Abstract
The tachykinins, exemplified by substance P, are one of the most intensively studied neuropeptide families. They comprise a series of structurally related peptides that derive from alternate processing of three Tac genes and are expressed throughout the nervous and immune systems. Tachykinins interact with three neurokinin G protein-coupled receptors. The signaling, trafficking, and regulation of neurokinin receptors have also been topics of intense study. Tachykinins participate in important physiological processes in the nervous, immune, gastrointestinal, respiratory, urogenital, and dermal systems, including inflammation, nociception, smooth muscle contractility, epithelial secretion, and proliferation. They contribute to multiple diseases processes, including acute and chronic inflammation and pain, fibrosis, affective and addictive disorders, functional disorders of the intestine and urinary bladder, infection, and cancer. Neurokinin receptor antagonists are selective, potent, and show efficacy in models of disease. In clinical trials there is a singular success: neurokinin 1 receptor antagonists to treat nausea and vomiting. New information about the involvement of tachykinins in infection, fibrosis, and pruritus justifies further trials. A deeper understanding of disease mechanisms is required for the development of more predictive experimental models, and for the design and interpretation of clinical trials. Knowledge of neurokinin receptor structure, and the development of targeting strategies to disrupt disease-relevant subcellular signaling of neurokinin receptors, may refine the next generation of neurokinin receptor antagonists.
I. INTRODUCTION
Substance P (SP), the first member of the tachykinin family of peptides, has been called a “pioneering neuropeptide,” since knowledge gained from studies of tachykinins has informed our understanding of many neuropeptides. Indeed, the discovery of SP as an activity in extracts of horse brain and intestine with effects on intestinal contractility and blood pressure marked the identification of the first of many “brain-gut neuropeptides,” which are present in enteric neurons and enteroendocrine cells as well as in neurons of the brain (341). SP belongs to a large family of structurally related peptides, the tachykinins, that derive from alternative processing of three Tac genes. The tachykinins interact with three neurokinin receptors (NKRs) encoded by three Tacr genes. Knowledge of the structure, function, signaling, and trafficking of these receptors has guided studies of other GPCRs and, in this sense, the NKRs may be considered “pioneering receptors.”
The tachykinins are expressed throughout the nervous and immune systems, regulate an extraordinarily diverse range of physiological processes, and have been implicated in important pathological conditions. The realization that tachykinins mediate pathological processes that underlie important human disorders spurred enormous efforts by the pharmaceutical industry to develop NKR antagonists. These efforts have been highly successful. There are multiple NKR antagonists, with varying degrees of selectivity. Many antagonists are effective in preclinical studies of disease in experimental animals. Some have progressed to clinical trials, where the results have been generally disappointing. At present, there is but a single success: the approval of NK1R antagonists to treat nausea and vomiting after chemotherapy or surgery. However, there are many plausible explanations for these failures, including an inadequate understanding of disease mechanisms, the poor predictive value of animal models, and the inherent redundancy of the tachykinin system. Moreover, new information about the participation of tachykinins in disease processes, and a deeper understanding of the NKRs, has served to maintain interest in this field, and multiple clinical trials are still in progress.
In this review, we discuss the contributions of tachykinins and NKRs to pathophysiological control. We summarize the discovery, structure, and function of tachykinins and their receptors, review their roles in major organ systems (gastrointestinal, respiratory, urogenital, dermal, nervous, immune) and pathological processes (inflammation, pain, cancer), and discuss the successes and failures of NKR antagonists in clinical trials. Throughout, we highlight the challenges of defining functions of tachykinins in health and disease and identify key gaps in our understanding of this system. However, the tachykinin literature is vast, and some aspects are not discussed, including the development of antagonists and an in-depth discussion of tachykinins in the central nervous system (reviewed in Ref. 127).
II. TACHYKININ PEPTIDES AND GENES
A. Overview
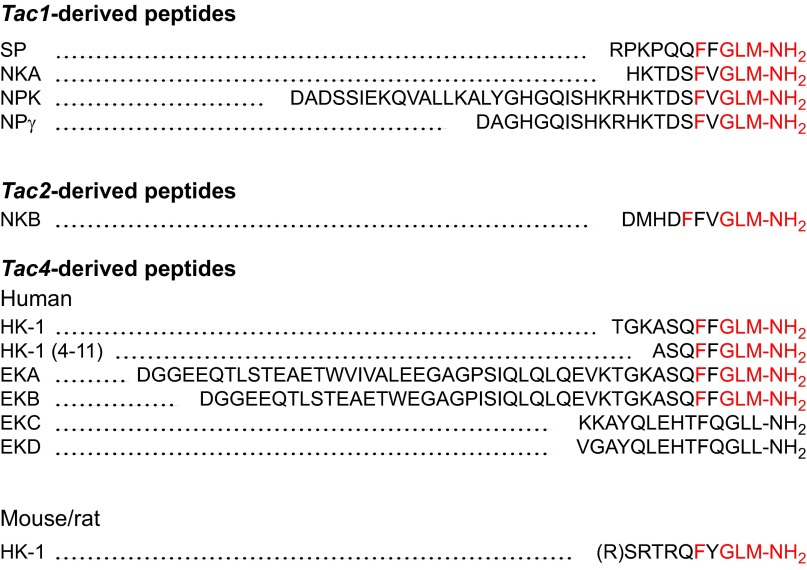
The tachykinins are named for their ability to rapidly stimulate contraction of intestinal muscle, in contrast to the slower acting bradykinins. They possess a conserved COOH-terminal sequence (-Phe-X-Gly-Leu-Met-NH2, X hydrophobic), which is required for receptor activation. The major mammalian tachykinins are SP, neurokinin A (NKA), and neurokinin B (NKB), together with NH2-terminally extended forms of NKA, including neuropeptide K (NPK) and neuropeptide γ (NPγ) (Figure 1). SP, NKA, NKB, and NPK were discovered as biological activities in tissue extracts and were subsequently identified by isolation, sequence, synthesis, and analysis of the Tac genes. Other tachykinins were first identified in the Tac genes, and were subsequently purified from tissues. These include NPγ, hemokinin-1 (HK-1) and the NH2-terminally extended forms of HK-1, endokinin A (EKA), and EKB (Figure 1). Three genes encode the tachykinins: Tac1 (pre-pro-tachykinin-A, Ppt-a), Tac3 (Ppt-b), and Tac4 (Ppt-c) (Figure 2). Tac1 encodes SP, NKA, NPK, and NPγ; Tac3 encodes only NKB; and Tac4 encodes HK-1 and EKA, EKB, EKC, and EKD. Although Tac2 was initially assigned to the gene encoding the NKA precursor, it was subsequently found to be identical to Tac1.
FIGURE 1.
The amino acid sequences of the tachykinins and tachykinin gene-related peptides. Note the presence of the signature tachykinin sequence X-Phe-X-Gly-Leu-Met-NH2 (red) in all of the tachykinins. This sequence is lacking in EKC and EKD, which are not tachykinins although they derive from the Tac4 gene. HK-1 peptides are unique since they differ between mammalian species, whereas other tachykinins are conserved. The existence of an NH2-terminal Arg residue of rat/mouse HK-1 is debatable.
FIGURE 2.

Structures of the human (h) Tac genes and the existence of mRNA splice variants. The peptide products are indicated by the horizontal black bars beneath the mRNA structures. [Adapted from Shimizu et al. (305).]
B. Tac1-Derived Tachykinins
1. SP
In the course of investigating the tissue distribution of acetylcholine, von Euler and Gaddum (341) reported the discovery of an activity in extracts of horse intestine and brain that induced atropine-resistant contraction of isolated rabbit jejunum and fall in blood pressure of anesthetized rabbits (Figure 3A). Extraction of tissues with acid and alcohol allowed the preparation of a water-soluble powder that retained high activity and was called “preparation P” and later “substance P.” Activity was highest in intestinal muscle, which contains enteric nerves, and in the brain, suggesting a neuronal origin. Incubation of extracts with trypsin destroyed biological activity, leading von Euler to conclude that “the active principal is of protein nature.”
FIGURE 3.

Landmark events in the history of tachykinins and neurokinin receptors. A: the discovery of substance P (SP). The kymograph tracings show contractions of the rabbit jejunum in response to acetylcholine (a.c.) (A, C) and SP (P) (B, D). The addition of atropine abolished the effects of acetylcholine (C) but not SP (D). [From von Euler and Gaddum (341). Copyright Wiley, Inc.] B: the isolation of SP. The trace of optical density (O.D.) shows the elution profile of extracts of bovine hypothalami, previously fractionated by gel filtration, from a column of carboxy-methyl cellulose. Fractions were assayed for their ability to stimulate salivary secretion in rats (dashed line). [From Chang and Leeman (60). Copyright American Society of Biochemistry and Molecular Biology.] C: a snake diagram of the human NK1R. D: the structure of aprepitant, the first NK1R antagonist to be approved for treatment of a human disorder (CINV).
Thirty years after its discovery, SP was identified as peptide from an unrelated line of investigation. While purifying corticotrophin-releasing factor from bovine hypothalamus, Leeman (180) identified fractions that stimulated atropine-resistant salivation in rats. This activity, named sialogen, was destroyed by proteases and had an estimated mass of 8,000–10,000 Da. Shortly thereafter, Lembeck (181) reported that preparations of SP also stimulated salivary secretion and suggested that sialogen and SP were the same. Starting with 20 kg of bovine hypothalami, Chang and Leeman isolated 0.15 mg of pure SP and determined its amino acid composition (Figure 3B) (60) and sequence (61), enabling synthesis of SP with the expected biological activity (337).
2. NKA
NKA (substance K, neurokinin α, neuromedin L), which is highly homologous to SP, was identified as an activity in extracts of porcine spinal cord that stimulated contraction of guinea pig ileum (151) (Figure 1). The existence of NKA was predicted from the Tac1 gene structure (233).
3. NPK
NPK, a 36-amino acid peptide with the COOH-terminal sequence of NKA (Figure 1), was isolated from the porcine brain using methods for detecting COOH-terminal amides of bioactive peptides (332). NPK stimulated gall bladder contraction, plasma extravasation, hypotension, and bronchial smooth muscle spasm. The existence of NPK was predicted from the Tac1 gene (233).
4. NPγ
NPγ, a 21-amino acid peptide, was predicted from the rat Tac1 gene (168) (Figure 1). NPγ was subsequently isolated from the rabbit intestine (142). Like NPK, NPγ is an NH2-terminally extended form of NKA, but NPγ lacks residues 3–17 of NPK (Figure 1).
5. Tac1
Two splice variants of Tac1 (αTac1, βTac1) were first cloned from bovine striatum (233) (Figure 2). Both mRNAs encoded SP. The COOH terminus of SP was followed by Gly, which donates the amide group of the COOH-terminal Met, and Gly was followed by a dibasic Lys-Arg processing site. An Arg-Arg sequence formed the NH2-terminal processing site. Whereas αTac1 encoded SP alone, βTac1 encoded an additional peptide with the tachykinin signature sequence (-Phe-X-Gly-Leu-Met-NH2) and appropriate residues for processing. The peptide was named substance K (NKA) due to homology with amphibian kassinin. Tac1 has been cloned from several species, which confirmed existence of αTac1 and βTac1, and identified the γTac1 and δTac1 splice variants (51, 168). These splice variants lack various exons; encode a combination of SP, NKA, NPK, and NPγ (Figure 2); and are differentially expressed in various tissues.
C. Tac3-Derived Tachykinins
1. NKB
NKB (neurokinin β, neuromedin K), which resembles SP and NKA, was isolated from porcine spinal cord as an activity that stimulates contraction of the guinea pig ileum (143) (Figure 1).
2. Tac3
Tac3 was first identified in the bovine as a gene that encoded neuromedin K (NKB) (164). There are two forms of Tac3, both of which encode a single tachykinin, NKB (Figure 2).
D. Tac4-Derived Tachykinins
1. HK and EK
The HKs and EKs were discovered by analysis of the Tac4 gene rather than by extraction and bioassays of tissue extracts (Figure 1) (248, 362).
2. Tac4
The appreciation that tachykinins control the myeloid lineage and regulate lymphoid differentiation led to the search for tachykinins in the hematopoietic system (362). A cDNA was identified that encoded an open reading frame of 128 amino acids, including a stretch of 11 amino acids with tachykinin signature sequence flanked by dibasic processing sites and with a Gly adjacent to the COOH-terminal Met. The gene was designated Ppt-c (Tac4), and the peptide named HK-1 to reflect its presence in hematopoietic cells (Figure 1). Although mouse and rat Tac4 are homologous, the human precursor is truncated to 68 amino acids, and human HK-1 differs from the mouse/rat form within the NH2-terminal region. Thus HK-1 differs from the other tachykinins, which are conserved across mammals. Moreover, the human precursor contains two monobasic cleavage sites and has the potential to generate truncated HK-1(4–11) as well as full-length HK-1. Four splice variants of human Tac4 have been identified (α, β, γ, δ), which are generated from a combination of five exons (Figure 2) (248). These splice variants are capable of encoding other peptides, named endokinins in view of their proposed role in endocrine tissues. EKA and EKB are NH2-terminally extended forms of HK-1 and are true tachykinins. As expected, HK-1, EKA, and EKB have SP-like biological actions and can interact with NKRs (248, 362). EKC and EKD lack the tachykinin sequence, have minimal tachykinin-like actions, and show negligible affinity for the NKRs. They are tachykinin gene-related peptides, not tachykinins.
III. NEUROKININ RECEPTORS AND GENES
A. Overview
The first suggestion of multiple receptors for tachykinins came from comparisons of the potencies of mammalian and nonmammalian tachykinins in bioassays. Cloning of three NKRs (NK1R, Tacr1; NK2R, Tacr2; NK3R, Tacr3) confirmed this proposal, representing a major advance in this field. The Tacr genes possess five exons and four introns, which interrupt the protein coding sequences. They encode GPCRs with seven membrane-spanning domains, three extracellular and intracellular loops, and extracellular NH2 and intracellular COOH termini. The availability of the cloned receptors enabled studies of receptor structure, function, and regulation and facilitated the identification of selective antagonists. However, there is still much to learn about this family of receptors, especially the NK2R and NK3R, which have been less thoroughly studied. Importantly, to our knowledge, the crystal structures of the NKRs have not been reported, which would provide key information about receptor activation and signaling.
B. NKR Structure
1. NK1R
The NK1R (SP receptor) was cloned from a rat brain cDNA library by electrophysiological assessment of receptor expression in Xenopus oocytes and cross-hybridization to bovine NK2R (substance K receptor) (355), which had been cloned earlier (203). A clone of 3,408 nucleotides was identified encoding a GPCR of 407 residues (Figures 3C and 4A). When expressed in monkey kidney COS cells, the clone conferred high-affinity binding for SP, that was displaced by SP, NKA, and NKB (IC50 SP > NKA > NKB). Although there is a high degree of similarity of NK1Rs between different species (94.5% identity between rat and human), differences at key residues can affect interaction with antagonists.
FIGURE 4.
Comparison of regulation and signaling of the full-length and truncated human NK1R. A: the full-length NK1R is phosphorylated by GRKs and PKC within the COOH terminus and interacts with β-arrestins, which mediate desensitization, endocytosis, and endosomal signaling (75). The receptor activates Ca2+, ERK1/2, NF-κB, and PKC-δ and stimulates IL-8 secretion (175). B: the truncated NK1R lacks most of the COOH terminus, is not phosphorylated, and does not interact with β-arrestins. As a result, it is defective in desensitization and endocytosis and does not assemble β-arrestin signalosomes (75, 183). The truncated receptor does not couple robustly to Ca2+ and NF-κB and inhibits PKCδ phosphorylation and IL-8 secretion (175).
A splice variant of the human and guinea pig NK1R missing exon five encodes a COOH-terminally truncated NK1R of 311 residues that lacks most of the intracellular C-tail (Figure 4B) (19, 98). The truncated human receptor has been detected in monocytes and macrophages (173), discrete brain regions (cortex, cerebellum) (172), and colonic epithelial cells of patients with colitis-associated cancer, where the short form is preferentially upregulated (108). Since the truncated receptor signals differently from the full-length NK1R (see sect. IIIC), this differential expression is of probable functional importance.
2. NK2R
The NK2R (substance K receptor) was the first tachykinin receptor to be cloned. Nakanishi's group used the new approach of expression cloning, in which mRNAs from a bovine stomach cDNA library were expressed in Xenopus oocytes (203). From ∼3 × 105 clones, a single clone was identified that conferred an electrophysiological response to NKA. The 2,458-nucleotide sequence encoded a GPCR of 384 residues. Oocytes expressing this clone responded to tachykinins with a potency ranking of NKA>NKB>SP.
3. NK3R
The NK3R (NMB receptor) cDNA was cloned from rat brain by hybridization with bovine NK2R cDNA, and predicted to encode a GPCR of 452 residues (304). When expressed in Xenopus oocytes, the clone conferred electrophysiological responses to tachykinins, and ligand-binding experiments on membranes from NK3R-expressing COS cells revealed the rank order of potency of NKB > NKA > SP.
C. NKR Signaling
Although NKR signaling has been thoroughly studied, many aspects warrant further attention. First, NKR signaling has been studied mostly in cell lines rather than primary cells, where the intricacies of signaling depend on the level of NKR expression, the compliment of signaling proteins, and the cellular environment. Second, most information derives from studies of high-affinity ligands (SP for NK1R). Since agonists can stabilize distinct GPCR conformations that transmit unique signals (biased agonism, reviewed in Ref. 281), individual tachykinins may signal differently via the same NKR, with diverse outcomes. Third, NKR signaling is usually studied by measuring total cellular levels of second messengers, rather than second messenger generation in subcellular compartments. Compartmentalized signaling can also lead to divergent outcomes, which explains how GPCRs that couple to the same G proteins can transduce specific signals (223). Finally, most studies have examined signaling by full-length unmodified receptors. Variants of the same receptor, which can be generated by alternative splicing or by posttranslational mechanisms, can signal by distinctly different mechanisms. This differential signaling may be important in pathophysiological situations where alternative receptor processing can occur.
1. Initiation of NKR signaling
The major proximal pathways that are activated by tachykinins in NKR-transfected Chinese hamster ovary (CHO) and rat kidney epithelial (KNRK) cells include the following: 1) activation of phospholipase C, leading to formation of inositol triphosphate, which mobilizes intracellular stores of Ca2+, and diacylglycerol, which activates protein kinase C (PKC); 2) activation of adenylate cyclase, resulting in accumulation of cAMP and stimulation of protein kinase A (PKA); and 3) activation of phospholipase A2 and generation of arachidonic acid, a precursor of lipid inflammatory mediators (Figure 5). SP-induced activation of the NK1R in human embryonic kidney (HEK293) cells also causes a rapid change in morphology, including the formation of blebs in the plasma membrane, which involves the Rho-associated ROCK system and phosphorylation of the myosin regulatory light chain (212). SP-evoked blebbing is not related to apoptosis, which often accompanies blebbing, but rather to formation of microparticles, a mechanism of intercellular communication that has been implicated in disease (63).
FIGURE 5.

Mechanisms of neurokinin receptor signaling from the plasma membrane. 1: SP activation of the NK1R at the plasma membrane initiates G protein-mediated signaling events that include activation of phospholipase C (PLC), formation of inositol trisphosphate (IP3) and diacylglycerol (DAG), mobilization of intracellular stores of Ca2+, and activation of PKC; activation of adenylyl cyclase (AC), formation of cAMP, and activation of PKA; activation of phospholipase A2 (PLA2), formation of arachidonic acid (AA), and generation of PGs, leukotrienes (LX), and thromboxane A2 (TXA2); and activation of ROCK and phosphorylation of myosin regulatory light chain (MLC). 2: the NK1R also transactivates the EGFR by a mechanism that involves G protein-dependent activation of members of the a disintegrin and metalloproteinase (ADAM) domain-containing proteases that cleave and liberate membrane-tethered EGFR agonists. The EGFR dimerizes, phosphorylates (P), and assembles a SHC/Grb2 complex that leads to activation of ERK1/2. There are many interactions between these pathways, and the precise details of activation vary between cell types. 3: NK1R signaling leads to diverse and sometimes cell type-specific effects that include inflammation, proliferation, anti-apoptosis, neuronal excitation, and migration.
SP signaling has been extensively studied in U373MG human astrocytoma cells, which express endogenous NK1R, and in NK1R-transfected NCM460 human colonocytes. In both cell types, SP and the NK1R transactivate the epidermal growth factor receptor (EGFR), which leads to activation of mitogen-activated protein kinases (MAPK), extracellular signal regulated kinases (ERK) 1 and 2, DNA synthesis, and proliferation (Figure 5) (54, 158). This mechanism partially mediates the ability of the NK1R to promote healing of the inflamed colonic epithelium (52). Mucosal healing also depends on the anti-apoptotic effect of SP, which involves Janus kinase 2 (JAK-2) and phosphoinositide 3-kinase (PI3K)-mediated activation of the anti-apoptotic molecule Akt (protein kinase B) (159). SP also activates Akt in glioblastoma cells (3).
In view of the proinflammatory actions of SP (see sect. IV), NK1R inflammatory signaling has been extensively studied. In U373MG cells, SP and the NK1R activate p38 and generate proinflammatory cytokines, including interleukin (IL)-6 and IL-8 (79, 94). SP activates the master proinflammatory transcription factor nuclear factor kappa B (NF-κB) in NCM460 cells by mechanisms that involve activation of Rho family kinases and PKCδ, leading to formation of IL-6, IL-8 and tumor necrosis factor α (TNF-α) (161, 363). SP induces cyclooxygenase-2 expression and prostaglandin (PG) E2 production in these cells via PKC-θ and JAK3/STAT3/5 pathways (160).
In addition to their role in GPCR desensitization and endocytosis, β-arrestins recruit signaling proteins to internalized GPCRs and mediate sustained signaling from endosomes (223). In NK1R-transfected KNRK cells and dermal endothelial cells that naturally express the NK1R, SP induces the assembly of an endosomal signaling complex (signalosome) comprising NK1R, β-arrestin, Src, MEKK, and ERK1/2 (Figure 6) (75). This complex promotes the nuclear translocation of activated ERK1/2, which is necessary for the proliferative and anti-apoptotic effects of SP. The truncated NK1R lacks phosphorylation sites that are necessary for high-affinity β-arrestin interactions, and thus cannot assemble the complex (Figure 4B). Although protease-activated receptor-2 (PAR2) also assembles β-arrestin-dependent signalosomes, activated ERK1/2 is retained in the cytosol (75). This difference in the subcellular fate of ERK1/2 depends on the affinities of the NK1R and PAR2 for β-arrestins, with PAR2 exhibiting a higher affinity and assembling a more stable complex that retains activated ERK1/2 in the cytosol (251).
FIGURE 6.

Compartmentalized signaling of the NK1R from endosomal membranes. 1: β-Arrestins (β-arrs) recruit the NK1R, Src, MEKK, and ERK1/2 to endosomes and thereby assemble a signalosome that mediates ERK1/2 phosphorylation and activation. β-Arrestin-activated ERK1/2 translocate to the nucleus. 2: Under normal circumstances, ERK1/2 mediate the proliferative and anti-apoptotic actions of SP. 3: When ERK1/2 activation is abnormally sustained, as occurs in cells that lack active ECE-1, ERK1/2 phosphorylate and induce Nur77, which induces cell death.
There are other differences in signaling of the full-length and truncated NK1R. Whereas SP stimulates NF-κB activity and IL-8 expression in cells expressing full-length NK1R, SP stimulation of the truncated receptor inhibits IL-8 expression (Figure 4B) (175). These differences may have implications for tachykinin signaling under pathological conditions where selective upregulation of the truncated receptor is observed (108).
