Abstract
PURPOSE
Sleep duration among breast cancer survivors correlates with fatigue, depression, and health-related quality of life (HRQOL); however, this has not been studied longitudinally. This study investigated patterns of sleep duration change across the early breast cancer survivorship period, their demographic and clinical predictors, and their relationships with subsequent cancer-related symptoms and HRQOL.
METHODS
Breast cancer survivors (n=572), were assessed 6 months post-diagnosis (current sleep & retrospective reports of pre-diagnosis sleep), 30 months post-diagnosis (sleep), and 39 months post-diagnosis (symptoms, HRQOL). Sleep duration change was determined by examining sleep at each time point in relation to published norms. Analysis of variance and logistic regression models tested demographic and clinical differences between the sleep change groups; linear regression models tested differences in symptoms and HRQOL.
RESULTS
Half of the survivors reported no sleep duration change over time; however, 25% reported sleep changes indicating a temporary (5.6%), late-occurring (14%), or sustained (5.9%) change. Survivors reporting sustained or temporary sleep changes were more likely to have been treated with chemotherapy (OR=2.62, p<.001) or gained weight after diagnosis (OR=1.82, p=.04) than those with no sleep change. Sustained sleep changes were related to greater subsequent severity, affective, and sensory aspects of fatigue (βs=2.0, 2.3, 1.8; all p <.0001) and lower vitality (β=−10.8, p=.005).
CONCLUSIONS
Survivors treated with chemotherapy and those who gain weight after diagnosis may have increased risk for sustained sleep duration changes, which may increase their fatigue. These results point to the need for routine assessment of sleep as part of survivorship care.
Keywords: Breast Cancer, Survivors, Long-term Effects, Sleep, Quality of Life
Introduction
Sleep disturbance occurs in 20–70% of breast cancer survivors [1, 2], and can include delayed sleep onset, mid-cycle or early morning awakening, or a combination of problems[3, 4] that either reduce total sleep duration, or increase daytime napping and overall sleep time[4–7]. In cross-sectional studies, breast cancer survivors report sleep problems before, during, and after primary cancer treatment[1] which may be new problems or pre-existing sleep problems exacerbated by treatment[3].
Sleep disturbance is a multidimensional construct encompassing sleep quality, latency, efficiency, duration, and daytime dysfunction. Most prior research has measured sleep quality. However, studying sleep duration in breast cancer survivors is important since survivors report sleep duration changes compared to healthy women even though overall sleep quality may be similar in the two groups[5] and since sleep duration is associated with mortality in general samples [8–13]. No longitudinal study of sleep duration in breast cancer survivors has been conducted; thus, the patterns of sleep duration change through and beyond cancer treatment are unknown.
Sleep duration change among cancer survivors has been associated with cancer treatment, especially chemotherapy[14, 15]. However, research identifying risk factors for sleep disturbance where sleep duration is a component of the sleep measure suggests that psychological stress, lower or higher education, unemployment, younger age, low physical activity, nocturnal hot flashes and night sweats, and poor physical or psychological health may also play a role in sleep[3, 4, 16–23]. Without longitudinal data, it is unknown how these demographic or clinical characteristics may relate to patterns of sleep duration change across survivorship.
Sleep duration among breast cancer survivors has been correlated with depression[5, 24] and fatigue symptoms[25, 26], and interventions that modify sleep duration document corresponding improvements in fatigue, depression, and anxiety[27, 28]. These symptoms may cluster together due to a shared etiology, notably elevated systemic inflammation after cancer treatment[17, 29, 30]. Without longitudinal data, whether sleep disturbance is a cause or a consequence of these other symptoms (or bidirectionally-determined) remains unknown.
Interventions that modify sleep duration have also shown improvements in health-related quality of life (HRQOL) among cancer survivors[27, 28]. However, it is unknown how patterns of sleep duration change relate to HRQOL.
The purpose of this paper was to determine 1) the patterns of sleep duration change across the early breast cancer survivorship period; 2) demographic or clinical characteristics that distinguished the sleep patterns; and 3) relationships between sleep duration change patterns and subsequent cancer-related symptoms and HRQOL.
Methods
The Health, Eating, Activity, and Lifestyle (HEAL) Study is a multicenter, multiethnic, prospective study of women diagnosed with in situ or Stages I to IIIA breast cancer[31]. Participants provided written or documented verbal informed consent. All study protocols were approved by the Institutional Review Boards of participating centers.
Eligibility, Recruitment, and Data collection
Patients diagnosed with their first primary breast cancer (n=1,183) were recruited from three Surveillance Epidemiology and End Results (SEER) registries in New Mexico, Western Washington, and Los Angeles County, California. However, sleep was not assessed at baseline at the California site so these women (n=366) were excluded from these analyses and are not described here. In New Mexico, we recruited 615 women aged 18 years or older diagnosed with in situ to regional breast cancer between 1996–1999, living in Bernalillo, Santa Fe, Sandoval, Valencia, or Taos counties. In Western Washington, we recruited 202 women aged 40 to 64 years diagnosed with in situ to regional breast cancer between 1997–1998, living in King, Pierce, or Snohomish counties. The age range for the Washington patients was restricted to avoid overlap with eligibility requirements of other accruing studies.
HEAL participants completed three assessments; the baseline interview (on average 6 months post-diagnosis; range=2–12), a follow-up assessment 30 months post-diagnosis (range=24–41; response rate=83%), and a third assessment 39 months post-diagnosis (range=24–59; response rate=78%). The baseline and 30-month follow-up assessed demographic and clinical variables, sleep, and physical activity. The 39-month follow-up assessed cancer-related symptoms and HRQOL (hereafter called the HRQOL assessment). For these analyses, we excluded 27 women diagnosed with recurrent or new primary breast cancer and 36 women missing data for the symptom,HRQOL, or sleep variables.. The final sample size was 572 women.
