Abstract
The solution phase synthesis of N-protected amino acids and peptides has been achieved through the Group-Assisted Purification (GAP) chemistry by avoiding disadvantages of other methods in regard to the difficult scale-up, expenses of solid and soluble polymers, etc. The GAP synthesis can reduce the use of solvents, silica gels, energy and manpower. In addition, the GAP auxiliary can be conveniently recovered for re-use and is of environmentally friendly benign by substantially reducing waste production in academic labs and industry.
Peptides and peptidomimetic drugs are involved in nearly all disciplines of chemical and biomedical sciences.1–5 The search for environmentally friendly synthetic approaches to these products for academic research and pharmaceutical production has been pursued for over five decades.6–11 A notable accomplishment in peptide synthesis is shown by the solid-phase-peptide synthesis (SPPS) invented by the late Nobel Laureate, Bruce Merrifield, in 1980's.12–13 This milestone discovery can overcome shortcomings of traditional solution-phase-peptide synthesis in regard to tedious purification, consumption of large amounts of materials including coupling reagents, solvents and silica gels, and can reduce waste generation; it has also greatly accelerated peptide and protein research. SPPS has also been imposing a huge impact on general chemical synthesis for drug discovery and development, particularly, on combinatorial screening.13 Since then several synthetic protocols have been developed to complement SPPS so as to minimize the difficulty of scale-up, the use of excess amounts of reagents and expensive resins for coupling reactions, particularly for synthesizing longer peptides.14–21 In this regard, an alternative method was developed by using soluble polymers as the templates for couplings of amino acid residues.22–23 This method makes the peptide synthesis to be performed in solution phase but the work-up in solid-phase manner, or, through convenient extractions. However, the latter method needs the soluble polymers of high molecular weights, which makes it inconvenient to produce large amounts of peptides of much lower molecular weights than polymeric templates. In addition, it often needs long periods to generate solid or crystalline products by carefully controlling solidification/crystallization conditions.
Recently, our labs have established the GAP (Group-Assisted Purification) chemistry concept through the design and synthesis of new reagents that were attached with unique achiral and chiral auxiliaries.24–31 By using GAP technology, organic synthesis can be performed efficiently and stereochemically without the use of traditional purifications of chromatography, recrystallization, etc. In fact, the stereoisomeric products can be obtained simply by washing the crude mixtures with inexpensive petroleum solvents or co-solvents. Therefore, it can substantially reduce the use of starting materials, silica gels, energy, manpower, etc. In addition, achiral and chiral auxiliaries can be conveniently recovered for re-use; and they can often be quantitatively recovered via a one-time extraction with n-butanol. It is believed that the GAP chemistry concept will have a huge impact on chemical synthesis and medicinal fields. In this report, we would like to disclose that the GAP chemistry strategy can advance peptide synthesis, which has advantages of both solid-phase-peptide synthesis (SPPS) and liquid-phase synthesis of peptides by avoiding their shortcomings in regard to the factors mentioned above.22–23