Posttranslational modifications, including phosphorylation (see below) and glycosylation, can influence NK1R signaling. The NK1R has two potential sites for N-linked glycosylation: Asn-14 and Asn-18. The importance of glycosylation has been examined by expressing a mutated human NK1R lacking these sites in NCM460 colonocytes (331). Mutation prevented NK1R glycosylation, accelerated SP-induced NK1R endocytosis, and suppresses SP-stimulated IL-8 secretion (331). Thus glycosylation stabilizes the NK1R in the plasma membrane and controls proinflammatory signaling. Whether NK1R glycosylation is regulated under pathophysiological conditions is unknown.
The endogenous NK1R signals differently in primary neurons (190). Nociceptive dorsal root ganglia (DRG) neurons release SP, which mediates neurogenic inflammation and pain (see sect. IVF). However, SP can also act in a poorly understood autocrine fashion to activate the NK1R on DRG neurons and sensitize transient receptor potential vanilloid 1 (TRPV1), leading to hyperalgesia but not spontaneous pain. Although SP activates PLC in DRG neurons, resulting in PKC-mediated sensitization of TRPV1, it does not robustly elevate intracellular Ca2+. Instead, the NK1R induces a Gi/o-dependent release of reactive oxygen species (ROS), leading to oxidation and activation of M-type K+ channels and consequent neuronal hyperpolarization, which explains why SP does not cause spontaneous pain. Importantly, when overexpressed in neurons, the NK1R induces a robust Ca2+ signal and inhibits M-currents. These results highlight the importance of understanding signaling by endogenous receptors in primary cells.
2. Termination of NKR signaling
GPCR signaling is terminated by mechanisms that remove agonists from the extracellular fluid (reuptake, degradation) and that restrict the capacity of the receptor to couple to signaling machinery (uncoupling, desensitization) (Figure 7).
FIGURE 7.
The regulation and NK1R signaling and trafficking. 1: The cell-surface peptidase neprilysin (NEP) degrades and inactivates SP in the extracellular fluid and thereby limits NK1R activation. 2: The SP-occupied NK1R is a substrate for GRK 2, 3, and 5, which phosphorylate Ser and Thr residues in intracellular loop 3 and the COOH-terminal tail. Phosphorylation increases affinity of the NK1R for β-arrestins (βarrs), which translocate to the plasma membrane, interact with the NK1R, and mediate G protein uncoupling and receptor desensitization. β-Arrestins also recruit protein phosphate 2A (PP2A), which can dephosphorylate and thereby resensitize the cell-surface receptor. 3: β-Arrestins are adaptor proteins for clathrin and AP2 and thereby promote dynamin-dependent endocytosis of the SP and the NK1R. 4: By recruiting the NK1R together with Src, MEKK, and ERK1/2 to endosomes, β-arrestins assemble signalosomes that allow the endocytosed NK1R to continue to signal. Rab5a mediates trafficking to early endosomes. 5: The NK1R can rapidly recycle from superficially located endosomes by rab4a- and rab11a-dependent mechanisms. 6: Alternatively, the NK1R traffics to endosomes in a perinuclear location that contain ECE-1. ECE-1 degrades SP in acidified endosomes and thereby destabilizes the SP/NK1R/β-arrestin/Src/MEKK and ERK1/2 signaling complex. 7: The NK1R then slowly recycles, which also mediate resensitization (8). 9: After sustained stimulation with high concentrations of SP, the NK1R is ubiquitinated and traffics to lysosomes, where degradation downregulates SP signaling.
Neprilysin is a cell-surface metalloendopeptidase that degrades SP in the extracellular fluid and thereby terminates NK1R activation. Neprilysin disruption suppresses SP degradation and causes widespread NK1R-dependent plasma extravasation (195). Neprilysin-deficient mice are more susceptible to SP-dependent inflammation of the intestine (24, 325) and skin (296), which illustrates the importance of this mechanism of restricting SP signaling.
After stimulation with SP, subsequent responses usually fade (desensitize) and then recover (resensitize). G protein-coupled receptor kinases (GRKs) and β-arrestins mediate desensitization of the NK1R (Figure 7). GRK2, GRK3, and GRK5 interact with and can phosphorylate the NK1R (23, 141, 170). The NK1R is extensively phosphorylated, which promotes high-affinity interactions with β-arrestins at the plasma membrane and in endosomes. β-Arrestins sterically uncouple the NK1R from G proteins and desensitize G protein-mediated signaling. The truncated NK1R is resistant to desensitization because it lacks GRK phosphorylation sites and does not interact with β-arrestins (183).
Mechanisms that control NK1R signaling from plasma membranes (SP degradation, NK1R interaction with β-arrestins) similarly control NK1R signaling from endosomal membranes (Figure 7). Endothelin-converting enzyme-1 (ECE-1) is a membrane metalloendopeptidase that degrades SP in endosomes and thereby disassembles the SP/NK1R/β-arrestin/Src signalosome, which attenuates ERK1/2 signaling (68, 259, 285). The importance of this mechanism is illustrated by the finding that ECE-1 inhibition causes sustained SP-induced ERK1/2 activation, leading to activation of the death receptor Nur77 and neurotoxicity (Figure 6) (68).
Resensitization of SP responses requires NK1R endocytosis, dissociation of β-arrestins, and NK1R recycling (see sect. IIID). However, after stimulation with SP, a substantial proportion of desensitized NK1Rs remain at the plasma membrane, where they resensitize by a mechanism that involves recruitment of protein phosphatase 2A, a β-arrestin binding partner that can dephosphorylate and resensitize the NK1R (Figure 7) (224).
D. NKR Trafficking
NK1R endocytosis is widely used to detect sites of SP release and receptor activation in the nervous system. Although endocytosis is also a key component of signal transduction (223, 342), the importance of NK1R trafficking for signaling in the nervous system is not fully understood.
1. Pathways of NKR trafficking
In NK1R-transfected KNRK and HEK293 cells, SP stimulates rapid NK1R endocytosis (Figure 7) (103, 110, 210). However, the fate of the internalized NK1R depends on the stimulation conditions (286). After brief exposure to low concentrations of SP, the NK1R traffics to rab5a-positive endosomes. Endosomal acidification dissociates SP from the NK1R, SP is degraded in endosomes by ECE-1, and the NK1R recycles (259, 285). After sustained incubation with high SP concentrations, as may occur during inflammation, the NK1R is ubiquitinated and degraded (67). Brief stimulation with SP evokes NK1R endocytosis and recycling in myenteric (109, 316) and spinal (202) neurons.
SP also stimulates NK1R trafficking in vivo. In rats, SP stimulates endocytosis and recycling of the NK1R in endothelial cells of tracheal postcapillary venules, which coincides with desensitization and resensitization of plasma extravasation (41). Stimuli that promote SP release from the central projections of DRG neurons in the dorsal horn of the spinal cord, including electrical stimulation of dorsal roots and intraplantar injection of the TRPV1 agonist capsaicin, trigger NK1R endocytosis in neurons in superficial laminae of the dorsal horn (199, 202). Stroking of the mucosa of the guinea pig ileum evokes NK1R endocytosis in myenteric neurons, which suggests that stimulation of enteric afferent mechanoreceptors promotes SP release and NK1R activation (317). Intestinal inflammation causes NK1R endocytosis in myenteric and spinal neurons, which reflects sustained release of SP from primary spinal afferent neurons in the inflamed intestine and dorsal horn (198, 361). Thus NK1R endocytosis occurs under pathophysiological conditions associated with SP release. The endocytosed NK1R has been used to deliver toxins, allowing ablation of NK1R-expressing spinal neurons and determination of their contribution to nociception (200). However, despite the extensive studies of NK1R endocytosis in the nervous system, the functional importance of this trafficking is unknown.
Like the NK1R, the NK2R internalizes and recycles (102). However, in neurons of the paraventricular hypothalamic nucleus, endogenous NKB stimulates internalization and trafficking of the NK3R to the nucleus (136). Importins mediate nuclear trafficking of the NK3R, which then associates with transcriptionally active chromatin and thereby controls gene transcription (97, 137).
2. Mechanism and function of NKR trafficking
The NK1R internalizes in cell lines and neurons by a clathrin-dependent mechanism that requires dynamin, rab5a, and β-arrestins (Figure 7) (109, 110, 210, 259, 294). Rab5a also mediates NK1R trafficking to endosomes in a perinuclear location, and rab4a and rab11a mediate NK1R recycling (286, 294). The NK1R has also been detected in lipid rafts of HEK293 cells, suggesting the existence of non-clathrin-dependent trafficking mechanisms (169).
Endocytosis does not mediate NK1R or NK2R desensitization (102), which instead depends on β-arrestins. However, NK1R resensitization requires endocytosis, intracellular processing, and recycling. Thus disruption of the mechanisms of NK1R endocytosis (294) or recycling (286) blocks the resensitization of SP-induced Ca2+ signals. By degrading SP in endosomes, ECE-1 disassembles the NK1R/β-arrestin signalosome, which allows the receptor, freed from β-arrestins, to recycle and resensitize (259, 285). By preventing the NK1R resensitization, ECE-1 inhibitors attenuate SP-stimulated plasma extravasation, which illustrates the importance of NK1R recycling for sustained inflammatory signaling of SP (55).
The NK1R can regulate trafficking and signaling of other GPCRs by mechanisms that include competition of receptors for β-arrestins and physical interactions between receptors. In KNRK cells and enteric neurons, the activated NK1R sequesters β-arrestins in endosomes and thereby impedes β-arrestin-dependent endocytosis and desensitization of the NK3R, which has a low affinity for β-arrestins (293). The activated NK1R similarly blocks agonist-stimulated trafficking of the μ-opioid receptor (356). The NK1R and μ-opioid receptor can also form heterodimers in HEK293 cells, where NK1R activation promotes μ-opioid receptor endocytosis, and delays μ-opioid receptor resensitization, presumably by causing its prolonged retention in endosomes with β-arrestins (261). Whether these complexes assemble in primary neurons is unclear. However, studies in living cells exclude the existence of NK1R homodimers or oligomers of the NK1R and suggest that NK1Rs concentrate in microdomains at the plasma membrane (213).
Conversely, other receptors can control NK1R trafficking. Transforming growth factor-β (TGF-β) delays SP-stimulated endocytosis of the NK1R in T cells of the inflamed intestine (30). Although the mechanism of this effect is unknown, it may be relevant to inflammation because TGF-β amplifies SP-induced inflammatory signaling, and the combination of TGF-β and SP stimulates release of interferon-γ and IL-17 from intestinal inflammatory T cells, whereas either agonist alone has no effect.
E. NKR Expression
The NKR system is remarkably plastic. Alterations in receptor expression influence responses to tachykinins and contribute to pathology, with implications for therapy with antagonists.
1. NK1R
The NK1R is upregulated in the inflamed organs, including in the intestine of patients with inflammatory bowel disease (IBD) (282), in the pancreas of mice with acute pancreatitis (33), and in mesenteric fat of mice with colitis (144), which may exacerbate the inflammatory effects of SP. However, the NK1R is also upregulated in noninflammatory states, including in spinal neurons of rats during chronic stress (43) and the cingulate cortex of HIV-positive patients (84).
NF-κB is a major regulator of NK1R expression during inflammation. A putative NF-κB binding site was noted in the promoter of the human NK1R gene (327) and was subsequently confirmed experimentally (307). NF-κB mediates IL-12- and IL-18-induced NK1R transcription in splenic T cells (347), IL-1β-stimulated NK1R expression in astrocytes (117), and expression of the truncated NK1R in breast cancer cells (275). SP and the NK1R can in turn activate NF-κB, leading to transcription of proinflammatory genes (26, 161).
The NK1R gene promoter contains putative binding sites for additional transcription factors, including AP-1, Sp1, and Oct-2, which regulate many inflammatory genes (327). Leukemia inhibitory factor, a cytokine of the IL-6 family, promotes NK1R expression in airway epithelial cells by JAK/STAT and MAPK/ERK pathways (128), and JNK upregulates the NK1R in acinar cells during pancreatitis (154). Notably, SP activates the JAK/STAT pathway in colonic epithelial cells leading to PGE2 secretion (160).
2. NK2R
The NK2R is upregulated in the ileum of rats with acute necrotizing enterocolitis (303), and in inflammatory cells in the colon of patients with IBD (282), which may explain the anti-inflammatory effects of NK2R antagonists. In fibroblasts, TGF-β1 and IL-1α stimulate NK2R expression, whereas IL-3 and granulocyte-macrophage colony stimulating factor (GM-CSF) have the opposite effect (21). Two p53 consensus sequences in the NK2R promoter are critical for the suppressive effects of NKA on the proliferation of hematopoietic progenitor cells (340).
3. NK3R
There is a complex pattern of NK3R regulation during inflammation. Whereas carrageenan-induced peripheral inflammation reduces responsiveness of spinal dorsal horn neurons to a NK3R agonist (2), intraplantar injection of Formalin or adjuvant upregulates NK3R mRNA expression in the spinal dorsal horn (209).
Alterations in the expression of the NK3R and NKB in the uterus during pregnancy point to important roles in reproduction and reproductive disorders. In the rat placenta, normal late pregnancy is associated with downregulation of NKB and NK3R (249). However, there are marked increases in the circulating concentrations of NKB in individuals with pre-eclampsia, NKB and NK3R levels in the placentas of women with pre-eclampsia are elevated, and NK3R expression in umbilical vein endothelial cells is upregulated in severe pre-eclampsia (193, 250). These results point to a major role of NKB and the NK3R in hypertension and pre-eclampsia, where NK3R blockade is a possible therapy.
Genetic studies suggest an important role for NKB and the NK3R in the hypothalamic-pituitary-gonadal axis in humans. A search for genetic associations that may cause isolated hypogonadotropic hypogonadism identified four pedigrees of Turkish descent with severe congenital gonadotropin deficiency and pubertal failure, with all affected subjects homozygous for loss-of-function mutations in the NKB or NK3R genes (335). A study of a larger and more ethnically diverse population revealed that mutations of NKB and NK3R occur in ∼5% of a normosmic isolated hypogonadotropic hypogonadism population (107). Thus NKB and NK3R may be critical regulators of gonadal function.
IV. PATHOPHYSIOLOGICAL FUNCTIONS OF TACHYKININS
A. Overview
Although knowledge of the localization of tachykinins and NKRs and the availability of selective antagonists and mice lacking Tac and Tacr genes have contributed enormously to our understanding, there are formidable challenges to defining the pathophysiological functions of tachykinins. The observation that exogenous tachykinins exert an effect does not imply that endogenous tachykinins have the same actions. Altered tachykinin and NKR expression in disease, and the inherent redundancy of the tachykinin system, with multiple peptides and receptors, can complicate interpretations. There are obvious limitations in extrapolating findings from inbred rodents under controlled conditions to diverse human subjects, and experimental animal models rarely recapitulate human diseases, especially those of unknown etiology. Despite these caveats, there is a wealth of information about the pathophysiological functions of tachykinins. This section discusses the functions of tachykinins in major organs systems (gastrointestinal, respiratory, urogenital, dermal, nervous, immune) and in important pathological processes (inflammation, pain, cancer).
B. Gastrointestinal Tract
The localization and function of tachykinins in the gastrointestinal tract have been reviewed (305). Although tachykinins are found in intestinal immune and enterochromaffin cells, the major sources in the gut are enteric neurons, followed by nerve fibers from dorsal root and vagal ganglia. Tachykinin-containing fibers surround enteric ganglia, ramify through muscle, form a perivascular mesh around submucosal arteries, and supply the mucosa. These fibers are in close proximity to cells expressing NKRs. The NK1R is expressed by enteric neurons, interstitial cells of Cajal, epithelial cells, and lymphocytes and macrophages of the lamina propria; the NK2R is expressed by myocytes, neuronal varicosities, and epithelial cells; and the NK3R is mostly neuronal. The locations of tachykinins and NKRs are consistent with the regulation of neuro-neuronal transmission, motility, secretion, inflammation, and pain.
1. Neuro-neuronal transmission
Evidence for neuro-neuronal transmission, whereby neuronal tachykinins activate NKRs on enteric neurons, derives from studies of receptor trafficking and synaptic transmission. Stimulation of enteric nerves induces endocytosis of the NK1R in myenteric neurons, which requires neuronal conduction, SP and NKA release, and NK1R activation (109, 316). Tachykinins generate slow excitatory postsynaptic potentials in enteric neurons by activating NK1R and NK3R (5).
2. Motility
SP and NKA mediate excitatory transmission via NK1Rs on interstitial cells of Cajal and NK2Rs on smooth muscle (305). In interstitial cells of Cajal, SP activates a nonselective cation channel that controls pacemaker functions (69) and a Na+-leak channel that mediates depolarization (149). Given the importance of interstitial cells of Cajal in motility, targeting these mechanisms could be treatment of motility disorders. HK-1, which is produced by immune cells, contracts circular muscle of mouse colon primarily by activating the neuronal NK1R, with a minor contribution of NK2Rs on myocytes (155). However, in segments of human colon, SP, NKA, and NKB all stimulate contraction of circular muscle by activating the NK2R on colonic myocytes (228).
Mechanical stimulation of the mucosa evokes release of SP and NKA, which partly mediate ascending contraction of peristalsis by NK1R- and NK2R-dependent mechanisms (76). Glial cell line-derived neurotrophic factor augments SP release to enhances its contractile actions (114). Given the role of SP in peristalsis, defects in tachykinin innervation could contribute to abnormal motility. There is a reduced density of SP-positive fibers in colonic circular muscle of children with slow transit constipation, which could explain the dysmotility (152). Conversely, the density of tachykinin-positive nerve fibers is increased in ileal myenteric ganglia of diabetic guinea pigs, which correlates with enhanced release of acetylcholine and tachykinins, and increased sensitivity of smooth muscle (58).
3. Secretion
Tachykinins stimulate electrolyte and fluid secretion from the intestinal epithelium by activating NKRs on epithelial cells, enteric neurons, or immune cells. This mechanism facilitates propulsion and mediates protective secretory responses to infection. Cryptosporidiosis is a diarrheal disorder caused by the protozoan parasite Cryptosporidium parvum. Although self-limiting in healthy subjects, diarrhea can be severe in immunocompromised patients. SP and the NK1R are upregulated in the jejunal mucosa of macaques infected with C. parvum and mediate increased Cl− secretion and glucose malabsorption (124). Thus NK1R antagonists may be useful to treat the symptoms of cryptosporidiosis and other infections of the intestine.
4. Inflammation
A wealth of evidence implicates tachykinins in intestinal inflammation, including plasma extravasation, granulocyte influx, generation of proinflammatory cytokines, and tissue damage (Figure 8). Inflammation correlates with NK1R activation (see sect. IIID) and upregulation (see sect. IIIE), and SP and the NK1R activate proinflammatory signaling pathways in colonocytes (see sect. IIIC). NK1R blockade or deletion abrogates intestinal inflammation induced by Clostridium difficile toxin A (53), TNBS (80), and piroxicam in IL-10 knockout mice (348). Tachykinins also participate in the inflammatory responses to infection, including formation of granulomas, sites of chronic inflammation that prevent spread of infectious agents (346). Together, these findings suggest a role of NKR antagonists in intestinal inflammatory diseases (see sect. VD).
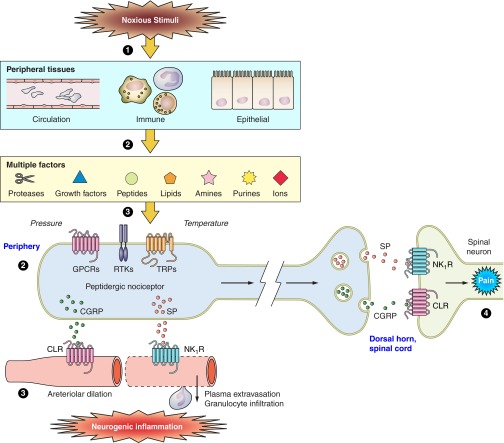
FIGURE 8.
Contributions of tachykinins and neurokinin receptors to neurogenic inflammation and pain. 1: Noxious stimulation of peripheral tissues leads to the release or generation of multiple factors that derive from the circulation, immune cells, and epithelial tissues. These can include proteases (e.g., mast cell tryptase), growth factors (NGF), peptides (bradykinin), lipids (prostaglandins), amines (5-hydroxytryptamine), purines (ATP), ions (protons), pressure, and elevated temperature. 2: These factors can activate several classes of receptors and channels expressed by peptidergic nociceptors, including GPCRs, TRP channels, and receptor tyrosine kinases (RTKs). 3: Activated nociceptors release neuropeptides in peripheral tissues, including SP and NKA, which stimulate NK1Rs on endothelial cells of postcapillary venules and cause plasma extravasation and granulocyte infiltration, and CGRP, which stimulates the calcitonin receptor-like receptor (CLR) on arterioles to cause hyperemia. Together, these changes constitute neurogenic inflammation. 4: If the factors excite nociceptors and generate action potentials, SP and CGRP are also released from the central projections of nociceptors in superficial laminae of the spinal cord dorsal horn, where neuropeptides activate receptors on spinal neurons to transmit painful stimuli centrally.
SP and the NK1R also contribute to the aftermath of inflammation, including fibrosis and healing. The NK1R is expressed by fibroblasts in the chronically inflamed mouse colon and in tissues from patients with Crohn's disease, where SP stimulates collagen synthesis (156). SP also protects colonocytes from apoptosis (159) and promotes expression of cysteine-rich angiogenic inducer 61 in the colonic epithelium, which contributes to healing (157). Consistent with these effects, NK1R deletion hampers healing of the mucosa after chronic colitis (52). Thus SP and the NK1R have dual roles in intestinal inflammation, orchestrating inflammation yet mediating repair. The potential beneficial effects of NK1R antagonists in IBD could, therefore, be offset by disrupted healing of inflamed tissues.
Tachykinins mediate intestinal inflammation induced by activation of TRP channels of primary spinal afferent neurons. The TRPV1 agonist capsaicin induces SP release and NK1R-mediated plasma extravasation in the mouse intestine (96). Components of the inflammatory soup that activate TRP ankyrin 1 (TRPA1) include 4-hydroxy-2-nonenal, formed when ROS peroxidate membrane phospholipids (338), and cyclopentenone metabolites of PGD and PGE (204). TRPA1 is expressed by primary sensory nerves innervating the intestine and pancreas, where activation promotes the release of SP and inflammation (56, 59). TNBS, commonly used to evoke inflammation, covalently binds to and activates TRPA1, which stimulates SP release and neurogenic inflammation in the colon (88). Thus TRPA1 antagonists could be a new therapy for colitis.
Mesenteric fat accumulates at sites of intestinal inflammation. In human mesenteric preadipocytes, SP induces NK1R-dependent proinflammatory signals and has proliferative and anti-apoptotic actions that could contribute to development of the mesenteric fat that is characteristic of Crohn's disease (144, 146). SP activation of the NK1R in adipose tissues may mediate pathologies that are associated with obesity, including glucose intolerance and insulin resistance, and NK1R antagonism has anti-obesity effects in mice (145).
Fibrous adhesions within the abdominal cavity invariably occur after surgical manipulation of the intestine. Although usually benign, adhesions can impede transit, lead to visceral pain and female infertility, and complicate further surgery. In line with the proinflammatory actions of SP, NK1R activation during surgical manipulation of the abdominal contents promotes formation of adhesions by limiting fibrinolytic activity, which allows fibrinous adhesions to persist (279). The NK1R antagonist aprepitant reduces adhesion formation in rats, supporting its therapeutic potential (188).
5. Pain
Colitis induces NK1R endocytosis in spinal neurons and nocifensive behavior that are blocked by intrathecal NK1R antagonist, consistent with SP release in the dorsal horn and NK1R activation on spinal neurons that transmit pain (361) (Figure 8) (see sect. IIID). The NK1R may also be activated in pain-processing areas of the brain of patients with IBD and irritable bowel syndrome (IBS) (135). A PET analysis of binding of the NK1R-selective ligand [18F]SPA-RQ revealed reduced NK1R binding in cortical and subcortical regions in patients with IBD and IBS. Several processes may account for decreased binding of the NK1R ligand, including release of endogenous SP and activation of the NK1R, displacement of the ligand by endogenous SP, or reduced expression of the receptor. Whatever the mechanism, the results suggest a role for the NK1R in the human brain during pain.
