrected with a conventional DCF (p<0.01). Perfusion defects could be clearly visualized with minimal off-resonance and dark-rim artifacts.
Conclusion
VD spiral pulse sequences using an apodized DCF produce high-quality first-pass perfusion images with minimal dark-rim and off-resonance artifacts, high SNR and CNR and good delineation of resting perfusion abnormalities.
Keywords: Myocardial Perfusion, Variable Density Spirals, Spiral Pulse Sequences, Saturation Recovery
Introduction
It is estimated that 7% of all Americans over age 20, nearly 16.5 million people, have coronary artery disease (CAD).(1) Given this high prevalence, nearly 10 million stress tests are performed in the US yearly to evaluate chest pain suspicious for CAD, placing a huge economic burden on the U.S. Healthcare system.(2) Having accurate techniques to determine which patients have obstructive CAD is of key importance as these patients are most likely to benefit from invasive evaluation with coronary catheterization and revascularization. Perhaps even more important is accurate identification of which patients do NOT have obstructive CAD, given that patients with falsely positive stress tests frequently undergo unnecessary cardiac catheterization, which is invasive and expensive. Adenosine stress CMR demonstrates high potential for improving detection of CAD; however, artifacts such as the dark-rim artifact (DRA) still limit the specificity of this technique.
Multiple factors contribute to DRA including Gibbs ringing from limited spatial resolution, myocardial motion during data acquisition, and magnetic susceptibility during first pass of gadolinium contrast agents.(3) Truncation of the k-space data in the phase encoding direction generates Gibbs ringing in MR imaging, and the effects of windowing the k-space data in the phase encoding direction to reduce artifact has been studied previously.(4) The extension of this concept to perfusion was first proposed by Di Bella et al., who demonstrated in an ex-vivo heart that Gibbs ringing alone is sufficient to explain DRA in perfusion images in the absence of myocardial motion, and demonstrated that applying a low-pass filter can effectively eliminate this artifact.(5) However, motion can also result in phase inconsistencies during data acquisition that can cause ringing artifacts similar to Gibbs ringing.(4) Story et al.(6) showed that an edge moving at a constant velocity causes induces a phase shift which is different for each successive phase encoding step of a rectilinear trajectory resulting in ringing artifact. It has been demonstrated that the dark-rim artifact has a different appearance at different phases of cardiac contraction due to the non-linear motion of the heart.(7)
As spiral trajectories are highly time efficient and robust to motion artifacts, they would appear to be a good candidate for CMR perfusion imaging provided that the effects from off-resonance can be managed. We have recently proposed an optimized pulse sequence for CMR perfusion imaging using spiral trajectories.(8) To minimize off-resonance artifacts we determined that the optimal readout duration should be less than 8ms for this application. The uniform density (UD) spiral trajectory described resulted in high resolution images with high SNR, but still suffered from some DRA and some off-resonance related blurring and dropout artifacts. Variable density (VD) spiral trajectories may offer multiple additional benefits as compared to UD spiral trajectories and could further mitigate these artifacts. For the same readout duration VD spiral trajectories are less susceptible to off-resonance artifacts and have higher spatial resolution. By using this increased efficiency to reduce the duration of the spiral interleaves, off-resonance artifacts can be lessened even further. Secondly, the high-frequency aliasing artifacts from VD spiral trajectories appear as incoherent noise-like artifacts. However, due to the non-uniform weighting of the noise during density compensation, VD spiral trajectories have a SNR penalty as compared to UD spiral trajectories. We hypothesize that intentionally “under-correcting” the density of a VD spiral pulse sequence could be used to shape the effective k-space weighting function (apodization) to reduce Gibbs-ringing and motion induced DRA while maintaining high spatial resolution and SNR similar to the UD spiral pulse sequence.
Methods
Theoretical Considerations
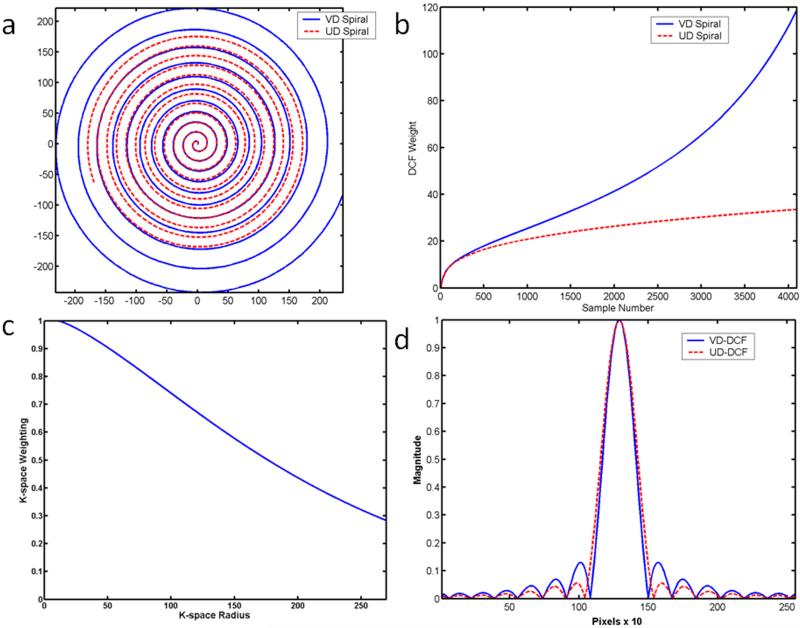
Conventional UD spirals have uniform spacing of 1/FOV between the spiral arms (Fig 1a, red), but non-uniform density along the spiral trajectory due to the finite slew of the gradient amplifiers of the scanner. This non-uniformity along the trajectory is generally compensated for during reconstruction with an appropriate density compensation function (DCF), which we will refer to as a UD-DCF (Fig 1b,red). VD spirals (Fig 1a,blue), in addition to the non-uniform density along the trajectory, also have non-uniform spacing of the spiral arms with decreasing density near the outer regions of k-space resulting in a higher resolution image for the same readout duration (or conversely an image with the same spatial resolution and decreased readout time and thus improved off resonance performance). If the VD data is compensated with a standard DCF that is the inverse of its local k-space density (Fig 1b, blue), which we will refer to as a VD-DCF, there will be uniform weighting of the k-space data. However, there will be a significant reduction in SNR due to the highly non-uniform weighting of noise in k-space.