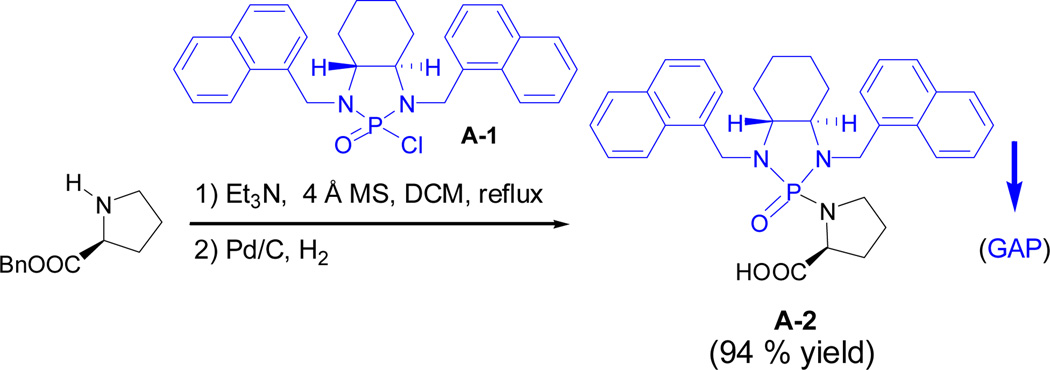
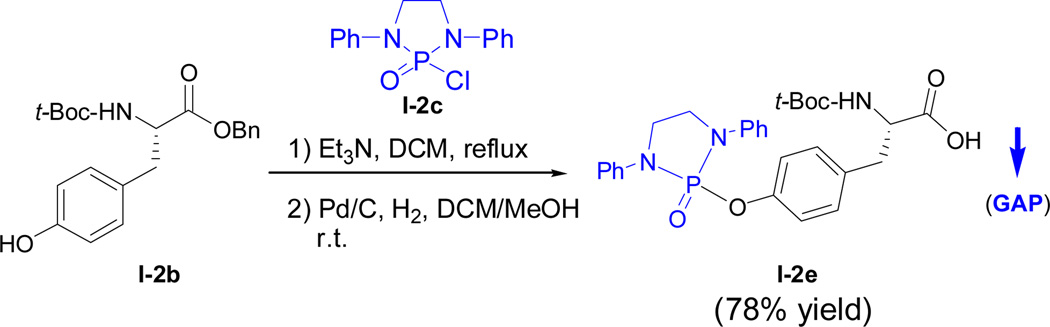
Amino acid residues are basic building blocks for the synthesis of peptides and proteins. We first explored if individual amino acids can be protected via the GAP chemistry protocol. N, N’-Di-(1-naphthylmethylene)-1,2-cyclohexyldiamino N-phosphonyl chloride A-1 was employed for the reactions with L-Pro-OBn·HCl, D-Ala-OMe·HCl and L-Phe-OMe·HCl. The solution of N-phosphonyl chloride in dichloromethane was added by triethylamine (2.5 equiv.), 4 Ǻ MS and amino ester; the resulting mixture was stirred at 90 °C overnight for complete consumption of major starting materials. The pure protected amino acid esters of above three products were obtained via the GAP work-up washing with hexane/DCM co-solvents by avoiding the use of column chromatography or recrystallization to give yields of 96%, 92% and 86%, respectively. For this protection experiment, 4 Ǻ MS was necessary for achieving higher yields. Interestingly, the protection of secondary amino acid ester (L-Pro) occurred more smoothly to give better yields than other primary amino cases (Scheme 1). The following catalytic hydrogenation afforded corresponding N-phosphonyl amino acids with free carboxylic acid groups.32 The next attention was turned to the protection on hydroxy group of Tyr which is among the most important aromatic amino acids5,33 so as to explore if the GAP chemistry strategy is suitable for peptide synthesis. The simpler achiral N-phosphonyl chloride was utilized for the protection which was followed by catalytic hydrogenation to afford corresponding N-phosphonyl tyrosine (Scheme 2). At this initial stage, this concise and achiral protection is being continued for peptide synthesis, although the GAP cases of using chiral ones is also showing promising in this laboratory.
Scheme 1.
GAP protection of L-proline benzyl ester and its deprotection
Scheme 2.
GAP protection of L-tyrosine benzyl ester and its deprotection
The new GAP strategy is presented by the synthesis of biphalin peptides that belong to opioid pain killer series.33–37 The original biphalin is a highly potent analgesic candidate but show low selectivity between δ and μ receptors.35–39 Its structural modifications are anticipated to generate new pain killers of high potency and selectivity so as to reduce unwanted side effects. The use of phosphoric acid moiety for modifying peptides would meet the purpose above; In fact, this strategy has not been well documented yet. The present GAP peptide synthesis will concisely generate a series of new biphalin derivatives and greatly accelerate the opioid peptide research.
