Abstract
The cellular and circuit mechanisms generating the rhythm of breathing in mammals have been under intense investigation for decades. Here, we try to integrate the key discoveries into an updated description of the basic neural processes generating respiratory rhythm under in vivo conditions.
Breathing is one of the perpetual rhythms of life, metabolically supporting all physiological processes in the body. Although there are many facets to the problem of respiratory neural control, a preoccupation of neurophysiologists over the past several decades has been the quest to uncover the fundamental neural processes generating the respiratory rhythm at the core of the neural control system.
The current conundrum in this endeavor is deciphering how rhythmic activity in the brain stem respiratory network emerges from the dynamic interplay of cellular biophysical and circuit-based synaptic processes. This task is challenging, since it requires intracellular recordings from defined subsets of neurons to reveal how biophysical properties of neurons are integrated with synaptic interactions within the active networks. A specific aspect concerns the in vivo contributions of intrinsic rhythmogenic properties of neurons in the pre-Bötzinger complex (pre-BötC), which was discovered in the early 1990s (87) and is now established to be critical for inspiratory rhythm generation. This structure has been the focus of numerous studies that have yielded important mechanistic insights (e.g., see Refs. 23, 91). Interactions of pre-BötC neurons with other brain stem respiratory circuits in vivo, such as within the adjacent Bötzinger complex (BötC) (52), which generates post-inspiratory and expiratory activities (72, 79), must also be considered to understand rhythm generation within the context of cellular and network processes producing the inspiratory, post-inspiratory, and expiratory phases of the normal respiratory cycle in vivo (81, 89).
We review evidence based on cellular recordings suggesting that, although pre-BötC excitatory circuits have intrinsic rhythmogenic capabilities that may operate autonomously under certain conditions to pace inspiration, rhythm generation normally involves dynamic adjustment through network interactions. Within the core rhythm-generating circuitry in the ventrolateral medulla, inhibitory synaptic interactions involving BötC circuits exert an effective control of the membrane potentials of pre-BötC neurons so that endogenous “pacemaker” currents cannot automatically become active to drive inspiratory bursting activity. Synaptic inhibition is also critical for the basic three-phase organization of the respiratory cycle, during which glycinergic inhibition functions to reset activity of inspiratory, post-inspiratory, and expiratory neurons. The emerging picture is that respiratory rhythm generation involves an exquisite alliance of cellular biophysical and synaptic processes controlling rhythm-generating neurons during unconscious and conscious breathing in vivo.
The Eupneic Breathing Rhythm In Vivo
Neural respiratory control is far too sophisticated to simply drive an automatic pump device for lung ventilation, and respiratory rhythm generation in vivo involves more complex cellular and circuit operations than just producing inspiration. This is easily identifiable in the diverse patterns of respiratory neuronal activity in the brain stem during eupneic breathing that show multiple activity phases (FIGURES 1, A AND B, AND 2) during a normal respiratory cycle. Even phrenic nerve (72) (FIGURE 1, A AND B) activity reveals that inspiration is not plain. It starts with a synchronized onset of discharge that steadily accumulates to maximum but suddenly ends with a complete breakdown of activity. This is followed by a secondary declining bursting called post-inspiratory (post-I) “after-discharge.” Such post-I discharge represents the important active phase of the so-called “passive” exhalation controlled by upper airway adductor muscles (83) such as the thyroarytenoid (TA) muscle to narrow the airway, providing a mechanical brake of expiratory airflow allowing continued gas exchange in the lungs (FIGURE 1C) (28).*
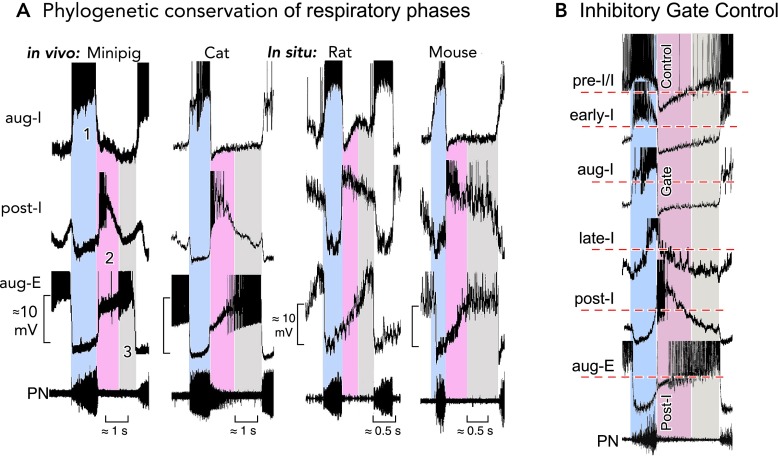
FIGURE 1.
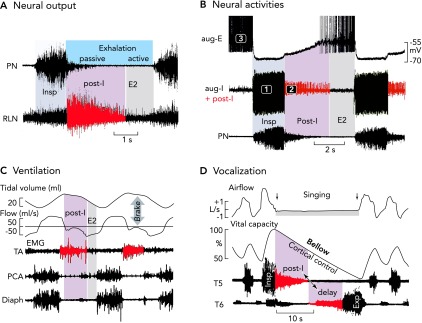
Neural and mechanical phases of a respiratory cycle in vivo
A: phrenic and recurrent laryngeal (RLN) nerve recordings in the anesthetized cat and rat reveal an augmenting inspiratory burst that ends abruptly. Thereafter, there appears a declining post-inspiratory discharge particularly strong in the RLN representing the neural control of laryngeal adductor muscles (83). B: simultaneous in vivo triplet recordings from three characteristic types of neuron in the anesthetized cat reveal a regular sequence of inspiratory (phase 1), post-inspiratory (phase 2), and late-expiratory (phase 3) discharges (action potentials are truncated) (77). C: the post-inspiratory discharge of laryngeal nerves activates a mechanical brake of the expiratory airflow and a holding of lung volume. Recordings are from the thyroarytenoid constrictor muscle (TA), the posterior cricoarythyoid dilator muscle (PCA), and the inspiratory diaphragm (Diaph) of halothane anesthetized lamb (see modified Figure 5 from Ref. 28). D: post-inspiratory activity controls vocalization. Recordings were from human inspiratory intercostal muscles in T5 and expiratory muscles in T6, and clearly show a significance of the post-inspiratory control of inspiratory and expiratory muscle activity during singing, which requires tight control of the breathing rhythm (85).
FIGURE 2.
Characteristic neuronal activities revealing a conserved respiratory network operation
A: the three-phased organization of respiratory activities in the ventrolateral medulla containing the pre-BötC and BötC regions is highly conserved in mammalian species. Characteristic patterns of neuronal discharges during each phase (1–3) and the underlying temporal fluctuations of membrane potentials revealed by intracellular recordings are very similar in mini-pig (41) and cat (72) in vivo, as well as in rat and mouse brain stem-spinal cord preparations in situ (9). Recordings are magnified in each panel to emphasize underlying membrane potential trajectories. Action potentials have been truncated. B: aligning the activities (truncated action potentials) of different types of respiratory neurons in the pre-BötC and BötC (from the cat in this illustration) to the rhythm defined by the output phrenic nerve discharges identifies post-I inhibition that produces dominant inhibitory synaptic volleys in pre-I/I, early-I, aug-I, and aug-E/E2 neurons and, therefore, exerts an effective control of rhythm generation by activity and voltage resetting, as originally described by Richter (75).
The neural processes engaged during eupneic breathing are thus designed to generate rhythmic patterns of coordinated inspiratory, post-inspiratory, and expiratory neural activity (77) (FIGURE 1B). As developed in the sections that follow, this three-phase organization and underlying circuit architecture, which have been conserved phylogenetically in mammals, appear to provide the format and a remarkably flexible neural substrate for rhythm generation in vivo. This organization also provides the conditions for coordinating the rhythm, especially via post-inspiratory phase activity, during various motor acts such as vocalization (FIGURE 1D, and see footnote) where rhythm generation must be tightly controlled.
