Abstract
Myocardial ischemia-reperfusion (IR) injury can cause ventricular cell death and is a major pathological event leading to morbidity and mortality in those with coronary artery disease. Interestingly, as few as five bouts of exercise on consecutive days can rapidly produce a cardiac phenotype that resists IR-induced myocardial injury. This review summarizes the development of exercise-induced cardioprotection and the mechanisms responsible for this important adaptive response.
Coronary artery disease remains a major cause of death worldwide (71). The major pathology associated with coronary artery disease is commonly the direct consequence of an ischemia-reperfusion (IR) insult. In this regard, the resultant myocardial damage due to IR injury is proportional to ischemic duration (98). Given the high incidence of coronary artery disease and the associated IR-induced cardiac injury, developing a countermeasure to protect the heart against IR-induced damage is important. In this regard, it is well established that exercise training provides robust cardioprotection against IR injury. For example, human epidemiological studies reveal that regular exercise reduces the risk of death during a myocardial IR insult (reviewed in Ref. 49). Importantly, numerous animal studies provide direct evidence that endurance exercise protects the heart from IR-induced injury (reviewed in Refs. 28, 56, 92).
This review summarizes the present understanding of the mechanisms responsible for exercise-induced cardioprotection against IR-induced injury. We begin with an introduction to IR-induced myocardial injury followed by a brief summary of the evidence demonstrating that exercise is cardioprotective. The remainder of this report focuses on the putative mechanisms responsible for exercise-induced cardioprotection with an emphasis on the important role that mitochondrial adaptations play in producing the protected phenotype.
Levels of IR-Induced Cardiac Injury
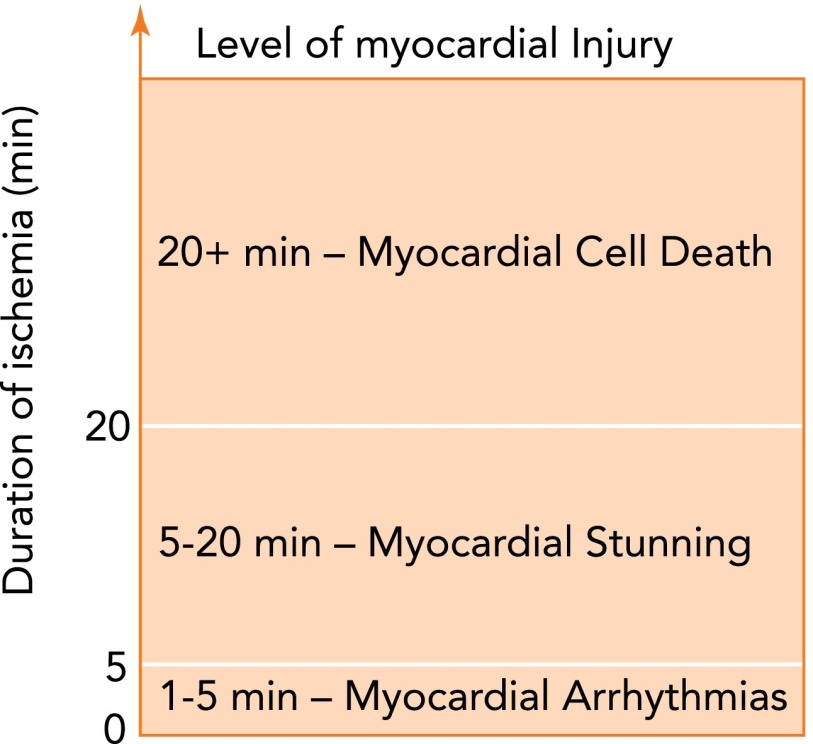
Depending on the duration of ischemia, three different levels of IR-induced cardiac injury ensue (FIGURE 1). The lowest level of IR-induced injury is the occurrence of cardiac arrhythmias. In this scenario, rapid reperfusion occurring after 1–5 min of ischemia often promotes ventricular tachycardia or fibrillation without depressed myocardial contractile performance or cardiac cell death (25). Reperfusion following 5–20 min of ischemia produces a higher level of myocardial injury termed “myocardial stunning” (25). Myocardial stunning is characterized by impaired ventricular contractility that occurs in the absence of cardiac cell death (9). Typically, IR-induced myocardial stunning results in cardiac contraction deficits that last 24–72 h following the insult (9). The final and most severe level of IR injury occurs when the duration of ischemia exceeds 20 min. During this most severe level of IR injury, cardiac myocytes are irreversibly damaged, and death occurs due to both apoptosis and necrosis (25). Additional evidence indicates that apoptotic tissue death can be either mitigated or potentiated by autophagic processes within ventricular myocytes exposed to ischemia (61, 100).
FIGURE 1.
Relationship between duration of ischemia and the level of IR-induced cardiac injury
Cellular Events Leading to IR-Induced Cardiac Injury
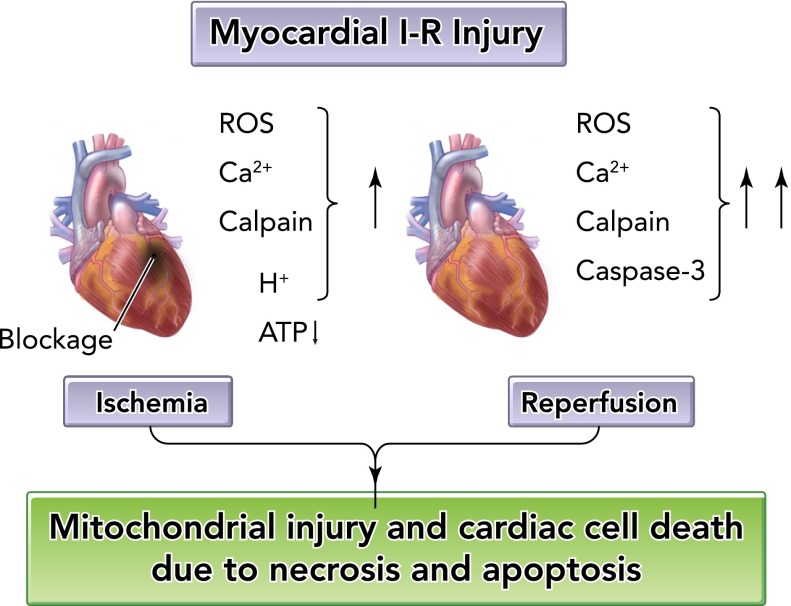
Despite the complexity of the events leading to IR-induced cardiac damage, the key factors responsible for IR-induced myocardial cell death are largely understood (for a review, see Refs. 33, 77, 91). During prolonged myocardial ischemia, numerous interrelated events occur resulting in decreased cellular [ATP], small increases in reactive oxygen species (ROS) production, accumulation of hydrogen ions, increased levels of cytosolic-free calcium, and activation of the calcium-activated protease calpain (FIGURE 2). During reperfusion, ROS production is exacerbated, cellular calcium overload continues, calpain remains active, and activation of caspase-3 occurs (50, 77). Furthermore, leukocyte infiltration to the area of cardiac injury occurs following severe IR damage to the heart (i.e., ischemia longer than 20 min) (77). Collectively, these factors promote mitochondrial injury and subsequent cardiac myocyte death (33, 77).
FIGURE 2.
Cellular events leading to ischemia-reperfusion injury in the heart
ROS, reactive oxygen species.
It is established that mitochondrial ROS production is central in IR-induced myocardial injury (124). Indeed, mitochondrial ROS are produced during both ischemia and reperfusion, with the majority of ROS production occurring during reperfusion (4, 124). The importance of ROS-mediated damage to the heart during an IR event is confirmed by reports demonstrating that delivery of exogenous antioxidants provide protection against IR-induced cardiac injury (2, 18, 44, 59). Although IR-induced ROS production occurs in several cellular locations, mitochondrial production of ROS plays a dominant role in IR-mediated oxidant injury in the heart (2, 59).