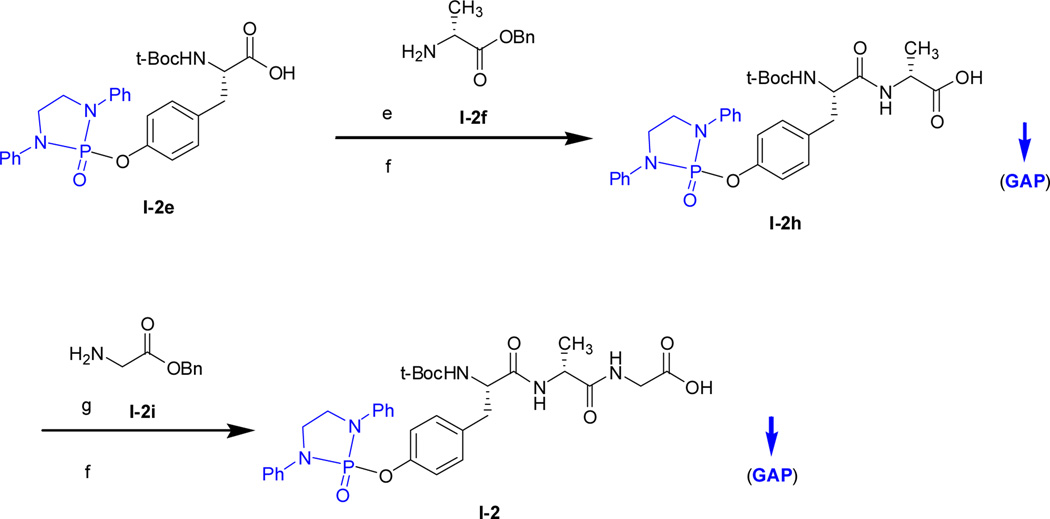
The GAP synthesis of dipeptide and tripeptide precursors to biphalins is represented by the example shown in Scheme 3. The coupling reactions were conducted under standard conditions of using TBTU and Et3N in DCM at room temperature.32 The catalytic hydrogenation to cleave benzyl group gives O-phosphonyl amino acid for the next coupling reaction. The individual N-t-Boc amino acid benzyl esters of D-Ala, Gly and Phe were prepared by following literature procedures.32 The dipeptide bridge precursors, I-3a to 3d and I-4a to 4d were also synthesized by following known methods under standard coupling systems.38
Scheme 3.
GAP synthesis of dipeptides and tripeptides
It should pointed out that that the GAP purification was successfully applied for the preparation of I-3a to 3d. In this preparation, the crude bis-coupling products were washed by co-solvent of EtOAc/hexanes (1:2, v/v) to give pure I-3a to 3d as white solids. Similarly, the crude products of I-4a to 4d via GAP procedure were washed with by co-solvent of dichloromethane/hexanes (1 : 100, v/v) to give pure I-4a to 4d as white solids as well.
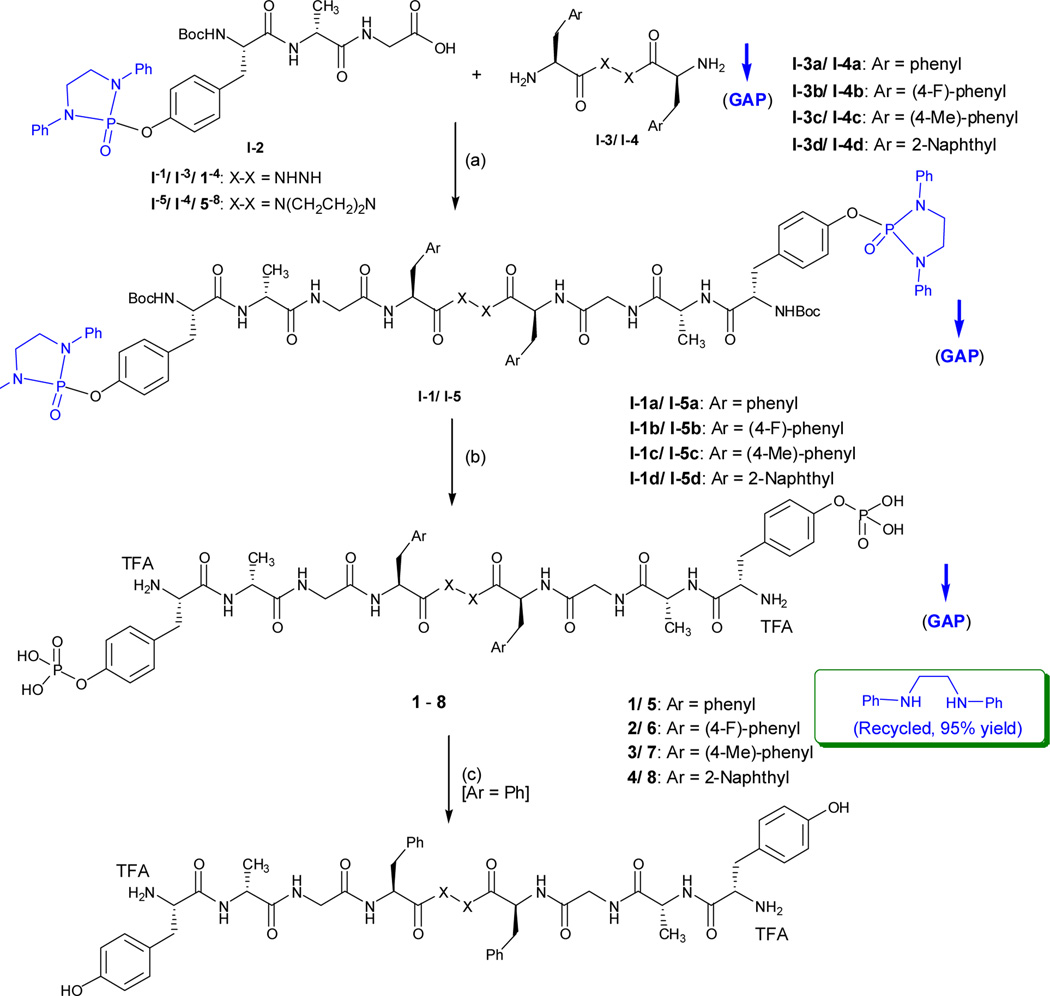