The Network Organization has been Conserved through Mammalian Phylogeny
It is not astonishing that the three-phased organization of the respiratory cycle is highly conserved in mammalian species. Comparison of characteristic neuronal discharges and underlying synaptic activities are essentially identical in mini-pig, cat, rat, and mouse despite major differences in breathing frequencies (FIGURE 2A; Refs. 9, 10, 42, 72). Always in vivo, as well as within “in situ” experimental preparations (60, 89) that preserve functionally intact brain stem-spinal cord circuitry, there is a sequence of integrated excitatory and inhibitory synaptic volleys (ESVs and ISVs) revealing a three-phased organization distinguishing augmenting inspiratory (aug-I) from the declining post-inspiratory (post-I) and augmenting expiratory (aug-E/E2) phases (FIGURE 1B).
The networks of all these species in the various experimental preparations show antagonistic synaptic inhibition between aug-I and aug-E neurons (7, 72, 82). They also reveal an efficient inspiration terminating inhibitory process, presumed to be mediated by glycinergic inhibitory postsynaptic potentials (IPSPs), during late-inspiration and Where are the critical interacting core circuits generating the three phases of the respiratory cycle? The respiratory network is not just a noeud vital (24) in the brain stem, but it developed into a distributed network connecting respiratory regions of the medulla and pons (50). Here, we give only a short summary of pertinent structures involved in rhythm generation and its control in the intact system as described in several publications (3, 89, 91) that have dealt in detail with the distributed organization of the respiratory network. The pre-BötC and BötC in the ventrolateral medulla are considered essential interacting core structures, the former for rhythmic inspiratory phase and the latter for post-I and E-2 phase generation (72, 79).
The pre-BötC functions as an excitatory “kernel,” consisting of bilaterally coupled excitatory circuits including endogenously active burster neurons (13, 36, 37, 61, 62, 68, 87, 93). Tracing and connectivity studies showed that this structure interconnects with the more rostrally localized BötC, containing most post-I and expiratory (aug-E/E2) neurons (4, 22, 52) and thus functions as the kernel structure for generating post-inspiratory and late expiratory activity. These excitatory and inhibitory circuits are controlled by convergent inputs, including from the parafacial region or retrotrapezoid nucleus, with its chemosensitive neurons providing tonic excitation (26, 57) [see Refs. 20, 97; see Congenital Central Hypoventilation Syndrome (CCHS)/Ondine Syndrome when it fails (20, 96)], and inputs from the dorsal group of respiratory neurons in the nuclei of the solitary tract. The latter receives ongoing tonic activation from arterial chemoreceptors, providing a vital excitatory drive (40, 43), and from rhythmically activated lung stretch receptor afferent inputs that, via transmission through the nuclei of the solitary tract (NTS), control inspiratory termination after lung inflation by late- and post-inspiratory synaptic inhibition (5, 7). More rostrally in the pons, the Kölliker-Fuse and parabrachial nuclei are relay nuclei for reflex and higher-order CNS commands regulating breathing, including control of post-I activity (19, 33, 56, 69, 89). This powerful control can be appreciated by considering, for example, the dive response that automatically stops breathing and closes the glottis when the face is submerged under water (21). We emphasize that, although rhythmic patterns of respiratory activity can be generated in their absence (see Ref. 91), the excitatory afferent inputs from the pons appear to be necessary for generating the normal three-phase respiratory cycle (1, 56). There are also many afferent inputs from cortical and subcortical brain regions, which control conscious and behavioral adjustment of breathing.
The Respiratory Network Operates Beyond Pacemakers
Endogenous rhythmic inspiratory bursting activity, albeit with a pattern that is different from the normal eupneic pattern, persists even when the pre-BötC is isolated in a slice from the neonatal rodent medulla in vitro (37, 87) and synaptic inhibition is blocked pharmacologically (e.g., Ref. 30). The immediate conclusion was that respiratory rhythm generation in the isolated pre-BötC could occur by excitatory circuits incorporating endogenous bursting neurons (eBNs), which were called “pacemakers” (FIGURE 3A1). All experimental observations to date indeed suggest that subpopulations of inspiratory neurons in the isolated pre-BötC have the tendency to burst intrinsically, particularly a core population of bilaterally connected excitatory (glutamatergic) neurons (36). The debates that have ensued since the discovery of eBNs in pre-BötC circuits (87) have centered on the cellular mechanisms underlying rhythmic bursting behavior of these cells, and particularly on their functional role when they are embedded within interacting circuits (77, 88, 90) of intact networks where neuronal activity is heavily controlled by ISVs and ESVs.
FIGURE 3.
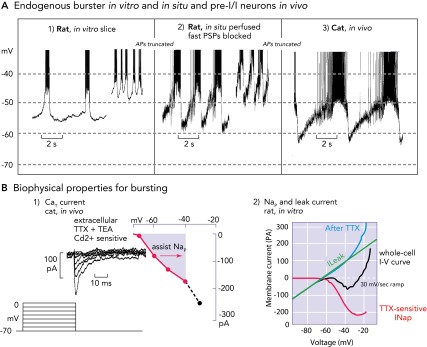
Biophysical properties and bursting behavior of pre-BötC inspiratory neurons
A: subpopulations of rodent pre-BötC neurons exhibit voltage-dependent endogenous rhythmic bursting in vitro (A1) after blockade of fast excitatory synaptic transmission (adapted from Ref. 36 and used with permission) or in situ (A2) after blockade of fast synaptic transmission (93). The membrane potentials for onset of rhythmic bursting in all cases is −60 mV, and the bursting frequency increases progressively with depolarization up to a baseline at approximately −45 mV. Identified eBNs reveal similar interburst voltage trajectories as recorded from pre-I/I neurons in vivo (A3), some of which may have intrinsic bursting behavior (58, 64). Recorded in vivo, these neurons also start to depolarize for bursting at a voltage range of approximately −60 mV. As synaptic interactions are intact in vivo, the membrane potential trajectory is controlled by postsynaptic inhibition during the post-inspiratory phase, during which endogenous bursting is suppressed. All action potentials are truncated. B1: when conditioned with hyperpolarizing pre-pulses, all groups of respiratory neurons generate a CaT current already at negative voltages of −80 mV that can depolarize neurons to spike activation threshold (64). B2: the underlying biophysical properties for in vitro and in situ endogenous bursting are primarily a TTX-sensitive persistent Na+ channel (Nap) and an ohmic-like Kleak conductance (34). The Nap current-voltage (I-V) relation (red curve), obtained from a slow voltage clamp ramp protocol applied to in vitro eBNs, is revealed after blocking the current with TTX and subtracting the resultant I-V relation from the whole cell I-V curve measured before TTX application. The K+-dominated leak I-V relation (green line) is obtained from the linear region of the whole-cell I-V curve (after Ref. 34).
Rhythm generation in the isolated pre-BötC involves cellular and circuit processes for regenerative initiation and termination of inspiratory bursts. The search for the biophysical basis for these processes has identified several ion channels contributing to rhythmic bursting of pre-BötC neurons and circuits. These include voltage-activated, slowly inactivating, “persistent” Na+ channels (NaP) (FIGURE 3B2) that have remarkable rhythmogenic capabilities (12, 13, 17, 34, 61, 93), and the K+-dominated leak currents (Kleak) (FIGURE 3B2) that contribute to burst termination and thus burst frequency control (13, 34, 35). Ca2+-activated nonselective cationic current (CAN) has been proposed to contribute to burst augmentation in vitro (54, 59, 62). Computer models (12, 29) have suggested how a heterogeneous network of coupled excitatory neurons incorporating these Na+-, Ca2+-, and K+-dependent conductance mechanisms can generate rhythmic inspiratory bursting activity with a frequency tunable by tonic input excitation. This in vitro voltage-dependent frequency control reflects an impressive mechanism but cannot be interpreted to represent the primary mechanism of frequency regulation in vivo (FIGURE 3, A1 AND A2).