Of the various pathological events that lead to IR injury, increased ROS production and increased cytosolic calcium levels play key roles. Furthermore, several lines of evidence indicate that cross talk occurs between ROS and calcium to magnify IR injury. For example, increased cytosolic calcium levels mediate cellular injury by activation of calpain, promotion of mitochondrial damage, and facilitating mitochondrial ROS production (33, 65, 86). Conversely, oxidative stress fosters cellular calcium overload in at least two ways. First, ROS-mediated formation of reactive aldehydes (i.e., 4-hydroxyl-2,3-trans-nonenal) attenuates plasma membrane calcium ATPase activity and diminishes calcium removal from the cell, resulting in increased cellular calcium levels (102). Second, ROS-mediated oxidation of the sarcoplasmic reticulum calcium release channel (i.e., ryanodine receptor) fosters release of calcium into the cytosol (3, 34).
Among the numerous factors that contribute to IR-induced cardiac cellular injury, mitochondria viability is a key arbitrator of cardiac cell life or death. Indeed, the degree to which aerobic ATP production is preserved in mitochondria following an IR insult determines whether the cell will live or die via necrosis or apoptosis (35, 36, 39, 65, 81). The main factor linking mitochondria to IR-induced cell death involves permeabilization of the mitochondrial outer membrane and/or opening of the mitochondrial permeability transition pore, which is linked to both apoptotic and necrotic cell death (81). Specifically, IR-induced apoptosis occurs during reperfusion by permeabilization of the outer mitochondrial membrane and the release of cytochrome c into the cytosol (65). Necrotic cell death can occur during both ischemia and reperfusion and involves the opening of the mitochondrial permeability transition pore followed by mitochondrial swelling and rupture (65).
In summary, numerous factors interact to produce cardiac cell injury during an IR insult. Of the various events responsible for IR injury, mitochondrial damage plays a central role, since these organelles are vital gate keepers of life and death in the cardiac myocyte. It follows that preventing IR-induced mitochondrial injury protects cardiac myocytes during an IR insult. Given the wealth of evidence indicating that exercise training produces robust protection against IR injury, it has been postulated that beneficial mitochondrial adaptations underpin these observations. A brief summary of the evidence that exercise provides protection against IR-induced myocardial injury follows.
Exercise Promotes Cardioprotection
It is well established that repeated bouts of exercise improve myocardial tolerance to IR in animals, which is commonly referred to as exercise-induced cardioprotection (reviewed in Refs. 28, 56, 92). Similar to the ischemic preconditioning (PC) phenomenon produced by a brief period of surgically induced ischemia, exercise-induced cardioprotection is also biphasic. The first phase of cardioprotection is acquired rapidly following an acute exercise bout (i.e., 0.5 h after exercise). However, this initial protection is rapidly lost within 3 h postexercise. The mechanisms responsible for the early phase of preconditioning are not well characterized but likely include allosteric activation of the endogenous antioxidant enzyme superoxide dismutase (SOD2) located within mitochondria of ventricular myocytes (46, 118). Given the similar time course for the early window of protection in both exercise-induced cardioprotection and PC, it is tempting to speculate that common mechanisms could mediate both forms of cardioprotection, although this conclusion requires scientific verification. The second or late phase of exercise-induced cardioprotection is achieved within 24 h after the exercise bout and persists for at least 9 days following a 5-day exercise routine and is far more robust than the aforementioned early protective window (66). This review is focused on the mechanisms responsible for this second window of exercise-induced cardioprotection.
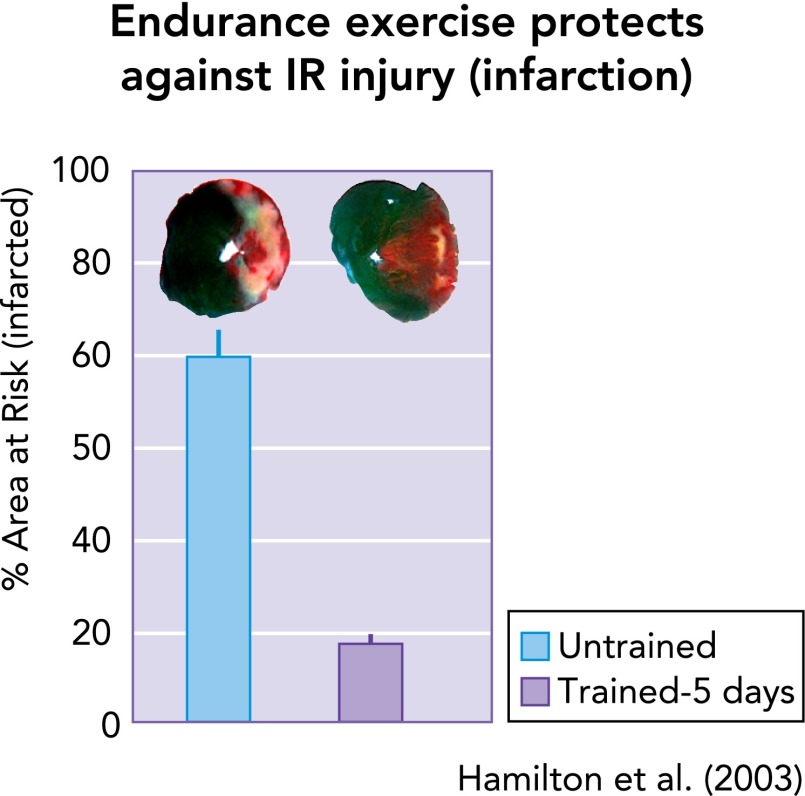
As mentioned earlier, repeated bouts of endurance exercise protect against IR-induced arrhythmias (29, 43, 75), myocardial stunning (11, 22, 23, 32, 42, 69, 70, 89, 113, 114), and myocardial infarction (29, 31, 95, 118). Interestingly, only 3–5 consecutive days of endurance exercise is required to achieve a significant level of cardioprotection against IR-induced myocardial infarction (FIGURE 3) (23, 31, 44). Complete details about the dose-response impact of aerobic exercise intensity on cardioprotection remain unknown. Findings from several studies provide insight into the influence of exercise intensity on resulting cardioprotection. One investigation into the role of intensity suggested that exercise below 55–60% V̇o2max did not achieve IR injury resistance (107), whereas another study concluded that both moderate- (i.e., 50% V̇o2max) and relatively high-intensity (i.e., 70% V̇o2max) exercise appear to be equally protective against IR-induced myocardial stunning (67). Regardless, it is feasible that there may be an exercise intensity threshold above which cardioprotection is achieved. Predictably, exercise-induced cardioprotection in rodents is lost rapidly (i.e., within 9–18 days) following the cessation of exercise training (66).
FIGURE 3.
As few as 5 consecutive days of exercise can provide significant protection against IR-induced myocardial infarction
Data are from Ref. 44.
Although it is clear that continuous aerobic exercise (e.g., 60 min) produces a cardioprotective phenotype, growing evidence suggests that high-intensity interval training (1 min of exercise at ≥V̇o2max) also produces a cardioprotective phenotype (69, 70). Furthermore, a recent study suggests that 12 wk of resistance exercise training provides protection against IR-induced myocardial infarction in rats (24, 103).
What Are the Mechanisms Responsible for Exercise-Induced Cardioprotection?