Stress-evoked activation of the NK1R in the spinal cord and intestine contribute to hyperalgesia and motility disorders. The chronic psychological stress of water avoidance in rats upregulates NK1R expression in neurons of superficial laminae in the spinal cord, and induces an NK1R-dependent visceromotor reflex to colorectal distention (42). Spinal microglial cells, p38 MAPK, and NF-κB contribute to these stress-induced changes. Thus water avoidance stress in rats activates spinal microglial cells, as determined by phosphorylation of p38 MAPK in microglial cells in laminae I and II of the spinal cord (43). Intrathecal administration of minocycline (inhibitor of microglial cell activation) suppressed stress-evoked p38 phosphorylation, NF-κB activation, and NK1R upregulation, whereas intrathecal SB203580 (inhibitor of p38 MAPK) blocked stress-evoked NF-κB activation but not NK1R upregulation. Notably, both minocycline and SB203580 suppressed the visceral hyperalgesia in stressed rats. Considered together, these results support a major role for spinal microglial cells and p38 MAPK in NK1R-dependent visceral hyperalgesia (43). Whether activated microglial cells release cytokines that upregulate the NK1R on spinal neurons and cause central sensitization requires further investigation.
C. Respiratory Tract
The contributions of tachykinins to inflammation, hyperreactivity, and secretion of the respiratory system have been reviewed (231). This section reviews recent insights into the debatable importance of tachykinins in the airways.
1. Neurogenic inflammation and airway hyperreactivity
There is a sparse innervation of the airways by peptidergic C- and Aδ-fibers, although nerve terminals containing SP and NKA supply the vasculature, smooth muscle, epithelium, and secretory glands. Although neuropeptide-containing sensory neurons innervating the nose and larynx contribute to sneezing and cough, it is the proinflammatory actions of SP and NKA that provided a major impetus for the development of NKR antagonists to treat inflammatory diseases of the airways.
As in many tissues, SP/NKA stimulate plasma extravasation and granulocyte infiltration in the airways by activating the NK1R in endothelial cells of postcapillary venules. Tachykinins also stimulate secretion from airway seromucous glands (283). Although these neurogenic inflammatory effects of tachykinins occur in the airways of most species, there are marked interspecies differences in the ability of tachykinins to alter tracheo-bronchial smooth muscle tone. In the isolated guinea pig and human bronchus, exogenous SP and NKA produce contraction that is mostly mediated by the NK2R and partly by NK1R, which are coexpressed by myocytes (7, 95). In guinea pigs, SP and NKA also induce relaxation due to activation of epithelial NK1R and release of nitric oxide and prostaglandins, although this effect is masked by the contractile component (95). However, in rats and mice, where the contractile response is absent because NKRs are not expressed by airway smooth muscle, NK1R-mediated epithelium-dependent relaxation prevails (201). After two decades of intense study, a role for endogenous tachykinins and the NK1R in airway hyperresponsiveness in allergen-stimulated mice has been proposed, providing evidence for a major role of SP and the NK1R in allergic hyperreactivity of the airways (123).
In the isolated guinea pig bronchus, electrical field stimulation (EFS) or capsaicin causes nonadrenergic and noncholinergic (NANC) bronchoconstriction that is mediated by tachykinins from sensory nerve endings. However, the situation is completely different in mice and rats, where EFS or capsaicin provoke an epithelium- and tachykinin-dependent bronchodilatation (106). Moreover, in the isolated human bronchus, there is no evidence that capsaicin affects motor functions or that EFS stimulates a NANC contractile and tachykinin-mediated effect. Although inhaled capsaicin causes cough in healthy human subjects that is exaggerated in asthmatic patients (318), there is no evidence that endogenous tachykinins cause bronchoconstriction in humans.
Despite this lack of evidence, the robust observation that exogenous SP and NKA constrict the isolated human bronchus has strengthened the proposal that NKR antagonists are a therapy for asthma and chronic obstructive pulmonary diseases (COPD) (see sect. VE). However, the finding that exogenous tachykinins cause bronchoconstriction does not imply that endogenous tachykinins have the same effect. The failure of endogenous tachykinins to increase bronchomotor tone in humans may be due to inadequate release of tachykinins, release from nerve fibers supplying cells that are distant from myocytes, or release from immune cells that are resistant to EFS and capsaicin. Whatever the explanation, the findings obtained by using exogenous tachykinins continue to confound our comprehension of the role of endogenous tachykinins in airway diseases.
2. TRP channels
A major advance in our understanding of neurogenic mechanisms of acute inflammation was provided by the report that α,β-unsaturated aldehydes, which are found in cigarette smoke, can activate TRPA1 on peptidergic primary sensory neurons of rodent airways (11). Activated TRPA1 triggers the release of SP and NKA that mediate neurogenic inflammation in response to inhaled cigarette smoke. These results are corroborated by the observations that other components of cigarette smoke, including acetaldehyde and nicotine, can also stimulate TRPA1 (22, 330). Indeed, TRPA1 is a major target of reactive oxygen, nitrogen, and carbonyl species. This unique sensitivity, coupled to the selective expression of TRPA1 by peptidergic nociceptors, suggests that TRPA1 is a neuronal sensor of oxidative stress. Oxidative stress is not only increased in the respiratory tract by environmental agents, such as cigarette smoke, but also occurs at sites of inflammation during the development of asthma (38) and COPD (273). In ovalbumin-sensitized mice, ovalbumin challenge induces airway hyperresponsiveness and inflammation that is blunted by deletion of TRPA1 (48). Thus TRPA1 is a neuronal sensor for factors that contribute to asthma and COPD, where activation triggers the release of neuropeptides, including tachykinins, that mediate neurogenic inflammation.
When inhaled, certain volatile anesthetics, including isoflurane and desflurane, induce airway irritation, inflammation and cough that can precipitate laryngospasm during anesthesia. TRPA1 has been implicated in these life-threatening adverse reactions, as it mediates the irritant and inflammatory effects of isoflurane and desflurane (87, 207, 292). NK1R and NK2R antagonists block these inflammatory effects, which depend on TRPA1-dependent release of tachykinins from primary afferent neurons in the airways (87, 207, 292).
The increased prevalence of asthma in infants and children in the last 50 years is a mystery that cannot be explained by the hygiene hypothesis. However, large epidemiological studies have identified an association between the increased prevalence of asthma with the growing use of acetaminophen in pregnant women, infants, and children (28). Although the pathway that links acetaminophen use with asthma is unknown, the N-acetyl-p-benzo-quinoneimine metabolite of acetaminophen can activate TRPA1 by virtue of its electrophilic nature, thereby provoking neurogenic inflammation of the airways (230). Although clinical doses of acetaminophen may promote moderate and reversible neurogenic inflammation of the airways, repeated use, especially in susceptible individuals with reduced levels of the endogenous ROS scavenger glutathione, may favor the development of the asthmatic phenotype (230). Of relevance for this hypothesis is the suggestion that the ability of N-acetyl-p-benzo-quinoneimine to stimulate and desensitize TRPA1 is the major mechanism for the analgesic action of acetaminophen (10).
In addition to the established pathway by which reactive molecules initiate tachykinin-mediated neurogenic inflammation in the airways via TRPA1, SP released from sensory nerve terminals increases ROS generation by a NK1R-dependent mechanism. Thus NK1R antagonism reduces ROS formation, epithelial damage, and subsequent remodeling in allergen-challenged guinea pigs (319), and SP induces NK1R-dependent neurogenic inflammation, oxidative stress, and proinflammatory responses in rats (185). Given the role of oxidative stress in thermal injury (221), it is of interest that an NK1R antagonist prevents pulmonary inflammation evoked by local burn injury (311).
Tachykinins may also participate in abnormalities of cough. Coughing subjects have elevated levels of calcitonin gene-related peptide (CGRP) and SP in nasal secretions (187). TRPA1 may contribute to abnormal cough since agonists cause cough in guinea pigs and humans (12, 36), and TRPA1 mediates the cough response to cigarette smoke (11). In common with other TRP channels, TRPA1 desensitizes after activation (290). The observations that smoking cessation leads to prompt enhancement of cough sensitivity, even after many years of smoking, and that suppression of cough reflex sensitivity is caused by resumption of cigarette smoking (312), may relate to the ability of cigarette smoke to activate and desensitize TRPA1. Although the role of tachykinins in these phenomena remains to be determined, an enhanced secretion of SP mediated by increased nitrosative stress could contribute to chronic cough hypersensitivity (18).
In addition to neurons, TRPA1 is expressed by airway epithelium, smooth muscle, and fibroblasts, which can contribute to inflammation by releasing cytokines (232). The possibility that tachykinins, which can also release cytokines from airway epithelial cells (165), synergize with TRPA1 to control cytokine production remains to be studied. Moreover, SP expression may not be restricted to sensory nerves, since airway epithelial cells express Tac1 (323), and cells of the hematopoietic lineage can secrete tachykinins. Indirect evidence of this possibility derives from the observation that immune complex-mediated and stretch-induced lung injury are enhanced by the presence of the Tac1 gene in hemopoietic-derived cells (62). Whatever their origin, tachykinins, acting on the NK2R, can activate dendritic cell-mediated type 1 immune responses, thereby increasing the production of IFN-γ and IL-2 production by CD4(+) T cells (153). Overdistension of lung tissue during mechanical ventilation can also evoke cytokine release, and neuronal and nonneuronal SP contributes to ventilator-induced lung inflammation and injury by an NK1R-mediated mechanism (45). In this context, it is not surprising that corticosteroids, the mainstream therapy of asthma, downregulate NK1R expression in airway myocytes of asthmatic rats (184). Remodeling of the sensory innervation of the airways may also contribute to inflammatory diseases. A TNF-α-mediated increase in the levels of nerve growth factor (NGF) results in the proliferation of tachykinin-containing sensory nerve endings (329). NGF also contributes to the tachykinin-mediated responses in rodent airways after ozone inhalation (246), including during early life (131). Finally, in a mouse model of allergic asthma induced by house-dust mite antigen, NGF, primarily expressed in the bronchial and alveolar epithelium, mediates the enhanced release of SP, sensory innervation, and airway hyperresponsiveness (240).
3. Fluid secretion
The mortality of patients with cystic fibrosis is mainly due to chronic bacterial infections of the airway that are favored by the impaired reflex stimulation of mucus secretion from submucosal glands. SP mediates the local responses to capsaicinoids through a mechanism involving coordinated activation of cystic fibrosis transmembrane regulator and K+ channels, which eventually results in secretion of fluid from seromucous glands (132). While seromucous glands from noncystic fibrosis patients respond to SP with increased secretion, glands from cystic fibrosis patients are unresponsive to SP (65). Similar findings were obtained in a pig model of cystic fibrosis, where SP was unable to cause glandular secretion (140). Thus defective secretory responses to SP may contribute to the abnormalities in airway secretion that underlie the pathology of cystic fibrosis.
D. Urogenital Tract
The role of tachykinins in urinary and reproductive tracts and the therapeutic potential of NKR antagonists have been reviewed (49, 247).
1. Urinary tract
Primary spinal afferent nerves containing SP and NKA innervate the renal pelvic wall, ureter, and urinary bladder, including the urothelium, muscle, and blood vessels (49). In the wall of the renal pelvis, endothelin 1 (350) and PGE2 (163) stimulate SP release from sensory nerves, whereas angiotensin counteracts the effects of PGE2 (162).
Tachykinins stimulate smooth muscle contraction of the human ureter, mostly by activating the NK2R (138, 227, 255), suggesting the potential use of NK2R antagonists for ureteral disease. In the urinary bladder, the NK1R is found in blood vessels, the urothelium and muscle layers, with some inter-species differences, and the NK2R is expressed by detrusor muscle of all mammalian species studied, including humans (49). In the rat, the NK1R couples to Rho kinase, linking this receptor to smooth muscle contraction (349). Patients with multiple sclerosis have increased density of SP-containing fibers in the urinary bladder, which may contribute to detrusor overactivity in these patients (271). NK1R antagonists are a potential therapy for overactive bladder syndrome in postmenopausal women (299) (see sect. VF), whereas NK2R blockade controls neurogenic detrusor overactivity in rats with a spinal cord injury (1).
SP and NK1R have proinflammatory effects in the bladder. In rats, bladder inflammation during early life induces an upregulation of SP that persists into adulthood and is associated with elevated micturition frequency, decreased micturition volume, and enhanced vascular permeability (74, 300). Other proinflammatory actions of SP in the bladder include stimulation of plasma extravasation and leukocyte infiltration, mast cell degranulation, generation of ROS, and expression of proinflammatory cytokines, chemokines, adhesion molecules, and cyclooxygenase-2 (49). SP stimulates expression of glucose-regulated protein 78, a receptor for activated α2-macroglobulin, and blockade of this receptor prevents SP-mediated bladder and urothelial inflammation (339). The NK1R mediates the proinflammatory effects of tachykinins in the mouse urinary bladder, since NK1R blockade or deletion attenuates antigen-induced cystitis (291). NK1R antagonists also prevent stress-induced urothelial degeneration and mast cell degranulation in the urinary bladder (89). Thus tachykinins contribute to the clinical manifestations of interstitial cystitis, and NK1R antagonists may be a treatment for inflammatory disorders of the bladder.
There is a high degree of comorbidity between genitourinary and gastrointestinal disorders that are characterized by chronic pelvic pain. For example, colitis in rats is associated with increased expression and release of SP and CGRP in the urinary bladder that requires activation of TRPV1 pathways (253).
2. Female reproductive system
Tachykinins and all NKRs are present in the uterus (57, 256). Their prevalence changes during pregnancy (256) and is regulated by ovarian steroids (263). The NK2R is the predominant tachykinin receptor involved in uterine contraction, and its activation is under tight regulation during pregnancy (260). However, the NK1R in the uterus can mediate inflammatory responses that may lead to abortion (93).
NKB is present in the human placenta, and increased placental levels of NKB and Tac3 could contribute to preeclampsia and hypertension during late pregnancy (249, 250). Although placental levels of NKB increase during normal pregnancy and decline after delivery, they increase even more after preterm labor (336), suggesting that regulation of NKB during pregnancy is important for normal gestation. NKB may induce NK1R-mediated vasodilation of the placenta during pregnancy and cause systemic NK3R-mediated vasoconstriction that leads to hypertension. Elevated NKB levels may be a diagnostic marker for preeclampsia, and NK3R antagonists could be a therapy for this common condition (247).
Within the ovary, tachykinins control steroid secretion and may have an ancient role in stimulating oocyte growth (14). Although tachykinins have been implicated in age-related decline in reproductive function (357), their contribution to control of reproductive capability remains to be fully defined.
3. Male reproductive system
Tac1, Tac3, and Tac4 are expressed by human sperm, and tachykinins increase sperm motility by NK1R- and NK2R-dependent mechanisms (264). Sperm also express the SP-degrading enzyme neprilysin, and neprilysin inhibition promotes motility by inhibiting degradation of endogenous tachykinins (264). The epididymis produces SP, which stimulates sperm motility, and tachykinins potentiate contractility of the vas deferens (49). The importance of tachykinins in testes development is illustrated by the finding of severe testicular atrophy in dogs treated with a mixed NKR antagonist at a young age (194).
The seminal vesicles are innervated by SP-containing fibers, and SP induces contractions and facilitates neurally mediated responses in this system (49). SP and NKA are present at low levels in the rat and guinea pig prostate, abundant in the dog prostate, but absent in humans (49). However, Tac1, Tac3, and Tac4 mRNAs have been detected in human prostate. The effects of tachykinins on prostate contraction involve the NK1R in all species, but NK2R is dominant in human (49).
SP is present in human erectile tissue and penile vessels and can contract human erectile tissue (49). However, its role in erection is unclear, since it has no effects in the cavernous artery and relaxes norepinephrine-induced contractions of corpus cavernosum and corpus spongiosum. NK1R antagonism suppresses ejaculation in rats in response to intraventricular administration of a dopamine D3 receptor agonist (66).
E. Skin
The contribution of tachykinins to homeostasis and diseases of the skin have been reviewed (Figure 9) (287).
FIGURE 9.
Functions of tachykinins in the skin. SP and NKA are released from the peripheral endings of primary sensory nerves in the skin. They act on keratinocytes via NK1R and NK2R to activate NF-κB and promote release of cytokines and chemokines. SP and NKA act within the vasculature to induce plasma extravasation and to upregulate adhesion factors that stimulate neutrophil adhesion and infiltration. During inflammation, SP and NKA activate mast cells, neutrophils, and Langerhans cells, which amplifies the inflammatory response. Centrally released tachykinins contribute to pain and itch transmission.
1. Neuronal tachykinins
SP and NKA are present in primary sensory nerves in the skin (70). Tachykinin-positive nerve fibers supply the dermis and epidermis as well as innervate dermal blood vessels, keratinocytes, mast cells, dendritic cells, and hair follicles. Many exogenous and endogenous factors can stimulate the release of tachykinins from peripheral nerves in the skin, including physical stimuli (heat, ultraviolet radiation, scratching), allergens, and inflammatory mediators (bradykinin, prostaglandins, proteases, cytokines). Other factors, for example, pituitary adenylate cyclase activating polypeptide-38, inhibit tachykinin release from cutaneous sensory nerves, in line with their anti-inflammatory effects (236).
2. Keratinocytes
Mouse and human keratinocytes express NK1R and NK2R (Figure 9) (315). The consensus of multiple studies is that SP and NKA control the capacity of keratinocytes to serve as cytokine factories by regulating production of proinflammatory cytokines (315). SP also upregulates NGF production by keratinocytes and may thereby control the regeneration of cutaneous nerves under normal conditions and during wound healing (47). In contrast to CGRP, SP has only a moderate effect on keratinocyte proliferation (284). Neprilysin is expressed by keratinocytes and endothelial cells, where it dampens the actions of tachykinins (245).
3. Cutaneous blood vessels
SP and NKA-positive nerve fibers innervate the vasculature of the superficial dermis, where tachykinins activate the NK1R on endothelial cells of postcapillary venules to stimulate plasma extravasation, granulocyte infiltration, and release of proinflammatory mediators (neurogenic inflammation) (Figure 8). However, endothelial cells can also produce tachykinins, and NGF upregulates SP expression and release from human dermal microvascular endothelial cells (215). Although SP and NKA promote plasma extravasation from postcapillary venules, resulting in edema, recent evidence suggests that while tachykinins maintain basal cutaneous microcirculation, pituitary adenylate cyclase activating polypeptide-38 mediates neurogenic inflammatory vasodilation of arterioles and neuropathic mechanical hyperalgesia (40). In human dermal microvascular endothelial cells, SP induces an NK1R-dependent upregulation of adhesion molecules through activation of the transcription factors NF-AT and NF-κB, which leads to the influx of inflammatory cells (269, 270).
4. Fibroblasts
Compared with other tissues, such as the airways, the role of tachykinins in dermal fibroblasts is poorly understood. Human dermal fibroblasts in culture express Tac1, which is upregulated by exogenous SP (17), as well as the NK1R, which is upregulated by interferon-γ (192). Thus tachykinins may regulate fibroblasts by autocrine and neuronal mechanisms to regulate proliferation and wound healing. Indeed, SP induces an NK1R-dependent proliferation of human dermal fibroblasts (129). Human dermal fibroblasts express neprilysin, which is augmented by IL-1β and IL-22 (351). Notably, neprilysin is upregulated in the skin and in ulcers of patients with diabetes, which, combined with the peripheral neuropathy, could contribute to impaired wound healing (13).
5. Dermatitis and pruritus
The peripheral nervous system has long been implicated in the pathophysiology of inflammation and itch in dermatitis. Tachykinins are upregulated in the lesional skin of humans and mice with atopic dermatitis (242). Studies in mice implicate tachykinins and NKRs in the sensitization and inflammatory phases of allergic contact dermatitis. In a model of allergic contact dermatitis in mice, NK1R deletion or antagonism attenuates the sensitization and inflammatory responses to dinitrofluorobenzene (297). SP acting within lymph nodes mediates the sensitization phase (302). Surprisingly, NK2R antagonists enhanced the inflammatory response, whereas NK2R agonists had the opposite effect, suggesting a protective role of for the NK2R. Similarly, repeated SP challenge resulted in an anti-inflammatory response by modulating T cell and dendritic cell function in a chronic stress-induced model of allergic contact dermatitis (258). Whereas neprilysin disruption exacerbates allergic contact dermatitis, it has no effect on irritant dermatitis in mice (296). In summary, the contributions of tachykinins to allergic contact dermatitis are not fully understood, with evidence for pro- and anti-inflammatory functions.
SP is a major mediator of pruritus of atopic dermatitis, and NKR antagonists have been proposed as a therapy for itch (see sect. VG). SP induces expression of artemin, a member of glial cell line-derived neurotrophic factors, by human dermal fibroblasts, and artemin evokes warmth-evoked scratching and thermal hyperalgesia in mice (222). As a novel therapeutic strategy, ointment containing the nerve repulsion factor semaphorin 3A reduces the density of innervation of mouse skin with SP-containing nerve fibers, attenuates inflammation, and suppresses pruritus in a mouse model of allergic dermatitis (234).
TRP channels of sensory neurons innervating the skin also contribute to inflammation and pruritus of contact dermatitis. Notably, TRPA1 mediates the inflammation and scratching behavior of mice exposed to haptens (oxazolone, urushiol) and the allergen of poison ivy, and SP-induced scratching behavior is not observed in Trpa1 knockout mice (191).
6. Wound healing
Tachykinins have been implicated in wound healing, and deficits in tachykinin innervation (266) and upregulation of neprilysin (13) may contribute to the abnormal wound healing that occurs in patients and animals with diabetes. In a laser-induced wound healing model in rats, exogenous SP was found to promote neurite outgrowth and wound healing (77), and capsaicin also causes a NK1R-dependent increase in NGF biosynthesis in the rat skin (8).
7. Stress-induced hair loss
The hair follicle apparatus expresses tachykinins, NKRs, and endopeptidases, and tachykinins have been implicated in stress- and autoimmune-evoked hair loss. Stress-induced premature induction of catagen and hair follicle apoptosis in mice requires expression of the NK1R and the presence of mast cells (15). Observations of human skin biopsies and hair follicles in culture indicate that SP downregulates production of prolactin, which is important for hair growth (178). SP, NK1R, and neprilysin regulate the inflammatory response in a murine model of alopecia areata, an autoimmune disorder of the hair follicle associated with inflammatory cell influx around growing hair follicles (306).
F. Neurogenic Inflammation and Nociceptive Transmission
Primary sensory neurons innervate most tissues and can release tachykinins from peripheral and central endings to induce neurogenic inflammation and pain transmission. This section discusses the mechanisms and importance of tachykinins in these processes.
1. Tachykinins in primary sensory neurons
The localization of tachykinins in a subset of primary sensory neurons of the trigeminal, dorsal root, and vagal ganglia has been a topic of great interest for the past 60 years since SP and NKA have been proposed to play a major role in transmission at the level of the first synapse in the nociceptive pathway. Tachykinin-containing neurons in sensory ganglia have small cell bodies with unmyelinated C-fibers or thinly myelinated Aδ-fibers and slow conduction velocities. These neurons mediate nociceptive responses to physical (thermal, mechanical) and chemical stimuli. In addition, by releasing neuropeptides from peripheral endings, they generate “neurogenic inflammation,” which includes arteriolar dilatation and plasma extravasation and granulocyte infiltration from postcapillary venules (Figure 8). SP- and NKA-expressing neurons, representing 30–50% of neurons of the rat DRG, coexpress multiple neuropeptides that have been detected by immunochemical techniques. However, convincing evidence for neuropeptide release from the central or peripheral nerve terminals, a prerequisite for physiological function, is available for only a limited number of neuropeptides, notably SP and CGRP (44).