Measures
Sleep duration
Participants reported their average total sleep time (excluding naps) on weekend days and weekdays via the Modifiable Activity Questionnaire which has been shown to be a reliable and valid measure of activity [32, 33]. The baseline questionnaire assessed sleep for the year prior to diagnosis (retrospective reports of pre-diagnosis sleep) and for the past month (sleep at 6-months post-diagnosis). The 30-month follow-up questionnaire collected similar information for the year prior to the interview (sleep at 30-months). The questions asked “During a typical 24-hour weekday (or weekend day) in the past year (or past month), how many hours did you spend sleeping at night?” Total daily sleep time variables were averaged across the week.
Outcome variables
Fatigue was measured with the 22-item Piper Fatigue Scale, a reliable and valid measure of subjective fatigue [34]. Four subscales (coded 0–10, increasing scores=greater fatigue) measured the behavioral changes (behavioral/severity subscale), emotional meaning (affective meaning subscale), and physical (sensory subscale) and emotional (cognitive/mood subscale) symptoms related to fatigue. We changed the response time frame to assess fatigue over the past month rather than the past week to assess the survivor's general fatigue.
Fear of recurrence was measured with the 5-item version of the Fear of Recurrence scale[35]. Likert scale responses were summed to create a scale ranging from 5–25 (increasing scores=greater distress and preoccupation with cancer recurrence).
Perceived stress was measured with the 4-item version of the Perceived Stress Scale that has been shown to be reliable and valid when compared to the 14-item PSS[36]. Items were coded 1–5 and responses were summed into a scale ranging from 4–20 (increasing scores=increased stress).
Health-related quality of life was measured with the well-validated SF-36[37, 38]. The 36 items were summarized into eight subscales, each ranging from 0–100 (increasing scores=better functioning).
Correlates/covariates
Physical activity levels before diagnosis, 6 months post-diagnosis, and at the 30-month follow-up were collected using the Modifiable Activity Questionnaire[32, 33] described above. Data from the three time points were coded identically: Hours per week spent in each activity were estimated by multiplying the frequency by the duration reported and converted to MET hours per week of sports/recreation activity. Diet variables were assessed at the 30-month assessment by a validated self-administered food frequency questionnaire [39] including caffeine (mg/day) and alcohol intake (g/day). The severity of hot flashes, night sweats, and self-reported weight gain (all 0=not at all to 4=extremely) were assessed with a modified form of the Breast Cancer Prevention Trial hormone-related symptom checklist[40]. Body mass index (kg/m2) was calculated as weight in kilograms divided by height in meters squared, computed from clinic measures at baseline (height) and 30-months (weight). Weight change from baseline to the 30-month follow-up was also computed from clinic measures (gained ≥ 5% of baseline weight vs. not).
Demographic and clinical characteristics included standard measures of age, education and race/ethnicity from baseline questionnaires and marital status from the 30-month assessment. Stage of disease was based on SEER records. Both estrogen receptor and progesterone receptor status were assessed using SEER data and coded as: hormone receptor positive, receptor negative, and borderline or unknown status. Breast cancer treatment data were abstracted from medical records and SEER data. Tamoxifen use was abstracted from medical records and self-reported at the baseline and 30-month assessments and coded as: use between baseline and 30-months, use at or before baseline only, or no use. Menopausal status was determined at the 30-month assessment using an algorithm (see[41]), that defined women as pre, post, or unclassifiable menopausal status using age, date of last menstruation, and hysterectomy/oophorectomy status. A comorbidity summary score was generated based on the number of self-reported medical conditions that limited current activities, categorized as 0, 1, or ≥2 conditions.
Analysis Methods
Sleep duration change prevalence and patterns were explored using the average nightly total sleep time variables from the three time points. At each point, survivors' sleep was categorized as undersleeping, oversleeping, or normal sleeping using three methods: 1) total sleep time was compared to norms for women by age[42]; over- and undersleeping were defined as being outside of ± one standard deviation of sleep norms; 2) oversleeping was defined as 8 hours or more per night, and undersleeping as 6 hours or fewer per night, consistent with studies linking these sleep amounts to excess mortality[8–13]; and 3) survivors were classified at the baseline and 30-month follow-up time points by change relative to their pre-diagnosis sleep time. Then, the over-, under-, and normal sleeping categories (as defined relative to norms) were examined across the three study time points to identify patterns of sleep duration change and categorize the sleep change groups.
Unadjusted AVOVA or logistic regression models tested differences among the sleep duration change groups on demographic and clinical characteristics, diet and exercise variables, and weight change. Specific contrasts tested the sleep change groups against the no change group or against the other sleep groups combined when they did not differ from the no change group.
Multivariable linear regression models tested differences among the sleep duration change groups on symptoms (fatigue, fear of recurrence, perceived stress) and HRQOL. Potential confounders (age, education, race/ethnicity, breast cancer stage & treatment type, tamoxifen, menopausal status, comorbidity, and time between diagnosis and symptom/HRQOL measurement) were modeled using backward elimination, with a 10–15% change in the sleep coefficient indicating confounding[43, 44]. Bonferroni-adjusted p-values were used to limit a potentially inflated type-1 error from multiple comparisons (p< .008 for symptoms; p< .006 for HRQOL).
Results
Participant Characteristics
As Table 1 shows, most women in the sample were Non-Hispanic White (80.4%) and the average age at baseline was 56.5 years. Over half of the sample (59.3%) had locally-staged breast cancers; 23% had in situ cancers. The majority of women were treated with surgery and radiation (42.3%) or surgery, radiation, and chemotherapy (21%). At the 30-month follow-up, 76.2% of women were post-menopausal and 54.9% had taken tamoxifen therapy.