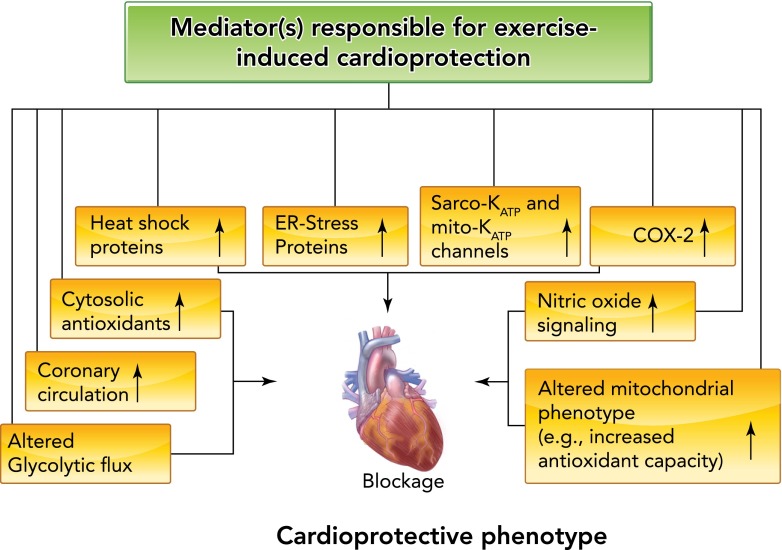
The mechanism(s) responsible for exercise-induced myocardial protection against IR injury remains a debated issue as numerous putative mediators have been proposed. In theory, exercise-induced cardioprotection could be achieved by any physiological adaptation that attenuates one or more of the damaging events that occur during ischemia and/or reperfusion. For example, exercise-induced cardioprotection could be acquired by changes in the coronary arteries (i.e., increased collateral circulation) and/or intrinsic changes in the cardiac myocyte. Potential intrinsic changes in the cardiac myocyte that could provide cellular protection against IR injury include increased glycolytic flux, altered nitric oxide (NO) signaling, increased levels of heat shock proteins (HSPs), amplified myocardial cyclooxygenase-2 (COX-2) activity, elevated endoplasmic reticulum (ER) stress proteins, enhanced function of sarcolemmal and/or mitochondrial ATP-sensitive potassium channels, increased cytosolic antioxidant capacity, and/or altered mitochondrial antioxidant capacity (FIGURE 4). An overview of the evidence to support or deny the role for each of these potential cardioprotective mediators is highlighted in the next segments.
FIGURE 4.
A list of proposed mediators of exercise-induced cardioprotection
See text for details.
Exercise-Induced Changes in Coronary Circulation Are Not Required for Cardioprotection
Endurance exercise-induced structural changes in the coronary circulation include increased conduit artery diameters and increased arteriolar densities and diameters (reviewed in Ref. 62). Specifically, in both young rats and humans, several months of endurance exercise increases the volume of the coronary vasculature because of increased conduit artery size (45, 68). Furthermore, regular aerobic exercise induces functional adaptations in the coronary circulation, including enhanced endothelium-dependent vasodilation (62). Although both of these training-induced adaptations within the coronary circulation could protect the heart during an IR event, at present, no experimental data exists to directly link these exercise-induced changes to short-term (e.g., 3–5 days) exercise-mediated cardioprotection. Indeed, a few consecutive days of exercise training can provide cardioprotection against IR injury (23, 42, 113), but structural changes in the coronary circulation do not occur within this short period of exercise training (118). Furthermore, evidence indicates that cardiac myocytes isolated from the heart of exercise-trained animals are protected against hypoxia-reoxygenation injury (55). Therefore, these results indicate that exercise can directly modify the cardiac myocyte to achieve a cardioprotective phenotype, and, consequently, a change in the coronary circulation is not required to achieve exercise-induced cardioprotection. Indeed, although exercise training has the potential to increase coronary flow during reperfusion (62), studies of isolated perfused hearts demonstrate that short-term exercise provides cardioprotection independent of improvements in coronary flow (11, 66, 93). Thus the remainder of this review will focus on exercise-induced endogenous changes in the cardiac myocyte that can contribute to cardioprotection.
Altered Glycolytic Flux as a Potential Mediator of Exercise-Induced Cardioprotection
Myocardial survival during an IR insult is dependent, at least in part, on cellular energy status. To limit the energy deficit during ischemia and hypoxia, cardiac energy production switches from the preferential use of fatty acids to carbohydrates; this switch in fuel utilization is essential to sustain ATP production via glycolysis (116). However, whether increased glycolytic flux is advantageous or detrimental during ischemia depends on the duration of the ischemic insult. Indeed, increased glycolysis in the ischemic heart is a double-edged sword since the accumulation of glycolytic end-products (e.g., lactate) during prolonged ischemia are detrimental to postischemic recovery (104). Hence, limiting glycolysis during long-duration myocardial ischemia could be a protective strategy to minimize IR injury (116). In this regard, it has been reported that PC results in a decrease in glycolytic flux in the ischemic heart (116). Similarly, evidence indicates that endurance exercise training decreases the rate of glycolysis in the rat heart during ischemia (14). Although the mechanism(s) by which exercise training alters the metabolic phenotype of the heart to produce this response is unknown, it is feasible that a reduction in glycolysis during ischemia could be cardioprotective. Nonetheless, to date, no direct evidence exists to mechanistically connect exercise-induced changes in myocardial glycolytic flux to cardioprotection.
Altered NO Signaling as a Facilitator of Exercise-Induced Cardioprotection
NO is produced in tissues from L-arginine, oxygen, and NADPH by NO synthase (NOS) enzymes (90). Numerous studies reveal that endurance exercise training results in increased phosphorylation and activity of endothelial NOS (eNOS) in both humans and animals (19, 38, 41). This exercise-induced rise in eNOS activity is associated with increased production of NO, as evidenced by augmented levels of both nitrite and nitrosothiols in tissue and blood. In this regard, nitrite is produced by the oxidation of NO in aerobic conditions (117), whereas nitrosothiols are formed when cysteine thiols in proteins are modified by NO via a process known as S-nitrosylation (27). It follows that circulating levels of nitrite and nitrosthiols are commonly used as biomarkers of NO availability (15). Importantly, nitrite is a potentially important storage form of NO in both blood and tissues because nitrite can be converted to NO by either acid reduction or nitrite reductases during ischemia (64).
A recent review summarized the possible role that NO metabolites play in the cardioprotective effects of exercise (15). In regard to the mechanisms linking NO to cardioprotection, increased NO levels can increase the S-nitrosylation of cardiac proteins during myocardial IR, which can attenuate injurious processes such as apoptosis (15). Indeed, caspase-3 activity can be inhibited via protein S-nitrosylation, and NO production of nitrosthiols can modify complex I of the mitochondrial electron transport chain, resulting in reduced mitochondrial ROS production during an IR event (15). Together, these changes could protect cardiac myocytes against IR-induced damage (117), and a recent study employing eNOS knockout mice concluded that increased production of NO is essential to achieve the cardioprotection associated with endurance exercise. However, it is also possible that some of the exercise-induced adaptations in the heart are not achieved in the eNOS knockout mouse. Indeed, a recent study reveals that several exercise-induced adaptations within the heart require eNOS expression (20). Therefore, additional experiments are required to confirm or deny whether NO or other NO metabolites are a requirement to achieve exercise-induced cardioprotection.
Elevated Myocardial Heat Shock Proteins Are Not Essential for Exercise-Induced Cardioprotection
It is clear that transgenic overexpression of heat shock protein 72 (HSP72) protects the heart against IR-induced injury (48, 51, 111). Furthermore, repeated bouts of endurance exercise result in a three- to fivefold increase in cardiac HSP72 levels (21, 44, 89, 113). In theory, elevated cellular levels of HSP72 can protect the myocardium against IR injury by augmenting myocardial antioxidant capacity, protecting mitochondria against IR injury, and preventing apoptosis (51, 109, 111).
Although exercise promotes the accumulation of HSP72 in the heart and HSP72 is cardioprotective, three independent studies demonstrate that an exercise-induced increase in HSP72 is not essential for exercise-induced cardioprotection (42, 95, 113). Therefore, although overexpression of HSP72 is sufficient to promote cardioprotection, increases in myocardial HSP72 are not a requirement to achieve exercise-induced cardioprotection.
Increased Myocardial Cyclooxygenase-2 is not Responsible for Exercise-Induced Cardioprotection
Cyclooxygenase-2 (COX-2) is the rate-limiting enzyme in prostaglandin biosynthesis catalyzing the conversion of arachidonic acid to prostaglandins. Over the past decade, COX-2 has emerged as an obligatory mediator of the late phase of ischemic preconditioning-induced cardioprotection, and it follows that COX-2 could also be a candidate molecule to explain exercise-induced cardioprotection (8, 10, 101).