Figure 1.
(a) Comparison of UD and VD spiral interleaves of the same readout duration. Near the center of k-space the trajectories are very similar due to low k-space velocity. VD spirals cover a larger k-space radius for the same imaging time. (b) This under sampling results in a DCF with increased amplitude at high k-space locations. If the UD-DCF is used to correct data from the VD trajectory, the data will effectively be windowed by the function shown in (c). This results in a significant reduction in side-lobe amplitude with minimal broadening of the PSF (d) which reduces Gibbs-ringing in the image.
When a DCF that is the inverse of the local k-space density is used, the k-space weighting at any k-space radius is unity. However, the k-space data can be weighted by an arbitrary density function such that the product of the DCF and the k-space density is equal to a desired k-space filter. This concept can be utilized to perform apodization of the data to reduce Gibbs-ringing as has been applied to spiral chemical shift imaging.(9) If the VD spiral data is instead weighted by the corresponding UD-DCF (Fig 1b,red) which “under corrects” the density, there will be more uniform weighting of noise in k-space and the images will have higher SNR. In the case of under-sampled VD-spirals there will be an additional increase in the apparent SNR as the aliased high-frequency data that contributes to the apparent noise in the images will also be reduced. This correction will cause the effective k-space weighting shown in Fig. 1c, which results in a smooth windowing of the k-space data that should reduce Gibbs ringing and thus potentially reduce DRA at the cost of some reduction in image resolution. The general approach that we employ in this work is to use VD sampling to reach a larger k-space radius (and hence higher nominal spatial resolution) than a UD spiral of the same readout duration, and then intentionally under-correct the k-space density to both reduce the sidelobe amplitude of the PSF, which reduces Gibbs ringing, and improve SNR as compared to using the standard VD-DCF.(10)
Pulse Sequence Design and Evaluation
Trajectory Design
Linear-in-time variable density, slew-limited spiral gradient trajectories were created using the using the optimal spiral design of Meyer et. al.(11) Briefly, the process consists of specifying the desired number of interleaves, sampling time, number of points per trajectory, nominal field of view (FOV), maximum gradient and slew rate parameters, and a function w(t) describing the relative density as a function of time where (1 corresponds to Nyquist sampling density) to determine the desired k-space trajectory.
| (1) |
An algorithm that maximizes τ, an arbitrary function of time, for each subsequent k-space step subject to the above constraints is repeated until the desired number of gradient points is achieved.
We evaluated 3 different spiral trajectories for a nominal FOV of 320-340 mm2 (Table 1): (1) 6 interleaves, readout duration 8.2 ms, starting density 1.2× Nyquist, ending density 0.4× Nyquist; (2) 8 interleaves, readout duration 6.1 ms, starting density 1.2× Nyquist, ending density 0.4× Nyquist; and (3) 8 interleaves, readout duration 6.1 ms, starting density 1.2× Nyquist, ending density 0.2× Nyquist. The readout duration of trajectory 1 was chosen to be 8.2 ms as our prior work demonstrated this to be the maximal readout duration per interleaf with an acceptable amount of off-resonance blurring and signal drop-out. Trajectory 2 has the same spatial and temporal resolution as trajectory 1, but has reduced readout duration to further explore the effect of readout duration per interleave on off-resonance artifacts. Trajectory 3 has the same readout duration as trajectory 2, but the outer regions of k-space are undersampled by an additional factor of two as compared to trajectory 2. The goal of this comparison was to evaluate SNR and perceived image blurring when the UD-DCF was applied to the data. Additional common sequence parameters included: saturation recovery (SR) time of 80 ms, TE 1.0 ms, slice thickness 10 mm, nominal FOV 320-340mm. Low-resolution field maps were obtained using two single-shot spiral images for off resonance correction with each perfusion image. The field map was acquired during the first two TR’s after the SR time with the same readout duration and TR as the rest of the perfusion image data. The flip angle was chosen to have nearly constant magnetization on each interleaf for the given SR time as described previously.(8) Images were reconstructed with linear off-resonance correction using both the UD and VD DCFs. DCFs were calculated using the method of Meyer et. al. for UD trajectories (12), and the method of Morrell et. al. for VD trajectories.(13) Individual coil images were combined using the square root of sum-of-squares method.