Table 1.
Demographic and clinical characteristics of HEAL participants with baseline and 30-month sleep data (N=572).
| Characteristic | N | % |
|---|---|---|
| Baseline Characteristics | ||
| Location | ||
| New Mexico | 413 | 72.2 |
| Western Washington | 159 | 27.8 |
| Age (yr) | ||
| 29–49 | 151 | 26.4 |
| 50–59 | 220 | 38.5 |
| 60–69 | 126 | 22.0 |
| 70+ (mean ± sd) | 75 | 13.1 |
| (56.5 ± 10.6) | ||
| Education | ||
| HS or less | 123 | 21.6 |
| Some college | 195 | 34.1 |
| College grad | 128 | 22.4 |
| Grad school | 125 | 21.9 |
| Race/Ethnicity | ||
| Non-Hispanic White | 460 | 80.4 |
| Black | 1 | 0.2 |
| Hispanic | 88 | 15.4 |
| Other | 23 | 4.0 |
| Stage at diagnosis | ||
| in situ | 133 | 23.2 |
| Local | 339 | 59.3 |
| Regional | 100 | 17.5 |
| Treatment type | ||
| Surgery only | 171 | 29.9 |
| Surgery/Radiation | 242 | 42.3 |
| Surgery/Chemotherapy | 39 | 6.8 |
| Surgery/Radiation/Chemotherapy | 120 | 21.0 |
| Hormone receptor status | ||
| ER+ | 336 | 58.8 |
| ER− | 66 | 11.5 |
| ER borderline or unknown | 170 | 29.7 |
| PR+ | 278 | 48.6 |
| PR− | 113 | 19.8 |
| PR borderline or unknown | 181 | 31.6 |
| Months Since Diagnosis (mean ± sd) | ||
| Diagnosis to Baseline | 6.0 ± 1.7 | |
| Diagnosis to 30-month Follow-up | 29.2 ± 2.6 | |
| Diagnosis to HRQOL Questionnaire | 39.5 ± 6.4 | |
| 30-month Follow-up Assessment Characteristics Menopausal status | ||
| Pre | 106 | 18.6 |
| Post | 436 | 76.2 |
| Unclassifiable | 30 | 5.2 |
| Tamoxifen | ||
| Use between baseline & 30 mo | 264 | 46.2 |
| Use at or before baseline only | 50 | 8.7 |
| No use during study period | 258 | 45.1 |
| Comorbidity Index (# conditions that limit activity) | ||
| 0 | 432 | 75.5 |
| 1 | 101 | 17.7 |
| 2+ | 39 | 6.8 |
| Body Mass Index (mean ± sd) | 27.0 ± 5.6 |
The mean total sleep time was about 7 hours at each time point (SDs=1.2; range for each 3.5–9). At the HRQOL follow-up, mean fatigue scores ranged from 2.4 (behavioral/severity) to 3.8 (cognitive/mood), the mean fear of recurrence score was 16.2, and the mean perceived stress scale score was 8.3. Means on the SF-36 subscales ranged from 54.8 (vitality) to 81.8 (social functioning).
Prevalence of Sleep Duration Change
Figure 1 shows the prevalence of undersleeping and oversleeping at each study time period using the three methods. Across methods, the prevalence of oversleeping was greater at baseline relative to pre-diagnosis values and remained higher at the 30-month follow-up. By comparison to sleep norms, the percent of survivors reporting undersleeping was lower at baseline (25.4%) relative to pre-diagnosis (28.3%), then higher again (27.6%) at 30-months; whereas, the prevalence did not change substantially when undersleeping was defined as ≤ 6 hours per night.
Figure 1.
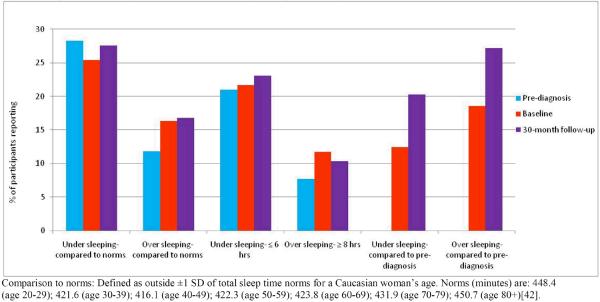
Sleep Duration Change Over Time in HEAL Study Participants (N=572)
Patterns of Sleep Duration Change
Overall, 77% of survivors fell into four distinct sleep duration change patterns (see Figure 2): 1) normal sleeping prior to diagnosis and at baseline, then either under- or oversleeping at the 30-month follow-up (Late-occurring sleep change, n= 80; 14.0%); 2) normal sleep before diagnosis but then either under or oversleeping at baseline that continued at the 30-month follow-up (Sustained sleep change, n= 34; 5.9%); 3) normal sleeping before diagnosis, then either under- or oversleeping at baseline, and normal sleeping again at the 30-month follow-up (Temporary sleep change, n=32; 5.6%); or 4) no change (No change over time, n=292; 51.0%). We defined a fifth group as those participants with sleep duration change patterns that did not fit any of the other groups (other change, n=134; 23%, most involving undersleeping prior to diagnosis).
Figure 2.