The PC cardioprotective actions of COX-2 appear to spring from PGE2 and/or PGI2 production, since this elevated prostanoid production improves vasodilation within the coronary vasculature (1, 47). Nonetheless, several lines of evidence suggest that COX-2 is not required for exercise-induced cardioprotection. For example, exercise does not elevate COX-2 levels in the rat heart (94). Similarly, pharmacological inhibition of COX-2 does not prevent exercise-induced protection against IR-induced cardiac arrhythmias (78). Together, these findings suggest that an increase in COX-2 is not essential to achieve exercise-induced cardioprotection against IR injury.
Elevated Endoplasmic Reticulum Stress Proteins do not Contribute to Exercise-Mediated Cardioprotection
Endoplasmic reticulum (ER) stress proteins represent a group of cardioprotective proteins that could contribute to exercise-induced cardioprotection, since recent evidence indicates that ER stress contributes to IR-induced myocardial injury (80). Indeed, IR-induced ER dysfunction can promote both mitochondrial-dependent and -independent cell death resulting from a disturbance in calcium homeostasis and/or impaired protein folding (115).
Two proteins that could protect against ER stress are the glucose-regulated proteins Grp78 and Grp94. Both Grp78 and Grp94 function in ER protein folding and also exhibit calcium-binding properties, and overexpression of Grp94 and Grp78 can protect cardiomyocytes against both calcium overload and oxidative damage (115, 120, 121). Moreover, increased Grp78 and Grp94 expression is linked to a reduction in IR-induced necrosis and apoptosis in the heart (74). Nevertheless, exercise does not elevate Grp78 and Grp94 (76). Therefore, the existing evidence indicates that increased ER stress proteins are not a requirement for exercise-induced cardioprotection against IR injury.
Sarcolemmal and/or Mitochondrial ATP-Sensitive Potassium Channels May Participate in Exercise Trained-Mediated Cardioprotection
ATP-sensitive potassium channels located in the sarcolemma (sarcoKATP) and mitochondria (mitoKATP) of cardiac myocytes are important regulators of cardiac myocyte function (79, 84). Both sarcoKATP and mitoKATP channels are composed of two protein complexes, an inwardly rectifying potassium channel pore and an associated sulfonylurea receptor subunit that governs channel opening. These energy-sensing channels are named according to a classical understanding of their function, in which potassium ion currents are inhibited by an abundance of cellular ATP. Channel activation also occurs in response to other activating factors such as acute ischemia, increases in adenosine and MgADP, along with activation of protein kinase C-ε (PKC-ε) (99). Independent of the activating stimulus, opening either sarcoKATP or mitoKATP channels before an IR insult confers cardioprotection (40, 60). The means by which KATP channels confer cardioprotection are not fully understood. Nonetheless, it is clear that KATP channel opening prevents IR injury through multiple biochemical alterations within the ventricular myocyte. For instance, opening of sarcoKATP channels is predicted to protect against IR injury by shortening the cardiac action potential duration via the acceleration of phase 3 repolarization (40). Shortening the cardiac action potential could inhibit Ca2+ entry into the cell via L-type Ca2+ channels and avert Ca2+ overload (40). Furthermore, the slowing of depolarization could also reduce Ca2+ entry by avoiding the reversal of the Na+/Ca2+ exchanger. Together, these actions could protect the cardiac myocyte during IR by reducing the cytosolic Ca2+ overload. Another role that sarcoKATP channels play is triggering the opening of the mitoKATP channels (84). The specific details of how mitoKATP channels protect the heart against IR injury remains controversial, but it is believed that opening the mitoKATP channels protects mitochondria against IR-induced Ca2+ overload and damage (5, 40).
The role that sarcoKATP channels play in exercise-induced protection against IR injury has received limited investigative attention, but two studies suggest that endurance exercise training increases the expression of sarcoKATP channels in the cardiac myocyte (12, 122). Furthermore, other reports reveal that pharmacological blockage of the sarcoKATP channels impairs the exercise-induced protective benefits against IR-induced myocardial necrosis (12, 96). Nonetheless, because of concerns associated with the pharmacological inhibitors used in these studies, it is difficult to form a firm conclusion regarding the mechanistic role that sarcoKATP channels play in exercise-induced cardioprotection.
Finally, using pharmacological inhibitors of the MitoKATP channel, it appears that MitoKATP channel activation protects the heart against IR-induced ventricular arrhythmias (97) but does not protect against IR-induced infarction (12). Nonetheless, the inability to detect the molecular identity of the MitoKATP channel and concerns associated with the specificity of the channel blocker used in these experiments does not permit firm conclusions regarding the role that the MitoKATP channel plays in exercise-induced cardioprotection.
Increased Cytosolic Antioxidant Capacity May Play a Required Role in Exercise-Induced Cardioprotection
Although the etiology of cardiac IR injury involves numerous factors and varies as a function of the duration of ischemia, mitochondrial-produced ROS are significant contributors to the necrotic and apoptotic cell death following an IR insult (reviewed in Refs. 77, 91). There is abundant evidence that endurance exercise training increases several components of the antioxidant buffering system in the heart. In particular, many studies agree that endurance exercise increases SOD2, which is the SOD isoform located in the mitochondrial matrix (reviewed in Refs. 28, 56, 92). Another SOD isoform (SOD1) is located in both the cytosol and the mitochondrial intermembrane space (90). Emerging evidence indicates that exercise training also elevates SOD1 in the mitochondria (63). In contrast, the impact of exercise on the cytosolic isoform of SOD1 in the heart remains equivocal.
SOD is a first line of defense against superoxide in cells, and SOD-mediated dismutation of superoxide results in formation of the nonradical ROS hydrogen peroxide (H2O2). Cardiac myocytes are equipped to eliminate H2O2 via several routes, including the enzymatic removal by catalase, thioredoxins, and glutathione peroxidase (90). However, most studies report that the activities of catalase, thioredoxins, and glutathione peroxidase are not increased in the heart following exercise (28, 30, 31, 53, 97, 105, 112). Nonetheless, growing evidence suggests that exercise training increases the activity of glutathione reductase in the heart via posttranslational modifications (29, 30). An increase in glutathione reductase activity would amplify the heart's ability to replenish cardiac levels of glutathione that is required for glutathione peroxidase to remove H2O2. In this regard, a recent report concludes that increases in glutathione reductase activity play an essential role in exercise-induced cardioprotection (29). However, it is currently unclear whether this exercise-induced increase in glutathione reductase activity in cardiac myocytes is confined to the cytosolic compartment alone or whether glutathione reductase activity also increases in other cellular compartments such as the mitochondrion. Regardless of the cellular location of this enzyme, it appears likely that increases in myocardial glutathione reductase activity contribute to exercise-induced cardioprotection.
Exercise-Induced Alterations in Mitochondrial Proteins and Phenotype Are Central to Exercise-Induced Cardioprotection
Growing evidence reveals that endurance exercise training results in alterations in mitochondrial phenotype, and this adaptation is required to achieve exercise-induced cardioprotection. In the following segments, we discuss exercise-induced changes in mitochondrial phenotype and evaluate evidence that exercise-induced increases in key mitochondrial proteins are required to achieve cardioprotection against an IR insult.
Exercise alters mitochondrial phenotype in the heart.
Emerging evidence reveals that exercise induces a mitochondrial phenotype that resists apoptotic stimuli and IR-induced mitochondrial damage (6, 57, 63, 73, 105). Indeed, both subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria undergo biochemical adaptations in response to endurance exercise that lead to decreased apoptotic susceptibility (57, 58). For example, in vitro experiments using isolated cardiac mitochondria reveal that exercise training results in a mitochondrial phenotype that resists cytochrome c release from both SS and IMF mitochondria exposed to ROS and/or calcium challenges (58). The concept that exercise training results in a mitochondrial phenotype that resists IR-mediated damage is also supported by experiments using isolated cardiac mitochondria exposed to anoxia followed by reoxygenation. These studies reveal that, following anoxia-reoxygenation, state 3 respiration is better preserved in mitochondria isolated from the hearts of exercised rats (6). This finding was associated with attenuated oxidative damage to mitochondrial proteins and is in contrast to the severe metabolic dysfunction and oxidative damage observed in cardiac mitochondria isolated from sedentary animals.