Peptidergic sensory neurons express TRP ion channels, including the thermosensors TRPV1, TRPV2, TRPV3, and TRPV4, the menthol sensor TRPM8, and the irritant sensor TRPA1. Once activated, TRP channels induce release of neuropeptides, including tachykinins. The observation that chronic treatment with the TRPV1 agonist capsaicin depletes neuropeptide from sensory nerve terminals implies that all peptidergic neurons express TRPV1. Moreover, TRPA1-positive neurons are a component of the TRPV1 neuronal population (324), with TRPA1 localized to peptidergic neurons (34). However, a proportion of nonpeptidergic neurons express TRPA1 (150). Mature sensory neuropeptides are produced from pre-pro-hormones synthesized in the neuronal cell body to be transported by active mechanisms to both central and peripheral nerve endings, where they are stored in dense-core vesicles. Neurotrophins, including NGF, regulate the expression of neuropeptides by sensory neurons, as well as the development of the neurons themselves (314).
Two modes of neuropeptide release from peripheral terminals of peptidergic sensory neurons have been identified. The first, induced in vitro by EFS and putatively in vivo by antidromic invasion of a propagated action potential to the terminal region of nerve fibers, results in a tetrodotoxin-sensitive and neuropeptide-mediated response. This pathway offers a neurochemical and ionic basis for the seminal hypothesis of Bayliss (27) and Lewis (182), whereby injury induces a neurogenic flare response, which we now know is mediated by CGRP released from cutaneous perivascular sensory nerves (309). The TRPV1 agonist capsaicin activates the second tetrodotoxin-insensitive pathway of neuropeptide release, providing insights into the function of peptidergic sensory neurons (326). An identical differentiation exists in the activation of neuropeptide release from central endings of primary sensory neurons in response to peripheral noxious stimuli (328). However, while there is no doubt that the release of neuropeptides from peripheral endings of sensory neurons causes neurogenic inflammation, there is uncertainty of the pathophysiological importance of centrally released tachykinins to nociceptive transmission.
1. NKR activation in DRG and spinal cord
NKRs are expressed by neurons of the DRG and dorsal horn of the spinal cord. Primary sensory neurons express both NK1R and NK2R that may serve as autoreceptors for SP and NKA released from the same neurons. Thus Ca2+-dependent release of SP enhances TRPV1 activity of DRG neurons in an autocrine manner by activating neuronal NK1R and NK2R (298, 360). PKC-ϵ mediates NK1R-dependent sensitization of TRPV1, probably by phosphorylating TRPV1 and altering channel gating (360). The NK1R and NK2R are also expressed by neurons of the dorsal horn, where SP/NKA released from the central projections of primary sensory neurons can activate NKRs on spinal neurons and induce endocytosis (see sect. IIID). Additionally, mediators released locally within the spinal cord may stimulate tachykinin release and thereby activate spinal NKRs. Thus spinal cytochrome P-450 activity can generate 5′,6′-epoxyeicosatrienoic acid, which activates TRPV4 on DRG neurons to release sensory neuropeptides (134, 344). One consequence of NK1R activation is the formation of ROS (104, 319). In DRG neurons, the intracellular ROS-mediated pathway augments M-type K+ channels and thereby counterbalances the ability of SP to sensitize TRPV1 and induce thermal hypersensitivity (190). This mechanism may explain the finding that SP causes hyperalgesia but not acute nociception. However, the sensitizing effect of SP on neurons is not confined to TRPV1, since NK1R activation also sensitizes P2X3 channels of trigeminal nociceptive, nonpeptidergic neurons (254).
2. Tachykinin-induced transmission in the dorsal spinal cord
A large body of evidence supports the view that SP and the NK1R contribute to nociception and hyperalgesia in experimental animals (Figure 8). Thus Tac1 deletion attenuates moderate to intense pain and prevents neurogenic inflammation (50), and NK1R deletion suppresses stress-induced pain (73). However, NK1R antagonists have failed as analgesics in clinical trials (126), and it is now recognized that of all the neuropeptides released by C-fibers, CGRP is a more likely contributor to pain typical of migraine headaches (31). However, despite the negative results of clinical trials, preclinical studies still support a role for SP and NKRs in the modulation of pain and hyperalgesia, although their role may be more subtle than that previously proposed.
Normally, Aδ and C fibers transmit pain while Aβ fibers signal touch. However, after nerve injury, Aβ fibers can transmit pain. In models of inflammatory and neuropathic pain, SP is upregulated in large-diameter neurons (237). Intraplantar injection of carrageenan leads to spinal production of 12-lipoxygenase-derived hepoxilin A3, which enhances release of SP and contributes to inflammatory hyperalgesia via TRPV1 and TRPA1 (113). In a chronic constriction model of nerve injury, the NK1R is upregulated in spinal neurons, which could also amplify pain (71). Studies of Tac1-deficient mice reveal a contribution of SP to pain-related behavior induced by intraplantar Formalin and capsaicin, although the contribution of SP is small and transient (29). In a mouse model of peripheral diabetic neuropathy, increased SP expression in the presynaptic sensory fibers innervating lamina I-III has been found in association with enhanced ERK1/2 phosphorylation and induction of other markers of activation of the nociceptive pathway (72).
Intraplantar and intrathecal pretreatment with the NK1R antagonist CP96345 inhibits mustard oil-induced thermal hyperalgesia and nocifensive behaviors (229). Peripheral inflammation induced by intraplantar Formalin activates a cascade of intracellular events in NK1R-positive spinal neurons that can lead to hyperalgesia, including PI3K, Akt, and the mammalian target of rapamycin (mTOR) (353), which have previously been observed in DRG and spinal cord neurons (352). Intraplantar carrageenan also induces NK1R-dependent activation of PI3K and Akt in dorsal horn neurons (64).
Several analgesics have been proposed to act by modulating peptidergic sensory neurons. Among a series of Ca2+ channel antagonists, only ziconotide reduced NK1R internalization evoked by noxious stimulation (328). The vesicular glutamate transporters in Nav1.8Cre-positive neurons and SP are required for Formalin-evoked nociception (171). Intrathecal botulinum neurotoxin B inhibits SP release from spinal primary afferent sensory fibers and attenuates the ensuing nociceptive response in models of inflammatory and neuropathic pain (130).
Finally, SP has been linked to intrinsic spinal cord pathways that modulate pain as indicated by the observation that descending facilitation from rostral ventral medial medulla contributes to facilitation of nociceptive transmission and hyperalgesia via NK1Rs (46). An opposing function has been reported for SP in the locus coeruleus, where NK1R activation facilitates spinal noradrenergic transmission and attenuates mechanical allodynia after chronic constriction injury (226).
G. Immune System
Tachykinins and their receptors are expressed by multiple immune cells, where they participate in inflammatory responses and immunity.
1. Neutrophils
SP regulates the activity and function of neutrophils and can control their production and infiltration into inflamed tissues. Thus SP stimulates the generation and release of cytokines, chemokines, matrix metalloproteases, and ROS from neutrophils and promotes bacterial phagocytosis (16, 244). Treatment of neonatal rats with capsaicin depletes the levels of SP in bone marrow, yet enhances neutrophil generation and release, which may be related to enhanced expression of Tac1 and Tacr1 by bone marrow cells (99). While some reports fail to demonstrate a role for the NK1R in cutaneous neutrophil recruitment (262), others have shown that NK1R antagonism attenuates neutrophil influx (295), suggesting selective involvement of the NK1R under different experimental conditions.
2. Monocytes and macrophages
There is convincing evidence for a role of tachykinins in regulating macrophage function during wound healing, inflammation, autoimmunity, and infection (266, 354). Moreover, NKR expression by macrophages is affected in certain disorders. For example, hypoxia in rats upregulates NK1R expression by pulmonary macrophages (359), and the NK1R is upregulated by threefold in macrophages of smokers compared with nonsmokers (26). In a patient with myelofibrosis, which is often associated with inflammatory and neoplastic conditions, increased levels of SP and TGF-β were observed (121). Finally, Tac4 mRNA, which encodes HK-1, is present in CD11b+ macrophages and CD11c+ dendritic cells in mice, suggesting a role of macrophage- or DC-derived HK-1 in immunoregulation (235).
3. Dendritic cells
Dendritic cells (DC) coordinate the activation of antigen-specific effector and memory lymphocytes, and thereby play a major role in acquired immunity. Both murine and human DCs express functional NK1R (238) and NK2R (153), where activation engages the NF-κB pathway and regulates T-cell function. SP and the NK1R are critically involved in controlling the immunoregulatory role of Langerhans DCs of the mouse skin (205). Human Langerhans cells also express SP (321), which may control T-cell proliferation (176) and regulate other dermal cells, such as keratinocytes. Tachykinins have been implicated in stress-evoked alterations in immunity. Thus acute immobilization stress of mice exacerbated cutaneous infection with Leishmania, which correlated with decreased numbers of epidermal Langerhans cells and greater SP immunoreactivity in skin (289). In the lung, SP regulates pulmonary responses to inhaled antigens by promoting the recruitment of DCs (166).
4. Mast cells
Mast cells are closely associated with SP-positive sensory nerves in many tissues, and there is a bidirectional communication between mast cells and primary sensory neurons, whereby sensory neuropeptides stimulate mast cells, and mast cell products control neuropeptide release. SP induces the release of TNF-α (78) and vascular endothelial growth factor (301) from mast cells, thereby contributing to inflammation, immunity, and angiogenesis. SP also induces Ca2+ signals and leukotriene B4 release from mast cells, but only when co-cultured with fibroblasts (243). However, other studies suggest that SP stimulates the release of preformed mediators, such as histamine, from skin mast cells, but does not affect the transcription of cytokine genes (115). The NK1R also mediates stress-evoked mast cell degranulation in rat skin (90).
5. Eosinophils
Like mast cells, eosinophils are also found in close proximity to SP-positive neurons in inflammatory diseases (313). However, the pathways that lead to eosinophil activation by tachykinins or the production of tachykinins by eosinophils are poorly understood and may involve epithelial cells (139). In the skin, SP-induced eosinophil infiltration is under the control of mast cell stimulation (239). In a model of allergic asthma in mice, NK1R activation promotes the release of leukotriene B4 and eosinophil recruitment (4), and in a guinea pig model of asthma, SP and NKA concentrations correlate positively with higher numbers of eosinophils (267).
6. Lymphocytes
T cells synthesize SP and express the NK1R during inflammation and infection, and SP regulates T-cell proliferation, cytokine and chemokine release, and killer activity (343). This regulation may be important in the development of autoimmune conditions such as type 1 diabetes, where T cell-mediated death of pancreatic beta cells causes insulin deficiency. For example, pancreatic sensory nerves that express TRPV1 are required for the development of islet inflammation and insulin resistance in diabetes-prone mice, whereas SP delivery to the pancreas reverses insulin resistance, inflammation, and diabetes (277). SP and the NK1R also regulate release of cytokines in autoimmune encephalomyelitis in mice (280). Both HK-1 and the NK1R are found in murine B cells and can regulate B-cell development and differentiation, probably in an autocrine fashion (111, 362).
7. Natural killer cells
Natural killer cells of rodents and humans express NK1R and respond to SP (92). SP enhances cytotoxicity of natural killer cells (177), promoting release of cytokines (186). The protective actions of SP against certain tumors and viruses may depend on activation of natural killer cells and T cells (197).
H. Cancer
Many types of tumor cells express NKRs, and tachykinins from tumor cells or infiltrating nerves or immune cells can influence proliferation, apoptosis, and metastasis of tumor cells in an autocrine, paracrine, or neurocrine manner.
1. Neural cancer
Neural tumors and cell lines often express tachykinins and the NK1R (119, 179, 219). The NK1R activates signaling pathways in U373MG cells that are related to growth and survival (see sect. IIIC). Notably, NK1R blockade reduces basal Akt phosphorylation, which suggests expression of a constitutively active receptor in glioblastoma cell lines (3). HK-1 also causes NK1R-mediated expression of the matrix metalloproteases by glioma cells, which promotes migration (217). NK1R antagonists may be a therapy for neural tumors since blockade reduces growth of U373MG cells xenografts (252).
Neuroblastoma is a sympathetic nervous system-derived childhood tumor with a poor prognosis. SY5Y and CHP212 neuroblastoma cell lines express full-length and truncated NK1R as well Tac1, and NK1R silencing blocks mitogenesis (218). Interestingly, stimulation of P2X7 nucleotide receptor triggers proliferation of neuroblastoma cell lines in part due to SP release, suggesting the existence of an autocrine pathway (272). Thus NK1R antagonists could be a therapy for neuroblastoma.
2. Colon cancer
Colon cancer is the leading cause of death among gastrointestinal diseases. Multiple observations support the involvement of SP and the NK1R in proliferation and tumorigenesis in the colonic epithelium. In human NCM460 colonic epithelial cells, SP activates multiple signaling pathways that are linked to proliferation (see sect. IIIC). The NK1R is expressed in SW-403 colorectal cancer cells, and the NK1R antagonist L-733,060 blocks proliferation in these cells with and without exogenous SP, suggesting existence of an autocrine mechanism (288). The truncated NK1R is preferentially upregulated in colonic epithelial cells of patients with ulcerative colitis who develop colonic carcinoma, suggesting a functional role for the truncated NK1R in transformation (108). The diminished desensitization and endocytosis of the truncated NK1R could amplify its tumorigenic potential.
The inactivation of tumor suppressor genes, often associated with promoter hypermethylation, can be exploited to identify novel tumor suppressor genes. Importantly, Tac1 was found to be a frequent target of methylation in primary colon cancers (47%) (216). The intensity of Tac1 methylation was higher in Dukes A/B than in Dukes C/D cancers and was associated with a small but significant downregulation of Tac1 mRNA in MSI-high colon tumors, indicating that Tac1 expression is reduced in colon carcinogenesis through hypermethylation.
3. Pancreatic cancer
Pancreatic cancer has the lowest survival rates of any gastrointestinal malignancy. The NK1R is upregulated in human pancreatic tumors, especially in advanced tumors with a poor prognosis (101). Several pancreatic cancer cell lines also express the NK1R, and SP exposure stimulates growth of these cells by an NK1R-dependent process (101).
4. Breast cancer
Cell lines and malignant breast biopsies have increased levels of Tac1, NK1R, and NK2R mRNA and produce high levels of SP (35, 310). Studies of human breast carcinoma cells in culture and after xenograft implantation into mice indicate that NK1R and NK2R antagonists inhibit growth by a cytostatic mechanism, in both the presence and absence of exogenous SP and NKA (35). The RE-1 silencer of transcription (REST) is a transcriptional regulator that, together with NF-κB, suppresses Tac1 gene expression in non-neuronal cells (112). REST inhibits Tac1 in breast cancer cell lines and in primary breast cancer cells, suggesting an important role for REST in the oncogenic function of SP (278). Immunoneutralization of SP strongly inhibits MAPK activation, cell cycle progression, and growth of breast cancer cell lines, while promoting apoptosis (208). Overexpression of the short form but not the full-length NK1R induces transformation of breast cancer cells, suggesting that targeting the truncated version of this receptor could be a therapeutic strategy, if achievable (257).
5. Lung cancer
Small cell lung cancers express a variety of neuropeptides that can act as autocrine growth factors. NKA has been detected in small cell lung cancers lines (32), and Tac1 and its products are present in some lung cancers (37). NK1R antagonism or deletion predisposes the development of bleomycin-associated lung adenocarcinoma (196). Blockade of the NK1R in mice treated with bleomycin also affects cytoplasmic translocation of the death receptor Nur77 and alters expression of Bcl2 and Bak, which are associated with apoptosis. Consistent with these observations, treatment of mice with aerosolized SP impedes cigarette smoke-induced lung damage and tumor development, and reverses cellular and genetic precursors of emphysema and malignancy (120).
6. Skin cancer
SP and the NK1R are expressed by melanomas and melanoma cell lines (148, 220). NK1R blockade inhibits growth and apoptosis of melanoma cells, in the presence or absence of SP, indicating an autocrine SP/NK1R system (220). While SP and the NK1R can directly promote melanoma cell growth, NK1R signaling may indirectly suppress melanoma development in vivo. SP infusion protects mice against melanoma cell growth, and this protection is lost in mice depleted of T and natural killer cells (197). The adoptive transfer of these cells from SP-treated mice also suppressed tumor growth, suggesting that SP can prime the immune system to defend against melanoma tumorigenesis.
V. THERAPEUTIC POTENTIAL OF NEUROKININ RECEPTOR ANTAGONISTS
A. Overview
Preclinical studies implicating tachykinins in diverse pathological processes (see sect. IV) spurred intense efforts by the pharmaceutical industry to develop NKR antagonists. These efforts were highly successful. Over 500 patents for NKR antagonists have been filed during the last 20 years, and some compounds have been evaluated in clinical trials. Despite intensive efforts, there is but one success: approval of the NK1R antagonist aprepitant (EMENDT) and its prodrug fosaprepitant (IVEMENDT) to treat chemotherapy-induced nausea and vomiting (CINV) and postoperative nausea and vomiting (Figure 3D) (118). Other large-scale trials of NKR antagonists for diverse conditions have either failed to demonstrate a benefit compared with existing therapies or placebo, or the results have been equivocal. In other areas, such as pruritus and viral infection, small-scale trials show promise, and large studies are necessary to determine the clinical utility. Finally, there are some indications, such as postsurgical formation of fibrous adhesions and cancer, where studies in animals show that NKR antagonists are effective treatments, but data from humans are lacking.
The challenges of developing NKR antagonists to treat human diseases are formidable, and there are multiple possible reasons for the failure. Many of the disease targets are poorly understood, with unknown etiology and complex pathology. There are obvious caveats in extrapolating the results from experimental animals to humans. Some animal models faithfully recapitulate human disorders, including models of nausea and emesis, where NK1R antagonists are effective in multiple species, including humans. However, this is not usually the case, and the difficulties of developing an animal model of a human disorder of unknown cause are daunting. The challenge of extrapolating from animal to human studies is complicated by differences in the potency with which certain antagonists interact with NKRs of different species (98). The molecular nature of the interactions between antagonists and receptors can markedly influence efficacy. Considerations include whether antagonists interact with the same sites as the endogenous agonists (orthosteric), or whether they bind to different sites and thereby alter receptor interactions with endogenous tachykinins (allosteric). Whereas orthosteric antagonists shut down signaling, allosteric antagonists fine-tune signaling by endogenous tachykinins and thereby exert more subtle and perhaps beneficial effects. Whether drugs are neutral antagonists (no effects in the absence of agonist), inverse agonists (decrease basal activity of constitutively active receptors), or insurmountable antagonists (sustained interactions with receptors) can also affect the outcome. The redundancy inherent in the tachykinin system also complicates therapeutic strategies. When one receptor is antagonized, the other two may compensate, and antagonists that target two or more NKRs, or even NKRs and other GPCRs, could be necessary. Interactions between NKRs and other GPCRs adds complexity since receptor heterodimers exhibit distinct signaling properties and likely pharmacology (261). Finally, there are challenges of targeting antagonists, not only to appropriate tissues but also to appropriate subcellular domains. PET imaging with labeled antagonists enables verification of central penetrance, allowing optimization of dosing regimens for effective drug occupancy of brain NK1Rs. However, the subcellular targeting of antagonists to block NKR signaling from specific subcellular domains has not been given similar attention. NKRs traffic to different subcellular compartments from which they can transmit unique signals with distinct outcomes (223). Thus targeting antagonists to defined subcellular compartments, possibly by lipidation of antagonists, could allow more selective antagonism, with fewer side effects than global antagonism of all signaling events.
Despite these disappointments, the pharmaceutical interest has not waned, as reflected by the numerous and continued efforts to develop antagonists of all three NKRs, and the large number of ongoing clinical trials. This section highlights the successes and failures of clinical trials of NKR antagonists. The development of NKR antagonists has been reviewed (6).
B. CINV
Cytotoxic chemotherapy is the mainstay treatment for cancer, with the debilitating and feared side effects of nausea and vomiting. Although steroids and antagonists of serotonin 5-HT3 and dopamine D2 receptors are effective treatments for the initial nausea, they are ineffective against the later phases. The realization that SP is concentrated in brain areas involved in vomiting (9), coupled with the findings that NK1R antagonists block emesis induced by peripherally and centrally acting emetics (345), provided the impetus for development of NK1R antagonists as treatments for CINV. Studies in humans substantiated these findings in animals, confirming the predictability of animal models (125). The development of PET tracers for brain imaging in humans verified central penetrance of peripherally administered NK1R antagonists and facilitated the design of dosing regimens for aprepitant that allowed blockade of >90% of the targeted receptors (118). In patients receiving cisplatin-based chemotherapy, aprepitant combined with the standard treatment of ondansetron (5-HT3 antagonist) and dexamethasone provided better antiemetic protection than the standard regimen alone (265). Aprepitant was approved in 2003 for oral administration in combination with the standard regimen. Fosaprepitant, a prodrug of aprepitant, was approved for intravenous use to treat CINV in 2008, and aprepitant was approved to treat postoperative nausea and vomiting in 2006. A recent review of multiple trials of NK1R antagonists for treatment of emesis confirmed their effectiveness but also revealed that use may be associated with increased rates of infection, suggesting that ongoing safety assessment is required (82).
C. Affective and Addictive Disorders
The development of NKR antagonists to treat the stress-related disorders has been reviewed (86). The presence of SP in brain areas involved in emotion and affective functions, and its colocalization with serotoninergic and noradrenergic transmitters that regulate emotions, suggest involvement of tachykinins in affective disorders. Thus immobilization stress in rats induces SP release in the medial nucleus of the amygdala, and central administration of an NK1R antagonist blocks stress-evoked anxiogenic-like behavior (85). A role for tachykinins in depression was provided by the report that NK1R blockade suppressed depressive behavior in guinea pigs and that the NK1R antagonist MK-869 had antidepressant effects in patients with moderate to severe major depression (167). Phase II trials confirmed effectiveness of NK1R antagonists to treat depression (86). However, a large placebo-controlled multisite phase III trial of patients with major depressive disorder did not confirm the efficacy of MK869, despite a dosing regimen that achieved near-maximum receptor occupancy in the brain (147). Although subsequent trials confirmed these results, the findings are difficult to interpret since the drugs selected as a positive control were also no more effective than the placebo. Recent trials also failed to show beneficial effects of NK1R antagonists in posttraumatic stress disorder and generalized anxiety (206, 214).
The lack of efficacy of antagonists in depression may relate to patient heterogeneity, assessment of end-points, and pharmacokinetic properties of the antagonists. A comparison of the NK1R antagonists aprepitant, CP-99994, and ZD6021 suggests that slow functional reversibility is associated with long-lasting in vivo efficacy of NK1R antagonists, and may be an important determinant of therapeutic potential (189). A recent clinical trial of the efficacy of the NK1R antagonist orvepitant to treat major depressive disorder supports this proposal (276).
Antagonists of other NKRs may be of value for stress-related disorders. NK3R antagonists may be effective treatments for schizophrenia and schizoaffective disorder (211), panic attacks, and Parkinson's disease (308). Antagonists of the NK2R have been shown to be effective in clinical trials for the treatment of major depression (86).
The NK1R has been implicated in addiction. NK1R deletion suppressed alcohol consumption in mice, and the NK1R antagonist LY686017 blunted the urge of detoxified alcoholic patients to consume alcohol (105). The NK1R antagonist CJ-11,974 decreases sucrose consumption in rats (322). NK1R deletion also attenuates the rewarding properties of opioids (225), and the NK1R antagonist L822429 reduces the acute reinforcing actions of heroin in rats (25). Thus NK1R antagonists could control several addictive behaviors.