The drawback for understanding rhythm generation in vivo, however, was that the voltage range for sufficient activation of the rhythmic burst-generating Nap channels is quite below −60 mV (FIGURE 3A1) (34). Under mature in vivo situations, the membrane potentials of many inspiratory neurons range below −60 mV and can reach a level of below −80 mV during ISVs (FIGURE 3A3) (72). A process that seems to compensate is a low voltage-activated CaT current (63) that recovers from inactivation during preceding hyperpolarizing ISVs and then produces an effective rebound response to elevate the voltage to NaP and even NaV activation threshold. But the critical, slow voltage-dependent inactivation property of Nap required for burst termination and associated slow recovery from inactivation, which requires several seconds during the interval between bursts at −60 mV (13), seems to be suppressed in the intact system. Furthermore, some eBNs, at least as analyzed at 31°C in situ, exhibit very low burst frequencies and burst durations that were much longer than those when the neurons are operating in the intact network (93). Consequently, eBNs alone might not be able to control inspiratory burst onset and subsequent termination under in vivo conditions, meaning that the term pacemaker is misleading. This conclusion originates from reviewing the data from experiments on the in situ perfused rat brain stem-spinal cord preparation clarifying that identified eBNs start rhythmic bursting already at −60 mV (personal communication by J. F. R. Paton) (FIGURE 3A2). Also, like in vivo (61), pharmacological block of NaP in this in situ preparation does not disrupt rhythmogenesis, except when the pre-BötC is physically disconnected from the BötC and more rostral brain stem circuits (89) or in pathological conditions such as hypoxia-induced gasping (61), when neurons depolarize due to failure of synaptic inhibition (74).
Therefore, other activity-dependent biophysical properties that were verified in vivo have to be considered in addition: there is a high-voltage-activated CaL current that is activated during burst discharges (63), generating a strong Ca2+ influx and intracellular Ca2+ accumulation (25), which may activate CAN (29, 59, 62, 66) that enhances inspiratory burst amplitudes, and also activates a big conductance Ca2+-activated K+ current (bKCa). The latter bKCa current causes significant spike frequency adaptation in all types of respiratory neurons (64) and contributes to termination of inspiratory bursts (11, 100).
The conclusions, important for the mechanisms discussed below, are that 1) inspiratory neurons in pre-BötC circuits of neonatal and mature preparations have combinations of biophysical properties that naturally promote bursting; 2) eBNs cannot operate like isolated “rhythmogenic pacemaker” neurons when embedded within intact circuits because they are under synaptic control, which includes excitatory interactions that synchronize multi-cell bursting activity and inhibitory control that can “gate” the onset and offset of bursting; and 3) intrinsically activated bursting activity can become operative when the excitatory drive to the network is strong enough to elevate membrane potentials above −60 mV.
Pre-I/I Neurons and Two Populations of Early-Inspiratory Neurons
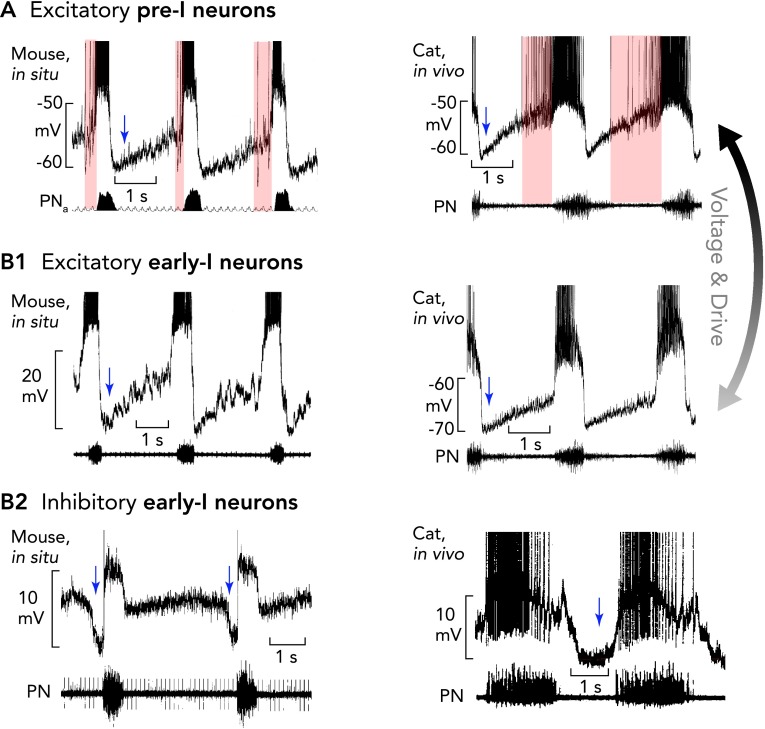
A current hypothesis is that, during normal breathing in vivo, a subpopulation of pre-BötC neurons with spiking that starts before and continues through inspiration, called pre-I/I neurons (FIGURE 3A3) (6, 8, 15, 101), are an essential component of the inspiratory rhythm generator that drives the onset of inspiration. Some of these neurons seem to have eBN properties. Indeed, models of a heterogeneous population of synaptically coupled excitatory neurons with NaP and receiving tonic excitation, show that the neurons with highest excitatory inputs will start exhibiting such pre-I/I spiking patterns (12, 89). In vivo, pre-I/I neurons/eBNs with less depolarizing excitatory input will, therefore, exhibit only an early-I spike discharge pattern (FIGURE 4, A–B1) (12, 64).
FIGURE 4.
Pre-I/I and two types of early-I neurons
The pre-BötC contains excitatory pre-I/I neurons (A) and two types of early-I spiking neurons [excitatory (B1) and inhibitory (B2)], which are considered to be important for inspiratory phase generation (pre-I/I and early-I excitatory neurons) and coordinating the inspiratory-expiratory phase transition (early-I inhibitory neurons). Recordings in mice are shown in at left and recordings in anesthetized cats at right. All action potentials are truncated. A and B1: characteristic of the pre-I/I neurons (onset and duration of their variable pre-I firing is indicated by pink areas) and early-I excitatory neurons is a lack of, or only weak, synaptic inhibition during stage 2 expiration. When hyperpolarized, pre-I/I neurons acquire an activity pattern similar to early-I excitatory neurons. The membrane potential trajectories and rapid onset of spiking of early-I excitatory neurons, which are thought to be part of the heterogenous excitatory neuron population critical for inspiratory phase generation, probably result from lower tonic excitation that regulate their busting behavior in the intact network. B2: inhibitory early-I neurons receive strong inhibition during the E2 phase (blue shaded region) and are important components of the inhibitory connectome (see FIGURE 6) controlling generation of expiratory phase activity. Periods of postsynaptic inhibition are indicated by blue arrows.
An essential characteristic of the pre-inspiratory spiking and early-I neurons that is not visible in plain extracellular recordings is their pronounced inhibition during post-inspiration (FIGURE 4A; also see Refs. 6, 8, 101). Another typical behavior of pre-I/I neurons is the large fluctuation of their timing of pre-inspiratory discharge (FIGURE 4A). Recordings for prolonged periods performed under normal (eupneic) conditions has revealed that these pre-inspiratory neurons may not always discharge before inspiration and appear as early-inspiratory spiking neurons. Intracellular analyses indicate that there is a heterogeneous population of pre-inspiratory and early inspiratory neurons that are characterized by a rapid onset and then declining early inspiratory discharge and a strong inhibition during post-inspiration thereafter. Typically, these neurons do not receive any obvious late-expiratory synaptic inhibition that would block all pre-inspiratory discharges in the pre-I/I neurons. Such E2 expiratory inhibition clearly characterizes a different population of early-I neurons that never discharge before the onset of inspiration and seem to belong to early inspiratory follower neurons that belong to the mutual inhibitory connectome of early-I, post-I, as well as E2 expiratory neurons (see FIGURE 6B2).
FIGURE 6.