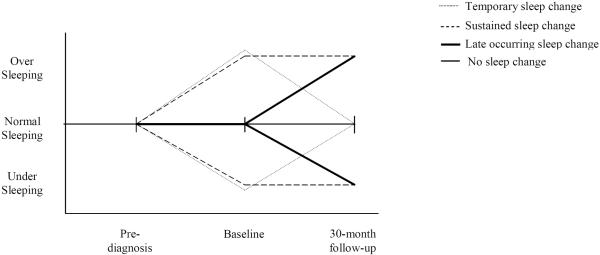
Patterns of Sleep Duration Change Over Time among HEAL participants
Correlates of sleep duration change
Table 2 presents the correlates of sleep duration change groups. Survivors with a temporary or sustained sleep duration change (categories combined since contrasts were similar) were 2.62 times more likely to have been treated with chemotherapy compared to the other sleep groups combined (p<.001). Survivors reporting a sustained sleep change reported greater weight gain after diagnosis compared to survivors in the no change group (2.0 vs. 1.3; p=0.03). Further, survivors with temporary or sustained sleep changes (categories combined since contrasts were similar) were 1.82 times more likely to have gained ≥ 5% of their baseline body weight compared to the other sleep groups combined (p=.04). No other characteristics differed by sleep change group.
Table 2.
Correlates of Sleep Duration Change Patterns
| Late-occurring change (n=80) Mean (SD) | Sustained change (n=34) Mean (SD) | Temporary change (n=32) Mean (SD) | Other change (n=134) Mean (SD) | No change (n=292) Mean (SD) | |
|---|---|---|---|---|---|
| Age | 56.2 (10.5) | 54.9 (10.2) | 56.0 (11.8) | 55.4 (10.2) | 57.3 (10.6) |
| Body Mass Index (kg/m2 at 30-month follow-up) | 28.0 (6.1) | 27.8 (4.8) | 26.8 (5.0) | 27.2 (5.4) | 26.6 (5.6) |
| Sports/recreational physical activity (MET-hours/week) | |||||
| Before diagnosis (retrospective from baseline) | 17.4 (27.5) | 13.2 (16.4) | 19.1 (20.1) | 15.4 (20.2) | 14.0 (18.1) |
| Baseline (6 months post-diagnosis) | 11.8 (19.0) | 8.2 (10.5) | 10.7 (13.5) | 9.8 (17.5) | 10.4 (15.5) |
| 30-month follow-up | 20.1 (34.0) | 14.8 (18.8) | 16.9 (19.3) | 13.3 (17.9) | 12.9 (15.2) |
| Hormone symptoms (0=not at all to 4=extremely severe) | |||||
| Hot Flashes | 2.0 (1.4) | 2.0 (1.4) | 1.9 (1.6) | 1.9 (1.5) | 1.7 (1.4) |
| Night Sweats | 1.4 (1.4) | 1.4 (1.3) | 1.2 (1.1) | 1.4 (1.4) | 1.3 (1.3) |
| Dietary variables (30-month follow-up) | |||||
| Caffeine intake (mg/day) | 169.4 (132.8) | 153.9 (120.2) | 135.7 (148.6) | 139.2 (118.1) | 137.1 (115.7) |
| Alcohol intake (g/day) | 3.9 (7.9) | 8.6 (14.2) | 6.3 (9.2) | 4.1 (8.6) | 5.2 (10.1) |
| Self-reported weight gain problem1 (0=not at all to 4=extremely severe) | 1.6 (1.4) | 2.0 (1.5) | 1.8 (1.3) | 1.7 (1.4) | 1.3 (1.3) |
| Late-occurring change (n=80) N (%) | Sustained change (n=34) N (%) | Temporary change (n=32) N (%) | Other change (n=134) N (%) | No change over time (n=292) N (%) | |
|---|---|---|---|---|---|
| Weight gain (baseline to 30-months)2 | |||||
| Gained ≥5 % of baseline weight | 21 (28.0) | 12 (40.0) | 11 (37.9) | 36 (28.6) | 61 (24.1) |
| Maintained or lost weight | 54 (72.0) | 18 (60.0) | 18 (62.1) | 90 (71.4) | 192 (75.9) |
| 1+ activity-limiting comorbid condition | 13 (16.2) | 11 (32.4) | 8 (25.0) | 33 (24.6) | 75 (25.7) |
| Race/Ethnicity | |||||
| Non-Hispanic White | 60 (75.0) | 26 (76.5) | 28 (87.5) | 107 (79.9) | 239 (81.1) |
| Hispanic/Black/Other | 20 (25.0) | 8 (23.5) | 4 (12.5) | 27 (20.1) | 53 (18.2) |
| Education | |||||
| HS or less | 21 (26.2) | 9 (26.5) | 3 (9.4) | 26 (19.5) | 64 (21.9) |
| Some college | 29 (36.2) | 16 (47.1) | 14 (43.8) | 41 (30.8) | 95 (32.5) |
| College grad | 17 (21.2) | 4 (11.8) | 6 (18.8) | 31 (23.3) | 70 (24.0) |
| Grad school | 13 (16.2) | 5 (14.7) | 9 (28.1) | 35 (26.3) | 63 (21.6) |
| Menopausal status | |||||
| Post | 58 (77.3) | 25 (89.3) | 26 (81.2) | 100 (78.1) | 227 (81.4) |
| Pre | 17 (22.7) | 3 (10.7) | 6 (18.8) | 28 (21.9) | 52 (18.6) |
| Marital Status (at 30-months) | |||||
| Married/living as married | 51 (63.7) | 20 (58.8) | 24 (75.0) | 81 (60.9) | 188 (64.8) |
| Divorced/Separated/Widowed/Single | 29 (36.2) | 14 (41.2) | 8 (25.0) | 52 (39.1) | 102 (35.2) |
| Treatment3 | |||||
| Surgery only | 25 (31.2) | 5 (14.7) | 10 (31.2) | 34 (25.4) | 97 (33.2) |
| Surgery/Radiation | 33 (41.2) | 11 (32.4) | 9 (28.1) | 58 (43.3) | 133 (44.9) |
| Any Chemotherapy | 22 (27.5) | 18 (52.9) | 13 (40.6) | 42 (31.3) | 64 (21.9) |
| Tamoxifen | |||||
| Use between baseline & 30-month follow-up | 41 (51.2) | 14 (41.2) | 16 (50.0) | 60 (44.8) | 133 (45.5) |
| Use at or before baseline only | 9 (11.2) | 5 (14.7) | 3 (9.4) | 11 (8.2) | 22 (7.5) |