D. Gastrointestinal Disorders
Preclinical studies and small-scale trials in IBS patients support the proposal that antagonists of all three NKRs may be useful therapies for IBS and related disorders. Analysis of two phase II studies of women with diarrhea-predominant IBS treated with the dual NK1R/NK2R antagonist DNK333 revealed relief of IBS symptoms compared with placebo (358). A small-scale study of female IBS patients evaluated the effectiveness of the NK1R antagonist AV608 on anxiety and visceral pain during painful and nonpainful visceral stimulation, with functional magnetic resonance imaging to evaluate brain activity (334). AV608 reduced anxiety and pain ratings, and suppressed activation of the amygdala, hippocampus, and anterior cingulate gyrus during visceral distension. IBS and IBD are associated with decreased binding of an NK1R PET agonist in the brain, which may reflect release of endogenous tachykinins and NK1R endocytosis, with a consequent reduction in binding of a PET ligand (135). Considered together, these studies support further exploration of NKR antagonists in IBS.
Although IBD is another potential target for NK1R antagonists, NK1R disruption delays healing of the inflamed colon, and antagonists could have the side effect of delayed resolution of colitis (see sect. IVB). Preclinical studies indicate that NK1R antagonists prevent the formation of fibrous adhesions after invasive abdominal surgery (see sect. IVB), which remains to be examined in clinical trials.
E. Respiratory Disorders
The contributions of tachykinins to neurogenic inflammation and hyperreactivity of the airways led to the suggestion that NKR antagonists could be treatments for asthma, bronchitis, and COPD (see sect. IVC). Although FK888, a peptidic NK1R antagonist, improved exercise-induced asthma (133), the small molecule NK1R antagonist CP99,994 had no protective effect on hypertonic saline-induced bronchial constriction or cough in patients with mild asthma (91). Moreover, the dual NK1R/NK2R antagonist AVE5883 did not protect asthmatics challenged with allergen, despite its antagonistic activity against inhaled NKA (39). A review of clinical trials of NKR antagonists as a treatment for asthma concluded that while there is a potential of NKR antagonist to decrease airway hyperresponsiveness and improve lung function, their effects on airway inflammation and asthma symptoms have not been studied in sufficient detail to warrant firm conclusions, suggesting the need for large-scale trials (274).
F. Urogenital Disorders
Preclinical studies indicate that tachykinins regulate contractility of smooth muscle in the urinary tract, suggesting that NKR antagonists may be treatments for bladder disorders (see sect. IVD). Overactive bladder, which is defined as urgency with or without incontinence, is a common disorder that severely compromises quality of life, and existing treatments with anticholinergic drugs have side effects such as constipation, dry mouth, and dry eyes. A large trial of patients with overactive bladder revealed that although the NK1R antagonist serlopitant decreased the daily number of urination episodes compared with placebo, it was no more effective than tolterodine (M2/M3 muscarinic receptor antagonist) and, unlike tolterodine, serlopitant did not reduce urgency or incontinence relative to placebo (100). Whether the NK2R, which is expressed by bladder detrusor muscle of all mammalian species studied, is a target for overactive bladder remains to be clinically evaluated.
G. Sensory Disorders of Pain and Pruritus
Extensive preclinical studies implicate SP and the NK1R in somatic and visceral pain (see sect. IV, B and F). Although the NK1R antagonist CP-99,994 reduced postsurgical pain of patients undergoing tooth extraction compared with placebo (81), NK1R antagonists were subsequently shown to be ineffective in other pain states in humans (126).
A novel strategy to develop improved analgesics has been to generate chimeric molecules composed of an NK1R antagonist and an opioid agonist, with the rationale that blockade of the algesic NK1R and activation of the analgesic opioid receptor would be a more effective therapy than targeting the individual receptors. A bifunctional chimeric peptide, [Dmt-d-Arg-Aba-Gly-NMe-30,50-(CF3)2-Bn], is a NK1R antagonist, a balanced agonist of μ- and δ-opioid receptors, and is algesic in mice (20, 116). The usefulness of such compounds in humans awaits evaluation.
Chronic pruritus accompanies diverse diseases that affect the skin (atopic dermatitis) and other organs (kidney, liver). Preclinical studies indicate that NK1R antagonism attenuates scratching behavior in a picrylchloride-induced dermatitis model in mice (241). A small study of patients with untreatable chronic pruritus revealed that the NK1R antagonist aprepitant caused a marked reduction of itch intensity (320). These studies justify a randomized, controlled clinical trial to fully evaluation the effectiveness of NK1R antagonists to treat chronic pruritus.
H. Viral and Bacterial Infections and Sepsis
In view of the major role of SP and the NK1R in regulation of immune functions (see sect. IVG), there has been considerable interest in use of NK1R antagonists to treat viral and bacterial infections. SP levels are elevated in the circulation of HIV-infected individuals (83), and SP enhances HIV replication in mononuclear phagocytes and macrophages by an NK1R-mediated mechanism (174). This effect of SP may depend on NK1R-induced expression of the chemokine receptor CCR5, a coreceptor for HIV. A small-scale trial of HIV patients revealed that aprepitant was safe but at the doses used did not show significant antiviral or immunologic improvement (333). Deletion of Tac1 or antagonism of the NK1R protects mice from polymicrobial sepsis and airway inflammation induced by cecal ligation and puncture (122, 268). Thus NK1R antagonist may be effective therapies for bacterial sepsis, which remains to be studied in humans.
I. Cancer
Multiple studies suggest that tachykinins induce proliferation and migration of tumor cells and that NKR blockade has antiproliferative and proapoptotic actions (see sect. IVH). Although these studies point to therapeutic utility of NKR antagonists in cancer, this possibility has yet to evaluated in clinical trials.
VI. CONCLUSIONS AND FUTURE PERSPECTIVES
Tachykinins are one of the most intensively studied families of neuropeptides. The early realization that tachykinins regulate important pathophysiological processes, such as inflammation, immunity, and nociception, provided the impetus for these studies. Knowledge of the structure, function, and regulation of tachykinins undoubtedly guided studies of other neuropeptides, confirming the importance of tachykinins as “pioneering neuropeptides.” The NKRs are also one of the most studied families of GPCRs. Information about the signaling, trafficking, and properties of NKRs certainly influenced studies of other GPCRs, establishing their position as “pioneering receptors.” Preclinical studies implicating involvement of NKRs in diverse disorders, including inflammatory diseases, pain, affective disorders, and vomiting, stimulated the pharmaceutical industry to develop antagonists of all three NKRs. On one hand, these efforts were successful: NKR antagonists are selective, potent, safe, and show good bioavailability. On the other hand, they were a major disappointment: apart from the usefulness of NK1R antagonists to treat nausea and vomiting after chemotherapy and surgery, large-scale trials failed to support further development of antagonists for the treatment of pain, depression, or bladder disorders.
Has the lack of widespread clinical utility dampened interest in tachykinins, and are NKRs still a viable therapeutic target? Judging from the ongoing reports of involvement of tachykinins in important physiological processes and diseases, including cancer and infection, the tachykinin field remains vibrant. There are also multiple ongoing clinical trials of NKR antagonists, suggesting that interest in industry is sustained. Moreover, recent preclinical studies of the involvement of tachykinins in fibrosis and pruritus support clinical studies of NKR antagonists in other disorders.
The reason why NKR antagonists failed in many clinical trials remains a mystery. Plausible explanations for failure include the use of inappropriate experimental models for preclinical development, the inappropriate selection of patients and end-points for efficacy in clinical trials, and a lack of understanding of the structural basis of antagonist interaction with NKRs. A deeper understanding of the molecular mechanisms of human disease is required for the development of suitable experimental models for evaluation of effectiveness of therapies, including NKR antagonists. This knowledge will inform trials of more homogeneous patient groups and permit the identification of more sensitive and specific end-points. Knowledge of the three-dimensional structure of NKRs in combination with agonists, antagonists, and signaling partners, including G proteins and β-arrestins, will facilitate drug design. The next generation of NKR antagonists could include allosteric modulators, which would permit finer control of signaling by endogenous tachykinins. Since tachykinins often act in concert with CGRP, drugs that simultaneously antagonize tachykinin and CGRP receptors may be more effective, and NKR antagonists in combination with opioid receptor agonists are already being studied for pain. Finally, the subcellular targeting of NKR antagonists to disrupt disease-relevant compartmentalized signaling may lead to increased specificity and fewer side-effects. Clearly, there is much still to learn from the study of tachykinins and NKRs.
GRANTS
The authors' laboratories are supported by the National Health and Medical Research Council, National Institutes of Health, Regione Toscana, and the Italian Institute of Technology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors. B. von Mentzer is the CEO of Pharmnovo AB, Erik Dahlbergsgatan 19, 411 26 Goteborg, Sweden. This is a new biotechnology company developing receptor antagonists.
ACKNOWLEDGEMENTS
We thank Drs. Nicholas Veldhuis, Daniel Poole, and Dane Jensen for helpful input.
Address for reprint requests and other correspondence: N. Bunnett, Monash Institute of Pharmaceutical Sciences, 381 Royal Parade, Parkville, Victoria 3052, Australia (e-mail: Nigel.Bunnett@Monash.edu).
REFERENCES
- 1.Abdel-Karim AM, Barthlow HG, Bialecki RA, Elhilali MM. Effects of M274773, a neurokinin-2 receptor antagonist, on bladder function in chronically spinalized rats. J Urol 174: 1488–1492, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Ackley MA, Asghar AU, Worsley MA, King AE. Peripheral inflammation reduces the response of spinal dorsal horn neurons to an NK3 receptor agonist. Neurosci Lett 308: 13–16, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Akazawa T, Kwatra SG, Goldsmith LE, Richardson MD, Cox EA, Sampson JH, Kwatra MM. A constitutively active form of neurokinin 1 receptor and neurokinin 1 receptor-mediated apoptosis in glioblastomas. J Neurochem 109: 1079–1086, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alessandri AL, Pinho V, Souza DG, Castro MS, Klein A, Teixeira MM. Mechanisms underlying the inhibitory effects of tachykinin receptor antagonists on eosinophil recruitment in an allergic pleurisy model in mice. Br J Pharmacol 140: 847–854, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alex G, Kunze WA, Furness JB, Clerc N. Comparison of the effects of neurokinin-3 receptor blockade on two forms of slow synaptic transmission in myenteric AH neurons. Neuroscience 104: 263–269, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Alvaro G, Di Fabio R. Neurokinin 1 receptor antagonists–current prospects. Curr Opin Drug Discov Devel 10: 613–621, 2007 [PubMed] [Google Scholar]
- 7.Amadesi S, Moreau J, Tognetto M, Springer J, Trevisani M, Naline E, Advenier C, Fisher A, Vinci D, Mapp C, Miotto D, Cavallesco G, Geppetti P. NK1 receptor stimulation causes contraction and inositol phosphate increase in medium-size human isolated bronchi. Am J Respir Crit Care Med 163: 1206–1211, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Amann R, Schuligoi R. Beta adrenergic inhibition of capsaicin-induced, NK1 receptor-mediated nerve growth factor biosynthesis in rat skin. Pain 112: 76–82, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Amin AH, Crawford TB, Gaddum JH. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J Physiol 126: 596–618, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, Bucher B, Galzi JL, Sterner O, Bevan S, Hogestatt ED, Zygmunt PM. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)-tetrahydrocannabiorcol. Nat Commun 2: 551, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118: 2574–2582, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andre E, Gatti R, Trevisani M, Preti D, Baraldi PG, Patacchini R, Geppetti P. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol 158: 1621–1628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antezana M, Sullivan SR, Usui M, Gibran N, Spenny M, Larsen J, Ansel J, Bunnett N, Olerud J. Neutral endopeptidase activity is increased in the skin of subjects with diabetic ulcers. J Invest Dermatol 119: 1400–1404, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Aoyama M, Kawada T, Fujie M, Hotta K, Sakai T, Sekiguchi T, Oka K, Satoh N, Satake H. A novel biological role of tachykinins as an up-regulator of oocyte growth: identification of an evolutionary origin of tachykininergic functions in the ovary of the ascidian, Ciona intestinalis. Endocrinology 149: 4346–4356, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Arck PC, Handjiski B, Kuhlmei A, Peters EM, Knackstedt M, Peter A, Hunt SP, Klapp BF, Paus R. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med 83: 386–396, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Augustyniak D, Jankowski A, Mackiewicz P, Skowyra A, Gutowicz J, Drulis-Kawa Z. Innate immune properties of selected human neuropeptides against Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Immunol 13: 24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae SJ, Matsunaga Y, Takenaka M, Tanaka Y, Hamazaki Y, Shimizu K, Katayama I. Substance P induced preprotachykinin-a mRNA, neutral endopeptidase mRNA and substance P in cultured normal fibroblasts. Int Arch Allergy Immunol 127: 316–321, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Bae YJ, Moon KA, Kim TB, Jang YJ, Lee YS, Park CS, Lee KY, Moon HB, Cho YS. The role of nitrosative stress in the pathogenesis of unexplained chronic cough with cough hypersensitivity. Am J Rhinol Allergy 26: e10–14, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Baker SJ, Morris JL, Gibbins IL. Cloning of a C-terminally truncated NK-1 receptor from guinea-pig nervous system. Brain Res 111: 136–147, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Ballet S, Feytens D, Buysse K, Chung NN, Lemieux C, Tumati S, Keresztes A, Van Duppen J, Lai J, Varga E, Porreca F, Schiller PW, Vanden Broeck J, Tourwe D. Design of novel neurokinin 1 receptor antagonists based on conformationally constrained aromatic amino acids and discovery of a potent chimeric opioid agonist-neurokinin 1 receptor antagonist. J Med Chem 54: 2467–2476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandari PS, Qian J, Oh HS, Potian JA, Yehia G, Harrison JS, Rameshwar P. Crosstalk between neurokinin receptors is relevant to hematopoietic regulation: cloning and characterization of neurokinin-2 promoter. J Neuroimmunol 138: 65–75, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Bang S, Kim KY, Yoo S, Kim YG, Hwang SW. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci 26: 2516–2523, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Barak LS, Warabi K, Feng X, Caron MG, Kwatra MM. Real-time visualization of the cellular redistribution of G protein-coupled receptor kinase 2 and beta-arrestin 2 during homologous desensitization of the substance P receptor. J Biol Chem 274: 7565–7569, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Barbara G, De Giorgio R, Stanghellini V, Corinaldesi R, Cremon C, Gerard N, Gerard C, Grady EF, Bunnett NW, Blennerhassett PA, Collins SM. Neutral endopeptidase (EC 3.42411) downregulates the onset of intestinal inflammation in the nematode infected mouse. Gut 52: 1457–1464, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbier E, Vendruscolo LF, Schlosburg JE, Edwards S, Juergens N, Park PE, Misra KK, Cheng K, Rice KC, Schank J, Schulteis G, Koob GF, Heilig M. The NK1 receptor antagonist l822429 reduces heroin reinforcement. Neuropsychopharm 38: 976–984, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardelli C, Gunella G, Varsaldi F, Balbo P, Del Boca E, Bernardone IS, Amoruso A, Brunelleschi S. Expression of functional NK1 receptors in human alveolar macrophages: superoxide anion production, cytokine release and involvement of NF-kappaB pathway. Br J Pharmacol 145: 385–396, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol 26: 173–209, 1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beasley R, Clayton T, Crane J, von Mutius E, Lai CK, Montefort S, Stewart A. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from Phase Three of the ISAAC programme. Lancet 372: 1039–1048, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Beaudry H, Dubois D, Gendron L. Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci 31: 13068–13077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beinborn M, Blum A, Hang L, Setiawan T, Schroeder JC, Stoyanoff K, Leung J, Weinstock JV. TGF-beta regulates T-cell neurokinin-1 receptor internalization and function. Proc Natl Acad Sci USA 107: 4293–4298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol 9: 9–14, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Bepler G, Rotsch M, Jaques G, Haeder M, Heymanns J, Hartogh G, Kiefer P, Havemann K. Peptides and growth factors in small cell lung cancer: production, binding sites, and growth effects. J Cancer Res Clin Oncol 114: 235–244, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci USA 95: 4760–4765, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya MR, Bautista DM, Wu K, Haeberle H, Lumpkin EA, Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc Natl Acad Sci USA 105: 20015–20020, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bigioni M, Benzo A, Irrissuto C, Maggi CA, Goso C. Role of NK-1 and NK-2 tachykinin receptor antagonism on the growth of human breast carcinoma cell line MDA-MB-231. Anticancer Drugs 16: 1083–1089, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med 180: 1042–1047, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bishop AE, Hamid QA, Adams C, Bretherton-Watt D, Jones PM, Denny P, Stamp GW, Hurt RL, Grimelius L, Harmar AJ. Expression of tachykinins by ileal and lung carcinoid tumors assessed by combined in situ hybridization, immunocytochemistry, and radioimmunoassay. Cancer 63: 1129–1137, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest 115: 2169–2179, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boot JD, de Haas S, Tarasevych S, Roy C, Wang L, Amin D, Cohen J, Sterk PJ, Miller B, Paccaly A, Burggraaf J, Cohen AF, Diamant Z. Effect of an NK1/NK2 receptor antagonist on airway responses and inflammation to allergen in asthma. Am J Respir Crit Care Med 175: 450–457, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Botz B, Imreh A, Sandor K, Elekes K, Szolcsanyi J, Reglodi D, Quinn JP, Stewart J, Zimmer A, Hashimoto H, Helyes Z. Role of pituitary adenylate-cyclase activating polypeptide and Tac1 gene derived tachykinins in sensory, motor and vascular functions under normal and neuropathic conditions. Peptides 43: 105–112, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, Bunnett NW, McDonald DM. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci USA 91: 8964–8968, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradesi S, Kokkotou E, Simeonidis S, Patierno S, Ennes HS, Mittal Y, McRoberts JA, Ohning G, McLean P, Marvizon JC, Sternini C, Pothoulakis C, Mayer EA. The role of neurokinin 1 receptors in the maintenance of visceral hyperalgesia induced by repeated stress in rats. Gastroenterology 130: 1729–1742, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Bradesi S, Svensson CI, Steinauer J, Pothoulakis C, Yaksh TL, Mayer EA. Role of spinal microglia in visceral hyperalgesia and NK1R up-regulation in a rat model of chronic stress. Gastroenterology 136: 1339–1348, e1331–1332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84: 903–934, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Bregeon F, Steinberg JG, Andreotti N, Sabatier JM, Delpierre S, Ravailhe S, Jammes Y. Substance P receptor blockade decreases stretch-induced lung cytokines and lung injury in rats. J Physiol 588: 1309–1319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brink TS, Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. Differential modulation of neurons in the rostral ventromedial medulla by neurokinin-1 receptors. J Neurophysiol 107: 1210–1221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burbach GJ, Kim KH, Zivony AS, Kim A, Aranda J, Wright S, Naik SM, Caughman SW, Ansel JC, Armstrong CA. The neurosensory tachykinins substance P and neurokinin A directly induce keratinocyte nerve growth factor. J Invest Dermatol 117: 1075–1082, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Caceres AI, Brackmann M, Elia MD, Bessac BF, Del Camino D, D'Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt SE. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA 106: 9099–9104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Candenas L, Lecci A, Pinto FM, Patak E, Maggi CA, Pennefather JN. Tachykinins and tachykinin receptors: effects in the genitourinary tract. Life Sci 76: 835–862, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature 392: 390–394, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Carter MS, Krause JE. Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide gamma. J Neurosci 10: 2203–2214, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castagliuolo I, Morteau O, Keates AC, Valenick L, Wang CC, Zacks J, Lu B, Gerard NP, Pothoulakis C. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. Br J Pharmacol 136: 271–279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard NP, Pothoulakis C. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest 101: 1547–1550, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castagliuolo I, Valenick L, Liu J, Pothoulakis C. Epidermal growth factor receptor transactivation mediates substance P-induced mitogenic responses in U-373 MG cells. J Biol Chem 275: 26545–26550, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Cattaruzza F, Cottrell GS, Vaksman N, Bunnett NW. Endothelin-converting enzyme 1 promotes re-sensitization of neurokinin 1 receptor-dependent neurogenic inflammation. Br J Pharmacol 156: 730–739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cattaruzza F, Johnson C, Leggit A, Grady E, Schenk AK, Cevikbas F, Cedron W, Bondada S, Kirkwood R, Malone B, Steinhoff M, Bunnett N, Kirkwood KS. Transient receptor potential ankyrin 1 mediates chronic pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol 304: G1002–G1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cejudo Roman A, Pinto FM, Dorta I, Almeida TA, Hernandez M, Illanes M, Tena-Sempere M, Candenas L. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril 97: 1213–1219, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Cellini J, Pommier R, Porter R, LePard KJ. Enhanced nerve-stimulated muscarinic and neurokinin contractions of ileum from streptozotocin guinea-pigs. Autonomic Autacoid Pharmacol 32: 23–39, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Ceppa E, Cattaruzza F, Lyo V, Amadesi S, Pelayo JC, Poole DP, Vaksman N, Liedtke W, Cohen DM, Grady EF, Bunnett NW, Kirkwood KS. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol 299: G556–G571, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang MM, Leeman SE. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem 245: 4784–4790, 1970 [PubMed] [Google Scholar]