N,N’-Di-phenyl-1,2-ethyldiamino O-phosphonyl attached biphalins, I-1 were synthesized by reacting tripeptide I-2 (1.0 eq) and dimeric dipeptide I-3 (0.5 eq) in dry dichloromethane (DCM) in the presence of triethyl amine (Et3N) (1.1 eq) and O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (TBTU) (1.1 eq) as the coupling reagent.12 The reaction mixture was cooled to 0 °C before TBTU was added and kept stirring for 2 hours at this temperature. The coupling reaction was completed after being stirred at room temperature overnight. The reaction mixture was diluted to double volume by DCM and washed by H2O, 0.5 M HCl, aq NaHCO3(sat.) and brine in order (Scheme 4).
Scheme 4.
GAP synthesis of biphalins
The new O-phosphoryl biphalin 1 was generated by treating I-1 with trifluoroacetic acid (TFA) in DCM at r.t. for 24 hours as monitored by 31P NMR. After the cleavage was finished, the resulting mixture was evaporated before H2O and DCM (1:1, v/v) were added. The resulting mixture was extracted by DCM for five times to completely remove the N,N`-biphenylethylenediamine auxiliary from the aqueous phase. Evaporating the aqueous phase to dryness afforded pure O-phosphoryl biphalin 1 as white solid in 88% yield. It’s noteworthy that the N,N`-biphenylethylenediamine auxiliary can be recovered from DCM in 95% recover rate.
Other O-phosphoryl biphalins 2 – 8 were conveniently obtained under the same conditions and worked-up by following the GAP washing without the use of column chromatography or recrystallization. They were obtained as white solids in good to excellent yields of 78% to 92%.