Table 1. Sequence Parameters.
| Sequence 1 | Sequence 2 | Sequence 3 | |
|---|---|---|---|
| Number of Interleaves | 6 | 8 | 8 |
| Readout Points | 4096 | 3072 | 3072 |
| Readout Duration | 8.2 ms | 6.1 ms | 6.1 ms |
| Starting Density | 1.2 | 1.2 | 1.2 |
| Ending Density | 0.4 | 0.4 | 0.2 |
| FOV | 320 mm | 320 mm | 320 mm |
| Flip Angle | 30° | 300° | 300° |
| TE | 1 ms | 1 ms | 1 ms |
| TR | 12 ms | 10 ms | 10 ms |
| Resolution (1/2Kmax) | 1.99 mm2 | 1.99 mm2 | 1.67 mm2 |
| Field Map Acquisition Time | 24 ms | 20ms | 20 ms |
| Total Image Acquisition Time | 176 ms | 180 ms | 180 ms |
Comparison of Point Spread Functions
In order to evaluate the effect of the combination of the above trajectories and density compensation strategies, a theoretical analysis of relative PSF width and side-lobe amplitude was performed. For each trajectory, unity values at each k-space location were gridded onto a 2× oversampled grid using the gridding algorithm. Prior to Fourier transformation, the data was zero-padded by a factor of 5, to effectively sinc interpolate the PSF by a factor of 10 in the image domain. Normalized PSFs were determined by dividing the PSF by its maximum value for comparison between the different strategies. The full-width at half maximum (FWHM) and the relative amplitude of the first sidelobes were determined. The PSF resulting from applying a Hanning filter to the gridded variable density k-space data with the standard VD-DCF was also evaluated for each of the trajectories. The PSF of a UD spiral with the same readout duration as each of the trajectories was also evaluated for comparison.
Evaluation of Gibbs-Ringing and motion-induced DRA
The effect of density correction with a UD-DCF versus a VD-DCF on the appearance of Gibbs-ringing and motion induced DRAs was evaluated in numerical simulations of the data acquisition in the absence and presence of motion. Using a technique we have previously described, a short-axis section of the heart was represented as two concentric circles (representing the left ventricle blood pool and myocardium) with a signal intensity ratio of 3:1 to simulate typical signal intensities when dark-rim artifacts occur most frequently in myocardial perfusion studies.(7,14) Data collection was simulated by sampling the k-space representation of these two circles (the sum of two jinc functions) at each k-space location according to the pulse sequence timing. To simulate the motion, the radii of the jinc functions were varied according to a cosinusoidal model to represent typical motion during mechanical systole and early diastole. Simulation parameters included, effective RR interval 1000 ms, time to peak systole 300 ms, wall thickness 10 mm at end diastole, LV end diastolic diameter 5.5 cm. Data was gridded onto an oversampled grid (512×512) to produce in an image matrix of 256×256 for all simulations. To isolate the effects of Gibbs and Motion induced ringing artifacts, the phantom did not include any other structures besides the heart model to avoid confounding from other types of artifacts. Furthermore, the simulation was performed without noise to exaggerate the visual appearance of any ringing artifacts. The effect of apodization on the reduction of DRA at rest (Gibbs-ringing) and during motion (Gibbs-ringing and motion-induced DRA) was assessed visually by a cardiologist (MS).
Imaging Protocol
Resting perfusion was performed in 25 subjects who were undergoing clinically ordered CMR studies with contrast under an IRB approved protocol. Imaging was performed on a 1.5T MR Scanner (Magnetom Avanto, Siemens Medical Solutions). The Siemens body matrix and spine matrix phased-array coils were used in the CP mode yielding between 3 and 6 effective coil channels depending on the size and positioning of the patient. Fifty images were obtained at 3 slice locations during injection of 0.1 mmol/kg Magnevist (Bayer Pharmaceuticals) via a peripheral IV at a rate of 4mL/sec using a saturation recovery-prepared VD spiral pulse sequence as described above.
To compare the appearance of dark-rim artifacts between pulse sequences without the confounding effects of true perfusion abnormalities, data from 18 subjects with low likelihood of coronary artery disease and no evidence of wall motion abnormalities or evidence of scarring by LGE were visually scored by two cardiologists. To evaluate the appearance of true resting perfusion abnormalities, 7 of the subjects who had myocardial fibrosis and would be expected to have perfusion abnormalities were also evaluated. An additional subject who was scheduled for coronary angiography underwent perfusion imaging during adenosine stress (140mcg/kg/min for 3 minutes) and at rest to evaluate for inducible ischemia.
Data analysis
For all studies the SNR was measured on each image in an ROI placed in the LV cavity, septal myocardium, and background noise. The SNRs at maximal LV and myocardial enhancement were recorded for each slice, and averaged over the three slice positions. The SNR was determined from the mean (μ) and standard deviation (s) of the background signal and accounted for multiple coil combination (Ncoils) according to the following:(15,16)
| (2) |
The mean SNRs of the LV cavity and myocardium for each of the above pulse sequences were compared using one-way ANOVA. The SNR was also compared between the UD-DCF and VD-DCF images. The contrast-to-noise ratio (CNR) was determined from an ROI in the myocardial septum as the difference between the SNR at peak myocardial enhancement minus the myocardial signal before contrast arrival.
All image series were evaluated for blurring, dark-rim, and signal dropout artifacts (graded as 0-none, 1-mild, 2-moderate, 3-severe). Image quality was graded on a 5-point scale (1-excellent, 5-poor) independently by two cardiologists. The image quality scores were compared using one-way ANOVA. The proportion of image series corrupted with blurring, dark-rim and signal dropout were compared using t-tests corrected for multiple comparisons.
Comparison to Uniform Density Spiral Perfusion
The results for the actual SNR of the LV and myocardium, CNR of the myocardium, and visual scores from the variable density spiral pulse sequence were compared to those of our previously published experience in 12 subjects undergoing uniform density spiral perfusion imaging.(8) The sequence parameters from that study were as follows: 8 interleaves, readout duration per interleaf 8.1ms, spatial resolution 2.18 mm2, TR 12ms, time to image one slice of 200ms. All other sequence parameters were the same as for the sequences used in this study.