| No use | 30 (37.5) | 15 (44.1) | 13 (40.6) | 63 (47.0) | 137 (46.9) |
| Stage | |||||
| in situ | 16 (20.0) | 4 (11.8) | 8 (25.0) | 33 (24.6) | 72 (24.7) |
| Local | 48 (60.0) | 24 (70.6) | 19 (59.4) | 73 (54.5) | 175 (59.9) |
| Regional | 16 (20.0) | 6 (17.6) | 5 (15.6) | 28 (20.9) | 45 (15.4) |
| Hormone-receptor status (ER) | |||||
| ER+ | 45 (56.2) | 23 (67.6) | 15 (46.9) | 84 (62.7) | 169 (57.9) |
| ER− | 8 (10.0) | 7 (20.6) | 4 (12.5) | 14 (10.4) | 33 (11.3) |
| Hormone-receptor status (PR) | |||||
| PR+ | 43 (53.8) | 18 (52.9) | 13 (40.6) | 72 (53.7) | 132 (45.2) |
| PR− | 11 (13.8) | 11 (32.4) | 6 (18.8) | 24 (17.9) | 61 (20.9) |
weight gain in the sustained sleep change group > no sleep change group; p=0.03;
In logistic regression models, temporary or sustained sleep change groups (combined since contrasts were similar) were 1.82 times more likely to have gained ≥5% of their baseline body weight compared to the other sleep groups (combined) (p=.04).
In logistic regression models, temporary or sustained sleep change groups (combined since contrasts were similar) were 2.62 times more likely to have been treated with chemotherapy compared to the other sleep groups (combined) (p<.001).
Relationships between sleep duration change patterns and cancer-related symptoms
Table 3 presents the results of multivariable linear regression models testing differences between the sleep duration change groups on fatigue, fear of recurrence, and stress. The sustained sleep change group reported greater behavioral/severity, affective/meaning, and sensory aspects of fatigue compared to the no change group (β=2.0, 2.3, 1.8 respectively; all p <0.0001). In each of the fatigue models, sleep change group explained only 3–4% of the variance in fatigue score. The sleep groups did not differ in their reports of the cognitive/mood aspects of fatigue, fear of recurrence, or stress.
Table 3.
Associations between sleep duration change patterns and cancer-related symptoms
| Symptom | B | SE | 95% Confidence Limits | P value for contrast |
|---|---|---|---|---|
| Fatigue (Behavioral/severity) | ||||
| Sustained change | 2.0 | 0.5 | (1.1, 2.9) | <.0001 |
| Temporary change | 0.5 | 0.5 | (−0.4, 1.5) | .29 |
| Late-occurring change | −0.05 | 0.3 | (−0.7, 0.6) | .89 |
| Other change | −0.9 | 0.3 | (−0.6, 0.5) | .76 |
| No change | --- | --- | --- | |
| Fatigue (Affective meaning) | ||||
| Sustained change | 2.3 | 0.6 | (1.2, 3.3) | <.0001 |
| Temporary change | 0.8 | 0.6 | (−0.3, 1.9) | .17 |
| Late-occurring change | −0.1 | 0.4 | (−0.9, 0.6) | .74 |
| Other change | −0.03 | 0.3 | (−0.7, 0.6) | .93 |
| No change | --- | --- | --- | |
| Fatigue (Sensory) | ||||
| Sustained change | 1.8 | 0.5 | (0.9, 2.7) | <.0001 |
| Temporary change | 1.1 | 0.5 | (0.2, 2.0) | .02 |
| Late-occurring change | 0.2 | 0.3 | (−0.5, 0.8) | .63 |
| Other change | −0.05 | .3 | (−0.6, 0.5) | .86 |
| No change | --- | --- | --- | |
| Fatigue (Cognitive/mood)a,h | ||||
| Sustained change | 0.9 | 0.4 | (0.2, 1.5) | .01 |
| Temporary change | 0.3 | 0.4 | (−0.4, 1.0) | .40 |
| Late-occurring change | 0.2 | 0.2 | (−0.1, 0.6) | .30 |
| Other change | 0.3 | 0.2 | (−0.1, 0.6) | .19 |
| No change | --- | --- | --- | |
| Fear of recurrencea,g,h,i | ||||
| Sustained change | −0.7 | 0.8 | (−2.3, 0.8) | .36 |
| Temporary change | −0.3 | 0.8 | (−1.9, 1.3) | .71 |
| Late-occurring change | −0.5 | 0.5 | (−1.6, 0.6) | .37 |
| Other change | −0.3 | 0.5 | (−1.2, 0.6) | .56 |
| No change | --- | --- | --- | |
| Perceived stress | ||||
| Sustained change | 1.0 | 0.5 | (−0.2, 2.0) | .05 |
| Temporary change | −0.3 | 0.5 | (−1.3, 0.8) | .59 |
| Late-occurring change | 0.1 | 0.4 | (−0.6, 0.8) | .72 |
| Other change | 0.3 | 0.3 | (−0.3, 0.9) | .29 |
| No change | --- | --- | --- | |
a-iindicate significant confounders:
age,
education,
race/ethnicity,
breast cancer stage and
treatment,
tamoxifen,
menopausal status,
comorbidity,
months between diagnosis and HRQOL follow-up.
Results in boldface type indicate statistical significance adjusted for multiple comparisons: p < 0.008. Fatigue: range 0–10, increasing scores=greater fatigue; Fear of recurrence: range 5–25, increasing scores=greater fear; Perceived stress: range 4–20, increasing scores=greater stress.