After we successfully synthesized the new biphalin analogs with the phosphoryl moiety attached on tyrosine ring that would lead to interesting binding and biological profiles in future, we further performed the mild cleavage to give free biphalins.32 We found that the treatment of O-phosphonyl biphalins with calf intestinal phosphatase (CIP) in a buffer solution of PH 7–8 at room temperature afforded free biphalin peptide in 87% of chemical yield.
The Group-Assisted Purification (GAP) strategy has been successfully utilized for the solution phase synthesis of N-protected amino acids and peptides by avoiding the use of traditional purification protocols such as chromatography and recrystallization. In the present GAP synthesis, pure products have been obtained simply by washing the crude mixtures with inexpensive petroleum solvents or co-solvents to give good to high yields. In addition, the GAP auxiliary can be conveniently recovered for re-use.
Supplementary Material
Acknowledgments
This work is supported by NIH (R33DA031860, USA), Robert A. Welch Foundation (D-1361, USA) and Jiangsu Innovation Team Program (P. R. China).
Footnotes
Electronic Supplementary Information (ESI) available: Spectroscopic spectra of all pure products shown in Scheme 1–3 and Fig 1.
Notes and references
- 1.Clardy J, Walsh C. Nature. 2004;432:829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 2.Szeto HH, Schiller PW. Pharm. Res. 2011;28:2669–2679. doi: 10.1007/s11095-011-0476-8. [DOI] [PubMed] [Google Scholar]
- 3.White CJ, Yudin AK. Nat. Chem. 2011;3:509–524. doi: 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
- 4.Assem N, Yudin AK. Nat. Protoc. 2012;7:1327–1334. doi: 10.1038/nprot.2012.066. [DOI] [PubMed] [Google Scholar]
- 5.Hruby VJ, Li G, HaskellLuevano C, Shenderovich M. Biopolymers. 1997;43:219–266. doi: 10.1002/(SICI)1097-0282(1997)43:3<219::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 6.Gustafson JL, Lim D, Miller SJ. Science. 2010;328:1251–1255. doi: 10.1126/science.1188403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pattabiraman VR, Bode JW. Nature. 2011;480:471–479. doi: 10.1038/nature10702. [DOI] [PubMed] [Google Scholar]
- 8.Ko E, Liu J, Perez LM, Lu G, Schaefer A, Burgess K. J. Am. Chem. Soc. 2011;133:462–477. doi: 10.1021/ja1071916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan W-K, Ho C-M, Wong M-K, Che C-M. J. Am. Chem. Soc. 2006;128:14796–14797. doi: 10.1021/ja064479s. [DOI] [PubMed] [Google Scholar]
- 10.Hirschmann R, Smith AB, III, Taylor CM, Benkovic PA, Taylor SD, Yager KM, Sprengeler PA, Benkovic SJ. Science. 1994;265:234–237. doi: 10.1126/science.8023141. [DOI] [PubMed] [Google Scholar]
- 11.Smith AB, III, Benowitz AB, Sprengeler PA, Barbosa J, Guzman MC, Hirschmann R, Schweiger EJ, Bolin DR, Nagy Z, Campbell RM, Cox DC, Olson GL. J. Am. Chem. Soc. 1999;121:9286–9298. [Google Scholar]
- 12.Merrifield RB. J. Am. Chem. Soc. 1963;85:2149–2154. [Google Scholar]
- 13.Sharma I, Crich D. J. Org. Chem. 2011;76:6518–6524. doi: 10.1021/jo200497j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eom KD, Miao ZW, Yang JL, Tam JP. J. Am. Chem. Soc. 2003;125:73–82. doi: 10.1021/ja020529r. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Pentelute BL, Kent SBH. Angew. Chem.-Int. Edit. 2012;51:993–999. doi: 10.1002/anie.201106060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullen DG, Weigel B, Barany G, Distefano MD. J. Pept. Sci. 2010;16:219–222. doi: 10.1002/psc.1223. [DOI] [PubMed] [Google Scholar]