Results
Simulation Results
PSF evaluation and SNR
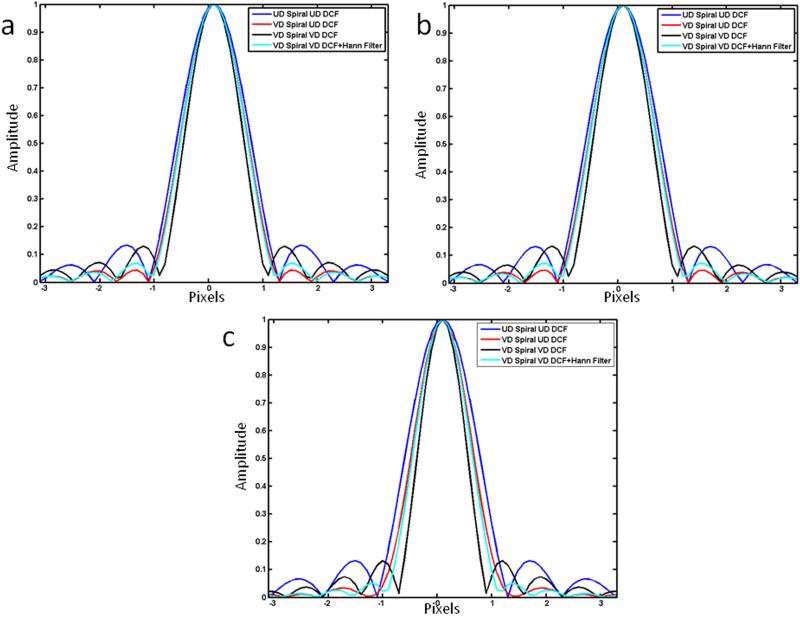
Figure 2 shows the comparison of PSFs for the three trajectory designs when the VD spiral trajectories are density compensated by the UD-DCF or VD-DCF. The PSF of a UD spiral with the UD-DCF and that of the VD spiral with the VD-DCF followed by apodization by a Hanning filter are included for reference. The UD-spiral has the widest PSF as it covers a smaller k-space radius than the VD spiral acquisition. The narrowest PSF is for the VD spiral acquisition with the conventional DCF (VD-DCF), since this DCF results in unity k-space weighting. The variable density spiral data corrected with the apodized (UD-DCF) has a main-lobe which is greater than that of the VD spiral corrected with the VD-DCF due to the low-pass filtering effect of the apodizing DCF, but narrower than a UD spiral acquisition with the same total sampling time.
Figure 2.
Comparison of theoretical PSF for (a) trajectory 1, (b) trajectory 2, and (c) trajectory 3 for data collected with the VD trajectory using either the conventional VD-DCF or the UD-DCF. Additionally the PSF from a UD trajectory with the same readout corrected with the UD DCF, and data from a VD trajectory windowed with a Hanning window are shown for comparison. The VD data corrected with the UD-DCF has a smaller FWHM than the UD spiral trajectory of the same readout duration, and has greater sidelobe amplitude suppression than the other proposed strategies.
As compared to a UD-Spiral with the same number of interleaves and readout duration, the FWHM of the PSF of the VD-spiral trajectory corrected by the UD-DCF is 8% narrower for trajectories 1 and 2 and 16% narrower for trajectory 3. As compared to the VD spiral data corrected with the VD-DCF (conventional) the PSF FWHM for the VD spiral data corrected with the UD-DCF (apodized) is 13% wider for trajectories 1 and 2 and 25% percent wider for trajectory 3. Notably there is a 66%, 68%, and 75% reduction in the amplitude of the first side-lobe of the PSF for trajectories 1, 2 and 3 respectively as compared to a UD spiral of the same number of interleaves and readout duration or for the VD-spiral corrected with the VD-DCF. The proposed strategy of using a UD-DCF to correct the VD-spiral data had similar PSF FWHM as compared to using a Hanning window, but has a further reduction in the amplitude of the first sidelobe amplitude.
Gibbs Ringing and Motion-Induced DRA
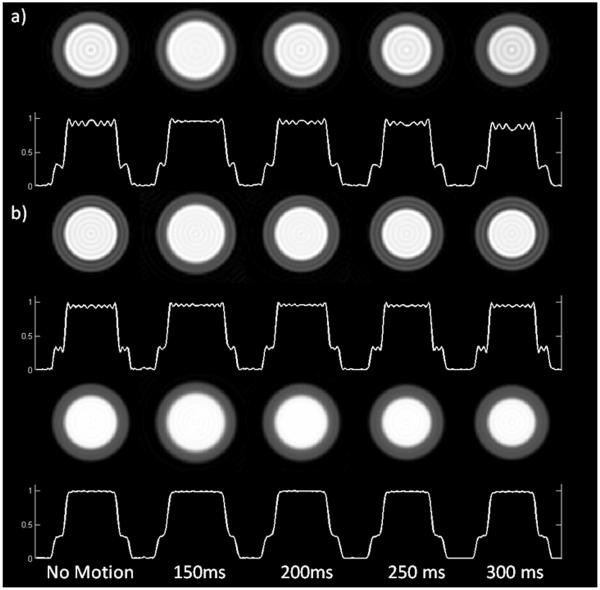
Figure 3 shows simulated short axis images of the left ventricle without motion, and at four time points from early systole (150ms) to end-systole (300ms) for the VD spiral data corrected with the VD-DCF (top row) and the UD-DCF (bottom row). In the absence of motion, there is a significant reduction in the visual appearance of Gibbs-ringing when the UD-DCF (Fig. 3b) as compared to when the standard VD-DCF is applied (Fig. 3a). Spiral trajectories are robust to motion-induced DRA and have only mild DRA when using the VD-DCF (Fig. 3a); however, the visual appearance of dark-rim artifact is further reduced using the UD-DCF (Fig. 3b). Thus employing the UD-DCF for data collected with a VD spiral appears to reduce the appearance of both Gibbs-ringing and motion-induced dark-rim artifacts.