- 61.Chang MM, Leeman SE, Niall HD. Amino-acid sequence of substance P. Nature New Biol 232: 86–87, 1971 [DOI] [PubMed] [Google Scholar]
- 62.Chavolla-Calderon M, Bayer MK, Fontan JJ. Bone marrow transplantation reveals an essential synergy between neuronal and hemopoietic cell neurokinin production in pulmonary inflammation. J Clin Invest 111: 973–980, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen P, Douglas SD, Meshki J, Tuluc F. Neurokinin 1 receptor mediates membrane blebbing and sheer stress-induced microparticle formation in HEK293 cells. PLoS One 7: e45322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi JI, Koehrn FJ, Sorkin LS. Carrageenan induced phosphorylation of Akt is dependent on neurokinin-1 expressing neurons in the superficial dorsal horn. Mol Pain 8: 4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi JY, Khansaheb M, Joo NS, Krouse ME, Robbins RC, Weill D, Wine JJ. Substance P stimulates human airway submucosal gland secretion mainly via a CFTR-dependent process. J Clin Invest 119: 1189–1200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clement P, Peeters M, Bernabe J, Laurin M, Alexandre L, Giuliano F. Role of the neurokinin-1 receptors in ejaculation in anesthetized rats. J Sex Med 6: 126–134, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Gehringer D, Grady EF, Bunnett NW. Ubiquitin-dependent down-regulation of the neurokinin-1 receptor. J Biol Chem 281: 27773–27783, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Cottrell GS, Padilla BE, Amadesi S, Poole DP, Murphy JE, Hardt M, Roosterman D, Steinhoff M, Bunnett NW. Endosomal endothelin-converting enzyme-1: a regulator of beta-arrestin-dependent ERK signaling. J Biol Chem 284: 22411–22425, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D'antonio C, Wang B, McKay C, Huizinga JD. Substance P activates a non-selective cation channel in murine pacemaker ICC. Neurogastroenterol Motil 21: 985–e979, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Dalsgaard CJ, Haegerstrand A, Theodorsson-Norheim E, Brodin E, Hokfelt T. Neurokinin A-like immunoreactivity in rat primary sensory neurons: coexistence with substance P. Histochemistry 83: 37–39, 1985 [DOI] [PubMed] [Google Scholar]
- 71.Datta S, Chatterjee K, Kline RHt, Wiley RG. Behavioral and anatomical characterization of the bilateral sciatic nerve chronic constriction (bCCI) injury: correlation of anatomic changes and responses to cold stimuli. Mol Pain 6: 7, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dauch JR, Yanik BM, Hsieh W, Oh SS, Cheng HT. Neuron-astrocyte signaling network in spinal cord dorsal horn mediates painful neuropathy of type 2 diabetes. Glia 60: 1301–1315, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 392: 394–397, 1998 [DOI] [PubMed] [Google Scholar]
- 74.DeBerry J, Randich A, Shaffer AD, Robbins MT, Ness TJ. Neonatal bladder inflammation produces functional changes and alters neuropeptide content in bladders of adult female rats. J Pain 11: 247–255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148: 1267–1281, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deiteren A, De Winter BY, Nullens S, Pelckmans PA, De Man JG. Role of tachykinin receptors in the modulation of colonic peristaltic activity in mice. Eur J Pharmacol 667: 339–347, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Delgado AV, McManus AT, Chambers JP. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model. Exp Biol Med 230: 271–280, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Delgado AV, McManus AT, Chambers JP. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides 37: 355–361, 2003 [DOI] [PubMed] [Google Scholar]
- 79.Derocq JM, Segui M, Blazy C, Emonds-Alt X, Le Fur G, Brelire JC, Casellas P. Effect of substance P on cytokine production by human astrocytic cells and blood mononuclear cells: characterization of novel tachykinin receptor antagonists. FEBS Lett 399: 321–325, 1996 [DOI] [PubMed] [Google Scholar]
- 80.Di Sebastiano P, Grossi L, Di Mola FF, Angelucci D, Friess H, Marzio L, Innocenti P, Buchler MW. SR140333, a substance P receptor antagonist, influences morphological and motor changes in rat experimental colitis. Dig Dis Sci 44: 439–444, 1999 [DOI] [PubMed] [Google Scholar]
- 81.Dionne RA, Max MB, Gordon SM, Parada S, Sang C, Gracely RH, Sethna NF, MacLean DB. The substance P receptor antagonist CP-99,994 reduces acute postoperative pain. Clin Pharmacol Ther 64: 562–568, 1998 [DOI] [PubMed] [Google Scholar]
- 82.Dos Santos LV, Souza FH, Brunetto AT, Sasse AD, da Silveira Nogueira Lima JP. Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting: a systematic review. J Natl Cancer Inst 104: 1280–1292, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Douglas SD, Ho WZ, Gettes DR, Cnaan A, Zhao H, Leserman J, Petitto JM, Golden RN, Evans DL. Elevated substance P levels in HIV-infected men. Aids 15: 2043–2045, 2001 [DOI] [PubMed] [Google Scholar]
- 84.Douglas SD, Lynch KG, Lai JP. Neurokinin-1 receptor mRNA expression differences in brains of HIV-infected individuals. J Neurol Sci 272: 174–177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ebner K, Rupniak NM, Saria A, Singewald N. Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc Natl Acad Sci USA 101: 4280–4285, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ebner K, Sartori SB, Singewald N. Tachykinin receptors as therapeutic targets in stress-related disorders. Curr Pharm Des 15: 1647–1674, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Eilers H, Cattaruzza F, Nassini R, Materazzi S, Andre E, Chu C, Cottrell G, Schumacher MA, Geppetti P, Bunnett NW. Pungent general anesthetics activate transient receptor potential-A1 to produce hyperalgesia and neurogenic bronchoconstriction. Anesthesiology 112: 1452–1463, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipovic MR, Izydorczyk I, Eberhardt M, Kichko TI, Mueller-Tribbensee SM, Khalil M, Siklosi N, Nau C, Ivanovic-Burmazovic I, Neuhuber WL, Becker C, Neurath MF, Reeh PW. TRPA1 and substance P mediate colitis in mice. Gastroenterology 141: 1346–1358, 2011 [DOI] [PubMed] [Google Scholar]
- 89.Ercan F, Akici A, Ersoy Y, Hurdag C, Erin N. Inhibition of substance P activity prevents stress-induced bladder damage. Regul Pept 133: 82–89, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Erin N, Ersoy Y, Ercan F, Akici A, Oktay S. NK-1 antagonist CP99994 inhibits stress-induced mast cell degranulation in rats. Clin Exp Dermatol 29: 644–648, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Fahy JV, Wong HH, Geppetti P, Reis JM, Harris SC, Maclean DB, Nadel JA, Boushey HA. Effect of an NK1 receptor antagonist (CP-99,994) on hypertonic saline-induced bronchoconstriction and cough in male asthmatic subjects. Am J Respir Crit Care Med 152: 879–884, 1995 [DOI] [PubMed] [Google Scholar]
- 92.Feistritzer C, Clausen J, Sturn DH, Djanani A, Gunsilius E, Wiedermann CJ, Kahler CM. Natural killer cell functions mediated by the neuropeptide substance P. Regul Pept 116: 119–126, 2003 [DOI] [PubMed] [Google Scholar]
- 93.Fest S, Zenclussen AC, Joachim R, Hagen E, Demuth HU, Hoffmann T. Stress and substance P but not the substance P-metabolite SP5–11 trigger murine abortion by augmenting TNF-alpha levels at the feto-maternal interface. Scand J Immunol 63: 42–49, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Fiebich BL, Schleicher S, Butcher RD, Craig A, Lieb K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-kappa B. J Immunol 165: 5606–5611, 2000 [DOI] [PubMed] [Google Scholar]
- 95.Figini M, Emanueli C, Bertrand C, Sicuteri R, Regoli D, Geppetti P. Differential activation of the epithelial and smooth muscle NK1 receptors by synthetic tachykinin agonists in guinea-pig trachea. Br J Pharmacol 121: 773–781, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Figini M, Emanueli C, Grady EF, Kirkwood K, Payan DG, Ansel J, Gerard C, Geppetti P, Bunnett N. Substance P and bradykinin stimulate plasma extravasation in the mouse gastrointestinal tract and pancreas. Am J Physiol Gastrointest Liver Physiol 272: G785–G793, 1997 [DOI] [PubMed] [Google Scholar]
- 97.Flynn FW, Jensen DD, Thakar A, Xu X, Flynn SW, Zhang Z. Neurokinin 3 receptor forms a complex with acetylated histone H3 and H4 in hypothalamic neurons following hyperosmotic challenge. Am J Physiol Regul Integr Comp Physiol 301: R822–R831, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fong TM, Anderson SA, Yu H, Huang RR, Strader CD. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol 41: 24–30, 1992 [PubMed] [Google Scholar]
- 99.Franco-Penteado CF, De Souza IA, Lima CS, Teixeira SA, Muscara MN, De Nucci G, Antunes E. Effects of neonatal capsaicin treatment in the neutrophil production, and expression of preprotachykinin-I and tachykinin receptors in the rat bone marrow. Neurosci Lett 407: 70–73, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Frenkl TL, Zhu H, Reiss T, Seltzer O, Rosenberg E, Green S. A multicenter, double-blind, randomized, placebo controlled trial of a neurokinin-1 receptor antagonist for overactive bladder. J Urol 184: 616–622, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Friess H, Zhu Z, Liard V, Shi X, Shrikhande SV, Wang L, Lieb K, Korc M, Palma C, Zimmermann A, Reubi JC, Buchler MW. Neurokinin-1 receptor expression and its potential effects on tumor growth in human pancreatic cancer. Lab Invest 83: 731–742, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Garland AM, Grady EF, Lovett M, Vigna SR, Frucht MM, Krause JE, Bunnett NW. Mechanisms of desensitization and resensitization of G protein-coupled neurokinin1 and neurokinin2 receptors. Mol Pharmacol 49: 438–446, 1996 [PubMed] [Google Scholar]
- 103.Garland AM, Grady EF, Payan DG, Vigna SR, Bunnett NW. Agonist-induced internalization of the substance P (NK1) receptor expressed in epithelial cells. Biochem J 303: 177–186, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gazzieri D, Trevisani M, Springer J, Harrison S, Cottrell GS, Andre E, Nicoletti P, Massi D, Zecchi S, Nosi D, Santucci M, Gerard NP, Lucattelli M, Lungarella G, Fischer A, Grady EF, Bunnett NW, Geppetti P. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free Radic Biol Med 43: 581–589, 2007 [DOI] [PubMed] [Google Scholar]
- 105.George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 319: 1536–1539, 2008 [DOI] [PubMed] [Google Scholar]
- 106.Geppetti P, Holzer P. Neurogenic Inflammation. Boca Raton, FL: CRC, 1996 [Google Scholar]
- 107.Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonca BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 95: 2857–2867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gillespie E, Leeman SE, Watts LA, Coukos JA, O'Brien MJ, Cerda SR, Farraye FA, Stucchi AF, Becker JM. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis-associated cancer. Proc Natl Acad Sci USA 108: 17420–17425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grady EF, Gamp PD, Jones E, Baluk P, McDonald DM, Payan DG, Bunnett NW. Endocytosis and recycling of neurokinin 1 receptors in enteric neurons. Neuroscience 75: 1239–1254, 1996 [DOI] [PubMed] [Google Scholar]
- 110.Grady EF, Garland AM, Gamp PD, Lovett M, Payan DG, Bunnett NW. Delineation of the endocytic pathway of substance P and its seven-transmembrane domain NK1 receptor. Mol Biol Cell 6: 509–524, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grassin-Delyle S, Buenestado A, Vallat L, Naline E, Marx S, Decocq J, Debre P, Bernard OA, Advenier C, Devillier P, Merle-Beral H. Expression and proliferative effect of hemokinin-1 in human B-cells. Peptides 32: 1027–1034, 2011 [DOI] [PubMed] [Google Scholar]
- 112.Greco SJ, Smirnov SV, Murthy RG, Rameshwar P. Synergy between the RE-1 silencer of transcription and NFkappaB in the repression of the neurotransmitter gene TAC1 in human mesenchymal stem cells. J Biol Chem 282: 30039–30050, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Gregus AM, Doolen S, Dumlao DS, Buczynski MW, Takasusuki T, Fitzsimmons BL, Hua XY, Taylor BK, Dennis EA, Yaksh TL. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci USA 109: 6721–6726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grider JR, Heuckeroth RO, Kuemmerle JF, Murthy KS. Augmentation of the ascending component of the peristaltic reflex and substance P release by glial cell line-derived neurotrophic factor. Neurogastroenterol Motil 22: 779–786, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guhl S, Lee HH, Babina M, Henz BM, Zuberbier T. Evidence for a restricted rather than generalized stimulatory response of skin-derived human mast cells to substance P. J Neuroimmunol 163: 92–101, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Guillemyn K, Kleczkowska P, Novoa A, Vandormael B, Van den Eynde I, Kosson P, Asim MF, Schiller PW, Spetea M, Lipkowski AW, Tourwe D, Ballet S. In vivo antinociception of potent mu opioid agonist tetrapeptide analogs and comparison with a compact opioid agonist-neurokinin 1 receptor antagonist chimera. Mol Brain 5: 4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo CJ, Douglas SD, Gao Z, Wolf BA, Grinspan J, Lai JP, Riedel E, Ho WZ. Interleukin-1beta upregulates functional expression of neurokinin-1 receptor (NK-1R) via NF-kappaB in astrocytes. Glia 48: 259–266, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hargreaves R, Ferreira JC, Hughes D, Brands J, Hale J, Mattson B, Mills S. Development of aprepitant, the first neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Ann NY Acad Sci 1222: 40–48, 2011 [DOI] [PubMed] [Google Scholar]
- 119.Harkins J, Roper M, Ham RG, Stewart JM. Biosynthesis of substance P in cultured mouse neuroblastoma and rat glioma cells. Brain Res 147: 405–409, 1978 [DOI] [PubMed] [Google Scholar]
- 120.Harris DT, Witten M. Aerosolized substance P protects against cigarette-induced lung damage and tumor development. Cell Mol Biol 49: 151–157, 2003 [PubMed] [Google Scholar]
- 121.Harrison JS, Corcoran KE, Joshi D, Sophacleus C, Rameshwar P. Peripheral monocytes and CD4+ cells are potential sources for increased circulating levels of TGF-beta and substance P in autoimmune myelofibrosis. Am J Hematol 81: 51–58, 2006 [DOI] [PubMed] [Google Scholar]
- 122.Hegde A, Zhang H, Moochhala SM, Bhatia M. Neurokinin-1 receptor antagonist treatment protects mice against lung injury in polymicrobial sepsis. J Leuk Biol 82: 678–685, 2007 [DOI] [PubMed] [Google Scholar]
- 123.Hens G, Raap U, Vanoirbeek J, Meyts I, Callebaut I, Verbinnen B, Vanaudenaerde BM, Cadot P, Nemery B, Bullens DM, Ceuppens JL, Hellings PW. Selective nasal allergen provocation induces substance P-mediated bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 44: 517–523, 2011 [DOI] [PubMed] [Google Scholar]
- 124.Hernandez J, Lackner A, Aye P, Mukherjee K, Tweardy DJ, Mastrangelo MA, Weinstock J, Griffiths J, D'Souza M, Dixit S, Robinson P. Substance P is responsible for physiological alterations such as increased chloride ion secretion and glucose malabsorption in cryptosporidiosis. Infect Immun 75: 1137–1143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hesketh PJ, Gralla RJ, Webb RT, Ueno W, DelPrete S, Bachinsky ME, Dirlam NL, Stack CB, Silberman SL. Randomized phase II study of the neurokinin 1 receptor antagonist CJ-11,974 in the control of cisplatin-induced emesis. J Clin Oncol 17: 338–343, 1999 [DOI] [PubMed] [Google Scholar]
- 126.Hill R. NK1 (substance P) receptor antagonists–why are they not analgesic in humans? Trends Pharmacol Sci 21: 244–246, 2000 [DOI] [PubMed] [Google Scholar]
- 127.Holzer P. Tachykinins. Berlin: Springer, 2004 [Google Scholar]
- 128.Hu CP, Feng JT, Tang YL, Zhu JQ, Lin MJ, Yu ME. LIF upregulates expression of NK-1R in NHBE cells. Mediators Inflamm 2006: 84829, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu D, Chen B, Zhu X, Tao K, Tang C, Wang J. [Substance P up-regulates the TGF-beta 1 mRNA expression of human dermal fibroblasts in vitro]. Chin J Plastic Surg 18: 234–236, 2002 [PubMed] [Google Scholar]
- 130.Huang PP, Khan I, Suhail MS, Malkmus S, Yaksh TL. Spinal botulinum neurotoxin B: effects on afferent transmitter release and nociceptive processing. PLoS One 6: e19126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hunter DD, Carrell-Jacks LA, Batchelor TP, Dey RD. Role of nerve growth factor in ozone-induced neural responses in early postnatal airway development. Am J Respir Cell Mol Biol 45: 359–365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ianowski JP, Choi JY, Wine JJ, Hanrahan JW. Substance P stimulates CFTR-dependent fluid secretion by mouse tracheal submucosal glands. Pflügers Arch 457: 529–537, 2008 [DOI] [PubMed] [Google Scholar]
- 133.Ichinose M, Miura M, Yamauchi H, Kageyama N, Tomaki M, Oyake T, Ohuchi Y, Hida W, Miki H, Tamura G, Shirato K. A neurokinin 1-receptor antagonist improves exercise-induced airway narrowing in asthmatic patients. Am J Respir Crit Care Med 153: 936–941, 1996 [DOI] [PubMed] [Google Scholar]
- 134.Iliff JJ, Fairbanks SL, Balkowiec A, Alkayed NJ. Epoxyeicosatrienoic acids are endogenous regulators of vasoactive neuropeptide release from trigeminal ganglion neurons. J Neurochem 115: 1530–1542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jarcho JM, Feier NA, Bert A, Labus JA, Lee M, Stains J, Ebrat B, Groman SM, Tillisch K, Brody AL, London ED, Mandelkern MA, Mayer EA. Diminished neurokinin-1 receptor availability in patients with two forms of chronic visceral pain. Pain 154: 987–996, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jensen D, Zhang Z, Flynn FW. Trafficking of tachykinin neurokinin 3 receptor to nuclei of neurons in the paraventricular nucleus of the hypothalamus following osmotic challenge. Neuroscience 155: 308–316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jensen DD, Sundstrom K, Flynn FW. Expression of the nuclear transport protein importin ss-1 and its association with the neurokinin 3 receptor in the rat hypothalamus following acute hyperosmotic challenge. Neuroscience 170: 1020–1027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jerde TJ, Saban R, Bjorling DE, Nakada SY. NK-2 is the predominant tachykinin receptor subtype in the swine ureter. BJU Int 83: 312–318, 1999 [DOI] [PubMed] [Google Scholar]
- 139.Jonsson M, Norrgard O, Forsgren S. Substance P and the neurokinin-1 receptor in relation to eosinophilia in ulcerative colitis. Peptides 26: 799–814, 2005 [DOI] [PubMed] [Google Scholar]
- 140.Joo NS, Cho HJ, Khansaheb M, Wine JJ. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J Clin Invest 120: 3161–3166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jorgensen R, Holliday ND, Hansen JL, Vrecl M, Heding A, Schwartz TW, Elling CE. Characterization of G-protein coupled receptor kinase interaction with the neurokinin-1 receptor using bioluminescence resonance energy transfer. Mol Pharmacol 73: 349–358, 2008 [DOI] [PubMed] [Google Scholar]
- 142.Kage RR, McGregor GPG, Thim LL, Conlon JMJ. Neuropeptide-gamma: a peptide isolated from rabbit intestine that is derived from gamma-preprotachykinin. J Neurochem 50: 1412–1417, 1988 [DOI] [PubMed] [Google Scholar]
- 143.Kangawa K, Minamino N, Fukuda A, Matsuo H. Neuromedin K: a novel mammalian tachykinin identified in porcine spinal cord. Biochem Biophys Res Commun 114: 533–540, 1983 [DOI] [PubMed] [Google Scholar]
- 144.Karagiannides I, Kokkotou E, Tansky M, Tchkonia T, Giorgadze N, O'Brien M, Leeman SE, Kirkland JL, Pothoulakis C. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci USA 103: 5207–5212, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Karagiannides I, Stavrakis D, Bakirtzi K, Kokkotou E, Pirtskhalava T, Nayeb-Hashemi H, Bowe C, Bugni JM, Nuno M, Lu B, Gerard NP, Leeman SE, Kirkland JL, Pothoulakis C. Substance P (SP)-neurokinin-1 receptor (NK-1R) alters adipose tissue responses to high-fat diet and insulin action. Endocrinology 152: 2197–2205, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karagiannides I, Torres D, Tseng YH, Bowe C, Carvalho E, Espinoza D, Pothoulakis C, Kokkotou E. Substance P as a novel anti-obesity target. Gastroenterology 134: 747–755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, Hargreaves R, Hietala J, Lines C, Beebe K, Reines S. Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biol Psychiatry 59: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 148.Khare VK, Albino AP, Reed JA. The neuropeptide/mast cell secretagogue substance P is expressed in cutaneous melanocytic lesions. J Cutan Pathol 25: 2–10, 1998 [DOI] [PubMed] [Google Scholar]
- 149.Kim BJ, Chang IY, Choi S, Jun JY, Jeon JH, Xu WX, Kwon YK, Ren D, So I. Involvement of Na+-leak channel in substance P-induced depolarization of pacemaking activity in interstitial cells of Cajal. Cell Physiol Biochem 29: 501–510, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim YS, Son JY, Kim TH, Paik SK, Dai Y, Noguchi K, Ahn DK, Bae YC. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J Comp Neurol 518: 687–698, 2010 [DOI] [PubMed] [Google Scholar]
- 151.Kimura S, Okada M, Sugita Y, Kanazawa I, Munekata E. Novel neuropeptides, neurokinin α, β isolated from porcine spinal cord. Proc Japan Acad Ser B: Phys Biol Sci 59: 101–104, 1983 [Google Scholar]
- 152.King SK, Sutcliffe JR, Ong SY, Lee M, Koh TL, Wong SQ, Farmer PJ, Peck CJ, Stanton MP, Keck J, Cook DJ, Chow CW, Hutson JM, Southwell BR. Substance P and vasoactive intestinal peptide are reduced in right transverse colon in pediatric slow-transit constipation. Neurogastroenterol Motil 22: 883–892, e234, 2010 [DOI] [PubMed] [Google Scholar]
- 153.Kitamura H, Kobayashi M, Wakita D, Nishimura T. Neuropeptide signaling activates dendritic cell-mediated type 1 immune responses through neurokinin-2 receptor. J Immunol 188: 4200–4208, 2012 [DOI] [PubMed] [Google Scholar]
- 154.Koh YH, Moochhala S, Bhatia M. Activation of neurokinin-1 receptors up-regulates substance P and neurokinin-1 receptor expression in murine pancreatic acinar cells. J Cell Mol Med 16: 1582–1592, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kong ZQ, Han M, Yang WL, Zhao YL, Fu CY, Tao Y, Chen Q, Wang R. In vitro characterization of the effects of rat/mouse hemokinin-1 on mouse colonic contractile activity: a comparison with substance P. Neuropeptides 43: 213–220, 2009 [DOI] [PubMed] [Google Scholar]
- 156.Koon HW, Shih D, Karagiannides I, Zhao D, Fazelbhoy Z, Hing T, Xu H, Lu B, Gerard N, Pothoulakis C. Substance P modulates colitis-associated fibrosis. Am J Pathol 177: 2300–2309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koon HW, Shih DQ, Hing TC, Chen J, Ho S, Zhao D, Targan SR, Pothoulakis C. Substance P induces CCN1 expression via histone deacetylase activity in human colonic epithelial cells. Am J Pathol 179: 2315–2326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Koon HW, Zhao D, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem 279: 45519–45527, 2004 [DOI] [PubMed] [Google Scholar]