Relationships between sleep duration change patterns and HRQOL
Table 4 describes the results of multivariable linear regression models testing differences between the sleep duration change groups on HRQOL subscales. The sustained sleep change group reported lower vitality compared to the no change group (β=−10.8, p < 0.005) and had lower scores on most other SF-36 subscales as well; however, these contrasts did not reach statistical significance. Sleep change explained only 1–2% of the variance in HRQOL scores.
Table 4.
Associations between sleep duration change patterns and health-related quality of life
| SF-36 subscale | B | SE | 95% Confidence Limits | P value for contrast |
|---|---|---|---|---|
| Vitality | ||||
| Sustained change | −10.8 | 3.9 | (−18.4, −3.2) | .005 |
| Temporary change | −4.6 | 4.0 | (−12.5, 3.2) | .25 |
| Late-occurring change | −1.5 | 2.7 | (−6.8, 3.8) | .58 |
| Other change | −0.5 | 2.2 | (−4.9, 3.9) | .83 |
| No change | --- | --- | --- | |
| Social Functioninge | ||||
| Sustained change | −8.3 | 4.0 | (−16.2, −0.3) | .04 |
| Temporary change | 4.4 | 4.1 | (−3.7, 12.5) | .28 |
| Late-occurring change | 0.4 | 2.8 | (−5.0, 5.9) | .88 |
| Other change | 0.2 | 2.0 | (−4.3, 4.7)) | .94 |
| No change | --- | --- | --- | |
| Role-Emotionala | ||||
| Sustained change | −6.6 | 6.5 | (−19.5, 6.4) | .31 |
| Temporary change | 2.1 | 6.8 | (−11.2, 15.4) | .76 |
| Late-occurring change | 1.3 | 4.6 | (−7.7, 10.3) | .77 |
| Other change | −1.2 | 3.8 | (−8.6, 6.3) | .75 |
| No change | --- | --- | --- | |
| Mental Healthe | ||||
| Sustained change | −5.6 | 3.0 | (−11.7, 0.9) | .06 |
| Temporary change | 1.0 | 3.1 | (−5.0, 7.1) | .74 |
| Late-occurring change | −1.3 | 2.1 | (−5.4, 2.9) | .55 |
| Other change | −1.2 | 1.7 | (−4.6, 2.2) | .50 |
| No change | --- | --- | --- | |
| Physical Functioninga,i | ||||
| Sustained change | −6.2 | 4.2 | (−14.5, 2.1) | .15 |
| Temporary change | 2.8 | 4.4 | (−5.7, 11.3) | .52 |
| Late-occurring change | −4.1 | 3.0 | (−9.9, 1.8) | .17 |
| Other change | 1.6 | 2.5 | (−3.3, 6.4) | .53 |
| No change | --- | --- | --- | |
| Role-Physicala | ||||
| Sustained change | −18.4 | 6.8 | (−31.9, −5.0) | .007 |
| Temporary change | −2.5 | 7.0 | (−16.3, 11.3) | .73 |
| Late-occurring change | −5.9 | 4.8 | (−15.3, 3.4) | .21 |
| Other change | −5.7 | 3.9 | (−13.4, 2.1) | .15 |
| No change | --- | --- | --- | |
| Bodily Paina | ||||
| Sustained change | −9.3 | 4.3 | (−17.9, −0.8) | .03 |
| Temporary change | 1.1 | 4.5 | (−7.7, 9.8) | .81 |
| Late-occurring change | 2.2 | 3.0 | (−3.7, 8.1) | .47 |
| Other change | 2.5 | 2.5 | (−2.4, 7.5) | .31 |
| No change | --- | --- | --- | |
| General Healthe | ||||
| Sustained change | −4.8 | 3.9 | (−12.5, 2.9) | .22 |
| Temporary change | 2.0 | 4.0 | (−5.9, 9.9) | .63 |
| Late-occurring change | 1.3 | 2.7 | (−4.1, 6.7) | .62 |
| Other change | 0.4 | 2.3 | (−3.9, 4.9) | .85 |
| No change | --- | --- | --- | |
a-iindicate significant confounders:
age,
education,
race/ethnicity,
breast cancer stage and
treatment,
tamoxifen,
menopausal status,
comorbidity,
months between diagnosis and HRQOL follow-up.
Results in boldface type indicate statistical significance adjusted for multiple comparisons: p < 0.006. SF-36 subscales: range 0–100, increasing scores=better HRQOL
Discussion
This study identified patterns indicating a late-occurring, temporary, or sustained sleep duration change through and beyond breast cancer treatment. To our knowledge, this is the first study to describe longitudinal patterns of sleep duration change in this period. These three patterns mirror patterns of other physical and psychosocial sequelae of cancer that can either be chronic long-term problems or late-occurring effects that emerge after cancer treatment[45]. The temporary sleep change mirrors the proposed trajectory of true sleep disturbance secondary to cancer that likely resolved once the cancer-related stress and acute effects of treatment resolved. Sleep duration change in the late-occurring group appears to be influenced by other stressors in addition to cancer. The sustained sleep duration change group could represent a true comorbid sleep change that developed from the cancer experience but then evolved into a self-sustaining problem.