Figure 3.
Simulated heart images without motion, and at various times during systole and early diastole, are shown for (a) a UD pulse sequence and VD pulse sequence 2 corrected with either (b) the VD-DCF or the (c) UD-DCF. Utilization of the UD-DCF significantly reduces the appearance of dark rim artifacts both at rest, and during simulated cardiac motion as compared to the UD spiral pulse sequence and the VD pulse sequence with the VD-DCF. Note that the total readout time and number of interleaves has been matched between the UD and VD images.
Imaging Results
SNR comparisons
The SNR of the LV cavity and myocardium for the three trajectories reconstructed with the standard VD-DCF sequences were 47.9 ± 8.0 and 17.4 ± 3.4, 47.7 ± 10.0 and 17.1 ± 3.2, and 36.7 ± 14.7 and 14.9 ± 4.3 respectively. The mean peak contrast to noise (CNR) radios for the myocardium were 10.7±2.6, 10.5±2.3, and 9.7±3.3 respectively. There were no differences in the SNR of the LV (p=0.18), the SNR of the myocardium (p=0.44), or CNR of the myocardium (p=0.78) between the three trajectories corrected with the VD-DCF. The SNR of the LV cavity and myocardium for the three trajectories reconstructed with the UD-DCF were 77.0 ±16.2 and 28.8 ± 6.9, 75.3 ± 21.4 and 27.3 ± 7.3, and 61.7 ± 24.1 and 25.3 ± 6.4 respectively. The mean peak contrast-to-noise (CNR) ratios for the myocardium were 18.0±4.9, 17.2±4.9, and 16.3±5.1 respectively. There were no differences in the SNR of the LV (p=0.38), the SNR of the myocardium (p=0.65), or CNR of the myocardium (p=0.96) between the three trajectories corrected with the UD-DCF. When comparing the images reconstructed with the UD-DCF versus those reconstructed with the VD-DCF the SNR of the LV cavity and myocardium, and the CNR of the myocardium were all significantly higher (p values for all comparisons <0.01). The myocardial SNR was increased by 64% for trajectory 1, 58% for trajectory 2, and 72% for trajectory 3 when using the UD-DCF. Thus, utilizing the UD-DCF results in a significant improvement in SNR and CNR.
Image Quality
The average image quality scores for the three trajectories reconstructed with the UD-DCF were 1.66, 1, and 1 respectively. The image quality scores were improved for the trajectories with the 6.1ms readout as compared to the 8.2 ms readout (p=0.025). This was largely driven by evidence of mild blurring and dropout of signal in the inferolateral wall due to off-resonance effects in the trajectory with the 8.2 ms readout duration. The average DRA scores were 0.58, 0.38, and 0.13 for the three trajectories respectively (p=0.507). We expected that the 3rd trajectory should have the least DRA as it has the most apodization. When comparing trajectory 1 trajectory 3, there was a significant difference in DRA artifact score (p=0.02). There was a difference in blurring score among the three trajectories (0.77, 0.11, 0.33 (p<0.001). Trajectory 1 had significantly more blurring than trajectory 2 (p=0.02). Although trajectory 3 produced the least DRA, the images visually appeared to have a lower effective spatial resolution as compared to trajectory 2.
Clinical Evaluation
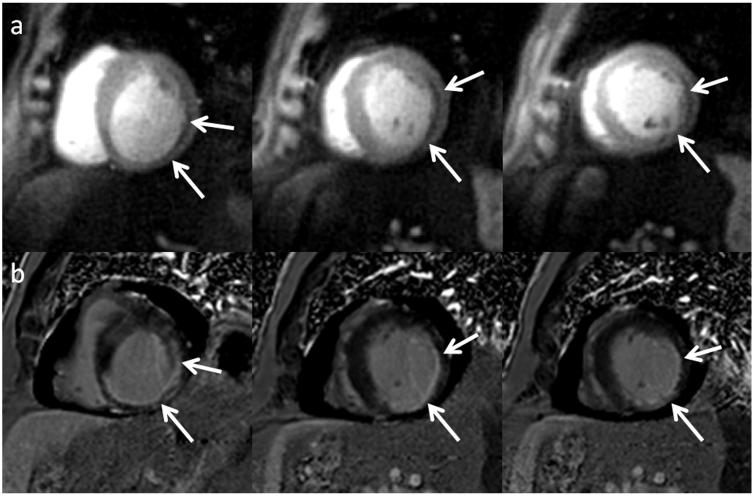
As a result of the SNR experiments and visual scoring, trajectory 2 was chosen as the best sequence by both reviewers and was chosen for subsequent evaluation in patients with expected perfusion abnormalities. Figure 4 shows resting perfusion images from a normal subject reconstructed with both the VD-DCF and UD-DCF. The images reconstructed with the UD-DCF have improved SNR as compared to the images corrected with the VD-DCF. In this particular subject, there is a mild DRA on the apical slice, which is evident on the VD-DCF image but reduced on the image where the UD-DCF was used for density compensation. This shows in-vivo evidence of further reduction of DRA utilizing an apodizing density correction function. Figure 5 shows resting perfusion images, reconstructed with both the VD-DCF (top) and UD-DCF (middle), and delayed enhancement images from a subject with evidence of both a chronic and acute myocardial infarction obtained with trajectory 2. A very thin perfusion abnormality is seen in the area of thinned myocardium which is consistent in position with the corresponding chronic myocardial infarction seen on LGE. There is also a larger perfusion abnormality in the lateral wall which nicely corresponds with a region of microvascular obstruction on LGE images. The improved SNR is evident with the UD-DCF as compared to the VD-DCF, and the perfusion defects are seen well with either DCF choice. Figure 6 shows resting perfusion images from another subject with evidence of prior myocardial infarction (MI). A subendocardial perfusion defect is visualized in the region of chronic MI. These images were obtained with spiral trajectory 2. Figure 7 shows resting perfusion and LGE images from a patient with sarcoid cardiomyopathy. There are perfusion abnormalities in the regions consistent with epicardial fibrosis on the LGE images. Figure 8 shows perfusion images during adenosine stress (a) and at rest from a patient undergoing adenosine stress imaging as part of a clinical research study. There are inducible perfusion abnormalities in the LAD and RCA territories. At cardiac catheterization the patient had a high grade stenosis in his LAD (c) and in a non-dominant RCA (d). The average visual score in the clinical patients was 1.33 with a DRA mean score of 0.42 and a blurring score of 0. There was no significant difference in visual score (p=0.11), DRA score (p=0.77), or blurring score (p=0.17) in the clinical patients as compared to normal subjects using the same trajectory (trajectory 2). In the patient studies, the cardiologists gave the same scores for image quality with either of the two DCFs. In cases where there was high SNR, the VD-DCF images, which have superior spatial resolution, were preferred by one of the two cardiologists. In cases of lower SNR, the VD-DCF which has increased SNR was preferred. All perfusion abnormalities were equally visualized by the images reconstructed with the VD-DCF or UD-DCF.