- 159.Koon HW, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci USA 104: 2013–2018, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C. Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J Immunol 176: 5050–5059, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koon HW, Zhao D, Zhan Y, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves protein kinase Cdelta activation. J Pharmacol Exp Ther 314: 1393–1400, 2005 [DOI] [PubMed] [Google Scholar]
- 162.Kopp UC, Cicha MZ, Smith LA. Angiotensin blocks substance P release from renal sensory nerves by inhibiting PGE2-mediated activation of cAMP. Am J Physiol Renal Physiol 285: F472–F483, 2003 [DOI] [PubMed] [Google Scholar]
- 163.Kopp UC, Cicha MZ, Smith LA. PGE2 increases release of substance P from renal sensory nerves by activating the cAMP-PKA transduction cascade. Am J Physiol Regul Integr Comp Physiol 282: R1618–R1627, 2002 [DOI] [PubMed] [Google Scholar]
- 164.Kotani H, Hoshimaru M, Nawa H, Nakanishi S. Structure and gene organization of bovine neuromedin K precursor. Proc Natl Acad Sci USA 83: 7074–7078, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Koyama S, Sato E, Nomura H, Kubo K, Nagai S, Izumi T. Acetylcholine and substance P stimulate bronchial epithelial cells to release eosinophil chemotactic activity. J Appl Physiol 84: 1528–1534, 1998 [DOI] [PubMed] [Google Scholar]
- 166.Kradin R, MacLean J, Duckett S, Schneeberger EE, Waeber C, Pinto C. Pulmonary response to inhaled antigen: neuroimmune interactions promote the recruitment of dendritic cells to the lung and the cellular immune response to inhaled antigen. Am J Pathol 150: 1735–1743, 1997 [PMC free article] [PubMed] [Google Scholar]
- 167.Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ, Reines SA, Liu G, Snavely D, Wyatt-Knowles E, Hale JJ, Mills SG, MacCoss M, Swain CJ, Harrison T, Hill RG, Hefti F, Scolnick EM, Cascieri MA, Chicchi GG, Sadowski S, Williams AR, Hewson L, Smith D, Carlson EJ, Hargreaves RJ, Rupniak NM. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 281: 1640–1645, 1998 [DOI] [PubMed] [Google Scholar]
- 168.Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci 84: 881–885, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kubale V, Abramovic Z, Pogacnik A, Heding A, Sentjurc M, Vrecl M. Evidence for a role of caveolin-1 in neurokinin-1 receptor plasma-membrane localization, efficient signaling, and interaction with beta-arrestin 2. Cell Tissue Res 330: 231–245, 2007 [DOI] [PubMed] [Google Scholar]
- 170.Kwatra MM, Schwinn DA, Schreurs J, Blank JL, Kim CM, Benovic JL, Krause JE, Caron MG, Lefkowitz RJ. The substance P receptor, which couples to Gq/11, is a substrate of beta-adrenergic receptor kinase 1 and 2. J Biol Chem 268: 9161–9164, 1993 [PubMed] [Google Scholar]
- 171.Lagerstrom MC, Rogoz K, Abrahamsen B, Lind AL, Olund C, Smith C, Mendez JA, Wallen-Mackenzie A, Wood JN, Kullander K. A sensory subpopulation depends on vesicular glutamate transporter 2 for mechanical pain, and together with substance P, inflammatory pain. Proc Natl Acad Sci USA 108: 5789–5794, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lai JP, Cnaan A, Zhao H, Douglas SD. Detection of full-length and truncated neurokinin-1 receptor mRNA expression in human brain regions. J Neurosci Methods 168: 127–133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lai JP, Ho WZ, Kilpatrick LE, Wang X, Tuluc F, Korchak HM, Douglas SD. Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc Natl Acad Sci USA 103: 7771–7776, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lai JP, Ho WZ, Zhan GX, Yi Y, Collman RG, Douglas SD. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc Natl Acad Sci USA 98: 3970–3975, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lai JP, Lai S, Tuluc F, Tansky MF, Kilpatrick LE, Leeman SE, Douglas SD. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci USA 105: 12605–12610, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lambrecht BN, Germonpre PR, Everaert EG, Carro-Muino I, De Veerman M, de Felipe C, Hunt SP, Thielemans K, Joos GF, Pauwels RA. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol 29: 3815–3825, 1999 [DOI] [PubMed] [Google Scholar]
- 177.Lang K, Drell TL, Niggemann B, Zanker KS, Entschladen F. Neurotransmitters regulate the migration and cytotoxicity in natural killer cells. Immunol Lett 90: 165–172, 2003 [DOI] [PubMed] [Google Scholar]
- 178.Langan EA, Vidali S, Pigat N, Funk W, Lisztes E, Biro T, Goffin V, Griffiths CE, Paus R. Tumour necrosis factor alpha, interferon gamma and substance P are novel modulators of extrapituitary prolactin expression in human skin. PLoS One 8: e60819, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee CM, Kum W, Cockram CS, Teoh R, Young JD. Functional substance P receptors on a human astrocytoma cell line (U-373 MG). Brain Res 488: 328–331, 1989 [DOI] [PubMed] [Google Scholar]
- 180.Leeman SE, Hammerschlag R. Stimulation of salivary secretion by a factor extracted from hypothalamic tissue. Endocrinology 81: 803–810, 1967 [DOI] [PubMed] [Google Scholar]
- 181.Lembeck F, Starke K. [Substance P and salivary secretion]. Naunyn-Schmiedebergs Arch Exp Pathol Pharmakol 259: 375–385, 1968 [PubMed] [Google Scholar]
- 182.Lewis ST. The nocifensor system of nerves and its reactions. BMJ 194: 431–435, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li H, Leeman SE, Slack BE, Hauser G, Saltsman WS, Krause JE, Blusztajn JK, Boyd ND. A substance P (neurokinin-1) receptor mutant carboxyl-terminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. Proc Natl Acad Sci USA 94: 9475–9480, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li M, Shang YX. Inhaled corticosteroids inhibit substance P receptor expression in asthmatic rat airway smooth muscle cells. BMC Pulm Med 12: 79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li PC, Chen WC, Chang LC, Lin SC. Substance P acts via the neurokinin receptor 1 to elicit bronchoconstriction, oxidative stress, and upregulated ICAM-1 expression after oil smoke exposure. Am J Physiol Lung Cell Mol Physiol 294: L912–L920, 2008 [DOI] [PubMed] [Google Scholar]
- 186.Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD. Substance P regulates natural killer cell interferon-gamma production and resistance to Pseudomonas aeruginosa infection. Eur J Immunol 35: 1567–1575, 2005 [DOI] [PubMed] [Google Scholar]
- 187.Lim KG, Rank MA, Kita H, Patel A, Moore E. Neuropeptide levels in nasal secretions from patients with and without chronic cough. Ann Allergy Asthma Immunol 107: 360–363, 2011 [DOI] [PubMed] [Google Scholar]
- 188.Lim R, Morrill JM, Prushik SG, Reed KL, Gower AC, Leeman SE, Stucchi AF, Becker JM. An FDA approved neurokinin-1 receptor antagonist is effective in reducing intraabdominal adhesions when administered intraperitoneally, but not orally. J Gastrointest Surg 12: 1754–1761, 2008 [DOI] [PubMed] [Google Scholar]
- 189.Lindstrom E, von Mentzer B, Pahlman I, Ahlstedt I, Uvebrant A, Kristensson E, Martinsson R, Noven A, de Verdier J, Vauquelin G. Neurokinin 1 receptor antagonists: correlation between in vitro receptor interaction and in vivo efficacy. J Pharmacol Exp Ther 322: 1286–1293, 2007 [DOI] [PubMed] [Google Scholar]
- 190.Linley JE, Ooi L, Pettinger L, Kirton H, Boyle JP, Peers C, Gamper N. Reactive oxygen species are second messengers of neurokinin signaling in peripheral sensory neurons. Proc Natl Acad Sci USA 109: E1578–1586, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA, Jordt SE. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J 27: 3549–3563, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu JY, Hu JH, Zhu QG, Li FQ, Sun HJ. Substance P receptor expression in human skin keratinocytes and fibroblasts. Br J Dermatol 155: 657–662, 2006 [DOI] [PubMed] [Google Scholar]
- 193.Liu Y, Chen X, Chen H. Placental and umbilical cord levels of neurokinin B and neurokinin B receptor in pre-eclampsia. Int J Gynaecol Obstet 107: 58–59, 2009 [DOI] [PubMed] [Google Scholar]
- 194.Losco PE, Leach MW, Sinha D, Davis P, Schmahai TJ, Nomier A, Kakkar T, Reyderman L, Lynch ME. Administration of an antagonist of neurokinin receptors 1, 2, and 3 results in reproductive tract changes in beagle dogs, but not rats. Toxicol Pathol 35: 310–322, 2007 [DOI] [PubMed] [Google Scholar]
- 195.Lu B, Figini M, Emanueli C, Geppetti P, Grady EF, Gerard NP, Ansell J, Payan DG, Gerard C, Bunnett N. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat Med 3: 904–907, 1997 [DOI] [PubMed] [Google Scholar]
- 196.Lucattelli M, Fineschi S, Geppetti P, Gerard NP, Lungarella G. Neurokinin-1 receptor blockade and murine lung tumorigenesis. Am J Respir Crit Care Med 174: 674–683, 2006 [DOI] [PubMed] [Google Scholar]
- 197.Manske JM, Hanson SE. Substance-P-mediated immunomodulation of tumor growth in a murine model. Neuroimmunomodulation 12: 201–210, 2005 [DOI] [PubMed] [Google Scholar]
- 198.Mantyh CR, Pappas TN, Lapp JA, Washington MK, Neville LM, Ghilardi JR, Rogers SD, Mantyh PW, Vigna SR. Substance P activation of enteric neurons in response to intraluminal Clostridium difficile toxin A in the rat ileum. Gastroenterology 111: 1272–1280, 1996 [DOI] [PubMed] [Google Scholar]
- 199.Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science 268: 1629–1632, 1995 [DOI] [PubMed] [Google Scholar]
- 200.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 278: 275–279, 1997 [DOI] [PubMed] [Google Scholar]
- 201.Manzini S. Bronchodilatation by tachykinins and capsaicin in the mouse main bronchus. Br J Pharmacol 105: 968–972, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neurosci 17: 8129–8136, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Masu Y, Nakayama K, Tamaki H, Harada Y, Kuno M, Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. Nature 329: 836–838, 1987 [DOI] [PubMed] [Google Scholar]
- 204.Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 105: 12045–12050, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mathers AR, Tckacheva OA, Janelsins BM, Shufesky WJ, Morelli AE, Larregina AT. In vivo signaling through the neurokinin 1 receptor favors transgene expression by Langerhans cells and promotes the generation of Th1- and Tc1-biased immune responses. J Immunol 178: 7006–7017, 2007 [DOI] [PubMed] [Google Scholar]
- 206.Mathew SJ, Vythilingam M, Murrough JW, Zarate CA, Jr, Feder A, Luckenbaugh DA, Kinkead B, Parides MK, Trist DG, Bani MS, Bettica PU, Ratti EM, Charney DS. A selective neurokinin-1 receptor antagonist in chronic PTSD: a randomized, double-blind, placebo-controlled, proof-of-concept trial. Eur Neuropsychopharm 21: 221–229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci USA 105: 8784–8789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mayordomo C, Garcia-Recio S, Ametller E, Fernandez-Nogueira P, Pastor-Arroyo EM, Vinyals L, Casas I, Gascon P, Almendro V. Targeting of substance P induces cancer cell death and decreases the steady state of EGFR and Her2. J Cell Physiol 227: 1358–1366, 2012 [DOI] [PubMed] [Google Scholar]
- 209.McCarson KE, Krause JE. NK-1 and NK-3 type tachykinin receptor mRNA expression in the rat spinal cord dorsal horn is increased during adjuvant or formalin-induced nociception. J Neurosci 14: 712–720, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McConalogue K, Dery O, Lovett M, Wong H, Walsh JH, Grady EF, Bunnett NW. Substance P-induced trafficking of beta-arrestins. The role of beta-arrestins in endocytosis of the neurokinin-1 receptor. J Biol Chem 274: 16257–16268, 1999 [DOI] [PubMed] [Google Scholar]
- 211.Meltzer HY, Arvanitis L, Bauer D, Rein W. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry 161: 975–984, 2004 [DOI] [PubMed] [Google Scholar]
- 212.Meshki J, Douglas SD, Lai JP, Schwartz L, Kilpatrick LE, Tuluc F. Neurokinin 1 receptor mediates membrane blebbing in HEK293 cells through a Rho/Rho-associated coiled-coil kinase-dependent mechanism. J Biol Chem 284: 9280–9289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Meyer BH, Segura JM, Martinez KL, Hovius R, George N, Johnsson K, Vogel H. FRET imaging reveals that functional neurokinin-1 receptors are monomeric and reside in membrane microdomains of live cells. Proc Natl Acad Sci USA 103: 2138–2143, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Michelson D, Hargreaves R, Alexander R, Ceesay P, Hietala J, Lines C, Reines S. Lack of efficacy of L-759274, a novel neurokinin 1 (substance P) receptor antagonist, for the treatment of generalized anxiety disorder. Int J Neuropsychopharm 16: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 215.Milner P, Bodin P, Guiducci S, Del Rosso A, Kahaleh MB, Matucci-Cerinic M, Burnstock G. Regulation of substance P mRNA expression in human dermal microvascular endothelial cells. Clin Exp Rheumatol 22: S24–27, 2004 [PubMed] [Google Scholar]
- 216.Mori Y, Cai K, Cheng Y, Wang S, Paun B, Hamilton JP, Jin Z, Sato F, Berki AT, Kan T, Ito T, Mantzur C, Abraham JM, Meltzer SJ. A genome-wide search identifies epigenetic silencing of somatostatin, tachykinin-1, and 5 other genes in colon cancer. Gastroenterology 131: 797–808, 2006 [DOI] [PubMed] [Google Scholar]
- 217.Mou L, Kang Y, Zhou Y, Zeng Q, Song H, Wang R. Neurokinin-1 receptor directly mediates glioma cell migration by up-regulation of matrix metalloproteinase-2 (MMP-2) and membrane type 1-matrix metalloproteinase (MT1-MMP). J Biol Chem 288: 306–318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mukerji I, Ramkissoon SH, Reddy KK, Rameshwar P. Autocrine proliferation of neuroblastoma cells is partly mediated through neurokinin receptors: relevance to bone marrow metastasis. J Neurooncol 71: 91–98, 2005 [DOI] [PubMed] [Google Scholar]
- 219.Munoz M, Rosso M, Covenas R, Montero I, Gonzalez-Moles MA, Robles MJ. Neurokinin-1 receptors located in human retinoblastoma cell lines: antitumor action of its antagonist, L-732,138. Invest Ophthalmol Vis Sci 48: 2775–2781, 2007 [DOI] [PubMed] [Google Scholar]
- 220.Munoz M, Rosso M, Robles-Frias MJ, Salinas-Martin MV, Rosso R, Gonzalez-Ortega A, Covenas R. The NK-1 receptor is expressed in human melanoma and is involved in the antitumor action of the NK-1 receptor antagonist aprepitant on melanoma cell lines. Lab Invest 90: 1259–1269, 2010 [DOI] [PubMed] [Google Scholar]
- 221.Murakami K, Enkhbaatar P, Yu YM, Traber LD, Cox RA, Hawkins HK, Tompkins RG, Herndon D, Traber DL. l-Arginine attenuates acute lung injury after smoke inhalation and burn injury in sheep. Shock 28: 477–483, 2007 [DOI] [PubMed] [Google Scholar]
- 222.Murota H, Izumi M, Abd El-Latif MI, Nishioka M, Terao M, Tani M, Matsui S, Sano S, Katayama I. Artemin causes hypersensitivity to warm sensation, mimicking warmth-provoked pruritus in atopic dermatitis. J Allergy Clin Immunol 130: 671–682 e674, 2012 [DOI] [PubMed] [Google Scholar]
- 223.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci USA 106: 17615–17622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Murphy JE, Roosterman D, Cottrell GS, Padilla BE, Feld M, Brand E, Cedron WJ, Bunnett NW, Steinhoff M. Protein phosphatase 2A mediates resensitization of the neurokinin 1 receptor. Am J Physiol Cell Physiol 301: C780–C791, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature 405: 180–183, 2000 [DOI] [PubMed] [Google Scholar]
- 226.Muto Y, Sakai A, Sakamoto A, Suzuki H. Activation of NK(1) receptors in the locus coeruleus induces analgesia through noradrenergic-mediated descending inhibition in a rat model of neuropathic pain. Br J Pharmacol 166: 1047–1057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nakada SY, Jerde TJ, Bjorling DE, Saban R. In vitro contractile effects of neurokinin receptor blockade in the human ureter. J Urol 166: 1534–1538, 2001 [PubMed] [Google Scholar]
- 228.Nakamura A, Tanaka T, Imanishi A, Kawamoto M, Toyoda M, Mizojiri G, Tsukimi Y. Bidirectional regulation of human colonic smooth muscle contractility by tachykinin NK(2) receptors. J Pharmacol Sci 117: 106–115, 2011 [DOI] [PubMed] [Google Scholar]
- 229.Nakamura Y, Une Y, Miyano K, Abe H, Hisaoka K, Morioka N, Nakata Y. Activation of transient receptor potential ankyrin 1 evokes nociception through substance P release from primary sensory neurons. J Neurochem 120: 1036–1047, 2012 [DOI] [PubMed] [Google Scholar]
- 230.Nassini R, Materazzi S, Andre E, Sartiani L, Aldini G, Trevisani M, Carnini C, Massi D, Pedretti P, Carini M, Cerbai E, Preti D, Villetti G, Civelli M, Trevisan G, Azzari C, Stokesberry S, Sadofsky L, McGarvey L, Patacchini R, Geppetti P. Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. FASEB J 24: 4904–4916, 2010 [DOI] [PubMed] [Google Scholar]
- 231.Nassini R, Materazzi S, De Siena G, De Cesaris F, Geppetti P. Transient receptor potential channels as novel drug targets in respiratory diseases. Curr Opin Invest Drugs 11: 535–542, 2010 [PubMed] [Google Scholar]
- 232.Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, Viscomi AR, Pisano AR, Stokesberry S, Brunmark C, Svitacheva N, McGarvey L, Patacchini R, Damholt AB, Geppetti P, Materazzi S. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One 7: e42454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nawa H, Hirose T, Takashima H, Inayama S. Nucleotide sequences of cloned cDNAs for two types of bovine brain substance P precursor. Nature 306: 32–36, 1983 [DOI] [PubMed] [Google Scholar]
- 234.Negi O, Tominaga M, Tengara S, Kamo A, Taneda K, Suga Y, Ogawa H, Takamori K. Topically applied semaphorin 3A ointment inhibits scratching behavior and improves skin inflammation in NC/Nga mice with atopic dermatitis. J Dermatol Sci 66: 37–43, 2012 [DOI] [PubMed] [Google Scholar]
- 235.Nelson DA, Marriott I, Bost KL. Expression of hemokinin 1 mRNA by murine dendritic cells. J Neuroimmunol 155: 94–102, 2004 [DOI] [PubMed] [Google Scholar]
- 236.Nemeth J, Reglodi D, Pozsgai G, Szabo A, Elekes K, Pinter E, Szolcsanyi J, Helyes Z. Effect of pituitary adenylate cyclase activating polypeptide-38 on sensory neuropeptide release and neurogenic inflammation in rats and mice. Neuroscience 143: 223–230, 2006 [DOI] [PubMed] [Google Scholar]
- 237.Nitzan-Luques A, Devor M, Tal M. Genotype-selective phenotypic switch in primary afferent neurons contributes to neuropathic pain. Pain 152: 2413–2426, 2011 [DOI] [PubMed] [Google Scholar]
- 238.O'Connell PJ, Pingle SC, Ahern GP. Dendritic cells do not transduce inflammatory stimuli via the capsaicin receptor TRPV1. FEBS Lett 579: 5135–5139, 2005 [DOI] [PubMed] [Google Scholar]
- 239.Oboki K, Morii E, Kitamura Y. Deficient eosinophil chemotaxis-promoting activity of genetically normal mast cells transplanted into subcutaneous tissue of Mitfmi-vga9/Mitfmi-vga9 mice: comparison of the activity and mast cell distribution pattern with KitW/KitW-vMice. Am J Pathol 165: 1141–1150, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ogawa H, Azuma M, Muto S, Nishioka Y, Honjo A, Tezuka T, Uehara H, Izumi K, Itai A, Sone S. IkappaB kinase beta inhibitor IMD-0354 suppresses airway remodelling in a Dermatophagoides pteronyssinus-sensitized mouse model of chronic asthma. Clin Exp Allergy 41: 104–115, 2011 [DOI] [PubMed] [Google Scholar]
- 241.Ohmura T, Hayashi T, Satoh Y, Konomi A, Jung B, Satoh H. Involvement of substance P in scratching behaviour in an atopic dermatitis model. Eur J Pharmacol 491: 191–194, 2004 [DOI] [PubMed] [Google Scholar]
- 242.Ohmura T, Tsunenari I, Hayashi T, Satoh Y, Konomi A, Nanri H, Kawachi M, Morikawa M, Kadota T, Satoh H. Role of substance P in an NC/Nga mouse model of atopic dermatitis-like disease. Int Arch Allergy Immunol 133: 389–397, 2004 [DOI] [PubMed] [Google Scholar]
- 243.Okabe T, Hide M, Hiragun T, Morita E, Koro O, Yamamoto S. Bone marrow derived mast cell acquire responsiveness to substance P with Ca2+ signals and release of leukotriene B4 via mitogen-activated protein kinase. J Neuroimmunol 181: 1–12, 2006 [DOI] [PubMed] [Google Scholar]
- 244.Okaya T, Holthaus R, Kato A, Lentsch AB. Involvement of the neuropeptide substance P in lung inflammation induced by hepatic ischemia/reperfusion. Inflamm Res 53: 257–261, 2004 [DOI] [PubMed] [Google Scholar]
- 245.Olerud JE, Usui ML, Seckin D, Chiu DS, Haycox CL, Song IS, Ansel JC, Bunnett NW. Neutral endopeptidase expression and distribution in human skin and wounds. J Invest Dermatol 112: 873–881, 1999 [DOI] [PubMed] [Google Scholar]
- 246.Oslund KL, Hyde DM, Putney LF, Alfaro MF, Walby WF, Tyler NK, Schelegle ES. Activation of neurokinin-1 receptors during ozone inhalation contributes to epithelial injury and repair. Am J Respir Cell Mol Biol 39: 279–288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Page NM. Neurokinin B and pre-eclampsia: a decade of discovery. Reprod Biol Endocrinol 8: 4, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Page NM, Bell NJ, Gardiner SM, Manyonda IT, Brayley KJ, Strange PG, Lowry PJ. Characterization of the endokinins: human tachykinins with cardiovascular activity. Proc Natl Acad Sci USA 100: 6245–6250, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Page NM, Dakour J, Morrish DW. Gene regulation of neurokinin B and its receptor NK3 in late pregnancy and pre-eclampsia. Mol Hum Reprod 12: 427–433, 2006 [DOI] [PubMed] [Google Scholar]
- 250.Page NM, Woods RJ, Gardiner SM, Lomthaisong K, Gladwell RT, Butlin DJ, Manyonda IT, Lowry PJ. Excessive placental secretion of neurokinin B during the third trimester causes pre-eclampsia. Nature 405: 797–800, 2000 [DOI] [PubMed] [Google Scholar]
- 251.Pal K, Mathur M, Kumar P, DeFea K. Divergent beta-arrestin-dependent signaling events are dependent upon sequences within G-protein-coupled receptor C termini. J Biol Chem 288: 3265–3274, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Palma C, Bigioni M, Irrissuto C, Nardelli F, Maggi CA, Manzini S. Anti-tumour activity of tachykinin NK1 receptor antagonists on human glioma U373 MG xenograft. Br J Cancer 82: 480–487, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pan XQ, Gonzalez JA, Chang S, Chacko S, Wein AJ, Malykhina AP. Experimental colitis triggers the release of substance P and calcitonin gene-related peptide in the urinary bladder via TRPV1 signaling pathways. Exp Neurol 225: 262–273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Park CK, Bae JH, Kim HY, Jo HJ, Kim YH, Jung SJ, Kim JS, Oh SB. Substance P sensitizes P2X3 in nociceptive trigeminal neurons. J Dent Res 89: 1154–1159, 2010 [DOI] [PubMed] [Google Scholar]
- 255.Patacchini R, Santicioli P, Zagorodnyuk V, Lazzeri M, Turini D, Maggi CA. Excitatory motor and electrical effects produced by tachykinins in the human and guinea-pig isolated ureter and guinea-pig renal pelvis. Br J Pharmacol 125: 987–996, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Patak E, Pinto FM, Story ME, Pintado CO, Fleming A, Page NM, Pennefather JN, Candenas ML. Functional and molecular characterization of tachykinins and tachykinin receptors in the mouse uterus. Biol Reprod 72: 1125–1133, 2005 [DOI] [PubMed] [Google Scholar]
- 257.Patel HJ, Ramkissoon SH, Patel PS, Rameshwar P. Transformation of breast cells by truncated neurokinin-1 receptor is secondary to activation by preprotachykinin-A peptides. Proc Natl Acad Sci USA 102: 17436–17441, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pavlovic S, Liezmann C, Blois SM, Joachim R, Kruse J, Romani N, Klapp BF, Peters EM. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J Immunol 186: 848–855, 2011 [DOI] [PubMed] [Google Scholar]