Furthermore, a recent in vivo study employing a physiologically relevant experimental model provides more evidence that endurance exercise protects cardiac mitochondria from IR-induced damage (63). This work confirms that exercise training protects both SS and IMF mitochondria against IR-induced functional impairment and corroborates the concept that exercise training attenuates IR-induced increases in ROS release from both SS and IMF mitochondria. Moreover, this investigation establishes that exercise training also retards the IR-induced release of proapoptotic proteins from cardiac mitochondria (63). This observation may explain previous findings that exercise training is associated with attenuated cardiac apoptosis following IR insults in vivo (31, 95, 96). The next segment discusses the potential molecular mechanisms responsible for exercise-induced protection of cardiac mitochondria.
Exercise-induced alterations in mitochondrial proteins.
To understand the mechanisms by which exercise training alters mitochondrial phenotype to resist pro-apoptotic stimuli and stress-induced by hypoxia-reoxygenation, studies have investigated exercise-induced changes in mitochondrial protein expression (13, 57, 82, 88, 110). Collectively, these reports identify both increased and decreased expression of 21 different mitochondrial proteins in hearts from exercise-trained animals. These exercise-induced changes in mitochondrial proteins can be classified into five functional categories: 1) fatty acid metabolism; 2) amino acid metabolism; 3) mitochondrial respiratory chain/TCA cycle; 4) creatine kinase phospho-transfer circuit; and 5) redox balance. Increased expression of mitochondrial proteins involved in both fatty acid and amino acid metabolism could result in improved outcomes following IR (54, 72). Moreover, it is feasible that exercise-induced expression of mitochondrial respiratory chain/TCA cycle proteins can assist cardiac myocytes in maintaining energy homeostasis during IR insults (11, 106, 108). It is also possible that exercise-induced increases in mitochondrial creatine kinase could benefit the heart during an IR insult (26). In this regard, one study suggests short-term endurance training in humans increases the activity of mitochondrial creatine kinase in the heart (123). Nonetheless, at present, there is no direct evidence demonstrating cause and effect between cardioprotection and exercise-induced increases in proteins involved in fatty acid metabolism, amino acid metabolism, respiratory chain/TCA cycle function, and/or changes in mitochondrial creatine kinase activity.
Again, ROS play a major role in IR-induced cardiac injury, and much of the IR-induced ROS production in the heart is due to superoxide production in the mitochondria (91). Most of this IR-induced increase in superoxide is dismutated enzymatically by SOD1 and SOD2 located in the mitochondrial intermembrane space and matrix, respectively (90). However, dismutation of superoxide results in H2O2 that is converted to H2O and O2 by other antioxidant enzymes to avoid oxidative damage in cells (90). Importantly, studies report that exercise increases the protein abundance of several mitochondrial antioxidant enzymes including SOD1 and SOD2, along with the H2O2 removing enzymes glutathione peroxidase-1 and catalase (reviewed in Ref. 92). Hence, this exercise-induced fortification of these antioxidant enzymes has the potential to increase the removal of ROS produced in cardiac mitochondria.
Furthermore, exercise training decreases the expression of the mitochondrial enzyme monoamine oxidase A (MAO-A) in both SS and IMF mitochondria in the rat heart (57). This observation is important because MAO-A catalyzes the oxidative deamination of several monoamines, resulting in increased ROS production (85). Importantly, ROS production by MAO-A is a contributor to myocardial apoptosis during postischemia reperfusion (7, 85). Therefore, decreased mitochondrial levels of MAO-A in exercised hearts could be a beneficial adaptation that contributes to cardioprotection. Nonetheless, additional experiments are required to provide direct evidence for this postulate.
To summarize, endurance exercise training benefits the mitochondrion through increased expression of beneficial antioxidant proteins and decreased expression of proteins with potentially deleterious functions. Collectively, these changes improve mitochondrial capacity to produce ATP, eliminate ROS, and maintain a healthy mitochondrial redox balance. By extension, these exercise-induced changes result in a mitochondrial phenotype that is resistant to IR-induced damage. Direct evidence to support this prediction is highlighted in the next section.
Experimental evidence linking mitochondrial proteins to cardioprotection.
Again, endurance exercise training alters the expression of numerous mitochondrial genes, resulting in a resilient mitochondrial phenotype that is less sensitive to apoptotic stimuli and more resistant to IR-induced mitochondrial damage. The key question becomes: Which of these differentially expressed mitochondrial proteins are required contributors to exercise-induced cardioprotection? Although a complete answer to this question is not currently available, experimental evidence reveals that increased expression of mitochondrial SOD2 plays a major role in exercise-induced cardioprotection. For example, transgenic overexpression of SOD2 alone is sufficient to provide protection against IR-induced cardiac injury in mice (17). Furthermore, two independent studies have shown that exercise-induced expression of SOD2 in the heart plays a vital role in exercise-induced protection against IR-mediated myocardial infarction (118). Explicitly, using antisense oligonucleotides to prevent exercise-induced increased SOD2 expression in the heart, Yamashita et al. first demonstrated that preventing the exercise-induced increase in SOD2 expression abolished the cardioprotection normally associated with exercise (118). These findings were confirmed in a study demonstrating that prevention of exercise-induced increases in SOD2 greatly diminished the exercise-induced protection against IR-induced cardiac cell death due to both necrosis and apoptosis (31). Furthermore, although it is established that the primary regulation of SOD2 is through gene expression, deacetylation of SOD2 via Sirt3 increases SOD2 activity (16). The finding that exercise increases the protein levels of Sirt3 in the heart suggests that increases in Sirt3 may also play a role in exercise-induced cardioprotection via regulation of SOD2 activity (83).
After confirming that exercise-induced increases in myocardial SOD2 are important for exercise-induced cardioprotection, French et al. then investigated the downstream mechanisms by which increased myocardial SOD2 activity contributes to cardioprotection (31). This work reveals that exercise-induced increases in mitochondrial SOD2 protects against IR-induced myocardial infarction in part by attenuating IR-induced oxidative damage of calcium handling proteins and prevention of calpain activation (31). Indeed, an IR insult results in calpain activation in the cardiac myocyte, which promotes the degradation of four vital calcium handling proteins, including the sarcoplasmic reticulum calcium ATPase (SERCA2a), phospholamban, L-type calcium channels, and Na+/Ca2+ exchanger. Exercise training, performed before the IR insult, successfully protected the heart against IR-induced calpain activation and breakdown of these calcium handling proteins (32). Importantly, exercise-induced protection was lost when the exercise-stimulated increase in cardiac SOD2 was blocked using antisense oligonucleotides. Together, these studies demonstrate that an exercise-induced increase in SOD2 plays a key role in protection against IR-induced cardiac injury.