Figure 4.
Resting perfusion images from a normal subject obtained with trajectory 2 reconstructed with both (a) the VD-DCF and (b) the UD-DCF. The images reconstructed with the UD-DCF have improved SNR and reduced DRA as compared to the images corrected with the VD-DCF.
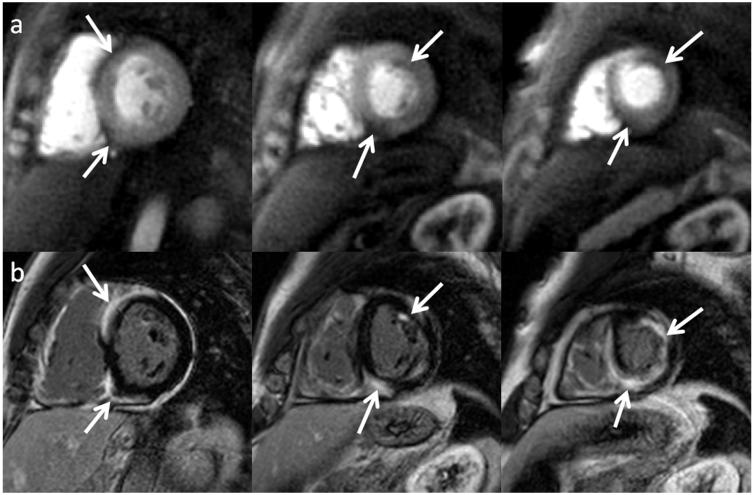
Figure 5.
Resting perfusion images reconstructed with both the VD-DCF (top) and UD-DCF (middle), and delayed enhancement images (bottom) from a subject with evidence of both a chronic and acute myocardial infarction obtained with trajectory 2 using the UD-DCF. A very thin perfusion abnormality is seen in the area of thinned myocardium (yellow arrows), which is consistent in position with the corresponding chronic myocardial infarction seen on LGE. There is also a larger perfusion abnormality in the lateral wall (white arrows), which nicely corresponds with a region of microvascular obstruction on LGE images. The improved SNR is evident with the UD-DCF as compared to the VD-DCF; however, the perfusion defects are seen well with either DCF choice.
Figure 6.
Resting perfusion images (top) and delayed enhancement images (bottom) from another subject with evidence of prior myocardial infarction, a sub-endocardial perfusion defect is visualized in the region of chronic myocardial infarction. These images were obtained with spiral trajectory 2.
Figure 7.
Resting perfusion (top) and LGE images (bottom) from a patient with sarcoid cardiomyopathy. There are perfusion abnormalities in the regions consistent with epicardial fibrosis on the LGE images.
Figure 8.
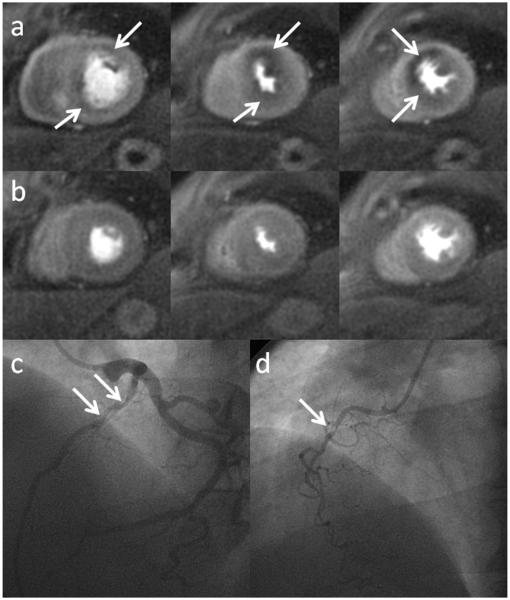
Perfusion images obtained during adenosine stress (a) and at rest (b) from a patient undergoing adenosine stress imaging as part of a clinical research study. There are inducible perfusion abnormalities in the LAD and RCA territories. At cardiac catheterization the patient had a high grade stenosis in his LAD (c) and in a non-dominant RCA (d).
Comparison to Uniform Density Spirals
As compared to the uniform density implementation, the variable density pulse sequence (trajectory 2) with the apoziding UD-DCF had increased SNR of the LV cavity (75.3 ± 21.4 vs 52.6 ± 13.7 p=0.03) and myocardium (27.3 ± 7.3 vs 21.3 ± 4.2 p=0.04), with similar CNR of the myocardium (17.2±4.9 vs 14.3±3.2 p=0.15), visual scores (1 vs 1.3 p=0.05), blurring scores (0 vs 0.03 p=NS) and dark-rim artifact scores (DRA score 0.44 vs 0.33 p=NS).