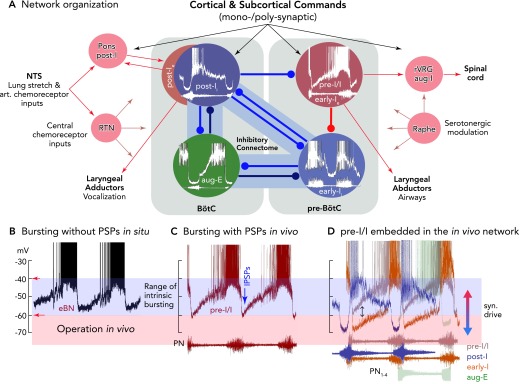
Functional model of the rhythmogenic respiratory network organization, including control by afferent inputs and output functions of neurons
A: the different patterns of synaptic inputs to the neurons of the network indicate that antagonistic (reciprocal) inhibition is controlling in vivo rhythm generation and coordinating inspiratory and expiratory activity phases, as well as operating under control of various input signals. There are various sorts of antagonism organized by inhibitory connectomes, as proposed by the authors (79, 90, 92) and supported by computational simulations (e.g., Refs. 58, 80, 89) as a “proof of principle.” The most important antagonistic connectivity involved in rhythm generation appears to exist between early-I and post-I inhibitory neurons and their connections with pre-I/I excitatory neurons, allowing inspiratory-post-inspiratory activity coordination, besides other connectomes of aug-E neurons to early-I and post-I neurons. Inhibitory connections are depicted as blue lines, excitatory connections as red lines, and other afferent inputs as gray lines. B: bursting patterns of eBNs recorded in situ, as revealed after block of synaptic transmission (without PSPs), illustrating membrane potential trajectories and approximate voltage range for intrinsic rhythmic bursting (see also FIGURE 4A). C: pre-I/I neurons/eBNs (red trace) embedded in the core circuitry in vivo and in situ and presumably receiving tonic excitation have depolarizing membrane potential trajectories and generate bursts (see FIGURE 4A) between voltages of approximately −60 to −40 mV, where the membrane potential trajectory is effectively controlled by post-I inhibition (IPSPs). D: using the onset and end of phrenic nerve bursts as a relative time coordinate shows the sequences of neuronal activities and membrane potential trajectories of pre-I/I, post-I, early-I, and aug-E neurons of the connectome shown in A. Potential pre-I/I neurons with stronger post-I and late-expiratory inhibition or receiving lower tonic excitation will behave as early-I bursting neurons (see color code for neuron types). All action potentials are truncated.
Synaptic Voltage and Conductance Clamp Control
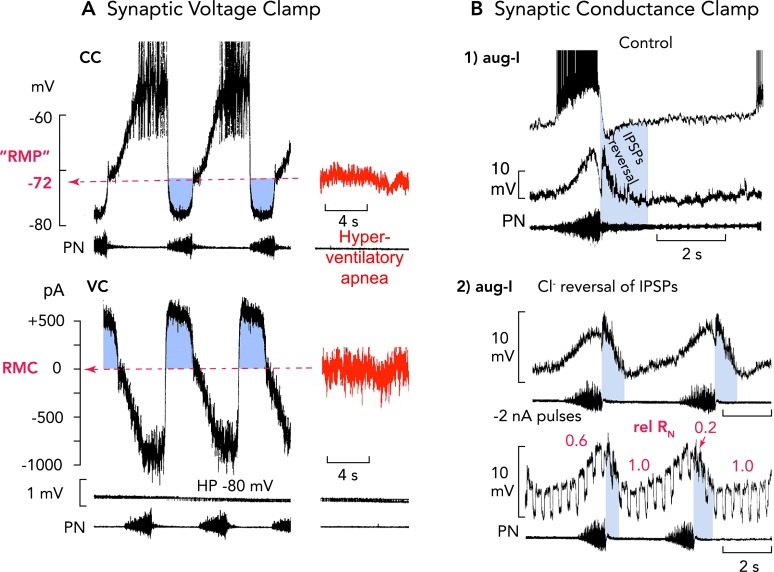
All endogenous biophysical properties of neurons are strongly controlled by spontaneous postsynaptic activities. In intact in vivo cat and also in situ rodent preparations, respiratory neurons do not show any resting membrane potential but reveal ongoing long-lasting voltage fluctuations generated by ESVs and ISVs summing up to more than ±10 mV in amplitude. ESVs provoke neuron-specific burst discharges, which are reliably followed by ISVs that hyperpolarize neurons toward the chloride equilibrium potential (ECl) at approximately −80 mV (FIGURE 5). A resting membrane potential (RMP) can only be estimated when in vivo animals are hyperventilated to reduce ongoing chemoreceptive excitatory drive and to provoke central apnea, revealing a fictive resting potential of about −70 mV (FIGURE 5A) (76).
FIGURE 5.
“Restless” respiratory neurons: a bombardment of excitatory and inhibitory synaptic inputs controls ongoing membrane potential oscillations
A: high-frequency alternating single-electrode current (CC) and voltage-clamp (VC) recordings [top and bottom, respectively; holding potential (HP)] from an expiratory neuron, as an example (76), reveals that neurons operating in the active respiratory network do not show any resting membrane potential. There are ongoing oscillations of membrane potential generated by alternating excitatory and inhibitory (blue regions) synaptic volleys. A fictive resting membrane potential (RMP) where the net current is 0 pA (RMC, bottom) at about −70 mV could only be estimated when animals were hyperventilated to provoke central apnea. Characteristic for inspiratory and expiratory neurons are very effective volleys of inhibitory currents causing a functional voltage clamp of neurons close to the chloride equilibrium potential (ECl) at approximately −80 mV. Action potentials are truncated. B: the prominent inhibitory synaptic volleys also produce a significant fall of neuronal input resistance (RN), which exerts a conductance clamp, because it effectively shunts endogenous and exogenous synaptic currents (72, 74). In all inspiratory neurons (examples of aug-I neurons shown), the most obvious RN fall by up to 80% occurs at the onset of post-I inhibition, as identified by IPSPs polarity reversal (blue regions) after intracellular chloride injection (71). Action potentials are truncated.
The strong volleys of synaptic input change the membrane potential and appear to set the conditions for activation, inactivation, and recovery of membrane conductances, as well as short-circuiting of their currents when there is synaptic inhibition. Synaptic inhibition is certainly not necessary for eBN-evoked respiratory rhythm generation per se in a slice but is essential under in vivo conditions, when eBNs are embedded and effectively controlled by synaptic inputs involving not only excitatory inputs that are necessary to synchronize activity (68) but also inhibitory inputs from post-I neurons. Therefore, it seems realistic to assume that ISVs hyperpolarize eBNs and keep them away from the voltage threshold of burst-generating currents such as Nap during the post-I phase. This functional voltage clamp is potentiated by a conductance clamp effect of ISVs producing ∼500-pA current strength that could easily block activation of Nap with 50- to 200-pA intensity when they are inhibited (17, 34). In essence, the widespread post-I synaptic inhibition produces an effective resetting and suppression of bursting capacities of all neurons until pre-I/I neurons or eBNs can escape synaptic inhibition (see below) to drive the onset of the inspiratory phase. The prominent ISVs are also effective by shunting intrinsic and extrinsic currents, because they produce significant changes in neuronal input resistance (RN) (FIGURE 5B) (72). During eupneic breathing, RN of aug-I neurons changes by ∼80% during post-I inhibition (FIGURE 5B). The significant membrane hyperpolarization and associated membrane conductance increase leaves a time window only after the end of the ISVs when burster or follower neurons become sensitive again to depolarizing drive from intrinsic and synaptic currents.
Network Model for Respiratory Rhythm Generation In Vivo
Comparison of the ESVs and ISVs of all different types of neurons in the pre-BötC and the BötC confirmed that synaptic interactions within an inhibitory connectome (FIGURE 6A) (79) are involved in the initiation, patterning, and termination of inspiration. This connectome, which dynamically interacts with pre-BötC excitatory circuits (FIGURE 6A), is presumed to be the core circuitry for generating a regular respiratory rhythm. Each type of neuron receives characteristic SVs in all phases of the cycle, and especially ISVs whenever other types of neurons take over to discharge a burst (FIGURE 6B). The most impressive effect is mediated by post-I neurons, which produce dominant ISVs in pre-I/I, early-I, aug-I and also aug-E/E2 neurons (FIGURE 6B) (72, 73). Thus network-mediated post-I synaptic inhibition is a mechanism for irreversible inspiratory phase termination that during quiet breathing also controls the time course of the membrane potential trajectory of pre-I/I neurons/eBNs (FIGURE 6C) during the expiratory interval (69) and may also provide time for the recovery from inactivation of the Nap conductance. Pharmacological alteration of cAMP levels affecting the abundantly expressed subtype α3 glycine receptor (GlyRα3; see below) leads to significant changes in burst frequency, and this indicates that the inhibitory glycinergic connectome is critical for respiratory frequency control (see FIGURE 8C) (47, 53). Importantly, an abnormally prolonged inspiration (called apneusis) occurs when network post-I activity is too weak to suppress retrieval of inspiratory bursting (FIGURE 3B) (38, 98).