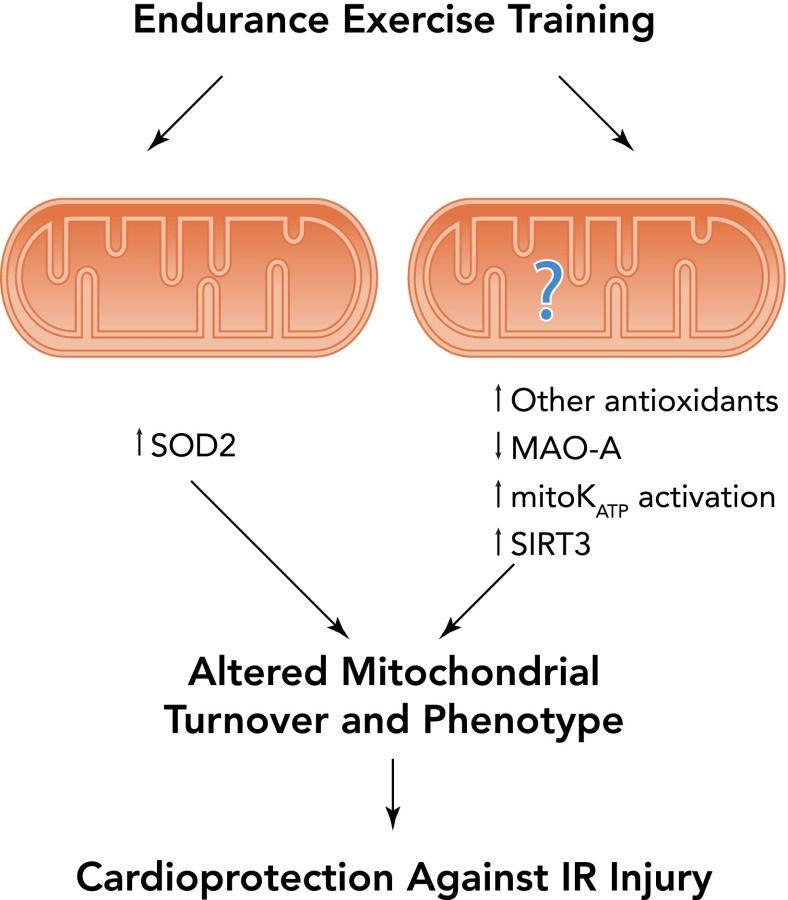
In synopsis, exercise training promotes the differential expression of numerous mitochondrial proteins, resulting in a mitochondrial phenotype that resists IR-induced injury (FIGURE 5). Present evidence suggests that an exercise-induced increase in SOD2 in cardiac mitochondria is essential to achieve the full benefit of exercise-induced cardioprotection. Furthermore, other exercise-induced mitochondrial proteins may also play a role in cardioprotection, but additional research is required to demonstrate cause and effect.
FIGURE 5.
Illustration of several exercise-induced mitochondrial alterations that promote cardioprotection against IR injury
Exercise increases mitochondrial levels of the important antioxidant enzyme superoxide dismutase 2 (SOD2). Exercise training could also increase the expression of mitochondrial ATP-sensitive potassium channels along with other mitochondrial proteins that could contribute to cardioprotection. MAO-A, monoamine oxidase; SIRT3, sirtuin 3; MitoKATP, mitochondrial potassium ATP-sensitive channel.
Conclusions and New Frontiers
Myocardial IR injury is a major cause of morbidity and mortality around the world, and, therefore, protecting the heart against IR injury is important. Currently, the only pragmatic method of providing sustainable cardioprotection against IR-induced myocardial injury is endurance exercise training, and regular participation in endurance exercise protects the heart against all levels of IR-induced injury.
The precise cellular adaptations responsible for exercise-induced cardioprotection remain a topic of debate. Nonetheless, there is evolving evidence that exercise training promotes alterations in mitochondrial protein expression and a change in mitochondrial phenotype that resists IR-induced damage. Furthermore, convincing evidence indicates that elevated myocardial SOD2 plays a major role in exercise-induced cardioprotection by protecting mitochondria and the cardiac myocyte against IR-induced oxidative damage. Moreover, it is feasible that other cardioprotective proteins exist in mitochondria that contribute to exercise training-induced cardioprotection. Moreover, there is reason to believe that both physiological and biochemical mechanisms exert synergistic protection in the exercised heart. Relative to the anti-arrhythmic protection afforded by elevated SOD and MitoKATP content of exercised ventricles, there is motive to suspect that autonomic changes associated with exercise may also contribute to the observed cardioprotection. This matter of synergistic protection against IR injury could help to explain clinical observations that exercise appears to be more protective than can be accounted for statistically using traditional risk factor analyses (52).
Although much has been learned about exercise-induced cardioprotection, many unanswered questions remain. For example, the role that posttranslational modifications play in exercise-induced cardioprotection against IR-injury is unknown. Indeed, posttranslational modifications to proteins such as phosphorylation, methylation, and acetylation are essential regulators of cell signaling pathways, and their contributions to cardioprotection are poorly understood (87).
Finally, another unanswered question regarding the mechanisms of exercise-induced cardioprotection relates to the impact of exercise training on mitochondrial turnover in cardiac myocytes. This type of mitochondrial quality control depends on the dynamic balance between mitophagy and mitochondrial biogenesis along with fission and fusion processes (37, 119). Theoretically, increased mitophagy of damaged mitochondria that produce high levels of ROS would benefit the exercised heart by leaving behind only the healthiest mitochondria. Therefore, these remaining mitochondria should have a higher threshold for opening of the mitochondrial permeability transition pore (37). This raises the question of “What role does mitochondrial quality in the cardiac myocyte play in cardioprotection?” Clearly, there is much more to be learned about the mechanisms responsible for exercise-induced cardioprotection against IR injury.
Footnotes
This work was supported by a grant from the National Heart, Lung, and Blood Institute (R01 HL-067855) awarded to S. K. Powers.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: S.K.P., A.J.S., A.N.K., and J.C.Q. conception and design of research; S.K.P., A.J.S., A.N.K., and J.C.Q. interpreted results of experiments; S.K.P., A.J.S., A.N.K., and J.C.Q. prepared figures; S.K.P., A.J.S., A.N.K., and J.C.Q. drafted manuscript; S.K.P., A.J.S., A.N.K., and J.C.Q. edited and revised manuscript; S.K.P., A.J.S., A.N.K., and J.C.Q. approved final version of manuscript.
References
- 1.Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem 274: 5038–5046, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19: 1088–1095, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14: 196–207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelos MG, Kutala VK, Torres CA, He G, Stoner JD, Mohammad M, Kuppusamy P. Hypoxic reperfusion of the ischemic heart and oxygen radical generation. Am J Physiol Heart Circ Physiol 290: H341–H347, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Ardehali H. Role of the mitochondrial ATP-sensitive K+ channels in cardioprotection. Acta Biochim Pol 51: 379–390, 2004 [PubMed] [Google Scholar]
- 6.Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA. Endurance training limits the functional alterations of rat heart mitochondria submitted to in vitro anoxia-reoxygenation. Int J Cardiol 109: 169–178, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 112: 3297–3305, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bolli R. Preconditioning: A paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 291: H19–H27, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79: 609–634, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res 55: 506–519, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowles DK, Starnes JW. Exercise training improves metabolic response after ischemia in isolated working rat heart. J Appl Physiol 76: 1608–1614, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol 569: 913–924, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budiono BP, See Hoe LE, Peart JN, Sabapathy S, Ashton KJ, Haseler LJ, Headrick JP. Voluntary running in mice beneficially modulates myocardial ischemic tolerance, signaling kinases, and gene expression patterns. Am J Physiol Regul Integr Comp Physiol 302: R1091–R1100, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Burelle Y, Wambolt RB, Grist M, Parsons HL, Chow JC, Antler C, Bonen A, Keller A, Dunaway GA, Popov KM, Hochachka PW, Allard MF. Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am J Physiol Heart Circ Physiol 287: H1055–H1063, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Calvert JW, Lefer DJ. Role of beta-adrenergic receptors and nitric oxide signaling in exercise-mediated cardioprotection. Physiology 28: 216–224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep 12: 534–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol 30: 2281–2289, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Coombes JS, Powers SK, Hamilton KL, Demirel HA, Shanely RA, Zergeroglu MA, Sen CK, Packer L, Ji LL. Improved cardiac performance after ischemia in aged rats supplemented with vitamin E and alpha-lipoic acid. Am J Physiol Regul Integr Comp Physiol 279: R2149–R2155, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Davis ME, Grumbach IM, Fukai T, Cutchins A, Harrison DG. Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J Biol Chem 279: 163–168, 2004 [DOI] [PubMed] [Google Scholar]