Discussion
We demonstrated that variable density spiral pulse sequences with an apodizing DCF (UD-DCF) produce high quality first-pass perfusion images with minimal artifacts which compare favorably to our prior UD spiral implementation but with improved scan time and similar high spatial resolution, and reduced PSF side lobe amplitude which may help reduce DRA. Furthermore, these sequences result in a 25% reduction in either the number of interleaves (trajectory 1) or readout duration (trajectories 2 and 3) as compared to the UD spiral pulse sequence with the same nominal 2mm spatial resolution over a 340 mm FOV. As the readout duration of the spiral pulse sequence is increased there is more time for additional phase accrual for off-resonance spins. Within the context of spiral perfusion imaging this effect manifests in two ways: (1) With longer readouts there are increased blurring artifacts, however this can largely be mitigated by off-resonance correction. (2) Longer readouts result in signal dropout artifact which is typically most evident in the inferolateral wall near the junction between the heart, lung and liver where there are severe off-resonance gradients, this effect more difficult to correct with traditional off-resonance correction. Furthermore, VD trajectories which move away from the k-space center more rapidly will be less sensitive to off-resonance effects as compared to a UD trajectory of the same readout duration.(17) In our experience, reducing the readout duration per interleaf from 8.1 ms to 6.1 ms resulted in improved off-resonance performance and image quality as compared to reducing the number of interleaves from 8 to 6.
We also show that for the perfusion application utilizing an apodizing DCF may be advantageous in terms of increasing SNR and reducing DRA. A similar concept has been applied to reduce Gibbs-ringing in chemical shift imaging.(9) In theory, applying such a DCF is equivalent to windowing the gridded k-space data which is known to reduce Gibbs ringing. For spiral imaging where the edges of k-space are undersampled relative to the center, this apodizing effect is inherent in the sampling density. Applying the UD-DCF effectively reduces the scaling of high frequency noise that occurs when traditional density compensation is performed.
As the VD pulse sequences used in this study intentionally under-sample the high spatial frequencies, the images may have some high frequency aliasing artifacts. These high-frequency artifacts have previously been shown to have the appearance of increased background noise in the images.(17) Utilizing the apodizing DCF which under-weights the high-spatial frequency data should also reduce the appearance of these high frequency artifacts and may play a role in the improved SNR using the apodizing UD-DCF. These artifacts can largely be overcome with either increasing the FOV, or shutting off remote coils which are only adding aliasing artifact to the region of interest.
The use of the DCF corresponding to the UD-spiral trajectory with the same starting density, readout duration and number of interleaves is somewhat arbitrary; however, it effectively compensates the density near the center of k-space as the UD and VD trajectories are very similar in this region. Furthermore, it was easily implemented within the context of our existing pulse sequence reconstruction pipeline. We compared the apodization inherent in this DCF to applying a Hanning window to the gridded k-space data and show that the UD-DCF results in a similar main lobe width of the PSF with even greater reduction in the sidelobe amplitude, justifying this as a reasonable choice for our application. Ideally, one could treat both the design of trajectory and DCF as an optimization problem for a desired k-space filter with design tradeoffs between mainlobe width, sidelobe amplitude, and SNR.
The efficiency of the VD-spiral trajectory without any parallel imaging has comparable imaging time to the Cartesian pulse sequences most often utilized clinically with rate 2-3 acceleration but enables higher spatial resolution. With low acceleration factor parallel imaging, complete coverage of the left ventricle should be feasible with VD spiral perfusion imaging. Non-Cartesian trajectories such as VD spirals or radial may have inherent advantages for the application of compressed-sensing techniques as they have more incoherent PSF’s as compared to Cartesian pulse sequences. We have successfully applied compressed sensing to achieve rate 4 acceleration of these VD spiral pulse sequences and we are currently evaluating these acceleration techniques clinically.(18) Currently our pulse sequence utilizes two extra interleaves for field map generation which reduces the overall efficiency of data acquisition. Recent developments in off-resonance deblurring without utilization of a field map show great potential for spiral imaging.(19) We have recently shown that for VD-spiral perfusion imaging automatic off-resonance deblurring without using an acquired field map performed equally to semi-automatic off-resonance deblurring utilizing an acquired field map for spiral perfusion imaging. This will potentially eliminate the need to obtain a field map with each image and will further increase the efficiency of the VD spiral technique. Spatial coverage may also be increased using 3D spiral trajectories such as stack-of-spirals as recently demonstrated.(20) Further clinical evaluation will be needed to determine whether the SNR advantages and the ability to image at one phase of the cardiac cycle with a 3D technique will dominate over the inherent high temporal resolution of 2D approaches.
This study has a few limitations. As this study was performed during a routine clinical examination, we were only able to obtain resting perfusion images with a single pulse sequence precluding the direct comparison of spiral and Cartesian pulse sequences in the same subject. With a more highly accelerated VD-spiral pulse sequence it may be feasible to obtain both Cartesian and VD-spiral data in an interleaved fashion within each heart beat. While within the context of this study we did not perform adenosine stress imaging in all subjects, we demonstrate that perfusion abnormalities in regions of myocardial infarction and scarring are readily visualized with the VD-spiral technique. This is true even in regions of thinned myocardium indicating that the technique is relatively robust to off-resonance induced image blurring. The example case provided using a spiral pulse sequence for adenosine stress CMR demonstrates excellent image quality with perfusion defects matching the location of obstructive coronary artery disease by cardiac catheterization. We have more recently been evaluating the utility of VD-spiral pulse sequences for adenosine stress imaging and have shown promising initial results.(21) A limitation of this study is that the VD spiral approach was not compared to the UD spiral approach in patients, so it is difficult to assess clinically the advantages and disadvantages of these two approaches. Further clinical studies and direct comparison to existing techniques will be necessary to demonstrate the potential utility of VD-spiral pulse sequences for this challenging application.