This study identified characteristics that may increase risk of sleep duration changes or suggest methods to reduce sleep change in survivors. Consistent with prior research, chemotherapy treatment was related to temporary and sustained sleep changes. Prior work identified disruptions in circadian rhythms from chemotherapy[46]. Chemotherapy also may disrupt sleep by increasing menopausal symptoms [3, 20, 21]; however vasomotor symptoms were not related to sleep changes in this cohort. Instead, this study suggests that weight gain after diagnosis may relate to sustained sleep duration changes. Post-diagnosis BMI levels alone did not differ by sleep change group; thus, it appears to be weight gain through and beyond treatment rather than body weight per se that was related to sustained sleep duration changes in this sample. Weight gain may contribute to sustained sleep duration changes by increasing risk of sleep disorders[47, 48] or increasing systemic inflammation [49] which may drive survivors' sleep problems and other symptoms[17, 29, 30]; or, sustained sleep changes may alter hormones leading to weight gain[50]. Alternatively, our findings linking chemotherapy and weight gain to sustained sleep duration changes may be related. Women treated with chemotherapy were 2.6 times more likely to gain ≥ 5% of their baseline body weight than those not treated with chemotherapy (p <.001; data not shown), suggesting that chemotherapy and its associated weight gain may be a potential mechanism of sleep duration change among breast cancer survivors.
Contrary to prior studies of sleep disturbance or quality[3, 4, 16–23], demographic and clinical variables were not different among the sleep duration change groups. It is unknown why demographic and clinical characteristics shown to increase risk of poor sleep quality in cross-sectional studies did not differentiate the longitudinal sleep change groups in this sample. Sleep duration change may not relate to diagnosable sleep problems or sleep quality defined using validated sleep questionnaires used in previous studies. Future studies of sleep in survivors should use a validated measure of sleep problems and objectively monitor sleep time (e.g, actigraphy).
This study adds to the evidence supporting a symptom cluster in breast cancer survivors. Prior cross-sectional studies have linked sleep duration with increased fatigue[25, 26]: these results extend prior work by finding that sustained sleep duration change is associated with fatigue. Although prior work has identified poor mental health as both a predictor of poor sleep and part of a comorbid symptom cluster with sleep problems[5, 16, 17, 19, 51–53], sleep duration change patterns were not related to the mental health symptom scales in this sample. The sleep change patterns may not relate to mental health or to these particular scales in the same way that diagnosable sleep problems or sleep quality do.
Interventions that modify sleep duration have shown improvements in HRQOL among cancer survivors[27, 28], and cross-sectional studies have linked sleep disturbance to poor HRQOL[4, 6, 18]. The current study found that the sustained sleep duration change group had significantly worse vitality and non-significant trends toward worse scores on almost all of the other SF-36 subscales as well. These results should be confirmed by future studies but they suggest that survivors with sustained sleep duration changes may experience the greatest decrements in vitality.
Strengths of this study include a large group of breast cancer survivors recruited through registries, multiple time points of sleep measurement to investigate sleep duration change patterns, and a prospective study design assessing symptoms and HRQOL. Limitations include self-reported recall of total sleep time rather than diagnosable sleep problems or sleep quality, a retrospective measure of pre-diagnosis sleep that is subject to recall bias, limited inclusion of racial minority women, and no adjustment for medication use that might affect sleep because too few survivors were taking such medications. Low levels of mental health symptoms potentially created a floor effect for detecting differences in symptoms. It remains unknown whether sleep change was a cause or consequence of fatigue or HRQOL because HRQOL was only measured once. Further, although the magnitude of fatigue and HRQOL differences observed is clinically meaningful, sleep duration change explained little of the variance in each factor, indicating the need for continued study of other explanatory factors. Finally, many survivors were undersleeping at each time point compared to norms; however, other survivors tended to oversleep at the baseline and 30-month follow-up points compared to their pre-diagnosis amount. It is not known whether these results reflect the effects of cancer on sleep before diagnosis, idiosyncratic responses to treatment or changes in lifestyle factors or medications, or depression in some individuals.
In sum, these results help to describe the overall picture of sleep duration change longitudinally over the early period of breast cancer survivorship. This study demonstrated four distinct patterns of sleep duration change and suggests that survivors reporting sustained sleep change during and after treatment may have greater fatigue and significantly worse vitality than other survivors. Results also suggest that survivors treated with chemotherapy and those who gain weight after diagnosis may represent a group at increased risk for sustained sleep duration changes. Assessment of sleep difficulty is not currently a standard part of survivorship care. Given the problems associated with sustained sleep change in this sample, screening for sleep difficulties should be considered a routine part of clinical care for all cancer survivors.
Acknowledgements
Supported by contracts from the National Cancer Institute: N01-CN-75036-20, N01-CN-05228, N01-PC-67010.
Footnotes
No authors declare conflicts of interest for this manuscript.