- 20.de Waard MC, van Haperen R, Soullie T, Tempel D, de Crom R, Duncker DJ. Beneficial effects of exercise training after myocardial infarction require full eNOS expression. J Mol Cell Cardiol 48: 1041–1049, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Demirel HA, Hamilton KL, Shanely RA, Tumer N, Koroly MJ, Powers SK. Age and attenuation of exercise-induced myocardial HSP72 accumulation. Am J Physiol Heart Circ Physiol 285: H1609–H1615, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Demirel HA, Powers SK, Caillaud C, Coombes JS, Naito H, Fletcher LA, Vrabas I, Jessup JV, Ji LL. Exercise training reduces myocardial lipid peroxidation following short-term ischemia-reperfusion. Med Sci Sports Exerc 30: 1211–1216, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, Naito H. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol 91: 2205–2212, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Doustar Y, Soufi FG, Jafary A, Saber MM, Ghiassie R. Role of four-week resistance exercise in preserving the heart against ischaemia-reperfusion-induced injury. Cardiovasc J Afr 23: 451–455, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downey JM. Free radicals and their involvement during long-term myocardial ischemia and reperfusion. Annu Rev Physiol 52: 487–504, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Dzeja PP, Hoyer K, Tian R, Zhang S, Nemutlu E, Spindler M, Ingwall JS. Rearrangement of energetic and substrate utilization networks compensate for chronic myocardial creatine kinase deficiency. J Physiol 589: 5193–5211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15: 391–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frasier CR, Moore RL, Brown DA. Exercise-induced cardiac preconditioning: how exercise protects your achy-breaky heart. J Appl Physiol 111: 905–915, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Frasier CR, Moukdar F, Patel HD, Sloan RC, Stewart LM, Alleman RJ, La Favor JD, Brown DA. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc Res 98: 47–55, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Frasier CR, Sloan RC, Bostian PA, Gonzon MD, Kurowicki J, Lopresto SJ, Anderson EJ, Brown DA. Short-term exercise preserves myocardial glutathione and decreases arrhythmias after thiol oxidation and ischemia in isolated rat hearts. J Appl Physiol 111: 1751–1759, 2011 [DOI] [PubMed] [Google Scholar]
- 31.French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J 22: 2862–2871, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.French JP, Quindry JC, Falk DJ, Staib JL, Lee Y, Wang KK, Powers SK. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol 290: H128–H136, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc Res 94: 168–180, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem 285: 28938–28945, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottlieb RA. Mitochondria and apoptosis. Biol Signals Recept 10: 147–161, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb RA. Mitochondrial signaling in apoptosis: mitochondrial daggers to the breaking heart. Basic Res Cardiol 98: 242–249, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb RA, Mentzer RM., Jr Cardioprotection through autophagy: ready for clinical trial? Autophagy 7: 434–435, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol 285: H921–H930, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, Mehta JL. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol 281: H1346–H1352, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Hamilton KL, Quindry JC, French JP, Staib J, Hughes J, Mehta JL, Powers SK. MnSOD antisense treatment and exercise-induced protection against arrhythmias. Free Radic Biol Med 37: 1360–1368, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Hamilton KL, Staib JL, Phillips T, Hess A, Lennon SL, Powers SK. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med 34: 800–809, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Haskell WL, Sims C, Myll J, Bortz WM, St Goar FG, Alderman EL. Coronary artery size and dilating capacity in ultradistance runners. Circulation 87: 1076–1082, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Hoshida S, Yamashita N, Otsu K, Hori M. Repeated physiologic stresses provide persistent cardioprotection against ischemia-reperfusion injury in rats. J Am Coll Cardiol 40: 826–831, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem 275: 11397–11403, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Hutter JJ, Mestril R, Tam EK, Sievers RE, Dillmann WH, Wolfe CL. Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation 94: 1408–1411, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Ignarro LJ, Balestrieri ML, Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res 73: 326–340, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Inserte J, Hernando V, Garcia-Dorado D. Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovasc Res 96: 23–31, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Latif N, Abunasra H, Murtuza B, Amrani M, Yacoub MH. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation 104: 303–307, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587: 5551–5558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol 289: R1564–R1572, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Julia P, Young HH, Buckberg GD, Kofsky ER, Bugyi HI. Studies of myocardial protection in the immature heart. II. Evidence for importance of amino acid metabolism in tolerance to ischemia. J Thorac Cardiovasc Surg 100: 888–895, 1990 [PubMed] [Google Scholar]
- 55.Kang PM, Yue P, Liu Z, Tarnavski O, Bodyak N, Izumo S. Alterations in apoptosis regulatory factors during hypertrophy and heart failure. Am J Physiol Heart Circ Physiol 287: H72–H80, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Kavazis AN. Exercise preconditioning of the myocardium. Sports Med 39: 923–935, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Kavazis AN, Alvarez S, Talbert E, Lee Y, Powers SK. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ Physiol 297: H144–H152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294: H928–H935, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Kloner RA, Hale SL, Dai W, Gorman RC, Shuto T, Koomalsingh KJ, Gorman JH, 3rd, Sloan RC, Frasier CR, Watson CA, Bostian PA, Kypson AP, Brown DA. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective Peptide. J Am Heart Assoc 1: e001644, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kong X, Tweddell JS, Gross GJ, Baker JE. Sarcolemmal and mitochondrial K(ATP)channels mediate cardioprotection in chronically hypoxic hearts. J Mol Cell Cardiol 33: 1041–1045, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 111: 1208–1221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol 302: H10–H23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee Y, Min K, Talbert EE, Kavazis AN, Smuder AJ, Willis WT, Powers SK. Exercise protects cardiac mitochondria against ischemia-reperfusion injury. Med Sci Sports Exerc 44: 397–405, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Lefer DJ. Nitrite therapy for protection against ischemia-reperfusion injury. Am J Physiol Renal Physiol 290: F777–F778, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lennon SL, Quindry J, Hamilton KL, French J, Staib J, Mehta JL, Powers SK. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol 96: 1299–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Lennon SL, Quindry JC, French JP, Kim S, Mehta JL, Powers SK. Exercise and myocardial tolerance to ischaemia-reperfusion. Acta Physiol Scand 182: 161–169, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Leon AS, Bloor CM. Effects of exercise and its cessation on the heart and its blood supply. J Appl Physiol 24: 485–490, 1968 [DOI] [PubMed] [Google Scholar]
- 69.Libonati JR, Gaughan JP, Hefner CA, Gow A, Paolone AM, Houser SR. Reduced ischemia and reperfusion injury following exercise training. Med Sci Sports Exerc 29: 509–516, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Libonati JR, Kendrick ZV, Houser SR. Sprint training improves postischemic, left ventricular diastolic performance. J Appl Physiol 99: 2121–2127, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics: 2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Lofgren B, Povlsen JA, Rasmussen LE, Stottrup NB, Solskov L, Krarup PM, Kristiansen SB, Botker HE, Nielsen TT. Amino acid transamination is crucial for ischaemic cardioprotection in normal and preconditioned isolated rat hearts: focus on L-glutamate. Exp Physiol 95: 140–152, 2010 [DOI] [PubMed] [Google Scholar]
- 73.Marcil M, Bourduas K, Ascah A, Burelle Y. Exercise training induces respiratory substrate-specific decrease in Ca2+-induced permeability transition pore opening in heart mitochondria. Am J Physiol Heart Circ Physiol 290: H1549–H1557, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res 98: 1186–1193, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Miller LE, Hosick PA, Wrieden J, Hoyt E, Quindry JC. Evaluation of arrhythmia scoring systems and exercise-induced cardioprotection. Med Sci Sports Exerc 44: 435–441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murlasits Z, Lee Y, Powers SK. Short-term exercise does not increase ER stress protein expression in cardiac muscle. Med Sci Sports Exerc 39: 1522–1528, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagy O, Hajnal A, Parratt JR, Vegh A. Delayed exercise-induced protection against arrhythmias in dogs: effect of celecoxib. Eur J Pharmacol 499: 197–199, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148, 1983 [DOI] [PubMed] [Google Scholar]