Conclusions
High quality perfusion images can be obtained using VD-spiral pulse sequence with an apodizing DCF which have minimal DRA or off-resonance artifacts and further evaluation may demonstrate that spiral pulse sequences may be of clinical utility for stress myocardial perfusion imaging.
Acknowledgments
Funding Sources: AHA 10SDG2650038, NIH R01 HL079110, NIH K23 HL112910-01, Siemens Medical Solutions
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the american heart association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Berrington de Gonzalez A, Kim KP, Smith-Bindman R, McAreavey D. Myocardial perfusion scans: projected population cancer risks from current levels of use in the United States. Circulation. 2010;122(23):2403–2410. doi: 10.1161/CIRCULATIONAHA.110.941625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerber BL, Raman SV, Nayak K, Epstein FH, Ferreira P, Axel L, Kraitchman DL. Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. J Cardiovasc Magn Reson. 2008;10(1):18. doi: 10.1186/1532-429X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker DL, Gullberg GT, Frederick PR. Gibbs artifact removal in magnetic resonance imaging. Med Phys. 1987;14(4):640–645. doi: 10.1118/1.596032. [DOI] [PubMed] [Google Scholar]
- 5.Di Bella EV, Parker DL, Sinusas AJ. On the dark rim artifact in dynamic contrast-enhanced MRI myocardial perfusion studies. Magn Reson Med. 2005;54(5):1295–1299. doi: 10.1002/mrm.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storey P, Chen Q, Li W, Edelman RR, Prasad PV. Band artifacts due to bulk motion. Magn Reson Med. 2002;48(6):1028–1036. doi: 10.1002/mrm.10314. [DOI] [PubMed] [Google Scholar]
- 7.Salerno MRW, Judd R, Kim R. Simulation of Banding Artifacts Resulting From Realistic Cardiac Motion During Single Shot Myocardial Perfusion Imaging; 10th Scientific Meeting of the Society of Cardiovascular Magnetic Resonance.2007. [Google Scholar]
- 8.Salerno M, Sica CT, Kramer CM, Meyer CH. Optimization of spiral-based pulse sequences for first-pass myocardial perfusion imaging. Magn Reson Med. 2011;65(6):1602–1610. doi: 10.1002/mrm.22746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adalsteinsson E, Star-Lack J, Meyer CH, Spielman DM. Reduced spatial side lobes in chemical-shift imaging. Magn Reson Med. 1999;42(2):314–323. doi: 10.1002/(sici)1522-2594(199908)42:2<314::aid-mrm14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Pipe JG, Menon P. Sampling density compensation in MRI: rationale and an iterative numerical solution. Magn Reson Med. 1999;41(1):179–186. doi: 10.1002/(sici)1522-2594(199901)41:1<179::aid-mrm25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Meyer CH, Hu P. Spiral parallel magnetic resonance imaging. Conf Proc IEEE Eng Med Biol Soc. 2006;1:369–371. doi: 10.1109/IEMBS.2006.259758. [DOI] [PubMed] [Google Scholar]
- 12.Meyer CH, Hu BS, Nishimura DG, Macovski A. Fast spiral coronary artery imaging. Magn Reson Med. 1992;28(2):202–213. doi: 10.1002/mrm.1910280204. [DOI] [PubMed] [Google Scholar]
- 13.Morrell G, Macovski A. Three-dimensional spectral-spatial excitation. Magn Reson Med. 1997;37(3):378–386. doi: 10.1002/mrm.1910370314. [DOI] [PubMed] [Google Scholar]
- 14.Salerno M, Kramer CM, Meyer CH. Simulation of Motion-Induced Dark-Rim Artifacts for Cartesian and Spiral Pulse Sequences; Proceedings of the 18th ISMRM.2010. [Google Scholar]
- 15.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47(2):372–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constantinides CD, Atalar E, McVeigh ER. Signal-to-noise measurements in magnitude images from NMR phased arrays. Magn Reson Med. 1997;38(5):852–857. doi: 10.1002/mrm.1910380524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai CM, Nishimura DG. Reduced aliasing artifacts using variable-density k-space sampling trajectories. Magn Reson Med. 2000;43(3):452–458. doi: 10.1002/(sici)1522-2594(200003)43:3<452::aid-mrm18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Chen X, Feng X, Wang M, Epstein FH, Meyer CH, Salerno M. Evaluation of Parallel Reconstruction Techniques for First-pass Perfusion Imaging Using Spiral Trajectories; Proceedings of the 20th ISMRM.2012. [Google Scholar]
- 19.Chen W, Meyer CH. Fast automatic linear off-resonance correction method for spiral imaging. Magn Reson Med. 2006;56(2):457–462. doi: 10.1002/mrm.20973. [DOI] [PubMed] [Google Scholar]
- 20.Shin T, Nayak KS, Santos JM, Nishimura DG, Hu BS, McConnell MV. Three-dimensional first-pass myocardial perfusion MRI using a stack-of-spirals acquisition. Magn Reson Med. 2012 doi: 10.1002/mrm.24303. epub ahead of print May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salerno M, Sica CT, Kramer CM, meyer CH. Variable-Density Spiral Adenosine Stress Perfusion Imaging Detects Coronary Artery Disease with High Diagnostic Accuracy; Proceedings of the 20th ISMRM.2012. [Google Scholar]