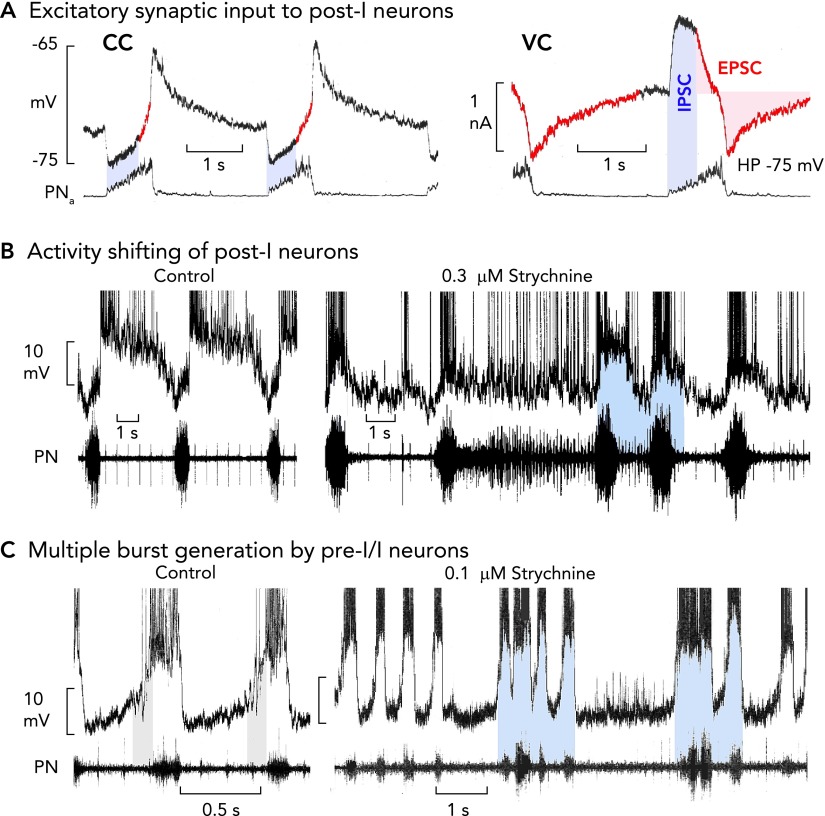
FIGURE 8.
Perturbations of medullary respiratory neuron and network activities during failure of glycinergic inhibition
A: post-I neurons receive an excitatory synaptic drive already during inspiration, shown in current clamp recording (CC). This becomes clear when neurons are voltage clamped [VC; holding potential (HP)] at normal voltages, revealing onset of excitatory inward currents during inspiration (see red colored traces; also see Refs. 47, 76). B: blockade of glycinergic inhibition provokes a shifting of post-I discharges into the period of inspiration due to the inspiratory excitatory synaptic input (9). The consequence is a recurring interruption of regular bursting leading to inspiratory and post-inspiratory doublet or multiple bursts, and persisting inspiratory (apneustic) activity is transmitted to phrenic nerve output (11). C: strychnine augments inspiratory burst amplitudes and discharge frequency of pre-I/I neurons due to the block of the normal glycine receptor-mediated steep membrane hyperpolarization after the burst. The activity shifting of post-I discharge into inspiration provokes a dynamic struggle between post-I neurons and their counterparts in the connectome, with pre-I/I bursters trying to start an inspiration against the shifted post-I neuron post-I inhibition that is reduced, but not completely blocked, by strychnine (11). All action potentials in B and C are truncated.
As noted earlier, during the E2 phase, the pre-I/I neurons appear to receive no or only weak (91) synaptic inhibition and, therefore, gain a more depolarized membrane potential. The associated gradually developing excitation, including excitatory drive to the local early-I neurons within the pre-BötC network, can then initiate the processes leading to the onset of inspiration. Other inspiratory “follower” neurons, such as inhibitory early-I and aug-I neurons (FIGURE 6B), cannot readily respond to pre-inspiratory excitatory drive because they receive strong inhibition from aug-E neurons during the late expiratory phase. Furthermore, aug-I neurons and also later spiking inspiratory neurons (late-I) (FIGURES 2B AND 6C) appear to receive a declining inhibition during the early inspiratory phase that, along with recurrent and tonic excitation, is shaping their steadily augmenting discharge patterns (72, 73).
Within the connectome of early-I and post-I neurons, the early-I pre-BötC inhibitory neurons with strong spike frequency adaptation also seem to play a particularly important role in coordinating the inspiratory to post-inspiratory phase transitions. It is postulated that these neurons function to provide feed-forward inhibition to all expiratory and inspiratory neurons in the network, including in the BötC (89, 91). Their rapid onset of bursting strongly inhibits post-I and aug-E neurons, whereas their strong spike frequency adaptation initiates the processes leading to late-inspiratory disinhibition of post-I neurons. Interestingly, there seem to be both early-I and post-I neurons in the pre-BötC (4, 84). It is therefore possible that the pre-BötC post-I neurons, in addition to the main population of BötC post-I inhibitory neurons, contribute to the local as well as widespread ISVs that control the timing of inspiratory and post-inspiratory activities. Thus we conclude that pre-BötC circuits are designed for multiple functions, including generating rhythmic excitatory drive and shaping patterns of inspiratory neuronal discharge.
Cooperativity Between Synaptic Control and Cellular Biophysics
The membrane biophysical properties of neurons within the core connectome represented in FIGURE 6A work synergistically with the circuit-based synaptic processes described above to coordinate the initiation, maintenance, and termination of active phases of the respiratory cycle. These concerted interactions of biophysical and synaptic processes promote disinhibition and rebound from inhibition (CaT), initiate (NaP), strongly amplify and sustain bursting (CaL and CAN) (67), and contribute to burst termination (bKCa, K+leak), operating in alliance with strong synaptic excitation and inhibition (11, 100). These intrinsic properties together have robust burst-generating and -terminating properties that may even underlie respiratory rhythm generation, for example, under severe hypoxia (61) when synaptic inhibition is suppressed in vivo (74). FIGURE 7 represents an attempt to summarize in simplified form the dynamic interplay of these circuit- and cellular-based mechanisms. Undoubtedly, other neuronal activity-/voltage-dependent biophysical mechanisms and more complex network interactions could contribute to this cellular and circuit synergy.
FIGURE 7.