- 17.Okada Y, Suzuki H, Nakae T, Fujita S, Abe H, Nagano K, Yamada T, Ebata N, Kim S, Chiba K. J. Org. Chem. 2012;78:320–327. doi: 10.1021/jo302127d. [DOI] [PubMed] [Google Scholar]
- 18.Moor WT. Solid-phase peptide synthesis. Methods in enzymology. 1997;289:520. [Google Scholar]
- 19.Li BC, Montgomery DC, Puckett JW, Dervan PB. J. Org. Chem. 2013;78:124–133. doi: 10.1021/jo302053v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields GB, Noble RL. International Journal of Peptide and Protein Research. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 21.Mullen DG, Veradi R, Porcelli F, Scaloni A, Barany G, Veglia G. Biopolymers. 2012;98:479–484. doi: 10.1002/bip.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer PM, Zheleva I. J. Pept. Sci. 2002;8:529–542. doi: 10.1002/psc.413. [DOI] [PubMed] [Google Scholar]
- 23.Kitada S, Takahashi M, Yamaguchi Y, Okada Y, Chiba K. Org. Lett. 2012;14:5960–5963. doi: 10.1021/ol302863r. [DOI] [PubMed] [Google Scholar]
- 24.Kattuboina A, Li G. Tetrahedron Lett. 2008;49:1573–1577. [Google Scholar]
- 25.Pindi S, Kaur P, Shakya G, Li G. Chem. Biol. Drug Des. 2011;77:20–29. doi: 10.1111/j.1747-0285.2010.01047.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaur P, Wever W, Pindi S, Milles R, Gu P, Shi M, Li GG. Green Chem. 2011;13:1288–1292. [Google Scholar]
- 27.Kattamuri PV, Ai T, Pindi S, Sun Y, Gu P, Shi M, Li GG. J. Org. Chem. 2011;76:2792–2797. doi: 10.1021/jo200070d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur P, Pindi S, Wever W, Rajale T, Li G. Chem. Comm. 2010;46:4330–4332. doi: 10.1039/c0cc00287a. [DOI] [PubMed] [Google Scholar]
- 29.Kaur P, Pindi S, Wever W, Rajale T, Li G. J. Org. Chem. 2010;75:5144–5150. doi: 10.1021/jo100865q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun H, Zhang HW, Han JL, Pan Y, Li G. Green Chem. submitted. [Google Scholar]
- 31.Pindi S, Wu JB, Li G. J. Org. Chem. accepted. [Google Scholar]
- 32.For HF cleavage see: Wuts PGM, Greene TW. Protective Groups in Organic Synthesis. Wiley; 2006.
- 33.Schiller PW. Biopolymers. 2005;80:492–492. [Google Scholar]
- 34.Sinha B, Cao Z, Murray TF, Aldrich JV. J. Med. Chem. 2009;52:7372–7375. doi: 10.1021/jm9007592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipkowski AW, Konecka AM, Sroczynska I. Peptides. 1982;3:697–700. doi: 10.1016/0196-9781(82)90173-5. [DOI] [PubMed] [Google Scholar]
- 36.Shimohigashi Y, Costa T, Chen HC, Rodbard D. Nature. 1982;297:333–335. doi: 10.1038/297333a0. [DOI] [PubMed] [Google Scholar]
- 37.Kawalec M, Kowalczyk JE, Beresewicz M, Lipkowski AW, Zablocka B. Neurochem. Res. 2011;36:2091–2095. doi: 10.1007/s11064-011-0568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G, Haq W, Xiang L, Lou BS, Houghes R, De Leon IA, Davis P, Gillespie TJ, Romanowski M, Zhu XY, Misicka A, Lipkowski AW, Porreca F, Davis TP, Yamamura HI, O’Brien DF, Hruby VJ. Bioorg. Med. Chem. Lett. 1998;8:555–560. doi: 10.1016/s0960-894x(98)00065-1. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Wang H, Shah K, Karamyan VT, Abbruscato TJ. Brain Res. 2011;1383:307–316. doi: 10.1016/j.brainres.2011.01.083. (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.