References
- 1.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26(5):768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10(6):419–429. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 4.Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24(5):471–480. doi: 10.1016/s0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter JS, Elam JL, Ridner SH, Carney PH, Cherry GJ, Cucullu HL. Sleep, fatigue, and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncol Nurs Forum. 2004;31(3):591–5598. doi: 10.1188/04.onf.591-598. [DOI] [PubMed] [Google Scholar]
- 6.Ancoli-Israel S, Liu L, Marler MR, Parker BA, Jones V, Sadler GR, Dimsdale J, Cohen-Zion M, Fiorentino L. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14(3):201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otte JL, Carpenter JS, Russell KM, Bigatti S, Champion VL. Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. J Pain Symptom Manage. 39(3):535–547. doi: 10.1016/j.jpainsymman.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 9.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: The effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002;3(4):305–314. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 10.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27(1):51–54. [PubMed] [Google Scholar]
- 11.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 12.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8(3):159–174. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Amagai Y, Ishikawa S, Gotoh T, Doi Y, Kayaba K, Nakamura Y, Kajii E. Sleep duration and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol. 2004;14(4):124–128. doi: 10.2188/jea.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne J, Piper B, Rabinowitz I, Zimmerman B. Biomarkers, fatigue, sleep, and depressive symptoms in women with breast cancer: a pilot study. Oncol Nurs Forum. 2006;33(4):775–783. doi: 10.1188/06.ONF.775-783. [DOI] [PubMed] [Google Scholar]
- 15.Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54(9):1309–1321. doi: 10.1016/s0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 16.Sateia MJ, Lang BJ. Sleep and cancer: recent developments. Curr Oncol Rep. 2008;10(4):309–318. doi: 10.1007/s11912-008-0049-0. [DOI] [PubMed] [Google Scholar]
- 17.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 18.Janz NK, Mujahid M, Chung LK, Lantz PM, Hawley ST, Morrow M, Schwartz K, Katz SJ. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health (Larchmt) 2007;16(9):1348–1361. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- 19.Stepanski EJ, Walker MS, Schwartzberg LS, Blakely LJ, Ong JC, Houts AC. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. Journal of Clinical Sleep Medicine. 2009;5(2):132–136. [PMC free article] [PubMed] [Google Scholar]
- 20.Bardwell WA, Profant J, Casden DR, Dimsdale JE, Ancoli-Israel S, Natarajan L, Rock CL, Pierce JP. The relative importance of specific risk factors for insomnia in women treated for early-stage breast cancer. Psychooncology. 2008;17(1):9–18. doi: 10.1002/pon.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couzi RJ, Helzlsouer KJ, Fetting JH. Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol. 1995;13(11):2737–2744. doi: 10.1200/JCO.1995.13.11.2737. [DOI] [PubMed] [Google Scholar]
- 22.Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med. 2007;20(5):434–443. doi: 10.3122/jabfm.2007.05.060225. [DOI] [PubMed] [Google Scholar]
- 23.Savard J, Davidson JR, Ivers H, Quesnel C, Rioux D, Dupere V, Lasnier M, Simard S, Morin CM. The association between nocturnal hot flashes and sleep in breast cancer survivors. J Pain Symptom Manage. 2004;27(6):513–522. doi: 10.1016/j.jpainsymman.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Palesh OG, Collie K, Batiuchok D, Tilston J, Koopman C, Perlis ML, Butler LD, Carlson R, Spiegel D. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75(1):37–44. doi: 10.1016/j.biopsycho.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sela RA, Watanabe S, Nekolaichuk CL. Sleep disturbances in palliative cancer patients attending a pain and symptom control clinic. Palliat Support Care. 2005;3(1):23–31. doi: 10.1017/s1478951505050042. [DOI] [PubMed] [Google Scholar]
- 26.Smets EM, Visser MR, Willems-Groot AF, Garssen B, Oldenburger F, van Tienhoven G, de Haes JC. Fatigue and radiotherapy: (A) experience in patients undergoing treatment. Br J Cancer. 1998;78(7):899–906. doi: 10.1038/bjc.1998.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J Clin Oncol. 2005;23(25):6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 28.Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, Douglas NJ, Engleman HM, Kelly HL, Paul J. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 29.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Jasmin C, Levi F. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11(5):1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 30.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. J Clin Oncol. 2005;23(25):6097–6106. doi: 10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]
- 31.Bowen DJ, Alfano CM, McGregor BA, Kuniyuki A, Bernstein L, Meeske K, Baumgartner KB, Fetherolf J, Reeve BB, Smith AW, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriska AM, Bennett PH. An epidemiological perspective of the relationship between physical activity and NIDDM: from activity assessment to intervention. Diabetes Metab Rev. 1992;8(4):355–372. doi: 10.1002/dmr.5610080404. [DOI] [PubMed] [Google Scholar]
- 33.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 Suppl):S1–205. [PubMed] [Google Scholar]
- 34.Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–684. [PubMed] [Google Scholar]
- 35.Northouse LL. Mastectomy patients and the fear of cancer recurrence. Cancer Nurs. 1981;4(3):213–220. [PubMed] [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 37.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE., Jr. The SF-36 health survey. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd edn. Lippincott-Raven; Philadelphia: 1996. pp. 337–345. [Google Scholar]
- 39.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 40.Ganz PA, Day R, Ware JE, Jr., Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87(18):1372–1382. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 41.Alfano CM, McGregor BA, Kuniyuki A, Reeve BB, Bowen DJ, Baumgartner KB, Bernstein L, Ballard-Barbash R, Malone KE, Ganz PA, et al. Psychometric properties of a tool for measuring hormone-related symptoms in breast cancer survivors. Psychooncology. 2006 doi: 10.1002/pon.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, Bush AJ. Epidemiology of Sleep: Age, Gender, and Ethnicity. Lawrence Erlbaum Associates, Inc; Mahwah, New Jersey: 2004. [Google Scholar]
- 43.Thompson WD. Statistical analysis of case-control studies. Epidemiol Rev. 1994;16(1):33–50. doi: 10.1093/oxfordjournals.epirev.a036143. [DOI] [PubMed] [Google Scholar]
- 44.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 45.Alfano CM, Rowland JH. Recovery issues in cancer survivorship: a new challenge for supportive care. Cancer J. 2006;12(5):432–443. doi: 10.1097/00130404-200609000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2009 doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 47.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40–64 years: a population-based survey. Sleep. 1997;20(1):65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel SR, Blackwell T, Redline S, Ancoli-Israel S, Cauley JA, Hillier TA, Lewis CE, Orwoll ES, Stefanick ML, Taylor BC, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32(12):1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 50.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12(4):289–298. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia: evidence for a symptom cluster in cancer. Semin Oncol Nurs. 2007;23(2):127–135. doi: 10.1016/j.soncn.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Goedendorp MM, Gielissen MF, Verhagen CA, Peters ME, Bleijenberg G. Severe fatigue and related factors in cancer patients before the initiation of treatment. Br J Cancer. 2008;99(9):1408–1414. doi: 10.1038/sj.bjc.6604739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]