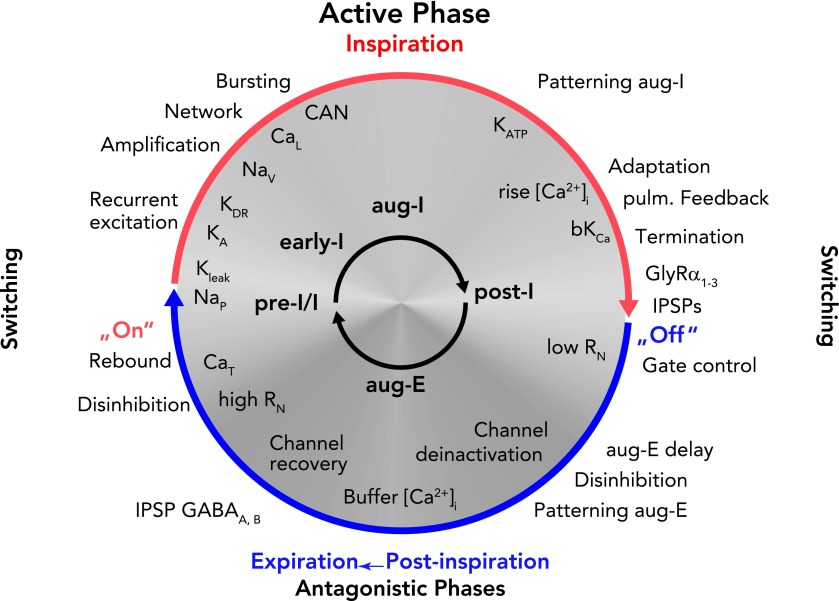
Schematic representation of the dynamic interplay of synaptic processes and neuronal biophysical properties determining rhythm generation and neuronal activity patterns during a respiratory cycle
The ongoing rhythmic cycling of respiratory phases results from a coalition between synaptic inputs to neurons and intrinsic neuronal biophysical properties (65). Network-related synaptic events [recurrent excitation, and inhibitory glycinergic (GlyRa1–3) or GABAergic (GABAA,B) neurotransmitter-related inhibition (IPSPs) or disinhibition] are indicated outside the circle. Note that there is a continuous inflow of synaptic activity from pulmonary and chemoreceptive afferents, as well as pontine and medullary reticular formation inputs. Intrinsic neuronal biophysical processes, including membrane channel activation, recovery from inactivation, deinactivation under membrane voltage control, as well as activity-related Ca2+-dependent processes, are indicated inside the circle in white lettering. Synaptic processes in the rhythmically active respiratory circuits control membrane potentials in neurons in the subthreshold voltage range to orchestrate expression of intrinsic membrane conductances, including burst-promoting conductances (CaT and NaP) as well as K+ conductances (KLeak, KA) that regulate onset of bursting. During neuronal spiking activity, Na+ and Ca2+ fluxes together with CAN augment neuronal activities to generate the active phases of the cycle. Ca2+ fluxes also activate bK(Ca) to induce spike-frequency adaptation that, in addition to synaptic inhibition, contributes to termination of active phases. The figure should be read clockwise. For further explanation, see text.
Our present understanding of how the respiratory network operates under normal in vivo conditions to organize a three-phased respiratory pattern explains how eBNs are integrated into the network, their potential spontaneous activity being under synaptic control that normally prevents them from endogenous bursting. However, eBNs could become endogenously active when there is weak synaptic inhibition but enough excitatory drive from sources such as the arterial chemoreceptors (43) or chemosensitive neurons in RTN (26, 57) to depolarize to a voltage range where intrinsic membrane currents become activated. Speculatively, such conditions may allow quiet breathing during slow-wave sleep.
Disturbances After Loss of Glycinergic Inhibition
Glycinergic inhibitory neurons and corresponding transmitter receptors are important components of the rhythm-generating circuitry in vivo. Transgenic mice expressing eGFP under the control of the GlyT2 promoter reveal that there is a dense concentration of glycinergic inhibitory neurons in the BötC and even in the pre-BötC, comprising >50% of the total neurons in these regions (48, 99). Even a subpopulation of pre-BötC inspiratory neurons with pacemaker properties were identified to be glycinergic inhibitory neurons (55). In neonatal rodents, there are also populations of GABAergic and co-expressing glycinergic-GABAergic neurons (36). An essential finding was that inhibitory neurons of the BötC and pre-BötC, and presumably pre-BötC excitatory neurons, express the specific GlyRα3. This GlyRα3 is a target for PKA phosphorylation that reduces inhibitory currents (48). Such modulation can be used to treat disturbances of inhibitory network control in translational medicine, since drugs reducing PKA phosphorylation can be used to reinforce synaptic inhibition in the network and to successfully treat apneustic apnea and breath-holdings (39, 78, 98).
One way to understand how the cellular- and circuit-based mechanisms depicted in FIGURE 7 function for rhythm generation and control is to consider how rhythmic activity is perturbed when inhibitory synaptic mechanisms are disrupted. Reduction of glycinergic inhibition in cases of genetic failure of glycine receptors (49), during hypoxia (74), or experimentally by systemic strychnine application (9) provokes a major reorganization of the network operation. This includes post-I neurons shifting their discharge into the inspiratory phase (FIGURE 8B). Voltage-clamp analysis has clarified that the underlying process primarily involves the sub-connectome of post-I and early-I neurons. This analysis showed that post-I neurons, besides receiving synaptic inhibition with a declining early-I pattern, also receive an augmenting pattern of excitatory drive during inspiration (FIGURE 8A) (46). Since inhibitory interactions are likely not completely blocked under glycine receptor-specific low strychnine concentrations (31), inhibitory post-I and early-I interactions were both partially maintained and provoke a struggle between inspiratory excitation and post-I inhibition, as also seen in the rapidly oscillating pre-I/I and post-I neuronal discharges and corresponding low-amplitude bursting of PN (FIGURE 8, B AND C). The shifting of post-I discharges into inspiration, their adaptation, and their subsequent fading of inhibition toward the end of inspiration provoke a rapid re-start of inspiratory activity, resulting in multiple high-frequency bursting. Such multiple burst generation provides additional evidence that pre-I/I neurons have eBN-like properties but normally are under vigorous control by synaptic inhibition (FIGURE 8C).
Similar disturbances of rhythmic breathing are often seen in pediatrics, mostly described clinically as (apneustic) apnea or breath-holdings (98) and also intractable hiccups (45). Glycinergic inhibition is, therefore, the primary target for clinical strategies to treat these patients. The rationale of such treatments is pharmacological (Buspirone: 5-HT1A receptor agonist mediated) dephosphorylation of Glyα3R to augment pathologically depressed glycinergic inhibition. This has rescued rhythmic breathing after surgical lesions of the ponto-medullary junction (obviously deleting pontine control, including the Hering-Breuer reflex) during fentanyl anesthesia (μ-opioid-induced depression of inhibitory synaptic interactions) or ischemic apneusis after brain stem stroke (hypoxia-induced depression of synaptic inhibition) (74). Synaptic inhibition is also disturbed in Rett Syndrome, which is a developmental disease (102) in humans starting after a healthy stage 1 of Early Onset Stagnation at an age of 6–9 mo with significant disturbances of breathing (32, 70, 86), whereas NMDA receptors are upregulated and type-A GABA receptors are downregulated already at birth in RTT mouse models (14, 51). An additional critical factor appearing after a comparable delay is a high expression level of the type 5B of 5-HT receptor depressing cAMP production to affect glycinergic inhibition (Manzke et al., unpublished observations), leading to severe irregularities in respiratory rhythm generation (2, 94, 95).
General Conclusions
We all would feel very inhibited if our speech would be regularly disrupted by pacemaker neurons and an autorhythmic behavior of pre-BötC excitatory networks that automatically pace breathing. Fortunately, this does not occur because in vivo respiratory rhythm generation is dynamically formatted within the three-phase respiratory cycle, which also allows adjustment of the ongoing rhythm, including integration with other motor acts. Adaptable inhibitory synaptic control from oscillating connectomes that are embedded within the intact network is a major feature of the rhythm-generating circuits. Various external excitatory and inhibitory drives to this circuitry are normally engaged for rhythm generation according to physiological demands and for motor behavioral control. Phasic synaptic inhibition is necessary to control stable rhythm generation by resetting the endogenous rhythmic processes of the pre-BötC whenever necessary. These cellular rhythm-generating processes are greatly disturbed when the pre-BötC excitatory network becomes functionally uncontrolled from synaptic inhibition. Under normal in vivo conditions, endogenous rhythmic bursting may even ensure quiet breathing whenever (post-inspiratory) glycinergic synaptic inhibition is reduced, possibly during sleep, allowing a more autonomous mode of rhythm generation for unconscious breathing. The ability of the respiratory network to potentially switch between states of autonomous operation vs. interaction of the pre-BötC with inhibitory circuits for effective control provides a high functional plasticity in vivo.