- 80.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110: 705–712, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal 19: 400–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Padrao AI, Ferreira R, Vitorino R, Alves RM, Figueiredo P, Duarte JA, Amado F. Effect of lifestyle on age-related mitochondrial protein oxidation in mice cardiac muscle. Eur J Appl Physiol 112: 1467–1474, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Milano) 1: 771–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patel HH, Gross ER, Peart JN, Hsu AK, Gross GJ. Sarcolemmal KATP channel triggers delayed ischemic preconditioning in rats. Am J Physiol Heart Circ Physiol 288: H445–H447, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res 100: 41–49, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann NY Acad Sci 1201: 183–188, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Porter K, Medford HM, McIntosh CM, Marsh SA. Cardioprotection requires flipping the ‘posttranslational modification’ switch. Life Sci 90: 89–98, 2012 [DOI] [PubMed] [Google Scholar]
- 88.Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL, Herb RA. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol Heart Circ Physiol 265: H2094–H2098, 1993 [DOI] [PubMed] [Google Scholar]
- 89.Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, Shanely RA, Jessup J. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol Regul Integr Comp Physiol 275: R1468–R1477, 1998 [DOI] [PubMed] [Google Scholar]
- 90.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Powers SK, Murlasits Z, Wu M, Kavazis AN. Ischemia-reperfusion-induced cardiac injury: a brief review. Med Sci Sports Exerc 39: 1529–1536, 2007 [DOI] [PubMed] [Google Scholar]
- 92.Powers SK, Quindry JC, Kavazis AN. Exercise-induced cardioprotection against myocardial ischemia-reperfusion injury. Free Radic Biol Med 44: 193–201, 2008 [DOI] [PubMed] [Google Scholar]
- 93.Quindry J, French J, Hamilton K, Lee Y, Mehta JL, Powers S. Exercise training provides cardioprotection against ischemia-reperfusion induced apoptosis in young and old animals. Exp Gerontol 40: 416–425, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Quindry JC, French J, Hamilton KL, Lee Y, Selsby J, Powers S. Exercise does not increase cyclooxygenase-2 myocardial levels in young or senescent hearts. J Physiol Sci 60: 181–186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quindry JC, Hamilton KL, French JP, Lee Y, Murlasits Z, Tumer N, Powers SK. Exercise-induced HSP-72 elevation and cardioprotection against infarct and apoptosis. J Appl Physiol 103: 1056–1062, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Quindry JC, Miller L, McGinnis G, Kliszczewicz B, Irwin JM, Landram M, Urbiztondo Z, Nanayakkara G, Amin R. Ischemia reperfusion injury, KATP channels, and exercise-induced cardioprotection against apoptosis. J Appl Physiol 113: 498–506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quindry JC, Schreiber L, Hosick P, Wrieden J, Irwin JM, Hoyt E. Mitochondrial KATP channel inhibition blunts arrhythmia protection in ischemic exercised hearts. Am J Physiol Heart Circ Physiol 299: H175–H183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reeve JL, Duffy AM, O'Brien T, Samali A. Don't lose heart: therapeutic value of apoptosis prevention in the treatment of cardiovascular disease. J Cell Mol Med 9: 609–622, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodrigo GC, Standen NB. ATP-sensitive potassium channels. Curr Pharm Des 11: 1915–1940, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr Cardiol 32: 275–281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA 97: 10197–10202, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siems W, Capuozzo E, Lucano A, Salerno C, Crifo C. High sensitivity of plasma membrane ion transport ATPases from human neutrophils towards 4-hydroxy-2,3-trans-nonenal. Life Sci 73: 2583–2590, 2003 [DOI] [PubMed] [Google Scholar]
- 103.Soufi FG, Saber MM, Ghiassie R, Alipour M. Role of 12-week resistance training in preserving the heart against ischemia-reperfusion-induced injury. Cardiol J 18: 140–145, 2011 [PubMed] [Google Scholar]
- 104.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res 34: 25–33, 1997 [DOI] [PubMed] [Google Scholar]
- 105.Starnes JW, Barnes BD, Olsen ME. Exercise training decreases rat heart mitochondria free radical generation but does not prevent Ca2+-induced dysfunction. J Appl Physiol 102: 1793–1798, 2007 [DOI] [PubMed] [Google Scholar]
- 106.Starnes JW, Beyer RE, Edington DW. Myocardial adaptations to endurance exercise in aged rats. Am J Physiol Heart Circ Physiol 245: H560–H566, 1983 [DOI] [PubMed] [Google Scholar]
- 107.Starnes JW, Choilawala AM, Taylor RP, Nelson MJ, Delp MD. Myocardial heat shock protein 70 expression in young and old rats after identical exercise programs. J Gerontol A Biol Sci Med Sci 60: 963–969, 2005 [DOI] [PubMed] [Google Scholar]
- 108.Starnes JW, Rumsey WL. Cardiac energetics and performance of exercised and food-restricted rats during aging. Am J Physiol Heart Circ Physiol 254: H599–H608, 1988 [DOI] [PubMed] [Google Scholar]
- 109.Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, Anderson RL. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem 279: 51490–51499, 2004 [DOI] [PubMed] [Google Scholar]
- 110.Sun B, Wang JH, Lv YY, Zhu SS, Yang J, Ma JZ. Proteomic adaptation to chronic high intensity swimming training in the rat heart. Comp Biochem Physiol D Genomics Proteomics 3: 108–117, 2008 [DOI] [PubMed] [Google Scholar]
- 111.Suzuki K, Murtuza B, Sammut IA, Latif N, Jayakumar J, Smolenski RT, Kaneda Y, Sawa Y, Matsuda H, Yacoub MH. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation 106: 270–276, 2002 [PubMed] [Google Scholar]
- 112.Taylor RP, Ciccolo JT, Starnes JW. Effect of exercise training on the ability of the rat heart to tolerate hydrogen peroxide. Cardiovasc Res 58: 575–581, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Taylor RP, Harris MB, Starnes JW. Acute exercise can improve cardioprotection without increasing heat shock protein content. Am J Physiol Heart Circ Physiol 276: H1098–H1102, 1999 [DOI] [PubMed] [Google Scholar]
- 114.Taylor RP, Olsen ME, Starnes JW. Improved postischemic function following acute exercise is not mediated by nitric oxide synthase in the rat heart. Am J Physiol Heart Circ Physiol 292: H601–H607, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabo R, Di Lisa F, Gorza L. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J 17: 923–925, 2003 [DOI] [PubMed] [Google Scholar]
- 116.Vogt AM, Poolman M, Ackermann C, Yildiz M, Schoels W, Fell DA, Kubler W. Regulation of glycolytic flux in ischemic preconditioning. A study employing metabolic control analysis. J Biol Chem 277: 24411–24419, 2002 [DOI] [PubMed] [Google Scholar]
- 117.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA 101: 13683–13688, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med 189: 1699–1706, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev 40: 159–164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang LQ, Zhang XQ, Ng YC, Rothblum LI, Musch TI, Moore RL, Cheung JY. Sprint training normalizes Ca2+ transients and SR function in postinfarction rat myocytes. J Appl Physiol 89: 38–46, 2000 [DOI] [PubMed] [Google Scholar]
- 121.Zhang PL, Lun M, Teng J, Huang J, Blasick TM, Yin L, Herrera GA, Cheung JY. Preinduced molecular chaperones in the endoplasmic reticulum protect cardiomyocytes from lethal injury. Ann Clin Lab Sci 34: 449–457, 2004 [PubMed] [Google Scholar]
- 122.Zingman LV, Zhu Z, Sierra A, Stepniak E, Burnett CM, Maksymov G, Anderson ME, Coetzee WA, Hodgson-Zingman DM. Exercise-induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation. J Mol Cell Cardiol 51: 72–81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zoll J, N'Guessan B, Ribera F, Lampert E, Fortin D, Veksler V, Bigard X, Geny B, Lonsdorfer J, Ventura-Clapier R, Mettauer B. Preserved response of mitochondrial function to short-term endurance training in skeletal muscle of heart transplant recipients. J Am Coll Cardiol 42: 126–132, 2003 [DOI] [PubMed] [Google Scholar]
- 124.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res 70: 181–190, 2006 [DOI] [PubMed] [Google Scholar]