- 259.Pelayo JC, Poole DP, Steinhoff M, Cottrell GS, Bunnett NW. Endothelin-converting enzyme-1 regulates trafficking and signalling of the neurokinin 1 receptor in endosomes of myenteric neurones. J Physiol 589: 5213–5230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pennefather JN, Patak E, Ziccone S, Lilley A, Pinto FM, Page NM, Story ME, Grover S, Candenas ML. Regulation of the stimulant actions of neurokinin a and human hemokinin-1 on the human uterus: a comparison with histamine. Biol Reprod 75: 334–341, 2006 [DOI] [PubMed] [Google Scholar]
- 261.Pfeiffer M, Kirscht S, Stumm R, Koch T, Wu D, Laugsch M, Schroder H, Hollt V, Schulz S. Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem 278: 51630–51637, 2003 [DOI] [PubMed] [Google Scholar]
- 262.Pinter E, Brown B, Hoult JR, Brain SD. Lack of evidence for tachykinin NK1 receptor-mediated neutrophil accumulation in the rat cutaneous microvasculature by thermal injury. Eur J Pharmacol 369: 91–98, 1999 [DOI] [PubMed] [Google Scholar]
- 263.Pinto FM, Pintado CO, Pennefather JN, Patak E, Candenas L. Ovarian steroids regulate tachykinin and tachykinin receptor gene expression in the mouse uterus. Reprod Biol Endocrinol 7: 77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pinto FM, Ravina CG, Subiran N, Cejudo-Roman A, Fernandez-Sanchez M, Irazusta J, Garrido N, Candenas L. Autocrine regulation of human sperm motility by tachykinins. Reprod Biol Endocrinol 8: 104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97: 3090–3098, 2003 [DOI] [PubMed] [Google Scholar]
- 266.Pradhan Nabzdyk L, Kuchibhotla S, Guthrie P, Chun M, Auster ME, Nabzdyk C, Deso S, Andersen N, Gnardellis C, Logerfo FW, Veves A. Expression of neuropeptides and cytokines in a rabbit model of diabetic neuroischemic wound healing. J Vasc Surg 58: 766–775, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Prado CM, Leick-Maldonado EA, Arata V, Kasahara DI, Martins MA, Tiberio IF. Neurokinins and inflammatory cell iNOS expression in guinea pigs with chronic allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol 288: L741–L748, 2005 [DOI] [PubMed] [Google Scholar]
- 268.Puneet P, Hegde A, Ng SW, Lau HY, Lu J, Moochhala SM, Bhatia M. Preprotachykinin-A gene products are key mediators of lung injury in polymicrobial sepsis. J Immunol 176: 3813–3820, 2006 [DOI] [PubMed] [Google Scholar]
- 269.Quinlan KL, Naik SM, Cannon G, Armstrong CA, Bunnett NW, Ansel JC, Caughman SW. Substance P activates coincident NF-AT- and NF-kappa B-dependent adhesion molecule gene expression in microvascular endothelial cells through intracellular calcium mobilization. J Immunol 163: 5656–5665, 1999 [PubMed] [Google Scholar]
- 270.Quinlan KL, Song IS, Naik SM, Letran EL, Olerud JE, Bunnett NW, Armstrong CA, Caughman SW, Ansel JC. VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by substance P. J Immunol 162: 1656–1661, 1999 [PubMed] [Google Scholar]
- 271.Radziszewski P, Crayton R, Zaborski J, Czlonkowska A, Borkowski A, Bossowska A, Majewski M. Multiple sclerosis produces significant changes in urinary bladder innervation which are partially reflected in the lower urinary tract functional status-sensory nerve fibers role in detrusor overactivity. Mult Scler 15: 860–868, 2009 [DOI] [PubMed] [Google Scholar]
- 272.Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V. The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res 66: 907–914, 2006 [DOI] [PubMed] [Google Scholar]
- 273.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166: 490–495, 2002 [DOI] [PubMed] [Google Scholar]
- 274.Ramalho R, Soares R, Couto N, Moreira A. Tachykinin receptors antagonism for asthma: a systematic review. BMC Pulmonary Med 11: 41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ramkissoon SH, Patel PS, Taborga M, Rameshwar P. Nuclear factor-kappaB is central to the expression of truncated neurokinin-1 receptor in breast cancer: implication for breast cancer cell quiescence within bone marrow stroma. Cancer Res 67: 1653–1659, 2007 [DOI] [PubMed] [Google Scholar]
- 276.Ratti E, Bettica P, Alexander R, Archer G, Carpenter D, Evoniuk G, Gomeni R, Lawson E, Lopez M, Millns H, Rabiner EA, Trist D, Trower M, Zamuner S, Krishnan R, Fava M. Full central neurokinin-1 receptor blockade is required for efficacy in depression: evidence from orvepitant clinical studies. J Psychopharmacol 27: 424–434, 2013 [DOI] [PubMed] [Google Scholar]
- 277.Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 127: 1123–1135, 2006 [DOI] [PubMed] [Google Scholar]
- 278.Reddy BY, Greco SJ, Patel PS, Trzaska KA, Rameshwar P. RE-1-silencing transcription factor shows tumor-suppressor functions and negatively regulates the oncogenic TAC1 in breast cancer cells. Proc Natl Acad Sci USA 106: 4408–4413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Reed KL, Fruin AB, Gower AC, Stucchi AF, Leeman SE, Becker JM. A neurokinin 1 receptor antagonist decreases postoperative peritoneal adhesion formation and increases peritoneal fibrinolytic activity. Proc Natl Acad Sci USA 101: 9115–9120, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Reinke EK, Johnson MJ, Ling C, Karman J, Lee J, Weinstock JV, Sandor M, Fabry Z. Substance P receptor mediated maintenance of chronic inflammation in EAE. J Neuroimmunol 180: 117–125, 2006 [DOI] [PubMed] [Google Scholar]
- 281.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol 52: 179–197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Renzi D, Pellegrini B, Tonelli F, Surrenti C, Calabro A. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol 157: 1511–1522, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rogers DF. Motor control of airway goblet cells and glands. Respir Physiol 125: 129–144, 2001 [DOI] [PubMed] [Google Scholar]
- 284.Roggenkamp D, Kopnick S, Stab F, Wenck H, Schmelz M, Neufang G. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol 133: 1620–1628, 2013 [DOI] [PubMed] [Google Scholar]
- 285.Roosterman D, Cottrell GS, Padilla BE, Muller L, Eckman CB, Bunnett NW, Steinhoff M. Endothelin-converting enzyme 1 degrades neuropeptides in endosomes to control receptor recycling. Proc Natl Acad Sci USA 104: 11838–11843, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. Recycling and resensitization of the neurokinin 1 receptor. Influence of agonist concentration and Rab GTPases. J Biol Chem 279: 30670–30679, 2004 [DOI] [PubMed] [Google Scholar]
- 287.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 86: 1309–1379, 2006 [DOI] [PubMed] [Google Scholar]
- 288.Rosso M, Robles-Frias MJ, Covenas R, Salinas-Martin MV, Munoz M. The NK-1 receptor is expressed in human primary gastric and colon adenocarcinomas and is involved in the antitumor action of L-733,060 and the mitogenic action of substance P on human gastrointestinal cancer cell lines. Tumour Biol 29: 245–254, 2008 [DOI] [PubMed] [Google Scholar]
- 289.Ruiz MR, Quinones AG, Diaz NL, Tapia FJ. Acute immobilization stress induces clinical and neuroimmunological alterations in experimental murine cutaneous leishmaniasis. Br J Dermatol 149: 731–738, 2003 [DOI] [PubMed] [Google Scholar]
- 290.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain 135: 271–279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Saban R, Saban MR, Nguyen NB, Lu B, Gerard C, Gerard NP, Hammond TG. Neurokinin-1 (NK-1) receptor is required in antigen-induced cystitis. Am J Pathol 156: 775–780, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Satoh J, Yamakage M. Desflurane induces airway contraction mainly by activating transient receptor potential A1 of sensory C-fibers. J Anesth 23: 620–623, 2009 [DOI] [PubMed] [Google Scholar]
- 293.Schmidlin F, Dery O, Bunnett NW, Grady EF. Heterologous regulation of trafficking and signaling of G protein-coupled receptors: beta-arrestin-dependent interactions between neurokinin receptors. Proc Natl Acad Sci USA 99: 3324–3329, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Schmidlin F, Dery O, DeFea KO, Slice L, Patierno S, Sternini C, Grady EF, Bunnett NW. Dynamin and Rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. J Biol Chem 276: 25427–25437, 2001 [DOI] [PubMed] [Google Scholar]
- 295.Scholzen TE, Brzoska T, Kalden D, Armstrong CA, Ansel JC, Luger TA. Angiotensin-converting enzyme and neutral endopeptidase terminate cutaneous inflammation. Arch Dermatol Res 292: 76, 2000 [Google Scholar]
- 296.Scholzen TE, Steinhoff M, Bonaccorsi P, Klein R, Amadesi S, Geppetti P, Lu B, Gerard NP, Olerud JE, Luger TA, Bunnett NW, Grady EF, Armstrong CA, Ansel JC. Neutral endopeptidase terminates substance P-induced inflammation in allergic contact dermatitis. J Immunol 166: 1285–1291, 2001 [DOI] [PubMed] [Google Scholar]
- 297.Scholzen TE, Steinhoff M, Sindrilaru A, Schwarz A, Bunnett NW, Luger TA, Armstrong CA, Ansel JC. Cutaneous allergic contact dermatitis responses are diminished in mice deficient in neurokinin 1 receptors and augmented by neurokinin 2 receptor blockage. FASEB J 18: 1007–1009, 2004 [DOI] [PubMed] [Google Scholar]
- 298.Sculptoreanu A, Aura Kullmann F, de Groat WC. Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur J Neurosci 27: 3171–3181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sellers DJ, McKay N. Developments in the pharmacotherapy of the overactive bladder. Curr Opin Urol 17: 223–230, 2007 [DOI] [PubMed] [Google Scholar]
- 300.Shaffer AD, Ball CL, Robbins MT, Ness TJ, Randich A. Effects of acute adult and early-in-life bladder inflammation on bladder neuropeptides in adult female rats. BMC Urol 11: 18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Shaik-Dasthagirisaheb YB, Varvara G, Murmura G, Saggini A, Potalivo G, Caraffa A, Antinolfi P, Tete S, Tripodi D, Conti F, Cianchetti E, Toniato E, Rosati M, Conti P, Speranza L, Pantalone A, Saggini R, Theoharides TC, Pandolfi F. Vascular endothelial growth factor (VEGF), mast cells and inflammation. Int J Immunopath Pharmacol 26: 327–335, 2013 [DOI] [PubMed] [Google Scholar]
- 302.Shepherd AJ, Beresford LJ, Bell EB, Miyan JA. Mobilisation of specific T cells from lymph nodes in contact sensitivity requires substance P. J Neuroimmunol 164: 115–123, 2005 [DOI] [PubMed] [Google Scholar]
- 303.Shi X, Gao NR, Guo QM, Yang YJ, Huo MD, Hu HL, Friess H. Relationship between overexpression of NK-1R, NK-2R and intestinal mucosal damage in acute necrotizing pancreatitis. World J Gastroenterol 9: 160–164, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shigemoto R, Yokota Y, Tsuchida K, Nakanishi S. Cloning and expression of a rat neuromedin K receptor cDNA. J Biol Chem 265: 623–628, 1990 [PubMed] [Google Scholar]
- 305.Shimizu Y, Matsuyama H, Shiina T, Takewaki T, Furness JB. Tachykinins and their functions in the gastrointestinal tract. Cell Mol Life Sci 65: 295–311, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Siebenhaar F, Sharov AA, Peters EM, Sharova TY, Syska W, Mardaryev AN, Freyschmidt-Paul P, Sundberg JP, Maurer M, Botchkarev VA. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata). J Invest Dermatol 127: 1489–1497, 2007 [DOI] [PubMed] [Google Scholar]
- 307.Simeonidis S, Castagliuolo I, Pan A, Liu J, Wang CC, Mykoniatis A, Pasha A, Valenick L, Sougioultzis S, Zhao D, Pothoulakis C. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci USA 100: 2957–2962, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Simonsen KB, Juhl K, Steiniger-Brach B, Nielsen SM. Novel NK(3) receptor antagonists for the treatment of schizophrenia and other CNS indications. Curr Opin Drug Discov Dev 13: 379–388, 2010 [PubMed] [Google Scholar]
- 309.Sinclair SR, Kane SA, Van der Schueren BJ, Xiao A, Willson KJ, Boyle J, de Lepeleire I, Xu Y, Hickey L, Denney WS, Li CC, Palcza J, Vanmolkot FH, Depre M, Van Hecken A, Murphy MG, Ho TW, de Hoon JN. Inhibition of capsaicin-induced increase in dermal blood flow by the oral CGRP receptor antagonist, telcagepant (MK-0974). Br J Clin Pharmacol 69: 15–22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Singh D, Joshi DD, Hameed M, Qian J, Gascon P, Maloof PB, Mosenthal A, Rameshwar P. Increased expression of preprotachykinin-I and neurokinin receptors in human breast cancer cells: implications for bone marrow metastasis. Proc Natl Acad Sci USA 97: 388–393, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sio SW, Puthia MK, Lu J, Moochhala S, Bhatia M. The neuropeptide substance P is a critical mediator of burn-induced acute lung injury. J Immunol 180: 8333–8341, 2008 [DOI] [PubMed] [Google Scholar]
- 312.Sitkauskiene B, Dicpinigaitis PV. Effect of smoking on cough reflex sensitivity in humans. Lung 188 Suppl 1: S29–32, 2010 [DOI] [PubMed] [Google Scholar]
- 313.Smyth CM, Akasheh N, Woods S, Kay E, Morgan RK, Thornton MA, O'Grady A, Cummins R, Sheils O, Smyth P, Gleich GJ, Murray FM, Costello RW. Activated eosinophils in association with enteric nerves in inflammatory bowel disease. PLoS One 8: e64216, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24: 1217–1281, 2001 [DOI] [PubMed] [Google Scholar]
- 315.Song IS, Bunnett NW, Olerud JE, Harten B, Steinhoff M, Brown JR, Sung KJ, Armstrong CA, Ansel JC. Substance P induction of murine keratinocyte PAM 212 interleukin 1 production is mediated by the neurokinin 2 receptor (NK-2R). Exp Dermatol 9: 42–52, 2000 [DOI] [PubMed] [Google Scholar]
- 316.Southwell BR, Seybold VS, Woodman HL, Jenkinson KM, Furness JB. Quantitation of neurokinin 1 receptor internalization and recycling in guinea-pig myenteric neurons. Neuroscience 87: 925–931, 1998 [DOI] [PubMed] [Google Scholar]
- 317.Southwell BR, Woodman HL, Royal SJ, Furness JB. Movement of villi induces endocytosis of NK1 receptors in myenteric neurons from guinea-pig ileum. Cell Tissue Res 292: 37–45, 1998 [DOI] [PubMed] [Google Scholar]
- 318.Spina D, Page CP. Regulating cough through modulation of sensory nerve function in the airways. Pulm Pharmacol Ther 5539: 00085–00080, 2013 [DOI] [PubMed] [Google Scholar]
- 319.Springer J, Groneberg DA, Dinh QT, Quarcoo D, Hamelmann E, Braun-Dullaeus RC, Geppetti P, Anker SD, Fischer A. Neurokinin-1 receptor activation induces reactive oxygen species and epithelial damage in allergic airway inflammation. Clin Exp Allergy 37: 1788–1797, 2007 [DOI] [PubMed] [Google Scholar]
- 320.Stander S, Siepmann D, Herrgott I, Sunderkotter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One 5: e10968, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Staniek V, Misery L, Dezutter-Dambuyant C, Claudy A, Schmitt D. Expression of neuropeptides on human epidermal Langerhans cells. Adv Exp Med Biol 378: 147–150, 1995 [DOI] [PubMed] [Google Scholar]
- 322.Steensland P, Simms JA, Nielsen CK, Holgate J, Bito-Onon JJ, Bartlett SE. The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol. PLoS One 5: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Stewart JP, Kipar A, Cox H, Payne C, Vasiliou S, Quinn JP. Induction of tachykinin production in airway epithelia in response to viral infection. PLoS One 3: e1673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003 [DOI] [PubMed] [Google Scholar]
- 325.Sturiale S, Barbara G, Qiu B, Figini M, Geppetti P, Gerard N, Gerard C, Grady EF, Bunnett NW, Collins SM. Neutral endopeptidase (EC 3.42411) terminates colitis by degrading substance P. Proc Natl Acad Sci USA 96: 11653–11658, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Szallasi A, Cruz F, Geppetti P. TRPV1: a therapeutic target for novel analgesic drugs? Trends Mol Med 12: 545–554, 2006 [DOI] [PubMed] [Google Scholar]
- 327.Takahashi K, Tanaka A, Hara M, Nakanishi S. The primary structure and gene organization of human substance P and neuromedin K receptors. Eur J Biochem 204: 1025–1033, 1992 [DOI] [PubMed] [Google Scholar]
- 328.Takasusuki T, Yaksh TL. Regulation of spinal substance p release by intrathecal calcium channel blockade. Anesthesiology 115: 153–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Takeda M, Miyake M, Muto T, Kamijima M, Sakamoto T. Proliferation of sensory C-fibers and subsequent neurogenic inflammation in rat airway induced by inhaled lipopolysaccharide. Neurotoxicology 32: 954–962, 2011 [DOI] [PubMed] [Google Scholar]
- 330.Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci 12: 1293–1299, 2009 [DOI] [PubMed] [Google Scholar]
- 331.Tansky MF, Pothoulakis C, Leeman SE. Functional consequences of alteration of N-linked glycosylation sites on the neurokinin 1 receptor. Proc Natl Acad Sci USA 104: 10691–10696, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Tatemoto K, Lundberg JM, Jörnvall H, Mutt V. Neuropeptide K: isolation, structure and biological activities of a novel brain tachykinin. Biochem Biophys Res Commun 128: 947–953, 1985 [DOI] [PubMed] [Google Scholar]
- 333.Tebas P, Tuluc F, Barrett JS, Wagner W, Kim D, Zhao H, Gonin R, Korelitz J, Douglas SD. A randomized, placebo controlled, double masked phase IB study evaluating the safety and antiviral activity of aprepitant, a neurokinin-1 receptor antagonist in HIV-1 infected adults. PLoS One 6: e24180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Tillisch K, Labus J, Nam B, Bueller J, Smith S, Suyenobu B, Siffert J, McKelvy J, Naliboff B, Mayer E. Neurokinin-1-receptor antagonism decreases anxiety and emotional arousal circuit response to noxious visceral distension in women with irritable bowel syndrome: a pilot study. Aliment Pharmacol Therapeut 35: 360–367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41: 354–358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Torricelli M, Giovannelli A, Leucci E, Florio P, De Falco G, Torres PB, Reis FM, Leoncini L, Petraglia F. Placental neurokinin B mRNA expression increases at preterm labor. Placenta 28: 1020–1023, 2007 [DOI] [PubMed] [Google Scholar]
- 337.Tregear GW, Niall H, Potts JT, Leeman S, Chang MM. Synthesis of substance P. Nature 232: 87–89, 1971 [DOI] [PubMed] [Google Scholar]
- 338.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104: 13519–13524, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Vera PL, Wang X, Bucala RJ, Meyer-Siegler KL. Intraluminal blockade of cell-surface CD74 and glucose regulated protein 78 prevents substance P-induced bladder inflammatory changes in the rat. PLoS One 4: e5835, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Vishalakumar S, Patel H, Moharita AL, Harrison JS, Rameshwar P. The anti-proliferative effect of neurokinin-A on hematopoietic progenitor cells is partly mediated by p53 activating the 5' flanking region of neurokinin-2 receptor. Cell Signal 18: 422–432, 2006 [DOI] [PubMed] [Google Scholar]
- 341.Von Euler US, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol 72: 74–87, 1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Von Zastrow M, Williams JT. Modulating neuromodulation by receptor membrane traffic in the endocytic pathway. Neuron 76: 22–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wang X, Douglas SD, Peng JS, Zhou DJ, Wan Q, Ho WZ. An in vitro model of morphine withdrawal manifests the enhancing effect on human immunodeficiency virus infection of human T lymphocytes through the induction of substance P. Am J Pathol 169: 1663–1670, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003 [DOI] [PubMed] [Google Scholar]
- 345.Watson JW, Gonsalves SF, Fossa AA, McLean S, Seeger T, Obach S, Andrews PL. The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol 115: 84–94, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Weinstock JV. The role of substance P, hemokinin and their receptor in governing mucosal inflammation and granulomatous responses. Front Biosci 9: 1936–1943, 2004 [DOI] [PubMed] [Google Scholar]
- 347.Weinstock JV, Blum A, Metwali A, Elliott D, Arsenescu R. IL-18 and IL-12 signal through the NF-kappa B pathway to induce NK-1R expression on T cells. J Immunol 170: 5003–5007, 2003 [DOI] [PubMed] [Google Scholar]
- 348.Weinstock JV, Blum A, Metwali A, Elliott D, Bunnett N, Arsenescu R. Substance P regulates Th1-type colitis in IL-10 knockout mice. J Immunol 171: 3762–3767, 2003 [DOI] [PubMed] [Google Scholar]
- 349.Wibberley A, Chen Z, Hu E, Hieble JP, Westfall TD. Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. Br J Pharmacol 138: 757–766, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Xie C, Wang DH. Ablation of transient receptor potential vanilloid 1 abolishes endothelin-induced increases in afferent renal nerve activity: mechanisms and functional significance. Hypertension 54: 1298–1305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Xie L, Takahara M, Nakahara T, Oba J, Uchi H, Takeuchi S, Moroi Y, Furue M. CD10-bearing fibroblasts may inhibit skin inflammation by down-modulating substance P. Arch Dermatol Res 303: 49–55, 2011 [DOI] [PubMed] [Google Scholar]
- 352.Xu JT, Zhao X, Yaster M, Tao YX. Expression and distribution of mTOR, p70S6K, 4E-BP1, and their phosphorylated counterparts in rat dorsal root ganglion and spinal cord dorsal horn. Brain Res 1336: 46–57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Xu Q, Fitzsimmons B, Steinauer J, O'Neill A, Newton AC, Hua XY, Yaksh TL. Spinal phosphinositide 3-kinase-Akt-mammalian target of rapamycin signaling cascades in inflammation-induced hyperalgesia. J Neurosci 31: 2113–2124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yaraee R, Ebtekar M, Ahmadiani A, Sabahi F, Ghazanfari T. The effect of substance P on nitric oxide production by HSV-1 infected macrophages. Int Immunopharmacol 7: 135–139, 2007 [DOI] [PubMed] [Google Scholar]
- 355.Yokota Y, Sasai Y, Tanaka K, Fujiwara T, Tsuchida K, Shigemoto R, Kakizuka A, Ohkubo H, Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem 264: 17649–17652, 1989 [PubMed] [Google Scholar]
- 356.Yu YJ, Arttamangkul S, Evans CJ, Williams JT, von Zastrow M. Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of mu-opioid receptors in CNS neurons. J Neurosci 29: 222–233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yuan M, Wen-Xia Z, Jun-Ping C, Yong-Xiang Z. Age-related changes in the oestrous cycle and reproductive hormones in senescence-accelerated mouse. Reprod Fertil Dev 17: 507–512, 2005 [DOI] [PubMed] [Google Scholar]
- 358.Zakko S, Barton G, Weber E, Dunger-Baldauf C, Ruhl A. Randomised clinical trial: the clinical effects of a novel neurokinin receptor antagonist, DNK333, in women with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 33: 1311–1321, 2011 [DOI] [PubMed] [Google Scholar]
- 359.Zee ED, Schomberg S, Carpenter TC. Hypoxia upregulates lung microvascular neurokinin-1 receptor expression. Am J Physiol Lung Cell Mol Physiol 291: L102–L110, 2006 [DOI] [PubMed] [Google Scholar]
- 360.Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, Zhao ZQ. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. J Neurosci 27: 12067–12077, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zhang MM, Ji W, Pei LY, Wang W, Chen T, Wang W, Li H, Zhang T, Wu SX, Li YQ. Acute colitis induces neurokinin 1 receptor internalization in the rat lumbosacral spinal cord. PLoS One 8: e59234, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat Immunol 1: 392–397, 2000 [DOI] [PubMed] [Google Scholar]
- 363.Zhao D, Kuhnt-Moore S, Zeng H, Pan A, Wu JS, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves Rho family small GTPases. Biochem J 368: 665–672, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]