Footnotes
When the post-I phase had been developed during phylogeny, it came under direct control of higher CNS regions to expand breathing to vocalization, which was necessary for reproductive survival and nesting (16). This required compulsory adjustment of the breathing rhythm and activation of various laryngeal muscles: abductor muscles to open airways during inspiration and adductor muscles such as the thyroarydenoid (TA) muscle to narrow vocal cords during post-inspiration (FIGURE 1A). An impressive example was given by the British neurophysiologist Tom Sears, who, in a self-experiment, recorded the EMG from various intercostal muscles, demonstrating that the post-I activity mediates a precise cortical control of inspiratory and even expiratory muscles during singing (FIGURE 1D; Ref. 85). It demonstrates that professional singers fully control inspiratory neurons so that they do not start automatically. Many other difficult motor actions are mostly performed during the post-inspiratory phase. An interesting example has been given by the German philosopher Eugen Herrigel when he described how to perform Japanese archery. He managed this task only by following the advice of his master saying that he should breathe properly: “inspiration” binds and combines, in “holding the breath” everything goes right, and “breathing out” releases and completes to overcome all limitations (27).
D. W. Richter was supported by DFG grants and the DFG Research Center for Molecular Physiology of the Brain (CMPB). J. C. Smith is supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: D.W.R. and J.C.S. conception and design of research; D.W.R. performed experiments; D.W.R. and J.C.S. analyzed data; D.W.R. and J.C.S. interpreted results of experiments; D.W.R. prepared figures; D.W.R. drafted manuscript; D.W.R. and J.C.S. edited and revised manuscript; D.W.R. and J.C.S. approved final version of manuscript.
References
- 1.Abdala AP, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JF. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir Physiol Neurobiol 168: 19–25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdala AP, Dutschmann M, Bissonnette JM, Paton JF. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 107: 18208–18213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol 143: 105–114, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol 164: 3–11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backman SB, Anders C, Ballantyne D, Röhrig N, Camerer H, Mifflin S, Jordan D, Dickhaus H, Spyer KM, Richter DW. Evidence for a monosynaptic connection between slowly adapting pulmonary stretch receptor afferents and inspiratory beta neurones. Pflügers Arch 402: 129–136, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Balis UJ, Morris KF, Koleski J, Lindsey BG. Simulations of a ventrolateral medullary neural network for respiratory rhythmogenesis inferred from spike train cross-correlation. Biol Cybern 70: 311–327, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne D, Richter DW. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol 348: 67–87, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi AL, Grélot L, Iscoe S, Remmers JE. Electrophysiological properties of rostral medullary respiratory neurones in the cat: an intracellular study. J Physiol 407: 293–310, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Büsselberg D, Bischoff AM, Paton JF, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflügers Arch 441: 444–449, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Büsselberg D, Bischoff AM, Becker K, Becker CM, Richter DW. The respiratory rhythm in mutant oscillator mice. Neurosci Lett 316: 99–102, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Busselberg D, Bischoff AM, Richter DW. A combined blockade of glycine and calcium-dependent potassium channels abolishes the respiratory rhythm. Neuroscience 122: 831–841, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. II. Populations of coupled pacemaker neurons. J Neurophysiol 82: 398–415, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. I. Bursting pacemaker neurons. J Neurophysiol 82: 382–397, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468: 263–269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connelly CA, Dobbins EG, Feldman JL. Pre-Botzinger complex in cats: respiratory neuronal discharge patterns. Brain Res 590: 337–340, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Dediu D, Levinson SC. On the antiquity of language: the reinterpretation of Neandertal linguistic capacities and its consequences. Front Psychol 4: 397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Botzinger complex inspiratory neurons in vitro. J Neurophysiol 88: 2242–2250, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Devor A. The great gate: control of sensory information flow to the cerebellum. Cerebellum 1: 27–34, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats. Brain Res 636: 259–269, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA 105: 1067–1072, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutschmann M, Paton JF. Trigeminal reflex regulation of the glottis depends on central glycinergic inhibition in the rat. Am J Physiol Regul Integr Comp Physiol 282: R999–R1005, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Ezure K, Tanaka I, Kondo M. Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J Neurosci 23: 8941–8948, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman JL. Chapter 14: looking forward to breathing. Prog Brain Res 188: 213–218, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Fluorens P. Note sur le point vital de la moelle allongée. CR Acad Sci 33: 437–439, 1851 [Google Scholar]
- 25.Frermann D, Keller BU, Richter DW. Calcium oscillations in rhythmically active respiratory neurones in the brainstem of the mouse. J Physiol 515: 119–131, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol 173: 244–255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrigel E. Zen in the Art of Archery. New York: Vintage, 1972 [Google Scholar]
- 28.Hutchison AA, Wozniak JA, Choi HG, Conlon M, Otto RA, Abrams RM, Kosch PC. Laryngeal and diaphragmatic muscle activities and airflow patterns after birth in premature lambs. J Appl Physiol 75: 121–131, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Jasinski PE, Molkov YI, Shevtsova NA, Smith JC, Rybak IA. Sodium and calcium mechanisms of rhythmic bursting in excitatory neural networks of the pre-Botzinger complex: a computational modelling study. Eur J Neurosci 37: 212–230, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson SM, Koshiya N, Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Botzinger complex “island”. J Neurophysiol 85: 1772–1776, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science 281: 419–424, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Julu PO, Kerr AM, Apartopoulos F, Al-Rawas S, Engerström IW, Engerström L, Jamal GA, Hansen S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch Dis Child 85: 29–37, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi S, Onimaru H, Inoue M, Inoue T, Sasa R. Localization and properties of respiratory neurons in the rostral pons of the newborn rat. Neuroscience 134: 317–325, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Koizumi H, Smith JC. Persistent Na+ and K+-dominated leak currents contribute to respiratory rhythm generation in the pre-Botzinger complex in vitro. J Neurosci 28: 1773–1785, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R, Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. J Neurosci 30: 4273–4284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koizumi H, Koshiya N, Chia JX, Cao F, Nugent J, Zhang R, Smith JC. Structural-functional properties of identified excitatory and inhibitory interneurons within pre-Botzinger complex respiratory microcircuits. J Neurosci 33: 2994–3009, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature 400: 360–363, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Lalley PM, Bischoff AM, Richter DW. Serotonin 1A-receptor activation suppresses respiratory apneusis in the cat. Neurosci Lett 172: 59–62, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Lalley PM, Pierrefiche O, Bischoff AM, Richter DW. cAMP-dependent protein kinase modulates expiratory neurons in vivo. J Neurophysiol 77: 1119–1131, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Lawson EE, Richter DW, Ballantyne D, Lalley PM. Peripheral chemoreceptor inputs to medullary inspiratory and postinspiratory neurons of cats. Pflügers Arch 414: 523–533, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Lawson EE, Richter DW, Bischoff A. Intracellular recordings of respiratory neurons in the lateral medulla of piglets. J Appl Physiol 66: 983–988, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Lawson EE, Richter DW, Czyzyk-Krzeska MF, Bischoff A, Rudesill RC. Respiratory neuronal activity during apnea and other breathing patterns induced by laryngeal stimulation. J Appl Physiol 70: 2742–2749, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Lipski J, McAllen RM, Spyer KM. The carotid chemoreceptor input to the respiratory neurones of the nucleus of tractus solitarus. J Physiol 269: 797–810, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liss B, Roeper J. A role for neuronal K(ATP) channels in metabolic control of the seizure gate. Trends Pharmacol Sci 22: 599–601; discussion 601–602, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Mandalà M, Rufa A, Cerase A, Bracco S, Galluzzi P, Venturi C, Nuti D. Lateral medullary ischemia presenting with persistent hiccups and vertigo. Int J Neurosci 120: 226–230, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Manzke T, Preusse S, Hülsmann S, Richter DW. Developmental changes of serotonin 4(a) receptor expression in the rat pre-Botzinger complex. J Comp Neurol 506: 775–790, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Manzke T, Dutschmann M, Schlaf G, Mörschel M, Koch UR, Ponimaskin E, Bidon O, Lalley PM, Richter DW. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos Trans R Soc Lond B Biol Sci 364: 2589–2602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manzke T, Niebert M, Koch UR, Caley A, Vogelgesang S, Hülsmann S, Ponimaskin E, Müller U, Smart TG, Harvey RJ, Richter DW. Serotonin receptor 1A-modulated phosphorylation of glycine receptor alpha3 controls breathing in mice. J Clin Invest 120: 4118–4128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markstahler U, Kremer E, Kimmina S, Becker K, Richter DW. Effects of functional knock-out of alpha 1 glycine-receptors on breathing movements in oscillator mice. Respir Physiol Neurobiol 130: 33–42, 2002 [DOI] [PubMed] [Google Scholar]
- 50.McCrimmon DR, Alheid GF, Jiang M, Calandriello T, Topgi A. Converging functional and anatomical evidence for novel brainstem respiratory compartments in the rat. Adv Exp Med Biol 551: 101–105, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Medrihan L, Tantalaki E, Aramuni G, Sargsyan V, Dudanova I, Missler M, Zhang W. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J Neurophysiol 99: 112–121, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Merrill EG. Where are the real respiratory neurons? Fed Proc 40: 2389–2394, 1981 [PubMed] [Google Scholar]
- 53.Mironov SL, Langohr K, Richter DW. A1 adenosine receptors modulate respiratory activity of the neonatal mouse via the cAMP-mediated signaling pathway. J Neurophysiol 81: 247–255, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Mironov SL. Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. J Physiol 586: 2277–2291, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgado-Valle C, Baca SM, Feldman JL. Glycinergic pacemaker neurons in preBotzinger complex of neonatal mouse. J Neurosci 30: 3634–3639, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos Trans R Soc Lond B Biol Sci 364: 2517–2526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nature Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Ogilvie MD, Gottschalk A, Anders K, Richter DW, Pack AI. A network model of respiratory rhythmogenesis. Am J Physiol Regul Integr Comp Physiol 263: R962–R975, 1992 [DOI] [PubMed] [Google Scholar]
- 59.Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBotzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol 582: 113–125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Meth 65: 63–68, 1996 [DOI] [PubMed] [Google Scholar]
- 61.Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci 9: 311–313, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Peña F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43: 105–117, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Pierrefiche O, Champagnat J, Richter DW. Calcium-dependent conductances control neurones involved in termination of inspiration in cats. Neurosci Lett 184: 101–104, 1995 [DOI] [PubMed] [Google Scholar]
- 64.Pierrefiche O, Haji A, Bischoff A, Richter DW. Calcium currents in respiratory neurons of the cat in vivo. Pflügers Arch 438: 817–826, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Ramirez JM, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Curr Opin Neurobiol 6: 817–825, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ, Richter DW. Respiratory rhythm generation in mammals: synaptic and membrane properties. Respir Physiol 110: 71–85, 1997 [DOI] [PubMed] [Google Scholar]
- 67.Ramirez JM, Koch H, Garcia AJ, 3rd, Doi A, Zanella S. The role of spiking and bursting pacemakers in the neuronal control of breathing. J Biol Phys 37: 241–261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rekling JC, Feldman JL. PreBotzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60: 385–405, 1998 [DOI] [PubMed] [Google Scholar]
- 69.Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP. Reflex prolongation of stage I of expiration. Pflügers Arch 407: 190–198, 1986 [DOI] [PubMed] [Google Scholar]
- 70.Rett A. [On a unusual brain atrophy syndrome in hyperammonemia in childhood]. Wien Med Wochenschr 116: 723–726, 1966 [PubMed] [Google Scholar]
- 71.Richter DW, Camerer H, Meesmann M, Röhrig N. Studies on the synaptic interconnection between bulbar respiratory neurones of cats. Pflügers Arch 380: 245–257, 1979 [DOI] [PubMed] [Google Scholar]
- 72.Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol 100: 93–107, 1982 [DOI] [PubMed] [Google Scholar]
- 73.Richter DW, Ballantyne D, Remmers JE. The differential organization of medullary post-inspiratory activities. Pflügers Arch 410: 420–427, 1987 [DOI] [PubMed] [Google Scholar]
- 74.Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. J Physiol 443: 231–256, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richter DW. Neural regulation of respiration: rhythmogenesis and afferent control. In: Comprehensive Human Physiology: From Cellular Mechanism to Integration, edited by Gregore R, Windhorts U. Berlin: Springer Verlag, 1996, p. 2079–2095 [Google Scholar]
- 76.Richter DW, Pierrefiche O, Lalley PM, Polder HR. Voltage-clamp analysis of neurons within deep layers of the brain. J Neurosci Meth 67: 121–123, 1996 [PubMed] [Google Scholar]
- 77.Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci 24: 464–472, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med 9: 542–548, 2003 [DOI] [PubMed] [Google Scholar]
- 79.Richter DW, Ballantyne D, Remmers JE. How is the respiratory rhythm generated? A model. News Physiol Sci 1: 109–112, 1986 [Google Scholar]
- 80.Rubin JE, Shevtsova NA, Ermentrout GB, Smith JC, Rybak IA. Multiple rhythmic states in a model of the respiratory central pattern generator. J Neurophysiol 101: 2146–2165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubin JE, Bacak BJ, Molkov YI, Shevtsova NA, Smith JC, Rybak IA. Interacting oscillations in neural control of breathing: modeling and qualitative analysis. J Comput Neurosci 30: 607–632, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmid K, Foutz AS, Denavit-Saubie M. Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res 710: 150–160, 1996 [DOI] [PubMed] [Google Scholar]
- 83.Schwarzacher SW, Wilhelm Z, Anders K, Richter DW. The medullary respiratory network in the rat. J Physiol 435: 631–644, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwarzacher SW, Smith JC, Richter DW. Pre-Botzinger complex in the cat. J Neurophysiol 73: 1452–1461, 1995 [DOI] [PubMed] [Google Scholar]
- 85.Sears T. Some Neural and Mechanical Aspects of Singing. Music and the Brain, edited by Critchley M, Henson RA. London: Heineman, 1977 [Google Scholar]
- 86.Smeets E, Schollen E, Moog U, Matthijs G, Herbergs J, Smeets H, Curfs L, Schrander-Stumpel C, Fryns JP. Rett syndrome in adolescent and adult females: clinical and molecular genetic findings. Am J Med Genet A 122A: 227–233, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith JC, Butera RJ, Koshiya N, Del Negro C, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir Physiol 122: 131–147, 2000 [DOI] [PubMed] [Google Scholar]
- 89.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci 364: 2577–2587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci 36: 152–162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.St-John WM, Stornetta RL, Guyenet PG, Paton JF. Location and properties of respiratory neurones with putative intrinsic bursting properties in the rat in situ. J Physiol 587: 3175–3188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stettner GM, Huppke P, Brendel C, Richter DW, Gärtner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol 579: 863–876, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stettner GM, Huppke P, Gärtner J, Richter DW, Dutschmann M. Disturbances of breathing in Rett syndrome: results from patients and animal models. Adv Exp Med Biol 605: 503–507, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Wall PD. The gate control theory of pain mechanisms. A re-examination and re-statement. Brain 101: 1–18, 1978 [DOI] [PubMed] [Google Scholar]
- 97.Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J Neurosci 33: 7756–7761, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J Pediatr 130: 89–94, 1997 [DOI] [PubMed] [Google Scholar]
- 99.Winter SM, Fresemann J, Schnell C, Oku Y, Hirrlinger J, Hülsmann S. Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflügers Arch 458: 459–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao MG, Hülsmann S, Winter SM, Dutschmann M, Richter DW. Calcium-regulated potassium currents secure respiratory rhythm generation after loss of glycinergic inhibition. Eur J Neurosci 24: 145–154, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Zheng Y, Barillot JC, Bianchi AL. Patterns of membrane potentials and distributions of the medullary respiratory neurons in the decerebrate rat. Brain Res 546: 261–270, 1991 [DOI] [PubMed] [Google Scholar]
- 102.Zoghbi HY. Rett syndrome: what do we know for sure? Nat Neurosci 12: 239–240, 2009 [DOI] [PubMed] [Google Scholar]